Abstract
The impact of Eph and ephrin signaling on cell behavior is complex and highly context dependent. Forward signaling initiated by Eph receptor activation and reverse signaling initiated by ephrin activation often mediate opposite effects. The apparent ligand-independent functions of Eph receptors recognized recently add another layer of complexity. This review will attempt to sort out the information generated recently on signaling by the A subfamily of Eph receptors and ephrin ligands. We will focus on EphA/ephrin-A signaling in the context of several physiological and disease processes, where new progresses have been made lately and unifying themes are emerging amid previous confusions. For more comprehensive survey of literature on Eph/ephrin signaling pathways and networks, readers are referred to outstanding reviews both in this volume and in other recent publications.
A. INTRODUCTION
The cloning of the first Eph receptor tyrosine kinase (RTK) from an erythropoietin-producing hepatoma cell line in 1987 set in motion of a rapid succession of identification of multiple other related receptors (1). The effort culminated in emergence of the Eph receptors as the largest subfamilies of RTKs in vertebrate systems with 16 distinct members including 14 in mammalian systems (2). The identification of ligands called ephrins lagged for several years, in part owning to the later realization of the membrane-bound nature of the ligands (3-5). The six ephrin-A ligands (A1-A6) are anchored to the cytoplasmic membrane through glycosyl phosphatidyl inositol (GPI) moiety, and generally bind to EphA receptors (A1-A10). The three ephrin-B ligands (B1-B3) are type I transmembrane proteins with highly conserved cytoplasmic tails and preferentially bind to EphB receptors (B1-B6). Notable exceptions are EphA4 binding to ephirn-B2/B3 (6-8), and EphB2 binding to ephrin-A5 (9). Ephs and ephrins are expressed in almost all embryonic tissues during development (10-13). While the expression dissipates after birth, most cell types of the adult tissues retain distinct repertoire of Eph receptor and ephrin ligand expression (14). The functional characterization of Eph/ephrin system in the last two decades revealed an astounding array of developmental, physiological and disease processes that are regulated by Eph/ephrin system (12,15-21). Matching the functional versatility is equally diverse signaling mechanisms, which are underscored by the unique bidirectional signaling properties of Eph/ephrin system: Not only are there signaling by Eph receptors, the ephrin ligands are also capable of receptor-like signaling into the interior of ligand-presenting cells. The bidirectional signaling by Eph/ligand system is intimately integrated with other cellular signaling networks via reciprocal regulation to exert effective control over cell behaviors (22,23).
The explosive expansion in the literature on Eph/ephrin function and signaling is marked with major discoveries as well as some controversies (19). This review will attempt to sort out the information generated recently on signaling by the A subfamily of Eph receptors and ephrin ligands. We will focus on EphA/ephrin-A signaling in the context of several physiological and disease processes, where new progresses have been made lately and unifying themes are emerging amid previous discrepancies. For more comprehensive survey of literature on Eph/ephrin signaling pathways and networks, readers are referred to outstanding reviews both in this volume and in many other recent publications. We apologize for unable to cite many original papers due to space constrains and the more focused themes of the review.
B. YIN AND YANG REGULATION OF CELL ADHESION AND MIGRATION BY EPHA/EPHRIN-A SIGNALING
The best documented function of EphA/ephrin-A signaling is the regulation of cell adhesion, positioning, and migration, processes critical for a wide variety of normal and pathological processes from embryonic development to tissue regeneration, immune surveillance, and tumor progression. Besides the highly context- and cell type-dependence, EphA and ephrin-A can use diverse means to impact the ability of a cell to adhere and migrate.
B1. Differential regulation of cell-matrix interaction by EphA/ephrin-A signaling
EphA receptors
Regulation of integrin activities has been demonstrated to be a key mechanism underpinning the effects of Eph/ephrin system on cell-matrix adhesion and migration. Earlier studies show that activation of EphA2 and EphB2 is linked to suppression of integrin function leading to the cell deadhesion and cell rounding effects (24,24,25). The latter effects on cell rounding have been further documented in more recent studies (26,27). The EphA2 activation-mediated outside-in signaling that triggers β1-integrin conformational changes and inactivation was associated with FAK and paxillin tyrosine dephosphorylation (25). Interestingly in a semi-in vivo setting, activation of EphA4 by ephrin-A3 in hippocampal slices also inhibits integrin downstream signaling, including FAK, Pyk2 and Crk dephosphorylation (28). In addition, EphA1 has been shown to be associated with integrin-linked kinse (ILK). Activation of EphA1 by ephrin-A1 inhibits ILK activity leading to the inhibition of cell spreading and migration (29). Despite the emerging theme pointing to the negative regulation of integrin function by EphA forward signaling, certain exceptions exist. The most notable example is EphA8. Ligand stimulation of EphA8 promotes integrin-mediated cell-matrix adhesion through PI3K. Interestingly, this effect is independent of EphA8 kinase activity, further suggesting its uniqueness (30).
More recent studies provide further support for negative regulation of integrins by EphA receptors. A large scale transcriptional profiling of ephrin-A-treated keratinocytes shows that ephrin-A treatment, presumably through Eph activation, causes decreased integrin gene expression, leading to the inhibition of cell migration (31). Similar observation has been made in Schwann cells, which express EphA2, -A4, and -A7 (32). Upon contact with astrocytes, which express most of ephrin-As, the interaction between EphAs and ephrin-As suppresses intermingling and mediate sorting of two cell types. Treatment with ephrin-A5 inhibits integrin activity and causes FAK dephosphorylation (32).
Ephrin-A ligands
In contrast with EphA receptors, whose forward signaling is often associated with inhibition of cell adhesion and migration as described above, the reverse signaling by ephrin-As appear to have generally opposite effects. Thus earlier studies showed that ephrin-A5 expressed in NIH 3T3 cells displayed increased adhesion to fibronectin and laminin upon ligation by EphA5-Fc through activation of β1-integrin (33). Similarly stimulation of ephrin-A2 on HEK 293 cells with EphA3-Fc promoted integrin-mediated cell adhesion and migration (34). This theme is further supported by recent studies. Thus, ephrin-A reverse signaling has recently been reported to induce phosphorylation of paxillin, a mediator of integrin signaling, and regulate hematopoietic stem cell adhesion and trafficking (35). Ephrin-As are co-expressed with EphAs on T cells and the reverse signaling initiated by ephrin-A activation stimulates integrin-mediated cell interaction and promotes T cell trafficking to lymph nodes in vivo (36).
It is worth pointing out that some of the seeming confusions and inconsistencies regarding EphA/ephrin-A regulation of cell adhesion and migration can be reconciled by differentiating between the receptor vs. the ligand signaling or by taking into consideration of the cellular context and different members of Ephs or ephrins involved.
B2. Differential regulation of cell-cell adhesion by EphA/ephrin-A signaling
Although EphA forward signaling negatively regulates cell-matrix interaction in many cases, it seems to exert different effects on cell-cell interactions. EphA/ephrin-A signaling regulates virtually all types of cell-cell adhesions. EphA2 has been reported to interact with claudin 4, a component of tight junction. Activation of EphA2 causes phosphorylation of claudin 4, leading to reduced association between claudin 4 and ZO-1 and delayed assembly of claudin 4 to tight junctions (37). EphA/ephrin-A signaling also regulates both Ca2+-dependent E-cadherin-based adherens junctions and Ca2+-independent Nectin-based cell-cell adhesions. Nectins are linked to the actin cytoskeleton through actin-binding protein afadin/AF-6. The nectin-afadin complex is important for the formation of not only adherens junctions, but also tight junctions in epithelial cells. Early study shows that AF-6 interacts with a subset of Eph receptors including EphA7 at cell-cell contacts in the brain. The interaction is mediated by the PDZ domain of AF-6 and is dependent on tyrosine phosphorylation of Eph kinases (38).
