Abstract
Fetal aortic balloon valvuloplasty (FAV) has shown promise in altering in utero progression of aortic stenosis to hypoplastic left heart syndrome. In patients who achieve a biventricular circulation after FAV, left ventricular (LV) compliance may be impaired. Echocardio-graphic indexes of diastolic function were compared between patients with biventricular circulation after FAV, congenital aortic stenosis (AS), and age-matched controls. In the neonatal period, patients with FAV had similar LV, aortic, and mitral valve dimensions but more evidence of endocardial fibroelastosis than patients with AS. Patients with FAV underwent more postnatal cardiac interventions than patients with AS (p = 0.007). Mitral annular early diastolic tissue velocity (E′) was lower in patients with FAV and those with AS and controls in the neonatal period and over follow-up (p <0.001). Septal E′ was similar among all 3 groups in the neonatal period. In follow-up patients, with FAV had lower septal E′ than patients with AS or controls (p <0.001). Early mitral inflow velocity/E′ was higher in patients with FAV as neonates and at follow-up (p <0.001). Mitral inflow pulse-wave Doppler-derived indexes of diastolic function were similar between groups. In conclusion, echocardiographic evidence of LV diastolic dysfunction is common in patients with biventricular circulation after FAV and persists in short-term follow-up. LV diastolic dysfunction in this unique population may have important implications on long-term risk of left atrial and subsequent pulmonary hypertension.
Fetal aortic balloon valvuloplasty (FAV) has shown promise in altering in utero progression of aortic stenosis (AS) to hypoplastic left heart syndrome.1–4 The postnatal course including size of the left-sided heart structures, left ventricular (LV) systolic function, and surgical management is variable in patients who have undergone FAV.2 Recent studies have reported promising results, with 35% to 40% of patients achieving a biventricular circulation.2,4 In children with other left heart obstructive lesions including congenital AS and aortic coarctation, LV diastolic dysfunction is common.5–8 However, LV diastolic function in patients who have undergone FAV has not been evaluated. This report describes echocardiographic indexes of LV diastolic function in patients with a biventricular circulation after FAV and compares them to patients with isolated congenital AS and to normal controls.
Methods
Records of all patients at our institution who underwent technically successful FAV for evolving hypoplastic left heart syndrome from January 2005 through July 2009 were reviewed. The technique for FAV and short-term clinical outcomes have been previously reported.1,9,10 Technically successful FAV was defined as FAV in which the aortic valve was crossed and a balloon inflated with clear evidence of increased flow across the valve by color Doppler. Only patients with a biventricular circulation at latest follow-up were included to minimize the effects of variable loading conditions. A biventricular circulation was defined as circulation in which the left ventricle was the sole source of systemic output with no intracardiac shunting except for an atrial septal defect or patent foramen ovale. To construct the infant AS cohort, we included patients diagnosed postnatally with congenital AS and who underwent balloon aortic valvuloplasty within the first 2 months of life from January 2005 through July 2009. Patients were excluded from the AS cohort if they had associated congenital heart disease except for aortic coarctation or if the LV was not apex forming. In the FAV and AS groups we included only patients who had complete assessment of diastolic function as defined later. Echocardiograms from age-matched patients (matched to FAV patients) with no structural or functional heart disease were used as controls. Separate control groups were selected for the neonatal period and follow-up age period (median age 23 months). Baseline patient characteristics, echocardio-graphic variables, and clinical course including cardiac interventions were collected and analyzed for each group. The committee for clinical investigation at Children's Hospital Boston approved the use of patient medical records for this retrospective review.
The first complete postnatal echocardiogram for each patient with FAV or AS was included in analysis of neonatal anatomic variables. All patients underwent complete echocardiography with 2-dimensional, spectral Doppler, and color flow Doppler analyses before the first postnatal intervention. LV variables and z scores for these variables (end-diastolic volume, long-axis dimension, mass, mass/volume, ejection fraction) and aortic and mitral valve dimensions and z scores were collected and compared. The first postnatal echocardiogram for each patient with FAV and with AS was reviewed and presence of endocardial fibroelastosis was qualitatively assessed.
