Abstract
AIM: To investigate the reliability of massive hepatectomy models by using clip techniques.
METHODS: We analyzed anatomical findings in 100 mice following massive hepatectomy induced by liver reduction > 70%. The impact of various factors in the different models was also analyzed, including learning curves, operative time, survival curves, and histopathological findings.
RESULTS: According to anatomical results, models with 75%, 80%, and 90% hepatectomy produced massive hepatectomy. Learning curves and operative times were most optimal with the clip technique. Each hepatectomy performed using the clip technique produced a reasonable survival curve, and there were no differences in histopathological findings between the suture and clip techniques.
CONCLUSION: Massive hepatectomy by the clip technique is simple and can provide reliable and relevant data.
Keywords: Hepatectomy, Animal model, Clip, Microsurgery, Surgical technique
INTRODUCTION
New insights into mechanisms in the hepatology field have been established from experimental animal models. The rodent hepatectomy model is mainly employed to examine liver regeneration, liver failure, and tumor metastasis, and the 70% hepatectomy by en bloc ligation of the lobes is well established in the rat[1,2]. However, mouse models allow for the use of gene-altered or knockout strains and for laboratory assays, due to the development of specific agents and antibodies[3]. At present, there are a number of murine hepatectomy models[4-10], of which the 70% hepatectomy (termed 2/3 partial hepatectomy) is most common. The introduction of microsurgical techniques has also enabled high rates of successful surgery, especially in individualized dissection and ligation of vessels[6,11,12]. However, murine hepatectomy remains challenging because of the delicate nature of the liver, the lack of intravenous access, and the risk of hemorrhage[9], resulting in high rates of mortality and morbidity[5,13]. In a previous paper, the rate of complications was reported as approximately 30%[6,14,15]. Currently, innovative methods for the hepatectomy model have been documented, such as the ligation method[7,9,14,15] and clip technique[5].
Despite the widespread use of the 70% hepatectomy[4-7], alternative hepatectomy models with resected volumes > 70% (so-called, “massive hepatectomy”) are required to provide more clinically relevant experiments on liver regeneration and hepatic failure. Nikfarjam et al[5] reported benefits of the hemostatic clip in hepatectomy models with 37% and 70% of resected volumes in the mouse. Herein, we describe detailed surgical procedures of our institutional hepatectomy models with > 70% resection using suture hemostatic clip in the mouse and compare various factors between suture and clip techniques. Then, we discuss the usefulness of this simple and reproducible technique in hepatectomy models of > 70% resection.
MATERIALS AND METHODS
Animals
Inbred C57BL/6 mice (male, 10-20 wk of age, approximately 25 g body weight) were used (C57BL/6NHsd; Harlan Laboratories, Indianapolis, IN, United States). All mice were maintained under specific pathogen-free conditions. All experimental protocols were approved by IACUC at Mayo Clinic (Protocol No. 33307 and 24907).
Hepatectomy by suture technique
General anesthesia was provided with an anesthesia system (VetEquip Inc., Pleasanton, CA, United States). Inhalational anesthesia was induced and maintained by isoflurane (Isoflurane USP, 250 mL; Webster Veterinary, Sterling, MA, United States). Isoflurane with oxygen flow at 5 L/min was used in the induction phase, and was reduced to 0.5-2.0 L/min in the maintenance phase. Body weight was measured after anesthesia. The abdominal wall was shaved and prepped with povidone-iodine. A transverse incision was performed and hemostasis was obtained using an electrocautery scalpel of bipolar type (Bipolar forceps, Martin ME 102 Electrosurgical unit and Bipolar Accessory Set for ME 102; Harvard Apparatus, Holliston, MA, United States) and that of monopolar type (Bovie, low temperature cautery kit; Aaron Medical, Clearwater, FL, United States). Mice that received only laparotomy served as a control group in the study. The gastrointestinal tract was moistened with warm saline, covered with gauze, and positioned to the outside of the left abdominal cavity. The liver was handled delicately with cotton-tipped applicators (Hardwood Products Company, Guilford, ME, United States). A surgical microscope at 5-10 × magnification (Surgical Scope M680, Type 10445496; Leica Microsystems Inc., Bannockburn, IL, United States) was used. Even though high magnification (12.5-20 × magnification) is not required, a sufficient light source was indispensable. We used fine-tipped and delicate scalpels which are suitable for ultra-microsurgery, i.e. structures including nerve or vessels < 0.5 mm in diameter[16]. The murine liver was divided into three lobes (right, left, and caudate lobes) and arranged into six segments[4-6,14,17]: right anterior segment (RAS), right middle segment (RMS), right posterior segment (RPS), left anterior segment (LAS), left posterior segment (LPS), and omental segment (OS) (Figure 1A and B). The falciform and triangular ligaments were sharply cut. The infra-hepatic vena cava runs into the RMS and RPS. The infra-hepatic inferior vena cava was skeletonized. The LPS was freed from the diaphragm and OS. The LAS and LPS were mobilized off the hepatic hilum and gastroduodenal tract.