While the evidence of the regulation of Nectin-based cell-cell adhesion by Eph/ephrin signaling is still relatively thin, there are mounting evidence demonstrates the mutual regulation between Eph receptor and E-cadherin-based cell-cell adhesion. A positive feedback loop between E-cadherin-mediated cell-cell adhesion and EphA2 forward signaling has been suggested in a recent study done by Miura and colleagues (39). In this study, EphA2 activation by ligand ephrin-A1 stimulation in MDCK cells suppresses Arf6, a GTPase primarily regulates recycling of plasma membrane components and remodeling of the membrane and actin cytoskeleton at the cell peripheries, and induces cell compaction, which is accompanied by the enhanced accumulation of E-cadherin to cell-cell contacts. Blocking E-cadherin function inhibits ephrin-A1-stimulation induced cell compaction. Therefore, E-cadherin-based cell-cell adhesion enhances EphA forward signaling, which in turn downregulates Arf6 activity to enhance E-cadherin-based cell-cell adhesion and apical-basal polarization of epithelial cells (39). This finding is consistent with early studies showing that the expression, localization, as well as function of EphA2 are regulated by E-cadherin (40,41). Disruption of E-cadherin-mediated cell-cell adhesion results in reduced phosphorylation and cell-cell contact localization of EphA2 In normal breast epithelial MCF-10A cells. Expression of E-cadherin into metastatic breast cancer MAD-MB-231 cells enhances the phosphorylation of EphA2 and redistribution of EphA2 from cell periphery to cell-cell contacts (41). It should be noted that in this study, reduced cell-matrix adhesion was also observed after EphA2 activation by ligand stimulation, which is consistent with many studies discussed in section A1 above.
A recent study by Lin et al further reinforced that EphA2 activation by ephrin-A1 stimulation strengthens inter-keratinocyte adhesion and induces stratification and terminal differentiation. In their model, although EphA2 and E-cadherin were both concentrated at nascent cell-cell contacts, activation of EphA2 caused no significant change in E-cadherin expression level. Instead it dramatically upregulated the expression of desmoglein 1 and desmocollin 1, the suprabasal desmosomal cadherin partners (42). The observation that EphA2 forward signaling induced terminal differentiation is in keeping with the research by Walsh and Blumenberg showing that treatment of keratinocytes with ephrin-A ligands causes differentiation (31). Both studies are consistent with previous report that EphA2 activation by ephrin-A1 simulation in primary keratinocytes results in the inhibition of Ras/ERK activities and cell growth (43). Deletion of EphA2 eliminates EphA2/ephrin-A1 interactions in the interface of the receptor-expressing and the ligand-expressing cells, which could contribute to the increased tumor susceptibility (43).
Interestingly, EphA2 forward signaling is also important for N-cadherin-mediated cell contacts and architectural integrity of tissues. Lens fiber epithelial cells express high levels of both EphA2 and ephrin-A5 (44,45). Ephrin-A5 activates EphA2, leading to the increased recruitment of β-catenin to N-cadherin. Loss of Efna5 or EphA2 genes results in the disorganization of N-cadherin in lens fiber cells and the development of cortical cataract (44,45). Moreover, human genetic studies revealed that EphA2 is associated with development of age-related cataract (44,46-49). While most of the SNPs are in the non-coding region, non-synonymous mutation was found in the kinase domain in one study that alters the expression and catalytic activities of EphA2 (44).
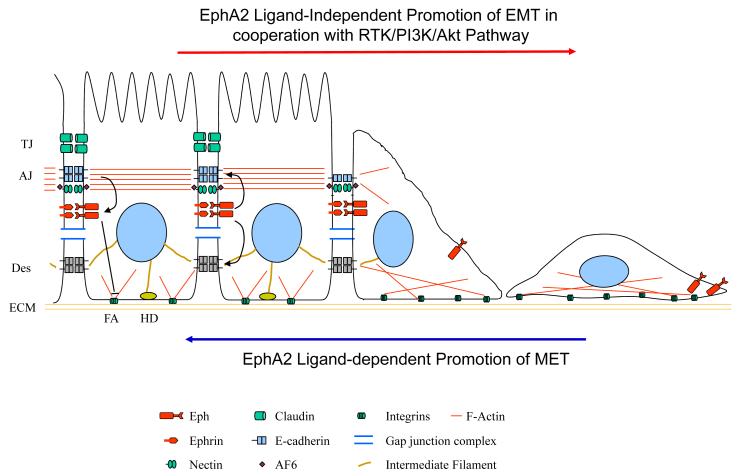
Making intercellular connections is a characteristic to epithelial cells. Disruption and reassembly of these adhesion features mark cell phenotype changes, defined as epithelia-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET), respectively. Both processes play key roles in embryonic development and cancer progression. Based on the findings discussed above, EphA/ephrin-A signaling differentially regulates cell-matrix and cell-cell adhesion, which may facilitate changes in cell phenotypes in developmental, physiological and disease processes (Fig. 1). It will be interesting to investigate how EphA/ephrin-A signaling coordinate these two types of adhesion and how EphA receptor activation facilitate the switch between cell-matrix adhesion and cell-cell adhesion during cell phenotype changes.
Fig. 1.
EphA2 signaling in epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET). EphA2 receptor activation by ligand stimulation enhances cell-cell adhesion and inhibits growth factor-mediated chemotaxis/scattering thereby promotes MET or inhibits EMT. In the absence ligand stimulation, EphA2 can function as an effector of oncogenic pathways and promote chemotaxis and EMT.
B3. EphA/ephrin-A signaling regulates cell adhesion and migration machinery through Rho GTPase-dependent and –independent pathways
Cytoskeleton reorganization is essential for cell shape change and movement. Regulation of cytoskeleton by EphA/ephrin-A signaling has been well-documented. Accumulating evidence shows that EphA /ephrin-A signaling is capable of regulating all three types of cytoskeleton including actin microfilament, microtubule, and intermediate filament through Rho GTPase-dependent and –independent pathways.
Through interacting and activating guanine exchange factors (GEFs), such as Ephexin and Vav and GTPase activating proteins (GAPs) such as α2- and β2-chimaerin, EphA receptor activation leads to activation of RhoA and/or inactivation of Rac1/Cdc42 in variety type of cells (18,50-54). As a result, actin filaments are stabilized and cell migration is inhibited. However there are exceptions. For example, ephrin-A1 binding to EphA2 may activate Rac1 through Tiam1, a Rac GEF, and stimulates neurite outgrowth from cortical neuron (55).
While activation of RhoA and inactivation of Rac/Cdc42 have been one of the common downstream effects of EphA kinase activation upon ligand stimulation, opposite effects can be achieved by EphA2 independent of ligand stimulation. It has been recently reported that Ephexin4, a GEF for RhoG, acts downstream from unligated EphA2. Activation of RhoG triggers a signaling cascade leading to activation of Rac and stimulation of breast cancer cell migration in the ephrin-A ligand-independent manner (56). This provides another possible mechanism underlying EphA2 ligand-independent migration-promoting effect that will be discussed in the next section. In addition, RhoG regulates cell proliferation and survival via PI3K independently of its ability to activate Rac and cytoskeleton assembly. Indeed EphA2/Ephexin4/RhoG signaling cascade has recently been implicated in mediating resistance to anoikis (57).
While Eph and ephrin are widely expressed during embryonic development of nervous system, they are often downregulated in most areas of adult CNS. Interestingly, reexpression or upregulation of EphA receptors has been found after nerve injury. For example, EphA4 is highly expressed on astrycyte at the sites of injury and is a major contributor to the inhibition of neuronal regeneration following spinal cord injury (58,59). The injury leads to cytoskeletal reorganization in astrocyte. Recently, EphA2/A4 receptor signaling by ephrin-A5 stimulation has been reported to regulate both actin stress fiber and glial fibrillary acidic protein (GFAP) intermediate filament formation in astrocytes in response to injury (60).
Mechanisms independent of Rho GTPases in EphA signaling regulation of cytoskeleton are also suggested. Cheng et al. have found that stimulation of EphA receptor by ephrin-A5 induces Cdk5 activation via Tyr15 phosphorylation, followed by phosphorylation of its substrate, tau, resulting in microtubule reorganization (61). More recently, Nie and colleagues have reported that EphA activation by ephrin-A inhibits local translation of β-actin and other mRNAs by inactivating mTOR pathway, which regulate actin cytoskeletal dynamics in the growth cone (62).