Indexes of diastolic function from the first postnatal echocardiogram that included tissue Doppler imaging and from the most recent follow-up echocardiogram with tissue Doppler imaging were collected and included in the analysis. Some patients in the 2 groups had tissue Doppler imaging data only in the neonatal period or at a follow-up visit and were included in the analysis of that age group only. Conventional pulse-wave Doppler indexes of diastolic function including peak early (E) and late (A) diastolic transmitral velocities, E/A ratio, A-wave duration, and E-wave deceleration time were measured from the spectral Doppler signal of the mitral valve inflow. Pulse-wave tissue Doppler imaging velocities were obtained from the lateral mitral annulus and the interventricular septum from the apical 4-chamber view. Tissue Doppler imaging measurements for each of the myocardial segments included peak early diastolic velocity (E′) and peak late diastolic velocity (A′). Only tracings that demonstrated a clear E′ were used. All measurements of diastolic variables were retrospectively remeasured by a single echocardiographer (K.F.) from images obtained at the time of the study. Each tissue Doppler imaging velocity was measured on 3 consecutive cardiac cycles and the average of these values was used for analysis. All examinations were performed using commercially available ultrasound equipment (Philips iE33, Koninklijke Philips Electronics, Netherlands).
Baseline anatomic variables for the AS and FAV groups are reported as median (range) and count for continuous and categorical variables, respectively. These 2 groups were compared using Wilcoxon rank-sum test and Fisher's exact test, as appropriate. Mean ± SD was used to describe mitral inflow and tissue Doppler imaging values because they were normally distributed. One-way analysis of variance was used to compare mean diastolic function parameters among the 3 groups during the neonatal and follow-up periods separately. Post hoc pairwise comparisons were performed with Bonferroni correction and Tamhane test to control for multiple comparisons and heterogeneity in variances across groups. Because tissue Doppler imaging values normally vary with age, we modeled the relation between tissue Doppler imaging values and age using linear regression with generalized estimating equation models to account for correlation between longitudinal measurements for each patient. In each generalized estimating equation model, 2 data points, the tissue Doppler imaging value from the initial neonatal echocardiogram and the value from the most recent echocardiogram, were included. All statistical analysis were 2-sided and type I error was controlled at a level of 0.05. Analyses were performed with SPSS 16.0 (SPSS, Inc., Chicago, Illinois) and STATA 10.1 (STATA Corporation, College Station, Texas).
Results
From January 2005 through July 2009 43 patients underwent FAV at our institution. Nineteen of these had a biventricular circulation at most recent follow-up. Eighteen of the 19 patients had adequate echocardiographic data to be included in analysis. Median age at FAV was 23 weeks of gestation (range 21 to 29). For the congenital AS cohort, 33 patients were identified, 19 of whom had sufficient echo-cardiographic data to be included in the analysis. Median age at aortic valvuloplasty in the AS cohort was 5 days (range 0 to 60), with 8 patients having critical AS.
Comparison of neonatal (preintervention) anatomic variables between patients with FAV and those with AS is presented in Table 1. Median sizes of left heart structures were similar and generally within normal range in the 2 groups except for smaller aortic valve dimension in patients with FAV. Patients with FAV had worse LV systolic function and were more likely to have echocardiographic evidence of endocardial fibroelastosis. In general, the FAV group underwent more postnatal cardiac interventions than the AS group (p = 0.007; Table 2), with a similar median follow-up duration (23 months). Repeat transcatheter aortic valvuloplasty, surgical mitral valve interventions, and endocardial fibroelastosis resection were common in patients with FAV.
Table 1.
Neonatal echocardiographic variables
| Variable | FAV (n = 18) | AS (n = 19) | p Value |
|---|---|---|---|
| Aortic valve (cm) | 0.56 (0.35–0.83) | 0.59 (0.42–0.76) | 0.35 |
| Aortic valve z score | −2.4 (−4.8 to 0.1) | −1.8 (−2.5 to 0.8) | 0.25 |
| Mitral valve lateral dimension | 0.8 (0.7–1.3) | 0.9 (0.8–1.3) | 0.14 |
| Mitral valve lateral dimension z score | −1.6 (−3.0 to −2.6) | −1.2 (−2.5 to 1.9) | 0.34 |
| Left ventricular diastolic volume z score | −0.8 (−2.6 to 6.6) | −1.6 (−2.5 to 2.8) | 0.63 |
| Left ventricular long axis dimension z score | −0.7 (−2.7 to 1.8) | 0.2 (−4.6 to 3.2) | 0.06 |
| Left ventricular mass (g) | 8.6 (4.1–14.6) | 9.0 (4.3–24.4) | 0.91 |
| Left ventricular mass z score | 0.2 (−2.0 to 1.5) | 1.1 (−2.7 to 4.9) | 0.19 |
| Left ventricular mass/volume | 1.1 (0.8–1.8) | 1.2 (0.6–4.6) | 0.15 |
| Left ventricular mass/volume z score | 1.1 (−0.8 to 6.0) | 2.0 (−1.6 to 5.2) | 0.19 |
| Left ventricular ejection fraction (%) | 40 (18–71) | 56 (10–70) | 0.03 |
| Peak aortic stenosis gradient (mm Hg) | 30 (0–93) | 57 (42–111) | 0.04 |
| Aortic regurgitation grade | 0 (0–3+) | 0 (0–2+) | 0.78 |
| Endocardial fibroelastosis | 17 (94%) | 5 (26%) | <0.01 |
Values are expressed as median (range) or number of patients (percentage).