Figure 1.
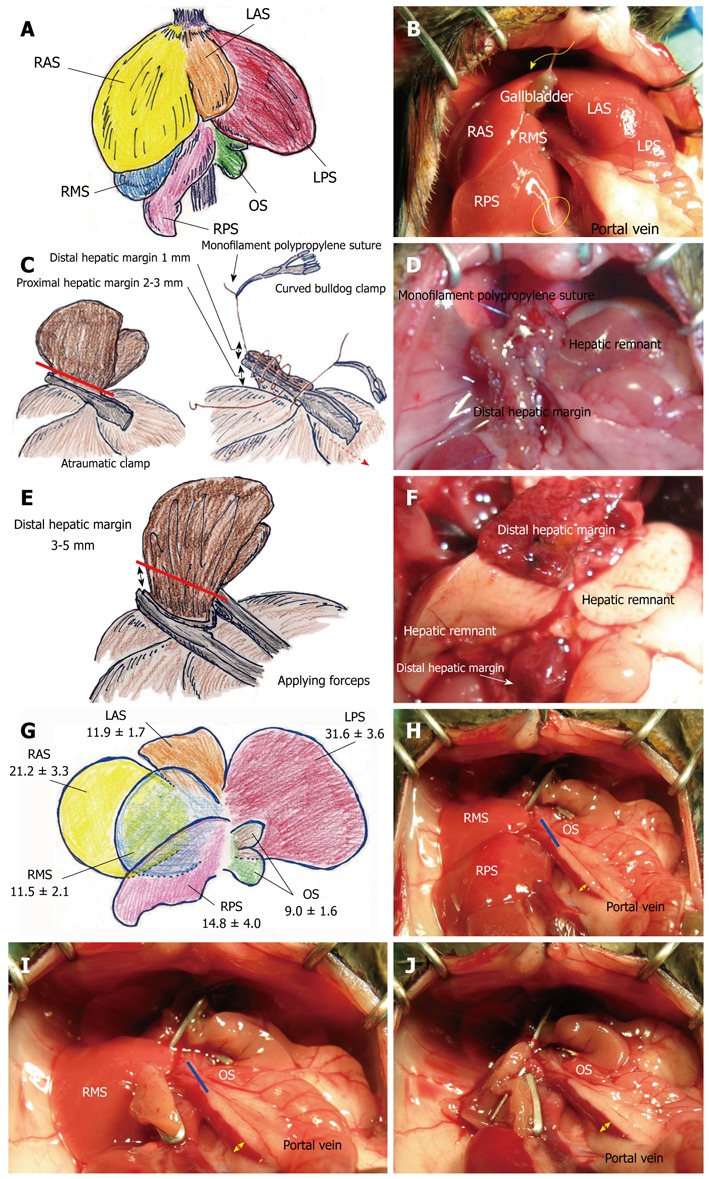
Hepatic segments and hepatectomy by suture or clip techniques. A: The murine liver is divided into six segments (RAS, RMS, RPS, LAS, LPS, and OS); B: The gallbladder is detected between the RAS and LAS; C: Atraumatic clamp was placed on hepatic segment, with optimal proximal hepatic margin of 2-3 mm from division of each segment. Clamped segment was sharply cut with 1 mm distal hepatic margin (red line). Sutures not involving the clamp were placed bilaterally. Bilateral sutures were ligated, and each suture was grasped with a curved bulldog clamp. By using suture of left-side ligation, cut surface of liver was sutured from left side to right side with loose continuous suture that involved a clamp, and the last suture reached the right side; D: Clamp was removed (dotted red arrow) and hepatic margin was immediately treated by tightening the continuous suture. The thread of tightened continuous suture was ligated with stay suture of right side without over-tightening. Continuous suture was made from right side to left side. The last suture was ligated with stay suture of left side without over-tightening; E: The relevant segment was cut with a 3-5 mm distal hepatic margin (red line); F: Ischemic change of distal hepatic margin at 24 h after 80% hepatectomy was shown. Although liver remnants after massive hepatectomy showed color change due to vacuolization, distal hepatic margins clearly showed necrotic changes. Color change between liver remnant and distal hepatic margin was more enhanced at autopsy; G: Percentages of total volume of each segment were 21.2% ± 3.3% for RAS, 11.5% ± 2.1% for RMS, 14.8% ± 4.0% for RPS, 11.9% ± 1.7% for LAS, 31.6% ± 3.6% for LPS, and 9.0% ± 1.6% for OS; H: Traditional 2/3 hepatectomy of RMS + RPS + OS (35.4% ± 4.0%) is shown. Additional resection of OS (blue line) will make a 75% hepatectomy (RMS + RPS, 26.4% ± 3.8 %); I: Hepatic remnant in 80% hepatectomy was RMS + OS (20.6% ± 2.6%). Additional resection of OS (blue line) will make an RMS-remnant 90% hepatectomy (RMS, 14.8% ± 4.0%); J: OS-remnant 90% hepatectomy is shown (OS, 9.0% ± 1.6%). Dilation of portal vein due to portal hypertension is confirmed in reverse proportion to volume of hepatic remnant (yellow arrows). LAS: Left anterior segment; LPS: Left posterior segment; OS: Omental segment; RAS: Right anterior segment; RMS: Right middle segment; RPS: Right posterior segment.