C. EXTENDING EPHA/EPHRIN-A SIGNALING NETWORK VIA CORSSTALK WITH OTHER RTKS AND PROTEIN TYROSINE PHOSPHATASES (PTPS)
C1. Reciprocal regulation between EphA/ephrin-A and growth factor receptor signaling
Eph receptor tyrosine kinases differ from conventional receptor tyrosine kinases such as growth factor receptors (GFR). Most GFP pathways have well-documented oncogenic activities whereas Eph receptor signaling often functions as tumor suppressor function in part by counteracting growth factor signaling (43,63-65). However, recent evidence has revealed that Eph receptors may have both pro- and anti-oncogenic functions depending on hardwiring of intracellular signaling networks and extracellular stimuli. Under normal condition, EphA/ephrin-A signaling plays a critical role in maintaining tissue homeostasis (13). In tumor cells particularly during malignant progression, EphA receptors can be co-opted by growth factor signaling and promote tumor cell invasion and metastasis (66-69).
EphA2 crosstalk with Ras/ERK pathway
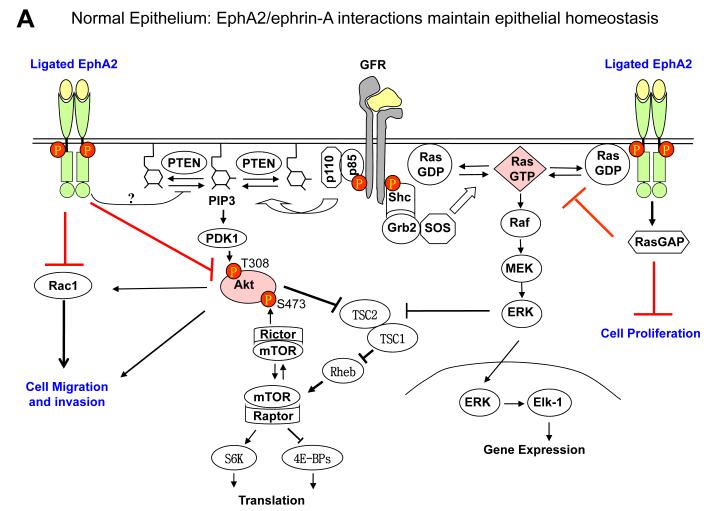
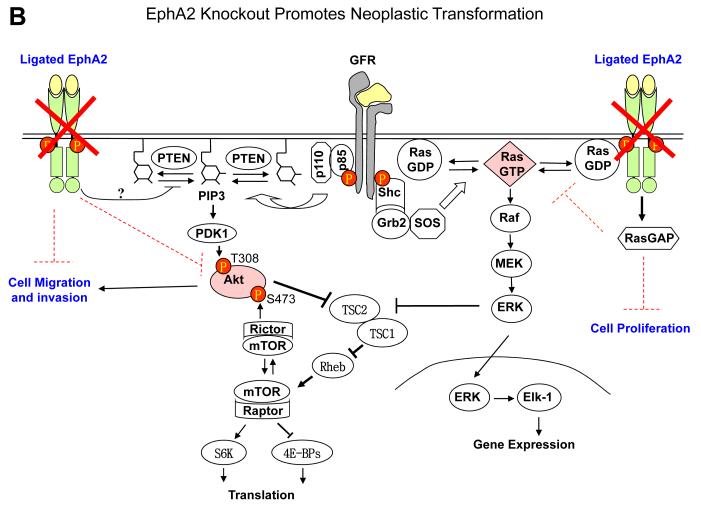
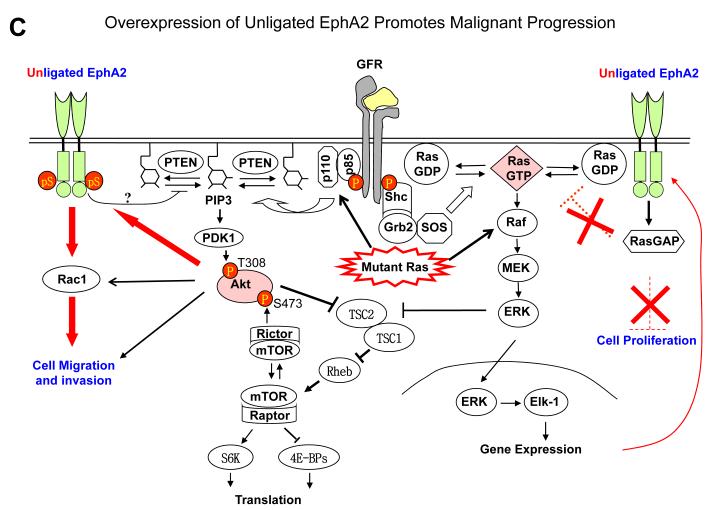
EphA receptor activation by ephrin-A ligands leads to inactivation of Ras/extracellular signaling-regulated kinase 1/2 (ERK1/2) pathway downstream from growth factor signaling in fibroblasts and epithelial cells (63). Similar observations have made in most normal and many tumor cells (43,62,63,70-72). Inhibition of Ras/ERK pathway is associated with cell growth inhibition in vitro and tumor suppressor activities in vivo. Interestingly growth factor–stimulated ERK1/2 activation can in turn upregulate EphA2 expression, completing a feedback loop (70). Under normal condition, the negative regulatory function of EphA2 forward signaling is important for maintaining epithelial tissue homeostasis. Because ligand-activated EphA2 receptor inhibits Ras by recruiting activating RasGAP (63,73), tumor cells with constitutively active Ras can bypass the inhibitory effects of the ligand-activated EphA kinases. During tumor development, the EphA2 forward signaling is often compromised due to hyperactivation of growth factor pathway, activation of Ras or downregulation of ephrin-A ligands (Fig. 2).
Fig. 2.
EphA2 signaling in normal epithelial cells and tumor cells. A. Under normal conditions, EphA2 and ephrin-A are properly expressed and engaged with each other. The signaling downstream from EphA2 activation by ligand engagement counteracts growth factor signaling by inhibiting activation of Ras/ERK and PI3K/Akt, which contributes to the maintenance of epithelial homeostasis. B. In EphA2-null epithelial cells, the negative regulator of growth factor signaling is disrupted, which renders epithelial cells more susceptible to carcinogen insults leading to tumor development. C. With tumor progression, EphA2 can be highly upregulated whereas ephrin-A is not or downregulated, which resulting in excess of unligated EphA2. Unligated EphA2 can serve as a substrate of Akt becoming an integral component of growth factor pathway to promote tumor cell migration and invasion. At the same time, unligated EphA2 is incapable of suppressing Ras/ERK pathway.
Reciprocal regulation between Akt and EphA2
In addition to the mutual regulation between EphA2 and Ras/ERK pathway, a novel regulatory loop has also been recently discovered between Akt and EphA2 (68). It turns out that EphA2 has diametrically opposite roles in regulating chemotactic cell migration and invasion depending on is ligand activation status. In the presence of the ligand, the activated EphA2 inhibits chemotactic cell migration and invasion, accompanied by strong suppression of Akt activities in variety of different cells (68,72). In contrast, when the ligands are absent, the unligated EphA2 promotes chemotactic cell migration and invasion instead. Interestingly the ligand-independent stimulation of cell motility and invasion was correlated with phosphorylation of EphA2 on a single serine residue (S897) by Akt (68). Therefore, in contrast to the role of EphA2/ephrin-A1 interaction in mediating repulsive response that prevents the invasion of tumor cells, EphA2 receptor when not occupied by its cognate ligand can become an integral part of growth factor receptor pathway and act as positive guidance molecule for migrating cells (Fig. 2).
The mutual regulation with growth factor pathway is also found in other EphA receptors. Activation of IGF-1R induces EphA3 expression in malignant T cells. On the other hand, EphA3 activation by ephrin-A5 stimulation resulted in a reduced integrin-mediated cell adhesion (69). The expression regulation goes both ways. EphA4 activation was found to induce FGF as well as it receptors and promote posterior protrusion in xenopus embryos (74). EphA4-FGFR1 heterodimer promotes FGFR1 signaling in glioma cell line. Ligand stimulation of EphA4 stimulates FGFR1 phosphorylation and signaling (67,67). On the other hand, Ephrin-A5 negatively regulates EGFR expression by promoting its ubiquitination through both reverse signaling and forward signaling by binding to EphA (75).
Ephrin-A ligands has also been reported to interact and function as integral components of growth factor signaling. For example, ephrin-A5 and –A6 bind to activated p75TrkB receptors to specifically enhance p75TrkB/BDNF signaling-induced PI3K/Akt activation, axon branching and synaptogenesis (76). Interestingly, the reverse signaling of ephrin-A5 by EphA7 stimulation can in turn suppress these processes (76).