Table 2.
Postnatal interventions
| Variable | FAV (n = 18) | AS (n = 19) |
|---|---|---|
| Number of postnatal interventions* | 3 (1–8) | 1 (1–3) |
| Neonatal aortic valvuloplasty | 17 (95%) | 19 (100%) |
| Repeat aortic valvuloplasty | 14 (78%) | 6 (32%) |
| Endocardial fibroelastosis resection | 13 (72%) | 0 (0%) |
| Mitral valvuloplasty or replacement | 9 (50%) | 1 (5%) |
| Aortic valve replacement or Ross | 7 (35%) | 2 (11%) |
Surgical or percutaneous.
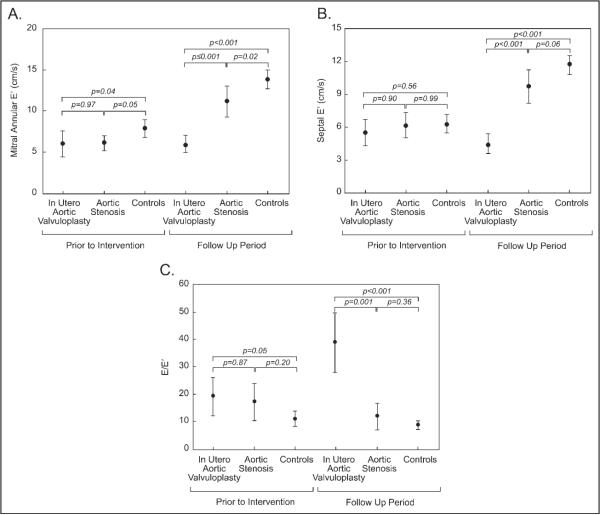
In the neonatal period, patients with FAV and with congenital AS had lower mitral annular E′ values than controls (Figure 1), whereas septal E′ values were similar between groups. Early mitral inflow pulse-wave Doppler velocity/early diastolic mitral annular tissue velocity ratio (E/E′) was higher in the FAV group than controls in the neonatal period.
Figure 1.
Error bar plots comparing mean diastolic properties (central dots) and 95% confidence interval (whiskers) among groups in the neonatal period and follow-up period. (A) Mitral annular early diastolic tissue velocity Doppler (before intervention p = 0.01, follow-up period p <0.001, analysis of variance). (B) Septal early diastolic tissue velocity (before intervention p = 0.18, follow-up period p <0.001, analysis of variance). (C) Early diastolic mitral inflow velocity/early diastolic tissue velocity (before intervention p = 0.05, follow-up period p <0.001, analysis of variance). The p values in the diagram represent post hoc pairwise comparisons with Bonferroni correction or Tamhane test.
On follow-up echocardiograms, mitral annular E′ and septal E′ values were lower in patients with FAV than in patients with congenital AS and controls (p <0.001; Figure 1). E/E′ was higher in patients with FAV than in patients with congenital AS or controls (p = 0.001).