We did not use Pringle’s maneuver during the operation. Hepatectomy by suture technique requires a proximal hepatic margin for suture bait. An atraumatic clamp (Micro Vascular Clip, RS-6470 or RS-6472; Roboz Surgical Instrument Co., Gaithersburg, MD, United States) was placed on the hepatic segment, and the optimal proximal hepatic margin was 2-3 mm from the division of each segment. The clamped segment was sharply cut with a 1 mm distal hepatic margin (Figure 1C). Monofilament polypropylene sutures (7-0 Prolene, BV-1, 8304H-X, or 8-0 Prolene, BV130-5, 8732H; Ethicon, Inc., Somerville, NJ, United States) were bilaterally placed without involvement of a clamp. These bilateral sutures were ligated, and each suture was grasped with a curved bulldog clamp (Cooley Bulldog clamps, curved, serrated, MB586R or MB587R; V.Mueller, Aesculap Inc., Center Valley, PA, United States). The liver was sutured by a loose continuous suture that initially involved the clamp, and the last suture reached the right side. The clamp was removed and the suture was tightened and ligated with the stay suture (Figure 1D). The presence of bleeding points was carefully checked using a cotton swab. An additional suture was made with a monofilament nylon suture (10-0 Ethilon, BV130-3, 2820G; Ethicon, Inc.) for well-focused hemostasis if required. Microfibrillar collagen (Avitene; C.R. Bard, Inc., Murray Hill, NJ, United States) was used in hemostasis.
The intraperitoneal cavity and organs were washed with warm saline. The peritoneum and fascia were closed with a continuous suture using absorbable thread (5-0 Coated Vicryl Plus; Ethicon, Inc.). The skin layer was closed separately using the same method.
Hepatectomy by hemostatic clip technique
The preparation and mobilization of each liver segment was similarly performed as described above. M-sized hemostatic clips were used (Horizon Ligation System; Teleflex Medical, Durham, NC, United States). The hepatic segment was retracted using tissue forceps when the clip was applied. A hemostatic clip was applied near the point of division of each segment (Figure 1E), and the proximal hepatic margin was approximately 1 mm from a segmental division. The segment was then cut with a distal hepatic margin of 3-5 mm. An additional suture for complete hemostasis was not typically required. Even during surgery, color change of the clipped segment due to ischemia was confirmed, and the color change between remnant liver and distal hepatic margin was observed at autopsy (Figure 1F).
Post-operative care
A warmer (RightTemp, RTHS-SM; Kent Scientific Co., Torrington, CT, United States) was used to maintain body temperature immediately after surgery, and a heating pad (E12107; Sunbeam, Gainesville, FL, United States) was used to warm the cage until mice completely recovered from anesthesia and surgery. Each mouse was kept separately. Postoperative observation was performed every 2 h for the first 12 h after surgery, and thereafter every 4 h. An analgesic agent (0.1 μg/g body weight, i.m.; buprenorphine 100 μg/mL; Cerilliant, Round Rock, TX, United States) was routinely given intramuscularly every 12 h for 5 d after surgery. Antibiotics (30 μg/g body weight, i.p.; Cephalexin Hydrate; MP Biomedicals, Cleveland, OH, United States) was administered every 12 h for 24 h after surgery. For each mouse, 1 mL of lactate Ringer’s solution (Lactated Ringer’s Injection USP; B. Braun Medical Inc., Irvine, CA, United States) was routinely administered every 6 h for 24 h.
Assessments and data analysis
We weighed each segment in a total of 100 mice, and segmental percentages were calculated as segmental weight (g)/whole liver weight (g) for each mouse. The extent of the hepatectomy is based on the segmental volumes. Of note, the gallbladder can be easily detected in the mouse between the RAS and the LAS (Figure 1B), while the rat does not have a gallbladder.