C2. Protein phosphatases take parts in both upstream and downstream of the EphA/ephrin-A signaling network
Not only do EphA receptors have mutual regulatory relationship with other RTKs, they also cross-talk with different types of protein phosphatases including receptor-like tyrosine phosphatases such as LAR subfamily PTP-3 and Ptpro, non-receptor tyrosine phosphatase such as SHP-2, PTP1B, and LMW-PTP, lipid phosphatase such as PTEN and SHIP2, as well as yet unidentified serine/threonine protein phosphatases(25,72,77-83).
The only C. elegans Eph receptor Vab interacts genetically with PTP-3, a LAR subfamily PTPs. ptp-3 and Eph signaling mutations show specific synergistic effects on morphogenesis. Ptpro determines the sensitivity of retinal axons to ephrin-A2 as a repellent in vitro and that the retinotectal projection is modified by Ptpro along both axes through control of the activity of EphA receptors (81).
Increasing evidence points to the tyrosine dephosphorylated EphA2 in oncogenic process. One of the phosphatases, LMW-PTP, has been reported to interact with EphA2. Overexpression of this PTP is sufficient to induce transformation of epithelial cells, which is dependent on the dephosphorylation of EphA2 (79). EphA2 tyrosine phosphorylation level was dramatically decreased by overexpression of LMW-PTP, and increased by expression of dnLMW-PTP, suggesting tyrosine-phosphorylated EphA2 is a substrate of LMW-PTP (78). This observation is in keeping with previous reports that EphA2 is prominently tyrosine phosphorylated in nontransformed cells and largely dephosphorylated in transformed cells (41,84).
The phosphorylation of EphA3 is controlled by specific protein tyrosine phosphatases (PTPs) in nonadherent leukemia cells and normal adherent cells. In typically nonadherent pre-B ALL cells that express EphA3 receptors, ephrin-A5 stimulation can cause either repulsion or adhesion. Interestingly, the molecular switch between this two diametrically opposite responses relies on the PTPs activity. While tyrosine phosphorylation of EphA3 causes adhesion, PTPs attenuates the response (82). In normal adherent cells, PTP1B was found to negatively regulate ephrin-A5-induced EphA3 phosphorylation. Upon contact with ephrin-A5-expressing cells, EphA3 recruits PTP1B to the cell surface there PTP1B in turn controls activity, trafficking, and function of EphA3 (80). In glioma and normal epithelial cell co-culture, EphA-ephrin-A interaction causes cell segregation. Inhibition of PTP1B by gene silencing or chemical inhibitor increases the segregation. In contrast, treatment with EphA3 kinase inhibitor causes the intermingling and cell dispersion between the two cell populations (80).
In addition to being regulated by protein phosphatases, EphA2 also utilizes protein phosphatases to dephosphorylate its target molecules. For example, SHP2 has been found to interact with EphA2 and mediate dephosphorylation of focal adhesion kinase (FAK) in PC-3 cells (25). An unidentified serine/threonine phosphatase function downstream EphA2 in dephosphorylating Akt and suppressing Akt-mTOR pathway (72). In C. elegans, Eph interacts with PTEN (phosphatase and tensin homolog) and inhibits its phosphatase activity (77).
D. EVER EVOLVING ROLE OF EPHA/EPHRIN-A SIGNALING IN TUMOR BIOLOGY
As we have already touched upon in previous sections, the complex signaling of EphA receptor and ephrin-A ligand plays a pivotal role in tumor development and progression by regulating many aspects of tumor biology from tumor initiation and growth to invasion and metastases. New mechanistic insights on how the same Eph kinase can function either as an oncogenic and tumor suppressive protein depending on the cellular context have reconciled some of persistent inconsistencies in the field. Here we will discuss recent advances in the intricate signaling networks by EphA/ephrin-A system in several different types of cancers to highlight some of the emerging themes. For a comprehensive survey of literature on Eph/ephrin bidirectional signaling in cancer the readers are referred to a recent review (21).
D1. Epithelial cancer
EphA1 was the first member of the Eph family to be identified (1) and is mainly expressed in epithelial tissues (85). Downregulation of EphA1 has been reported in malignant tumors (14,86). The reduced expression of EphA1 in breast carcinoma cells is associated with invasive behavior of the cells. In colorectal cancer, while overexpression of EphA1 is more prevalent in stage II CRCs, loss of EphA1, probably due to epigenetic silencing, is significantly associated with advanced stage and poor survival (87). Therefore, majority evidence suggests that EphA1 as a tumor suppressor. The mechanisms underlying its tumor suppressor function is largely understudied. Yamazaki and colleagues have demonstrated that EphA1 activation by ligand stimulation can inhibit ILK and suppress cell spreading and migration of breast cancer cells (29). Both Akt and Rho GTPase are downstream effectors of ILK, which could be regulated by EphA1 forward signaling and provide one of the possible mechanisms for tumor suppression.
Although most closely related with EphA1 compared to with other members of the family, EphA2 seems to function differently in tumorigenesis and tumor progression. It is one of the most affected members among Eph receptor family in human cancer, which should not be so surprising given the reciprocal regulatory loops between EphA2 and Ras/ERK as well as PI3K/Akt signaling as discussed in the previous section. EphA2 is frequently overexpressed in a variety of human epithelial cancer (88-90). The overexpression often associates with aggressive phenotype of the cancer (84,91-94). At least in some epithelial cancers, the overexpression is caused by activation of Ras/ERK signaling (70). It is worth noting that Ras/ERK signaling differentially regulates EphA2 receptor and ephrin-A1 ligand. While EphA2 is upregulated, ephrin-A1 is downregulated by Ras/ERK (70). An imbalance between the receptor and ligand expression may compromise EphA2 ligand-dependent tumor suppressive function and promote the ligand-independent pro-oncogenic function (Fig. 2).
Of note, activation of EphA2 receptor by ephrin-A1 ligand stimulation induces repulsive response in multiple epithelial cancer cells, which can be variably interpreted as evidence of either increased tumor cell dissemination or reduced tumor cell motility and invasion (25,27,68,95). It may depend on where the interaction of EphA2 and ephrin-A takes place. In the periphery or the immediate vicinity of tumor mass, homotypic interactions between cancer cells through EphAs/ephrin-As could propel cell dispersal from the primary tumor mass (27,96). Alternatively or in addition, ephrin-As presented by tumor stromal cells could impede invading tumor cells. Further in vivo studies will be necessary to discern which mechanism predominates in appropriate animal models.
EphA3 is the most frequently mutated EphA receptors in multiple malignant cancer including lung, hepatocellular, breast, and pancreatic cancer (97-100). Although the functional analysis is incomplete, our unpublished work suggested that at least some of the mutations may result in altered cell behaviors including adhesion, migration, and growth in 3-D culture (Johnson and Wang, unpublished data).
New evidence points to abnormal expression of A type Eph receptors in additional malignant cancers involving additional mechanisms. EphA4 overexpression correlates with liver metastasis of colorectal cancer (101). Hypermethylation of EphA5 and EphA7 is associated with high grade breast cancer and prostate cancer with high Gleason score, respectively (102,103). These studies add to the extensive previous literature on Eph/ephrin dysregulation in cancer. However, the pathological significance for many of these changes remains unclear, but is likely to be dependent on cancer types and Eph receptors in question.
The involvement of A type ligands in tumor progression is relatively less well studied. Complementary DNA microarray from human prostate cancer metastases to lymph nodes, liver, and bone revealed a decreased expression of EFNA1 (ephrin-A1) in bone metastases when compared to liver and lymph node metastases (104). This result suggests bone could be a perfect location for EphA receptors to exert ligand-independent effects. It should be noted that the ephrin-A1 has been identified as a target of tumor suppressor HIC1 in breast cancer (105), and ephrin-A1 signaling is also suggested to promote intestinal tumor invasive progression (106). It is important to dissect the functions of reverse signaling initiated by ephrin-A activation from that of forward signaling initiated by EphA receptor activation in order to better understand the ultimate role of EphA/ephrin-A system in tumor development and progression.