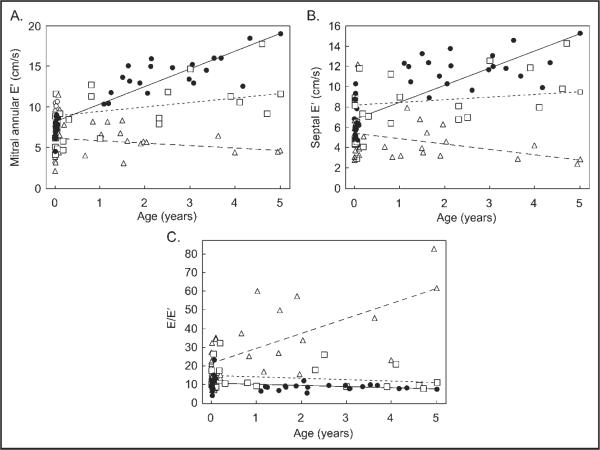
The relationship between age and diastolic function indexes (mitral annular E′, septal E′, and E/E′) for each group are shown in Figure 2. Mitral annular E′ increased with age in patients with congenital AS (p <0.001) and controls (p = 0.002), whereas in patients with FAV mitral annular E′ did not change with age (p = 0.16). Generalized estimating equation modeling demonstrated differences in slopes of regression lines for mitral annular E′ values between patient groups (p <0.001). Septal E′ values increased with age in controls (p <0.001) and patients with AS (p = 0.05), whereas in patients with FAV septal E′ values decreased with age (p = 0.01). Slopes of the regression line for septal E differed among the 3 groups (p <0.001). E/E′ values decreased slightly with age in controls (p <0.03), did not significantly change in patients with AS, and increased in the FAV group (p = 0.02).
Figure 2.
Scatter plots showing tissue Doppler imaging diastolic variable versus age in controls (circles), patients with fetal aortic valvuloplasty (triangles), and patients with aortic stenosis (squares). (A) Mitral annular early diastolic tissue velocity. (B) Septal early diastolic tissue velocity. (C) Early diastolic mitral inflow velocity/early diastolic tissue velocity. A generalized estimating equation regression line (straight lines) was determined for each group. For each plot the slope of the line representing patients with fetal aortic valvuloplasty differed (p <0.01) from patients with aortic stenosis and controls.
Evaluation of pulse-wave Doppler mitral inflow parameters showed no differences between groups in E/A ratio, proportion of patients with monophasic mitral inflow, A-wave velocity, or A-wave duration in the neonatal or follow-up period.
Discussion
In this study we evaluated LV diastolic function in patients with a biventricular circulation after FAV and compared them to patients diagnosed with AS in infancy and to controls. At latest follow-up (median age 23 months) FAV patients had lower mitral annular E′, lower septal E′, and higher E/E′ than patients with AS or controls, which are suggestive of LV diastolic dysfunction. In adults considerable data exist demonstrating the value of tissue Doppler indexes, primarily E′ and E/E′, as relatively load independent measurements of LV diastolic function and correlates of left atrial pressure with higher tissue velocity and a lower E/E′, respectively, indicating better diastolic function and lower left atrial pressure.11
In the FAV group significant abnormalities in LV diastolic function indexes were present in the neonatal period and through the follow-up period. In contrast, tissue Doppler imaging indexes of diastolic function improved over time in patients with congenital AS. Persistent LV diastolic dysfunction in patients who have undergone FAV may have important clinical and management implications. Diastolic dysfunction places these patients at increased risk for developing left atrial and pulmonary hypertension. As the FAV cohort reaches its second decade of life, long-term clinical follow-up will establish if these diastolic function abnormalities lead to progressive symptoms or significantly decreased exercise capacity.
Indexes of diastolic function improved over time in patients with congenital AS after relief of pressure load on the left ventricle, although they remained worse than controls. Previous studies have described improvement in diastolic function after relief of pressure load in several types of left heart obstructive lesions in adults and children.5,6,12–17 The extent of myocyte hypertrophy has been shown to play an important role in the mechanism of impaired LV early diastolic filling in adults and children with AS.18 In addition to hypertrophy, myocardial fibrosis contributes to diastolic dysfunction in many types of left heart obstructive lesions.19,20 Myocardial fibrosis has been demonstrated histopathologically and using cardiac magnetic resonance imaging in adults with severe aortic valve disease and has been implicated as a cause of diastolic dysfunction.17,21,22 In patients with hypoplastic left heart syndrome and those who have undergone FAV for evolving hypoplastic left heart syndrome, endocardial fibroelastosis is common.23–25 Endocardial fibroelastosis likely has an influence on diastolic function in its own right and may correlate with myocardial fibrosis.15 A possible explanation for the persistence of diastolic dysfunction despite normal LV mass in the FAV cohort is that myocardial fibrosis and endocardial fibroelastosis, rather than hypertrophy, are the primary mechanisms.