The learning curve for surgery is important for reliability of the hepatectomy model. To compare hepatectomy models using the suture technique vs the clip technique, we examined learning curves and operative time.
Rare complications of outflow block or biliary obstruction due to surgical clips were previously reported[18,19]. To confirm that the surgical issues were due to the clip itself, it was proposed that the clip may secondarily obstruct outflow or biliary ducts near the proximal hepatic margin or hepatic remnant. At first, we checked histopathological findings of the hepatic remnant near the proximal hepatic margin and the distal hepatic margin in the hepatectomy model by suture and clip techniques.
Focal and/or patchy necrosis is an important histopathological finding after hepatectomy[4,20]. To calculate the rate of the appearance of focal and/or patchy necrosis in hepatectomy models by the suture and clip techniques, we examined ten cases for each technique on the same day. Histopathological analyses and calculations were performed at 6 h after hepatectomy. These examinations were repeated three times.
In this study, data are presented as mean ± SD. Student’s t test was used for the comparisons of unpaired variables between the two groups. A survival curve was made by the Kaplan-Meier method, and the log-rank test was used for the comparisons of survival rates between two groups. Statistical calculations were performed using StatView Version 5.0 (SAS, Cary, NC, United States). A P value < 0.05 was considered statistically significant.
RESULTS
Measurement of the weight of each segment
The percentages of total volume for each of the segments were 21.2% ± 3.3% for RAS, 11.5% ± 2.1% for RMS, 14.8% ± 4.0% for RPS, 11.9% ± 1.7% for LAS, 31.6% ± 3.6% for LPS, and 9.0% ± 1.6% for OS (Figure 1G).
Hepatectomy models with > 70% resection in the mouse
Based on our results in the measurement of segmental weights, we calculated the liver volume of resection and the hepatic remnant. The hepatic remnant in the 75% hepatectomy was RMS + RPS (26.4% ± 3.8%); while for traditional 2/3 hepatectomy the remnant was RMS + RPS + OS (35.4% ± 4.0%) (Figure 1H). Hepatic remnant in the 80% hepatectomy was RMS + OS (20.6% ± 2.6%) (Figure 1I). We had two surgical options for 90% hepatectomy by sparing either OS (9.0% ± 1.6%) or RMS (11.5% ± 2.1%) (Figure 1J). Portal venous dilatation inversely proportional to the volume of the hepatic remnant was observed.
Learning curves
For each technique, ten cases were performed on the same day as one unit for training. The required units that showed no statistical differences in survival curves after hepatectomy with those at the next unit were analyzed. The survival curves of hepatectomy after the training period were compared between the two techniques.
Survival curves of each batch of 10 cases at early term in training in the 80% hepatectomy by the suture technique, and statistical results, are shown in Figure 2A. The P values between the first 10 cases and the second 10 cases, between the second 10 cases and the third 10 cases, between the third 10 cases and the fourth 10 cases, and between the fourth 10 cases and the fifth 10 cases were 0.0445, 0.0471, 0.4790, and 0.8941, respectively. The first and second units showed statistical differences in comparison with the next unit. Survival curves of each batch of 10 cases at early term in training in the 80% hepatectomy by the clip technique, and statistical results, are shown in Figure 2B. The P values between the first 10 cases and the second 10 cases, between the second 10 cases and the third 10 cases, between the third 10 cases and the fourth 10 cases, and between the fourth 10 cases and the fifth 10 cases were 0.0363, 0.6854, 0.9127, and 0.9007, respectively. Only the first unit showed statistical differences in comparison with the second unit.
Figure 2.