D2. Tumors of nervous system
Glioblastoma is the most common brain tumor in adults with medium survival of only 14 months after diagnosis, even with maximal treatment including surgical resection, radiotherapy, and concomitant chemotherapy. EphA2 and EphA4 are highly expressed in human glioma and the expression level is correlated with the tumor malignancy (67,94). Both EphA kinases have been shown to corporate with growth factor signaling in promoting tumor progression. While EphA2 promote growth factor signaling by serving as the Akt substrate in the absence of ephrin-A (68), EphA4 can do so by transphosphorylating growth factor receptors (67). In contrast to the alteration in EphA2 and EphA4, the ligand ephrin-A1 and ephrin-A5 are downregulated in the glioblastoma, which is consistent with the ligand-independent oncogenic role of EphA2 in glioblastoma where over 90% of which show overexpression of EphA2 (75,94,107).
EphA5 in plasma as dormancy-specific biomarker correlated with glioma stage (108). A significant increase in EphA5 level has been found in mice bearing microscopic dormant glioblastoma. It is significantly decreased in plasma of mice bearing angiogenic fast-growing glioblastoma. In human, high level of EphA5 was detected in normal brain tissue, which was decreased in low-grade glioma and further reduced in high-grade glioma tumor tissues. Therefore, EphA5 expression is decreased as the tumor stage advances, suggesting that EphA5 is tumor suppressor in glioma (108). This observation is consistent with previous report that activation of EphA5 on glioma cell line decreased cell proliferation instead of stimulating it (109).
D3. Hematological malignancies
Immune cell trafficking is an essential step of immune surveillance, during which a leukocyte has to navigate through many checkpoints to cross tissue barriers. EphA/ephrin-A system has emerged as an important regulator of immune trafficking either positively or negatively depending on the subtype of the immune cells. In primary T cells, activation of EphA receptors by ephrin-A1 inhibit stromal cell-derived factor (SDF)-1a-induced chemotaxis through activation of RhoA and simultaneously inactivation of Cdc42 (110). In CD4+ T cells, activation of EphA receptor by ephrin-A1 stimulate transendothelial migration of this subtype of T cells (111). Activation of EphA receptor by ephrin-A3 can also regulate dendritic cell interstitial migration (112). Recent studies revealed that EphA and ephrin-A are co-expressed on T cells and regulate integrin-mediated cell interaction in a diametrically opposing fashion, ephrin-A1 activation stimulates while EphA activation inhibits the interaction. Ephrin-A reverse signaling promotes T cell trafficking to lymph nodes in vivo (36).
Abnormal expression of EphA and ephrin-A proteins has been linked to hematological malignancies. For example, it has been reported that epigenetic silencing by hypermethylation of genes encoding EphA2, -A4, -A5, -A6, -A7, A10, ephrin-A1, -A3, -A5 contributes to acute lymphoblastic leukemia (ALL) (113). EphA3 was detected in T-cell lymphomas (69). Ephrin-A4 is highly expressed by chronic lymphocytic leukemia (CLL) cells and interacts with EphA2 that is expressed on endothelia cells. The reverse signaling of ephrin-A4 into CLL cells leads to reduced cell adhesion and impaired transendothelial migration (114).
D4. Other cancers
In addition to those extensively studied tumors discussed above, EphA receptors are also involved in the development and progression of other type of tumors. The best example was melanoma. Eph-ephrin signaling directs the migration of neural crest melanoblasts to the skin during development and their expression is low or absent in normal tissue melanocytes. During the melanocyte-to melanoma transition, tumor cells reacquire the expression of EphA2, EphA3, and ephrin-A1, which has been implicated in the invasive growth of tumor cells (19). A recent study suggested that the prooncogenic effect of EphA2 in melanoma is independent of ligand stimulation (115). Interestingly, the melanoma cells used in this study are either deleted or mutated for PTEN and overexpression of EphA2 promoted tumorigenesis(115), suggesting that Akt-EphA2 signaling axis discovered in glioma cells (68) might also be operated in melanoma cells.
It is important to point out that many observations regarding the involvement of Eph or ephrin in cancer are correlative not causative. For example, a particular expression pattern is frequently correlated with disease stage or outcome. While informative, rigorous in vitro and in vivo studies will be essential to draw a definitive conclusion.
E. CONCLUDING REMARKS AND FUTURE PERSPECTIVES
The signaling of EphA and ephrinA is very complex and the outcome is highly context- and cell type-dependent. We believe that the complexity of bidirectional signaling mechanisms provides a cell with flexible and versatile platform to orchestrate proper responses to its environment. As we discuss here that the forward signaling initiated by EphA receptor activation and reverse signaling initiated by ephrin-A activation often mediate opposite effects, which demands a better model system to dissect the signaling output. It also becomes increasingly clear that at least some of Eph kinases can function without ligand engagement (68,116). Paradoxically, this ligand-independent function is often opposite to its ligand-dependent function. The dual function is beginning to be recognized in tumor cells but remained to be tested in normal cells in the context of developmental process. Another issue yet to be addressed is if this ligand-independence is common to other Eph subfamily members.
Eph/ephrin system is increasingly implicated in variety of disease processes. In malignant diseases, the genetic and epigenetic instability as well as the cell-biological changes induced by stromal microenvironment generate multiple distinct subpopulations of cancer cells within a tumor. Currently, our knowledge is quite limited as to how the interactions between many Eph kinases and ephrins among different cell types may impact tumor cell behavior. Finally, given the critical role of A type Eph receptors and ephrin-A ligands in embryonic development, it is possible that they may also play a role in establishing or maintaining cancer stem-like cell properties as well. Studies in these areas can lead to not only new insight on Eph/ephrin signaling and function, but also new points of therapeutic intervention.
Highlights.
EphA signaling is cell-context dependent.
EphA receptors may have both ligand-dependent and -independent signaling mechanisms.
EphA signaling is intimately intertwined with other cellular signaling networks.
Signaling by EphA receptor and ephrin-A ligands often mediate opposing functions.
Acknowledgement
This work was supported by grants from National Institute of Health CA96533 (B.W.), CA92259 (B.W.), CA152371 (H.M. & B.W.), award to B.W. from FAMRI and Prayers From Maria Foundations, award to H.M. from American Heart Association (0465235B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- 2.Eph Nomenclature Committee Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee [letter] [In Process Citation] Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- 3.Bartley TD, Hunt RW, Welcher AA, Boyle WJ, Parker VP, Lindberg RA, Lu HS, Colombero AM, Elliott RL, Guthrie BA, et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature. 1994;368:558–560. doi: 10.1038/368558a0. [DOI] [PubMed] [Google Scholar]
- 4.Cheng A, Diller DJ, Dixon SL, Egan WJ, Lauri G, Merz KM., Jr. Computation of the physio-chemical properties and data mining of large molecular collections 39. J. Comput. Chem. 2002;23:172–183. doi: 10.1002/jcc.1164. [DOI] [PubMed] [Google Scholar]