The success of FAV in altering many aspects of hypoplastic left heart syndrome progression supports the hypothesis that there is plasticity in left heart growth and function. However, our data suggest that there may be a component of endocardial and myocardial damage and fibrosis that is less reversible. Fibrotic changes in the fetal myocardium and endocardium likely occur early in utero because of the flow aberrations leading to altered loading conditions and subendocardial ischemia.21,26,27 Extent of myocardial fibrosis and its clinical importance and prognostic value in patients who have undergone FAV and with congenital AS have not been well defined. Cardiac magnetic resonance imaging may be helpful in quantifying degree of myocardial fibrosis and play a role in determining which patients are most likely to progress successfully to biventricular circulation.21,22,28
This study should be viewed as an exploratory investigation because of limitations, including small cohort and potential selection bias introduced because of lack of universal collection of tissue Doppler imaging data. Since 2005, 60% of patients with AS and 95% of patients with FAV had echocardiograms with complete assessment of diastolic function. In addition, our results may be partly confounded by the significant difference in number and type of postnatal interventions between patients with AS and those with FAV.
References
- 1.Marshall AC, Tworetzky W, Bergersen L, McElhinney DB, Benson CB, Jennings RW, Wilkins-Haug LE, Marx GR, Lock JE. Aortic valvuloplasty in the fetus: technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535–539. doi: 10.1016/j.jpeds.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 2.McElhinney DB, Marshall AC, Wilkins-Haug LE, Brown DW, Benson CB, Silva V, Marx GR, Mizrahi-Arnaud A, Lock JE, Tworetzky W. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120:1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selamet Tierney ES, Wald RM, McElhinney DB, Marshall AC, Benson CB, Colan SD, Marcus EN, Marx GR, Levine JC, Wilkins-Haug L, Lock JE, Tworetzky W. Changes in left heart hemodynamics after technically successful in-utero aortic valvuloplasty. Ultrasound Obstet Gynecol. 2007;30:715–720. doi: 10.1002/uog.5132. [DOI] [PubMed] [Google Scholar]
- 4.McElhinney DB, Tworetzky W, Lock JE. Current status of fetal cardiac intervention. Circulation. 2010;121:1256–1263. doi: 10.1161/CIRCULATIONAHA.109.870246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eidem BW, McMahon CJ, Ayres NA, Kovalchin JP, Denfield SW, Altman CA, Bezold LI, Pignatelli RH. Impact of chronic left ventricular preload and afterload on Doppler tissue imaging velocities: a study in congenital heart disease. J Am Soc Echocardiogr. 2005;18:830–838. doi: 10.1016/j.echo.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Lam YY, Kaya MG, Li W, Gatzoulis MA, Henein MY. Effect of chronic afterload increase on left ventricular myocardial function in patients with congenital left-sided obstructive lesions. Am J Cardiol. 2007;99:1582–1587. doi: 10.1016/j.amjcard.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Nagueh SF, Lakkis NM, Middleton KJ, Killip D, Zoghbi WA, Quiñones MA, Spencer WH. Changes in left ventricular diastolic function 6 months after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. Circulation. 1999;99:344–347. doi: 10.1161/01.cir.99.3.344. [DOI] [PubMed] [Google Scholar]
- 8.Pacileo G, Pisacane C, Russo MG, Crepaz R, Sarubbi B, Tagliamonte E, Calabrò R. Left ventricular remodeling and mechanics after successful repair of aortic coarctation. Am J Cardiol. 2001;87:748–752. doi: 10.1016/s0002-9149(00)01495-8. [DOI] [PubMed] [Google Scholar]