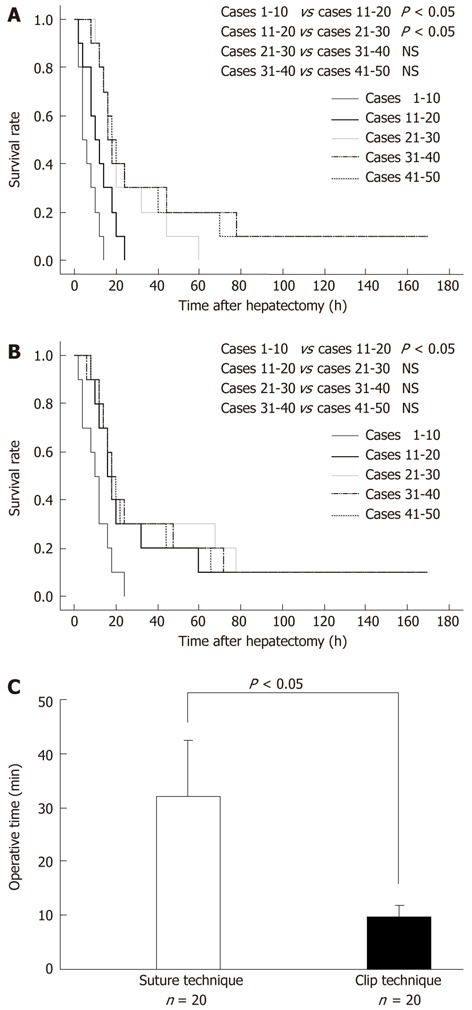
Survival outcomes in 80% hepatectomy after initial training. A: In 80% hepatectomy by suture technique, survival curves of each 10 cases at early term in training are shown; B: In 80% hepatectomy by the clip technique, survival curves of each 10 cases at early term in training are shown; C: In each technique, operative time was investigated in 20 cases of 80% hepatectomy. These data were corrected by experienced surgeons. Operative times of 80% hepatectomy by suture and clip techniques were significantly different (P < 0.0001). NS: Not significant.
In the 80% hepatectomy, the fifth batch of 10 cases (i.e., 10 cases after the experience of 40 cases) in the suture technique and the fourth batch of 10 cases (i.e., 10 cases after the experience of 30 cases) in the clip technique showed similar survival curves (P = 0.8516), even though 30 cases in the suture technique and 20 cases in the clip techniques seemed to be enough statistically. Our institution has similar results in the 90% hepatectomy model.
Operative time
Operative time data were also collected from experienced surgeons in 20 cases of 80% hepatectomy for each technique. Even with experienced surgeons, operative times for hepatectomy using the suture technique and the clip technique were significantly different (P < 0.0001) (Figure 2C). Operative times were clearly shortened in the clip techniques.
Survival curves after hepatectomy with > 70% resection
Actual survival curves of each hepatectomy are shown in Figure 3A and B. Twenty cases were followed for each hepatectomy by suture (Figure 3A) or clip technique (Figure 3B).
Figure 3.
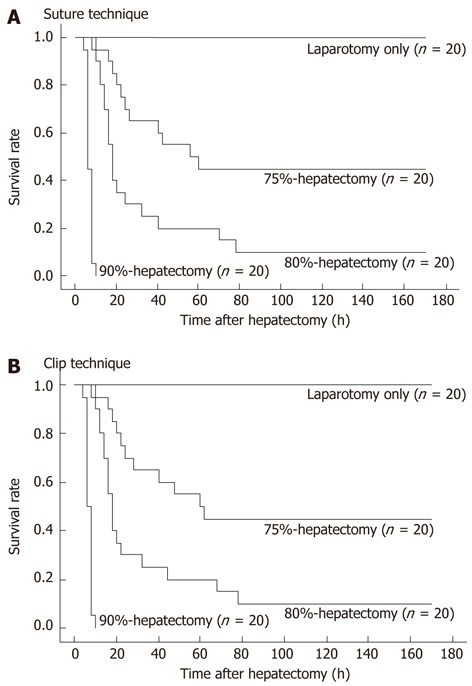
Actual survival curves after each hepatectomy with > 70% resection. A: Actual survival curves of each hepatectomy by suture technique; B: Actual survival curves of each hepatectomy by clip technique. NS: Not significant.
In the 90% hepatectomy, either the OS or RMS was left as the remnant. Although not shown, we observed no significant differences in survival curves between the OS-remnant and the RMS-remnant following the 90% hepatectomy using suture and clip techniques (P = 0.3784 and 0.3588, respectively).
In the 90% hepatectomy, although not shown, there were no significant differences in survival curves after hepatectomy by suture technique between the OS-remnant (n = 10) and the RMS-remnant (n = 10) (P = 0.3784). Similarly, there were no significant differences in survival curves after hepatectomy by clip technique between the OS-remnant (n = 10) and the RMS-remnant (n = 10) (P = 0.3588).
Histopathological findings of hepatic remnant and distal hepatic margin
Histopathological findings of the hepatic remnant near the proximal hepatic margin at 6 h after 80% hepatectomy by suture technique are shown in Figure 4A. No findings suggested obstructions by the suture, except for the changes consistent with hepatectomy. Histopathological findings of the distal hepatic margin showed severe ischemic and massive necroses at 6 h after 80% hepatectomy by the suture technique (Figure 4B).
Figure 4.