- 5.Beckmann MP, Cerretti DP, Baum P, Vanden Bos T, James L, Farrah T, Kozlosky C, Hollingsworth T, Shilling H, Maraskovsky E, et al. Molecular characterization of a family of ligands for eph- related tyrosine kinase receptors. EMBO Journal. 1994;13:3757–3762. doi: 10.1002/j.1460-2075.1994.tb06685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergemann AD, Zhang L, Chiang MK, Brambilla R, Klein R, Flanagan JG. Ephrin-B3, a ligand for the receptor EphB3, expressed at the midline of the developing neural tube. Oncogene. 1998;16:471–480. doi: 10.1038/sj.onc.1201557. [DOI] [PubMed] [Google Scholar]
- 7.Gale NW, Flenniken A, Compton DC, Jenkins N, Copeland NG, Gilbert DJ, Davis S, Wilkinson DG, Yancopoulos GD. Elk-L3, a novel transmembrane ligand for the Eph family of receptor tyrosine kinases, expressed in embryonic floor plate, roof plate and hindbrain segments. Oncogene. 1996;13:1343–1352. [PubMed] [Google Scholar]
- 8.Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17:9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- 9.Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat. Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- 10.Adams RH, Klein R. Eph receptors and ephrin ligands. essential mediators of vascular development. Trends Cardiovasc. Med. 2000;10:183–188. doi: 10.1016/s1050-1738(00)00046-3. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 1998;21:309–45. doi: 10.1146/annurev.neuro.21.1.309. 309-345. [DOI] [PubMed] [Google Scholar]
- 12.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 13.Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int. J. Biochem. Cell Biol. 2009;41:762–770. doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, Langmann T, Dietmaier W, Landthaler M, Vogt T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin. Chem. 2004;50:490–499. doi: 10.1373/clinchem.2003.026849. [DOI] [PubMed] [Google Scholar]
- 15.Flanagan JG. Neural map specification by gradients. Curr. Opin. Neurobiol. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Himanen JP, Saha N, Nikolov DB. Cell-cell signaling via Eph receptors and ephrins. Curr. Opin. Cell Biol. 2007;19:534–542. doi: 10.1016/j.ceb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr. Opin. Cell Biol. 2004;16:580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling 2. Nat. Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 19.Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight, or complexity? Sci. Signal. 2008;1:re2. doi: 10.1126/stke.115re2. [DOI] [PubMed] [Google Scholar]
- 20.Miao H, Wang B. Eph/ephrin signaling in epithelial development and homeostasis. Int. J. Biochem. Cell Biol. 2008 doi: 10.1016/j.biocel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arvanitis D, Davy A. Eph/ephrin signaling: networks. Genes Dev. 2008;22:416–429. doi: 10.1101/gad.1630408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Zou JX, Wang B, Kalo MS, Zisch AH, Pasquale EB, Ruoslahti E. An Eph receptor regulates integrin activity through R-Ras. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat. Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 26.Gopal U, Bohonowych JE, Lema-Tome C, Liu A, Garrett-Mayer E, Wang B, Isaacs JS. A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion. PLoS. One. 2011;6:e17649. doi: 10.1371/journal.pone.0017649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell Biol. 2010;12:1194–1204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- 28.Bourgin C, Murai KK, Richter M, Pasquale EB. The EphA4 receptor regulates dendritic spine remodeling by affecting beta1-integrin signaling pathways. J. Cell Biol. 2007;178:1295–1307. doi: 10.1083/jcb.200610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamazaki T, Masuda J, Omori T, Usui R, Akiyama H, Maru Y. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J. Cell Sci. 2009;122:243–255. doi: 10.1242/jcs.036467. [DOI] [PubMed] [Google Scholar]
- 30.Gu C, Park S. The EphA8 receptor regulates integrin activity through p110gamma phosphatidylinositol-3 kinase in a tyrosine kinase activity-independent manner. Mol. Cell Biol. 2001;21:4579–4597. doi: 10.1128/MCB.21.14.4579-4597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh R, Blumenberg M. Specific and shared targets of ephrin A signaling in epidermal keratinocytes. J. Biol. Chem. 2011;286:9419–9428. doi: 10.1074/jbc.M110.197087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afshari FT, Kwok JC, Fawcett JW. Astrocyte-produced ephrins inhibit schwann cell migration via VAV2 signaling. J. Neurosci. 2010;30:4246–4255. doi: 10.1523/JNEUROSCI.3351-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davy A, Robbins SM. Ephrin-A5 modulates cell adhesion and morphology in an integrin- dependent manner. EMBO J. 2000;19:5396–5405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huai J, Drescher U. An ephrin-A-dependent signaling pathway controls integrin function and is linked to the tyrosine phosphorylation of a 120-kDa protein. J. Biol. Chem. 2001;276:6689–6694. doi: 10.1074/jbc.M008127200. [DOI] [PubMed] [Google Scholar]
- 35.Ting MJ, Day BW, Spanevello MD, Boyd AW. Activation of ephrin A proteins influences hematopoietic stem cell adhesion and trafficking patterns. Exp. Hematol. 2010;38:1087–1098. doi: 10.1016/j.exphem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Sharfe N, Nikolic M, Cimpeon L, Van De Kratts A, Freywald A, Roifman CM. EphA and ephrin-A proteins regulate integrin-mediated T lymphocyte interactions. Mol. Immunol. 2008;45:1208–1220. doi: 10.1016/j.molimm.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J. Biol. Chem. 2005;280:42375–42382. doi: 10.1074/jbc.M503786200. [DOI] [PubMed] [Google Scholar]
- 38.Buchert M, Schneider S, Meskenaite V, Adams MT, Canaani E, Baechi T, Moelling K, Hovens CM. The junction-associated protein AF-6 interacts and clusters with specific Eph receptor tyrosine kinases at specialized sites of cell- cell contact in the brain. J. Cell Biol. 1999;144:361–371. doi: 10.1083/jcb.144.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miura K, Nam JM, Kojima C, Mochizuki N, Sabe H. EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol. Biol. Cell. 2009;20:1949–1959. doi: 10.1091/mbc.E08-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orsulic S, Kemler R. Expression of Eph receptors and ephrins is differentially regulated by E-cadherin. J. Cell Sci. 2000;113(Pt 10):1793–1802. doi: 10.1242/jcs.113.10.1793. [DOI] [PubMed] [Google Scholar]
- 41.Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-cadherin regulates the function of the EphA2 receptor tyrosine kinase [In Process Citation] Cell Growth Differ. 1999;10:629–638. [PubMed] [Google Scholar]
- 42.Lin S, Gordon K, Kaplan N, Getsios S. Ligand targeting of EphA2 enhances keratinocyte adhesion and differentiation via desmoglein 1. Mol. Biol. Cell. 2010;21:3902–3914. doi: 10.1091/mbc.E10-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo H, Miao H, Gerber L, Singh J, Denning MF, Gilliam AC, Wang B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- 44.Jun G, Guo H, Klein BEK, Klein R, Wang JJ, Mitchell P, Miao H, Lee KE, Joshi T, Buck M, Chugha P, Bardenstein D, Klein A, Bailey-Wilson JE, Gong X, Spector T, Hammond CJ, Elston RC, Iyengar SK, Wang B. EPHA2 is associated with age-related cortical cataract in mice and humans. 2009. Accepted ed. [DOI] [PMC free article] [PubMed]
- 45.Cooper MA, Son AI, Komlos D, Sun Y, Kleiman NJ, Zhou R. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16620–16625. doi: 10.1073/pnas.0808987105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan W, Hou S, Jiang Z, Hu Z, Yang P, Ye J. Association of EPHA2 polymorphisms and age-related cortical cataract in a Han Chinese population. Mol. Vis. 2011;17:1553–1558. [PMC free article] [PubMed] [Google Scholar]
- 47.Kaul H, Riazuddin SA, Shahid M, Kousar S, Butt NH, Zafar AU, Khan SN, Husnain T, Akram J, Hejtmancik JF, Riazuddin S. Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Mol. Vis. 2010;16:511–517. [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang T, Hua R, Xiao W, Burdon KP, Bhattacharya SS, Craig JE, Shang D, Zhao X, Mackey DA, Moore AT, Luo Y, Zhang J, Zhang X. Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum. Mutat. 2009;30:E603–E611. doi: 10.1002/humu.20995. [DOI] [PubMed] [Google Scholar]