- 9.Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, Colan SD, Benson CB, Lock JE, Perry SB. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins-Haug LE, Benson CB, Tworetzky W, Marshall AC, Jennings RW, Lock JE. In-utero intervention for hypoplastic left heart syndrome—a perinatologist. Ultrasound Obstet Gynecol. 2005;26:481–486. doi: 10.1002/uog.2595. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 12.Bauer F, Bénigno S, Lemercier M, Tapiéro S, Eltchaninoff H, Tron C, Baala B, Brunet D, Cribier A. Early improvement of left ventricular function after implantation of a transcutaneous aortic valve: a tissue Doppler ultrasound study. Arch Cardiovasc Dis. 2009;102:311–318. doi: 10.1016/j.acvd.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Jassal DS, Tam JW, Dumesnil JG, Giannoccaro PJ, Jue J, Pandey AS, Joyner CD, Teo KK, Chan KL. Clinical usefulness of tissue Doppler imaging in patients with mild to moderate aortic stenosis: a substudy of the aortic stenosis progression observation measuring effects of rosuvastatin study. J Am Soc Echocardiogr. 2008;21:1023–1027. doi: 10.1016/j.echo.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Steine K, Rossebø AB, Stugaard M, Pedersen TR. Left ventricular systolic and diastolic function in asymptomatic patients with moderate aortic stenosis. Am J Cardiol. 2008;102:897–901. doi: 10.1016/j.amjcard.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Pacileo G, Calabrò P, Limongelli G, Russo MG, Pisacane C, Sarubbi B, Calabrò R. Left ventricular remodeling, mechanics, and tissue characterization in congenital aortic stenosis. J Am Soc Echocardiogr. 2003;16:214–220. doi: 10.1067/mje.2003.10. [DOI] [PubMed] [Google Scholar]
- 16.de Kort E, Thijssen JM, Daniëls O, de Korte CL, Kapusta L. Improvement of heart function after balloon dilation of congenital valvar aortic stenosis: a pilot study with ultrasound tissue Doppler and strain rate imaging. Ultrasound Med Biol. 2006;32:1123–1128. doi: 10.1016/j.ultrasmedbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Villari B, Vassalli G, Monrad ES, Chiariello M, Turina M, Hess OM. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation. 1995;91:2353–2358. doi: 10.1161/01.cir.91.9.2353. [DOI] [PubMed] [Google Scholar]
- 18.Fifer MA, Borow KM, Colan SD, Lorell BH. Early diastolic left ventricular function in children and adults with aortic stenosis. J Am Coll Cardiol. 1985;5:1147–1154. doi: 10.1016/s0735-1097(85)80017-6. [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama H, Yutani C, Iida K, Arakaki Y, Yamada O, Kamiya T. The relation between right ventricular function and left ventricular morphology in hypoplastic left heart syndrome: angiographic and pathological studies. Pediatr Cardiol. 1999;20:422–427. doi: 10.1007/s002469900504. [DOI] [PubMed] [Google Scholar]
- 20.Villari B, Campbell SE, Hess OM, Mall G, Vassalli G, Weber KT, Krayenbuehl HP. Influence of collagen network on left ventricular systolic and diastolic function in aortic valve disease. J Am Coll Cardiol. 1993;22:1477–1484. doi: 10.1016/0735-1097(93)90560-n. [DOI] [PubMed] [Google Scholar]
- 21.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 22.Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlöhner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 23.McElhinney DB, Vogel M, Benson CB, Marshall AC, Wilkins-Haug LE, Silva V, Tworetzky W. Assessment of left ventricular endocardial fibroelastosis in fetuses with aortic stenosis and evolving hypoplastic left heart syndrome. Am J Cardiol. 2010;106:1792–1797. doi: 10.1016/j.amjcard.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 24.Sharland GK, Chita SK, Fagg NL, Anderson RH, Tynan M, Cook AC, Allan LD. Left ventricular dysfunction in the fetus: relation to aortic valve anomalies and endocardial fibroelastosis. Br Heart J. 1991;66:419–424. doi: 10.1136/hrt.66.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tworetzky W, del Nido PJ, Powell AJ, Marshall AC, Lock JE, Geva T. Usefulness of magnetic resonance imaging of left ventricular endocardial fibroelastosis in infants after fetal intervention for aortic valve stenosis. Am J Cardiol. 2005;96:1568–1570. doi: 10.1016/j.amjcard.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 26.Robinson JD, del Nido PJ, Geggel RL, Perez-Atayde AR, Lock JE, Powell AJ. Left ventricular diastolic heart failure in teenagers who underwent balloon aortic valvuloplasty in early infancy. Am J Cardiol. 2010;106:426–429. doi: 10.1016/j.amjcard.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 27.Lurie PR. Changing concepts of endocardial fibroelastosis. Cardiol Young. 2010;20:115–123. doi: 10.1017/S1047951110000181. [DOI] [PubMed] [Google Scholar]
- 28.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliot PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]