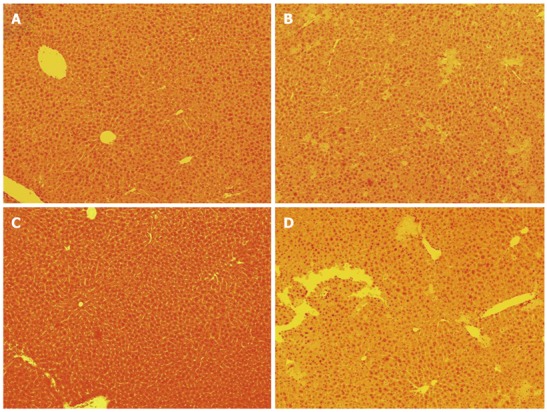
Histopathological findings of hepatic remnant near proximal hepatic margin and distal hepatic margins. Findings at 6 h after 80% hepatectomy are shown by hematoxylin and eosin staining at 100 × magnification. A: No findings suggested obstructions in hepatic remnant near proximal hepatic margin of hepatectomy by suture technique; B: In distal hepatic margin of hepatectomy by suture technique, severe ischemia and massive necrosis are confirmed; C: No findings suggested obstructions by hemostatic clip itself in clip-touched hepatic remnant near proximal hepatic margin of hepatectomy by clip technique; D: In distal hepatic margin of hepatectomy by clip technique, severe ischemia and massive necrosis are confirmed.
Histopathological findings of the clip-touched hepatic remnant near the proximal hepatic margin at 6 h after 80% hepatectomy by the clip technique are shown in Figure 4C. Again, no findings suggested obstructions by the hemostatic clip. Histopathological findings of the distal hepatic margin at 6 h after 80% hepatectomy by clip technique are shown in Figure 4D, showing severe ischemic and massive necroses as expected.
Focal and/or patchy necrosis after hepatectomy
Focal and patchy necrosis is usually observed as early as one hour after hepatectomy, as reported by others[20,21]. In our studies, we observed similar occurrence of focal necrosis following all types of massive hepatectomy. At 6 h after 70%, 80%, and 90% hepatectomies, there was no difference in the rate of appearance of focal and/or patchy necrosis between the suture technique and the clip technique (P = 0.6202; data not shown).
DISCUSSION
A number of different survival curves have been previously reported following hepatectomy with > 70% resection in the mouse. Differences in study design and institutional methodology, as well as various complications, may explain these discrepancies[4]. As such, we recommend that survival curves should be checked beforehand according to institutional methodology and study design in each laboratory. In our institution we have two types of 90% hepatectomy models available, while other models of approximately 90% hepatectomy exist[1,7]. Anatomical analysis of each institution’s animal strain is crucial for development of reliable models. All mice that receive 90% hepatectomy eventually die after surgery, although 90% hepatectomy has some advantages, especially in studies of liver failure and hepatic surgery. Inderbitzin et al[8] reported that the OS exhibits different behaviors according to the degree of liver-volume, and will not work in small-volume hepatectomy. Currently, our massive hepatectomy models employed the OS in only the 80% hepatectomy model, because we have a surgical option in the 90% hepatectomy model. In our experience, the OS of some mice exhibit few branches from veins, except for the portal vein, because swelling due to portal hypertension was often mild even after 90% hepatectomy, and color change was often not enhanced after ligation of the portal venous trunk. Overall, we consider that RMS-remnant 90% hepatectomy will provide more relevant data from the viewpoint of strict effects (i.e., liver damage) due to portal hypertension.
The outcomes of murine hepatectomy can vary greatly if surgical procedures are not performed properly. It is difficult to strictly estimate the stability of surgery, because the learning term may be still not enough, even if statistical analysis showed no differences. Based on our experience, we speculate that approximately 40 cases are required in hepatectomy by the suture technique for initial achievement of reliable survival curves, and that approximately 50 or more cases are required to start the study. By contrast, approximately 30 or 40 cases seem to be enough in hepatectomy by the clip technique for reliable samples. Our results of operative time for hepatectomy clearly indicated that the procedures in the suture technique were more complicated than those in the clip method. Overall, we suggest that the clip technique is advantageous even in hepatectomy models with > 70% resection because of its simplicity.
The hepatocyte is the first cell to start the proliferation reaction after hepatectomy[4]. Although histopathological injury can be confirmed from several hours after hepatectomy, some important signals for liver regeneration start in the later phase. As such, a suitable hepatectomy model must be selected according to experimental purpose. Jin et al[22] reported no focal and/or patchy necrosis even after hepatectomy models with 70% and approximately 90% resections, although this necrosis is an important finding after hepatectomy[4,20]. These histopathological findings are confirmed several hours after hepatectomy[4,20]. However, in our study a few mice that received 80% or 75% hepatectomy did not demonstrate necrosis, while all mice that received 90% hepatectomy showed necrosis in the early postoperative period. These discrepancies may be consistent with survival curves because a few mice that received 80% or 75% hepatectomy survived long term. These survivors can be used to provide liver samples with long-term observation after hepatectomy with > 70% resection and are useful for many studies that require long recovery times after hepatectomy with > 70% resection. Based on our results of survival curves and histopathological findings in comparison with suture technique, clip method seems to provide reliable and relevant data, even in murine hepatectomy models with > 70% resection.