- 49.Shiels A, Bennett TM, Knopf HL, Maraini G, Li A, Jiao X, Hejtmancik JF. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol. Vis. 2008;14:2042–2055. [PMC free article] [PubMed] [Google Scholar]
- 50.Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O’Connell S, Cowan CW, Hu L, Goldberg JL, Debant A, Corfas G, Krull CE, Greenberg ME. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 52.Shi L, Fu WY, Hung KW, Porchetta C, Hall C, Fu AK, Ip NY. Alpha2-chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16347–16352. doi: 10.1073/pnas.0706626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeuchi S, Yamaki N, Iwasato T, Negishi M, Katoh H. Beta2-chimaerin binds to EphA receptors and regulates cell migration. FEBS Lett. 2009;583:1237–1242. doi: 10.1016/j.febslet.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 54.Wegmeyer H, Egea J, Rabe N, Gezelius H, Filosa A, Enjin A, Varoqueaux F, Deininger K, Schnutgen F, Brose N, Klein R, Kullander K, Betz A. EphA4-dependent axon guidance is mediated by the RacGAP alpha2-chimaerin. Neuron. 2007;55:756–767. doi: 10.1016/j.neuron.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka M, Ohashi R, Nakamura R, Shinmura K, Kamo T, Sakai R, Sugimura H. Tiam1 mediates neurite outgrowth induced by ephrin-B1 and EphA2. EMBO J. 2004;23:1075–1088. doi: 10.1038/sj.emboj.7600128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hiramoto-Yamaki N, Takeuchi S, Ueda S, Harada K, Fujimoto S, Negishi M, Katoh H. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J. Cell Biol. 2010;190:461–477. doi: 10.1083/jcb.201005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harada K, Hiramoto-Yamaki N, Negishi M, Katoh H. Ephexin4 and EphA2 mediate resistance to anoikis through RhoG and phosphatidylinositol 3-kinase. Exp. Cell Res. 2011;317:1701–1713. doi: 10.1016/j.yexcr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 58.Fabes J, Anderson P, Yanez-Munoz RJ, Thrasher A, Brennan C, Bolsover S. Accumulation of the inhibitory receptor EphA4 may prevent regeneration of corticospinal tract axons following lesion. Eur. J. Neurosci. 2006;23:1721–1730. doi: 10.1111/j.1460-9568.2006.04704.x. [DOI] [PubMed] [Google Scholar]
- 59.Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res. Rev. 2006;52:327–345. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Puschmann TB, Turnley AM. Eph receptor tyrosine kinases regulate astrocyte cytoskeletal rearrangement and focal adhesion formation. J. Neurochem. 2010;113:881–894. doi: 10.1111/j.1471-4159.2010.06655.x. [DOI] [PubMed] [Google Scholar]
- 61.Cheng Q, Sasaki Y, Shoji M, Sugiyama Y, Tanaka H, Nakayama T, Mizuki N, Nakamura F, Takei K, Goshima Y. Cdk5/p35 and Rho-kinase mediate ephrin-A5-induced signaling in retinal ganglion cells. Mol. Cell Neurosci. 2003;24:632–645. doi: 10.1016/s1044-7431(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 62.Nie D, Di NA, Han JM, Baharanyi H, Kramvis I, Huynh T, Dabora S, Codeluppi S, Pandolfi PP, Pasquale EB, Sahin M. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat. Neurosci. 2010;13:163–172. doi: 10.1038/nn.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat. Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- 64.Batlle E, Bacani J, Begthel H, Jonkeer S, Gregorieff A, van de BM, Malats N, Sancho E, Boon E, Pawson T, Gallinger S, Pals S, Clevers H. EphB receptor activity suppresses colorectal cancer progression. Nature. 2005;435:1126–1130. doi: 10.1038/nature03626. [DOI] [PubMed] [Google Scholar]
- 65.Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat. Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- 66.Brantley-Sieders DM, Zhuang G, Hicks D, Fang WB, Hwang Y, Cates JM, Coffman K, Jackson D, Bruckheimer E, Muraoka-Cook RS, Chen J. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J. Clin. Invest. 2008;118:64–78. doi: 10.1172/JCI33154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukai J, Yokote H, Yamanaka R, Arao T, Nishio K, Itakura T. EphA4 promotes cell proliferation and migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol. Cancer Ther. 2008;7:2768–2778. doi: 10.1158/1535-7163.MCT-07-2263. [DOI] [PubMed] [Google Scholar]
- 68.Miao H, Li D-Q, Mukherjee A, Guo H, Petty A, Basilion J, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B. EphA2 Mediates Ligand-Dependent Inhibition and Ligand-Independent Promotion of Cell Migration and Invasion via a Reciprocal Regulatory Loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith LM, Walsh PT, Rudiger T, Cotter TG, Mc Carthy TV, Marx A, O’Connor R. EphA3 is induced by CD28 and IGF-1 and regulates cell adhesion. Exp. Cell Res. 2004;292:295–303. doi: 10.1016/j.yexcr.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 70.Macrae M, Neve RM, Rodriguez-Viciana P, Haqq C, Yeh J, Chen C, Gray JW, McCormick F. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Miao H, Nickel CH, Cantley LG, Bruggeman LA, Bennardo LN, Wang B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J. Cell Biol. 2003;162:1281–1292. doi: 10.1083/jcb.200304018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang NY, Fernandez C, Richter M, Xiao Z, Valencia F, Tice DA, Pasquale EB. Crosstalk of the EphA2 receptor with a serine/threonine phosphatase suppresses the AktmTORC1 pathway in cancer cells. Cell Signal. 2011;23:201–212. doi: 10.1016/j.cellsig.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tong J, Elowe S, Nash P, Pawson T. Manipulation of EphB2 regulatory motifs and SH2 binding sites switches MAPK signaling and biological activity. J. Biol. Chem. 2003;278:6111–6119. doi: 10.1074/jbc.M208972200. [DOI] [PubMed] [Google Scholar]
- 74.Park EK, Warner N, Bong YS, Stapleton D, Maeda R, Pawson T, Daar IO. Ectopic EphA4 receptor induces posterior protrusions via FGF signaling in Xenopus embryos. Mol. Biol. Cell. 2004;15:1647–1655. doi: 10.1091/mbc.E03-09-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li JJ, Liu DP, Liu GT, Xie D. EphrinA5 acts as a tumor suppressor in glioma by negative regulation of epidermal growth factor receptor. Oncogene. 2009;28:1759–1768. doi: 10.1038/onc.2009.15. [DOI] [PubMed] [Google Scholar]
- 76.Marler KJ, Becker-Barroso E, Martinez A, Llovera M, Wentzel C, Poopalasundaram S, Hindges R, Soriano E, Comella J, Drescher U. A TrkB/EphrinA interaction controls retinal axon branching and synaptogenesis. J. Neurosci. 2008;28:12700–12712. doi: 10.1523/JNEUROSCI.1915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brisbin S, Liu J, Boudreau J, Peng J, Evangelista M, Chin-Sang I. A role for C. elegans Eph RTK signaling in PTEN regulation. Dev. Cell. 2009;17:459–469. doi: 10.1016/j.devcel.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 78.Chiarugi P, Taddei ML, Schiavone N, Papucci L, Giannoni E, Fiaschi T, Capaccioli S, Raugei G, Ramponi G. LMW-PTP is a positive regulator of tumor onset and growth. Oncogene. 2004;23:3905–3914. doi: 10.1038/sj.onc.1207508. [DOI] [PubMed] [Google Scholar]
- 79.Kikawa KD, Vidale DR, Van Etten RL, Kinch MS. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J. Biol. Chem. 2002;277:39274–39279. doi: 10.1074/jbc.M207127200. [DOI] [PubMed] [Google Scholar]
- 80.Nievergall E, Janes PW, Stegmayer C, Vail ME, Haj FG, Teng SW, Neel BG, Bastiaens PI, Lackmann M. PTP1B regulates Eph receptor function and trafficking. J. Cell Biol. 2010;191:1189–1203. doi: 10.1083/jcb.201005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shintani T, Ihara M, Sakuta H, Takahashi H, Watakabe I, Noda M. Eph receptors are negatively controlled by protein tyrosine phosphatase receptor type O. Nat. Neurosci. 2006 doi: 10.1038/nn1697. [DOI] [PubMed] [Google Scholar]
- 82.Wimmer-Kleikamp SH, Nievergall E, Gegenbauer K, Adikari S, Mansour M, Yeadon T, Boyd AW, Patani NR, Lackmann M. Elevated protein tyrosine phosphatase activity provokes Eph/ephrin-facilitated adhesion of pre-B leukemia cells. Blood. 2008;112:721–732. doi: 10.1182/blood-2007-11-121681. [DOI] [PubMed] [Google Scholar]