Focal and patchy necroses are also observed as secondary findings after biliary stasis and/or congestion by outflow obstruction. Some surgical problems, including inflow and outflow obstructions, massive bleeding, and bile stasis, will invalidate results. All mice move well after complete recovery, and it is possible that the clip may slide following aggressive movements after recovery from anesthesia and surgery, although our results showed similar phenomena between the clip and suture techniques. During surgery, the hepatic margin may be changed according to clip size. Large-sized clips require more proximal hepatic margin for flow safety and more distal hepatic margin for complete hemostasis. Although we often treat the RAS and LAS in an en bloc manner using an ML-sized clip, only one of the smallest feasible clips to one segment should be used to prevent unexpected surgical issues. Surgeons should also note any unexpected findings after hepatectomy procedures. A dilated infra-hepatic inferior vena cava is a sign of disturbed flow of the inferior vena cava, because clipping of the RAS, LAS and LPS may cause a twist of the supra-hepatic inferior vena cava, while clipping of the RMS and RPS may cause stenosis of the hepatic vena cava. Segmental congestion and partial color change in the liver also suggest outflow and/or inflow obstruction. During autopsy, unexpected color changes of the liver, massive coagula, and bile leakage are informative for unstable postoperative course. To produce reliable samples, mice that indicate unexpected signs during surgery or autopsy should not be used to take samples.
In conclusion, our clip technique for murine hepatectomy models with > 70% resection (i.e., 75%, 80%, and 90% hepatectomy models) is simple and requires only basic surgical skills. Moreover, this technique provides reproducible results in comparison with the suture technique.
ACKNOWLEDGMENTS
We are grateful to Dennis W Dickson, Monica Castanedes-Casey, Virginia R Phillips, Linda G Rousseau, and Melissa E Murray (Department of Neuroscience, Mayo Clinic, Jacksonville, FL, United States) for their technical support in histopathological evaluations.
COMMENTS
Background
New insights into mechanisms in the hepatology field have been established from experimental animal models. Reliable models for massive hepatectomy in the mouse are required for experimental liver research.
Research frontiers
Despite the widespread use of the 70% hepatectomy, alternative hepatectomy models with resected volumes > 70% (so-called, “massive hepatectomy”) are required to provide more clinically relevant experiments on liver regeneration and hepatic failure.
Innovations and breakthroughs
In 2004, Nikfarjam et al reported benefits of the hemostatic clip in hepatectomy models with 37% and 70% of resected volumes in the mouse. Herein, the authors describe detailed surgical procedures of our institutional hepatectomy models with > 70% resection using suture hemostatic clips in the mouse and compare various factors between suture and clip techniques. The clip technique for murine hepatectomy models with > 70% resection is simple and requires only basic surgical skills.
Applications
The clip technique provides reproducible results in comparison with suture technique, even for massive hepatectomy in the mouse. This technique has an advantage in the simplicity of surgical procedures.
Peer review
The authors presented mouse experimental models for hepatectomy exceeding 70%. They evaluated two techniques of hepatectomy, which is suture technique and clip technique. They concluded that clip technique was superior to suture technique. The manuscript was well organized and well written.