- 83.Zhuang G, Hunter S, Hwang Y, Chen J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-Kinase-dependent Rac1 activation. J. Biol. Chem. 2007;282:2683–2694. doi: 10.1074/jbc.M608509200. [DOI] [PubMed] [Google Scholar]
- 84.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 85.Coulthard MG, Lickliter JD, Subanesan N, Chen K, Webb GC, Lowry AJ, Koblar S, Bottema CD, Boyd AW. Characterization of the Epha1 receptor tyrosine kinase: expression in epithelial tissues. Growth Factors. 2001;18:303–317. doi: 10.3109/08977190109029118. [DOI] [PubMed] [Google Scholar]
- 86.Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem. Biophys. Res. Commun. 2004;318:882–892. doi: 10.1016/j.bbrc.2004.04.102. [DOI] [PubMed] [Google Scholar]
- 87.Herath NI, Spanevello MD, Sabesan S, Newton T, Cummings M, Duffy S, Lincoln D, Boyle G, Parsons PG, Boyd AW. Over-expression of Eph and ephrin genes in advanced ovarian cancer: ephrin gene expression correlates with shortened survival. BMC. Cancer. 2006;6:144. doi: 10.1186/1471-2407-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis [In Process Citation] Oncogene. 2000;19:5614–5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 89.Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr. Cancer Drug Targets. 2005;5:149–157. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- 90.Nakamoto M, Bergemann AD. Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc. Res. Tech. 2002;59:58–67. doi: 10.1002/jemt.10177. [DOI] [PubMed] [Google Scholar]
- 91.Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int. J. Cancer. 2003;103:657–663. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- 92.Saito T, Masuda N, Miyazaki T, Kanoh K, Suzuki H, Shimura T, Asao T, Kuwano H. Expression of EphA2 and E-cadherin in colorectal cancer: correlation with cancer metastasis. Oncol. Rep. 2004;11:605–611. [PubMed] [Google Scholar]
- 93.Fang WB, Brantley-Sieders DM, Parker MA, Reith AD, Chen J. A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene. 2005:7859–7868. doi: 10.1038/sj.onc.1208937. [DOI] [PubMed] [Google Scholar]
- 94.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol. Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 95.Parri M, Buricchi F, Giannoni E, Grimaldi G, Mello T, Raugei G, Ramponi G, Chiarugi P. EphrinA1 activates a Src/focal adhesion kinase-mediated motility response leading to rho-dependent actino/myosin contractility. J. Biol. Chem. 2007;282:19619–19628. doi: 10.1074/jbc.M701319200. [DOI] [PubMed] [Google Scholar]
- 96.Wang B. Cancer cells exploit the eph-ephrin system to promote invasion and metastasis: tales of unwitting partners. Sci. Signal. 2011;4:e28. doi: 10.1126/scisignal.2002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bae HJ, Song JH, Noh JH, Kim JK, Jung KH, Eun JW, Xie HJ, Ryu JC, Ahn YM, Kim SY, Lee SH, Yoo NJ, Lee JY, Park WS, Nam SW. Low frequency mutation of the Ephrin receptor A3 gene in hepatocellular carcinoma. Neoplasma. 2009;56:331–334. doi: 10.4149/neo_2009_04_331. [DOI] [PubMed] [Google Scholar]
- 98.Balakrishnan A, Bleeker FE, Lamba S, Rodolfo M, Daniotti M, Scarpa A, van Tilborg AA, Leenstra S, Zanon C, Bardelli A. Novel somatic and germline mutations in cancer candidate genes in glioblastoma, melanoma, and pancreatic carcinoma. Cancer Res. 2007;67:3545–3550. doi: 10.1158/0008-5472.CAN-07-0065. [DOI] [PubMed] [Google Scholar]
- 99.Bonifaci N, Gorski B, Masojc B, Wokolorczyk D, Jakubowska A, Debniak T, Berenguer A, Serra MJ, Brunet J, Dopazo J, Narod SA, Lubinski J, Lazaro C, Cybulski C, Pujana MA. Exploring the link between germline and somatic genetic alterations in breast carcinogenesis. PLoS. One. 2010;5:e14078. doi: 10.1371/journal.pone.0014078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oshima T, Akaike M, Yoshihara K, Shiozawa M, Yamamoto N, Sato T, Akihito N, Nagano Y, Fujii S, Kunisaki C, Wada N, Rino Y, Tanaka K, Masuda M, Imada T. Overexpression of EphA4 gene and reduced expression of EphB2 gene correlates with liver metastasis in colorectal cancer. Int. J. Oncol. 2008;33:573–577. [PubMed] [Google Scholar]
- 102.Fu DY, Wang ZM, Wang BL, Chen L, Yang WT, Shen ZZ, Huang W, Shao ZM. Frequent epigenetic inactivation of the receptor tyrosine kinase EphA5 by promoter methylation in human breast cancer. Hum. Pathol. 2010;41:48–58. doi: 10.1016/j.humpath.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 103.Guan M, Xu C, Zhang F, Ye C. Aberrant methylation of EphA7 in human prostate cancer and its relation to clinicopathologic features. Int. J. Cancer. 2009;124:88–94. doi: 10.1002/ijc.23890. [DOI] [PubMed] [Google Scholar]
- 104.Morrissey C, True LD, Roudier MP, Coleman IM, Hawley S, Nelson PS, Coleman R, Wang YC, Corey E, Lange PH, Higano CS, Vessella RL. Differential expression of angiogenesis associated genes in prostate cancer bone, liver and lymph node metastases. Clin. Exp. Metastasis. 2008;25:377–388. doi: 10.1007/s10585-007-9116-4. [DOI] [PubMed] [Google Scholar]
- 105.Zhang W, Zeng X, Briggs KJ, Beaty R, Simons B, Yen R. W. Chiu, Tyler MA, Tsai HC, Ye Y, Gesell GS, Herman JG, Baylin SB, Watkins DN. A potential tumor suppressor role for Hic1 in breast cancer through transcriptional repression of ephrin-A1. Oncogene. 2010;29:2467–2476. doi: 10.1038/onc.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi L, Itoh F, Itoh S, Takahashi S, Yamamoto M, Kato M. Ephrin-A1 promotes the malignant progression of intestinal tumors in Apc(min/+) mice. Oncogene. 2008;27:3265–3273. doi: 10.1038/sj.onc.1210992. [DOI] [PubMed] [Google Scholar]
- 107.Li X, Wang L, Gu JW, Li B, Liu WP, Wang YG, Zhang X, Zhen HN, Fei Z. Up-regulation of EphA2 and down-regulation of EphrinA1 are associated with the aggressive phenotype and poor prognosis of malignant glioma. Tumour. Biol. 2010;31:477–488. doi: 10.1007/s13277-010-0060-6. [DOI] [PubMed] [Google Scholar]
- 108.Almog N, Ma L, Raychowdhury R, Schwager C, Erber R, Short S, Hlatky L, Vajkoczy P, Huber PE, Folkman J, Abdollahi A. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 109.Bruce V, Olivieri G, Eickelberg O, Miescher GC. Functional activation of EphA5 receptor does not promote cell proliferation in the aberrant EphA5 expressing human glioblastoma U-118 MG cell line. Brain Res. 1999;821:169–176. doi: 10.1016/s0006-8993(99)01112-9. [DOI] [PubMed] [Google Scholar]
- 110.Sharfe N, Freywald A, Toro A, Dadi H, Roifman C. Ephrin stimulation modulates T cell chemotaxis. Eur. J. Immunol. 2002;32:3745–3755. doi: 10.1002/1521-4141(200212)32:12<3745::AID-IMMU3745>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 111.Aasheim HC, Delabie J, Finne EF. Ephrin-A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood. 2005;105:2869–2876. doi: 10.1182/blood-2004-08-2981. [DOI] [PubMed] [Google Scholar]
- 112.de Saint-Vis B, Bouchet C, Gautier G, Valladeau J, Caux C, Garrone P. Human dendritic cells express neuronal Eph receptor tyrosine kinases: role of EphA2 in regulating adhesion to fibronectin. Blood. 2003;102:4431–4440. doi: 10.1182/blood-2003-02-0500. [DOI] [PubMed] [Google Scholar]
- 113.Kuang SQ, Bai H, Fang ZH, Lopez G, Yang H, Tong W, Wang ZZ, Garcia-Manero G. Aberrant DNA methylation and epigenetic inactivation of Eph receptor tyrosine kinases and ephrin ligands in acute lymphoblastic leukemia. Blood. 2010;115:2412–2419. doi: 10.1182/blood-2009-05-222208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trinidad EM, Zapata AG, Alonso-Colmenar LM. Eph-ephrin bidirectional signaling comes into the context of lymphocyte transendothelial migration. Cell Adh. Migr. 2010;4:363–367. doi: 10.4161/cam.4.3.11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Udayakumar D, Zhang G, Ji Z, Njauw CN, Mroz P, Tsao H. Epha2 is a critical oncogene in melanoma. Oncogene. 2011 doi: 10.1038/onc.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Noren NK, Yang NY, Silldorff M, Mutyala R, Pasquale EB. Ephrin-independent regulation of cell substrate adhesion by the EphB4 receptor. Biochem. J. 2009;422:433–442. doi: 10.1042/BJ20090014. [DOI] [PMC free article] [PubMed] [Google Scholar]