Footnotes
Supported by Grants to Justin H Nguyen from the Deason Foundation, Sandra and Eugene Davenport, Mayo Clinic CD CRT-II and NIH R01NS051646-01A2; partial grant to Tomohide Hori from the Uehara Memorial Foundation, No. 200940051, Tokyo 171-0033, Japan
Peer reviewers: Kenji Miki, MD, Department of Surgery, Showa General Hospital, 2-450 Tenjin-cho, Kodaira, Tokyo 187-8510, Japan; Misha Luyer, MD, PhD, Department of Surgery, Orbis Medical Centre, Postbus 5500, 6130 MB, Sittard, The Netherlands
S- Editor Cheng JX L- Editor Rutherford A E- Editor Zhang DN
References
- 1.Higgins G, Anderson R. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch path Lab Med. 1931;12:186–202. [Google Scholar]
- 2.Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg. 2006;244:89–98. doi: 10.1097/01.sla.0000218093.12408.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hori T, Nguyen JH, Zhao X, Ogura Y, Hata T, Yagi S, Chen F, Baine AM, Ohashi N, Eckman CB, et al. Comprehensive and innovative techniques for liver transplantation in rats: a surgical guide. World J Gastroenterol. 2010;16:3120–3132. doi: 10.3748/wjg.v16.i25.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 5.Nikfarjam M, Malcontenti-Wilson C, Fanartzis M, Daruwalla J, Christophi C. A model of partial hepatectomy in mice. J Invest Surg. 2004;17:291–294. doi: 10.1080/08941930490502871. [DOI] [PubMed] [Google Scholar]
- 6.Martins PN, Neuhaus P. Hepatic lobectomy and segmentectomy models using microsurgical techniques. Microsurgery. 2008;28:187–191. doi: 10.1002/micr.20478. [DOI] [PubMed] [Google Scholar]
- 7.Makino H, Togo S, Kubota T, Morioka D, Morita T, Kobayashi T, Tanaka K, Shimizu T, Matsuo K, Nagashima Y, et al. A good model of hepatic failure after excessive hepatectomy in mice. J Surg Res. 2005;127:171–176. doi: 10.1016/j.jss.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Inderbitzin D, Studer P, Sidler D, Beldi G, Djonov V, Keogh A, Candinas D. Regenerative capacity of individual liver lobes in the microsurgical mouse model. Microsurgery. 2006;26:465–469. doi: 10.1002/micr.20271. [DOI] [PubMed] [Google Scholar]
- 9.Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- 10.Boyce S, Harrison D. A detailed methodology of partial hepatectomy in the mouse. Lab Anim ( NY) 2008;37:529–532. doi: 10.1038/laban1108-529. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez G, Lorente L, Durán HJ, Aller MA, Arias J. A 70% hepatectomy in the rat using a microsurgical technique. Int Surg. 1999;84:135–138. [PubMed] [Google Scholar]
- 12.Aller MA, Mendez M, Nava MP, Lopez L, Arias JL, Arias J. The value of microsurgery in liver research. Liver Int. 2009;29:1132–1140. doi: 10.1111/j.1478-3231.2009.02078.x. [DOI] [PubMed] [Google Scholar]
- 13.Cornell RP, Liljequist BL, Bartizal KF. Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology. 1990;11:916–922. doi: 10.1002/hep.1840110603. [DOI] [PubMed] [Google Scholar]
- 14.Martins PN, Theruvath TP, Neuhaus P. Rodent models of partial hepatectomies. Liver Int. 2008;28:3–11. doi: 10.1111/j.1478-3231.2007.01628.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Zhang M, Xia L, Xia Q. The benefits of ligating the lobar portal triads before partial hepatectomy in the mouse. J Invest Surg. 2010;23:224–227. doi: 10.3109/08941930903469433. [DOI] [PubMed] [Google Scholar]
- 16.Mehdorn H, Muller H. Microsurgical exercises (basic techniques, anastomoses, refertilization, transplantation) New York: Thieme Medical Publishers; 1989. [Google Scholar]
- 17.Martins PN, Neuhaus P. Surgical anatomy of the liver, hepatic vasculature and bile ducts in the rat. Liver Int. 2007;27:384–392. doi: 10.1111/j.1478-3231.2006.01414.x. [DOI] [PubMed] [Google Scholar]
- 18.Kerr JM, NiMhuircheartaigh NM, McEntee GP, Fenlon HM. Extension of hepatic necrosis secondary to current arcing to surgical clips: a potential complication of radiofrequency ablation. Abdom Imaging. 2009;34:491–493. doi: 10.1007/s00261-008-9421-7. [DOI] [PubMed] [Google Scholar]
- 19.Herline AJ, Fisk JM, Debelak JP, Shull HJ, Chapman WC. Surgical clips: a cause of late recurrent gallstones. Am Surg. 1998;64:845–848. [PubMed] [Google Scholar]
- 20.Rudich N, Zamir G, Pappo O, Shlomai Z, Faroja M, Weiss ID, Wald H, Galun E, Peled A, Wald O. Focal liver necrosis appears early after partial hepatectomy and is dependent on T cells and antigen delivery from the gut. Liver Int. 2009;29:1273–1284. doi: 10.1111/j.1478-3231.2009.02048.x. [DOI] [PubMed] [Google Scholar]
- 21.Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142–149. doi: 10.1016/s0039-6060(97)90283-x. [DOI] [PubMed] [Google Scholar]
- 22.Jin X, Zhang Z, Beer-Stolz D, Zimmers TA, Koniaris LG. Interleukin-6 inhibits oxidative injury and necrosis after extreme liver resection. Hepatology. 2007;46:802–812. doi: 10.1002/hep.21728. [DOI] [PubMed] [Google Scholar]


