Abstract
AIM: To establish a simple model consisting of the routine laboratory variables to predict both minimal fibrosis and cirrhosis in chronic hepatitis B virus (HBV)-infected patients.
METHODS: We retrospectively investigated 114 chronic HBV-infected patients who underwent liver biopsy in two different hospitals. Thirteen parameters were analyzed by step-wise regression analysis and correlation analysis. A new fibrosis index [globulin/platelet (GP) model] was developed, including globulin (GLOB) and platelet count (PLT). GP model = GLOB (g/mL) × 100/PLT (× 109/L). We evaluated the receiver operating characteristics analysis used to predict minimal fibrosis and compared six other available models.
RESULTS: Thirteen clinical biochemical and hematological variables [sex, age, PLT, alanine aminotransferase, aspartate aminotransferase (AST), albumin, GLOB, total bilirubin (T.bil), direct bilirubin (D.bil), glutamyltransferase, alkaline phosphatase, HBV DNA and prothrombin time (PT)] were analyzed according to three stages of liver fibrosis (F0-F1, F2-F3 and F4). Bivariate Spearman’s rank correlation analysis showed that six variables, including age, PLT, T.bil, D.bil, GLOB and PT, were correlated with the three fibrosis stages (FS). Correlation coefficients were 0.23, -0.412, 0.208, 0.220, 0.314 and 0.212; and P value was 0.014, < 0.001, 0.026, 0.018, 0.001 and 0.024, respectively. Univariate analysis revealed that only PLT and GLOB were significantly different in the three FS (PLT: F = 11.772, P < 0.001; GLOB: F = 6.612, P = 0.002). Step-wise multiple regression analysis showed that PLT and GLOB were also independently correlated with FS (R2 = 0.237). By Spearman’s rank correlation analysis, GP model was significantly correlated with the three FS (r = 0.466, P < 0.001). The median values in F0-F1, F2-F3 and F4 were 1.461, 1.720 and 2.634. Compared with the six available models (fibrosis index, AST-platelet ratio, FIB-4, fibrosis-cirrhosis index and age-AST model and age-PLT ratio), GP model showed a highest correlation coefficient. The sensitivity and positive predictive value at a cutoff value < 1.68 for predicting minimal fibrosis F0-F1 were 72.4% and 71.2%, respectively. The specificity and negative predictive value at a cutoff value < 2.53 for the prediction of cirrhosis were 84.5% and 96.7%. The area under the curve (AUC) of GP model for predicting minimal fibrosis and cirrhosis was 0.762 [95% confidence interval (CI): 0.676-0.848] and 0.781 (95% CI: 0.638-0.924). Although the differences were not statistically significant between GP model and the other models (P all > 0.05), the AUC of GP model was the largest among the seven models.
CONCLUSION: By establishing a simple model using available laboratory variables, chronic HBV-infected patients with minimal fibrosis and cirrhosis can be diagnosed accurately, and the clinical application of this model may reduce the need for liver biopsy in HBV-infected patients.
Keywords: Globulin, Platelet, Globulin/platelet model, Liver fibrosis, Noninvasive fibrosis biomarker, Chronic hepatitis B virus
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a global public health problem and it is estimated that approximately 350 000 000 people are infected with this virus in the world. More often, it may progress into liver cirrhosis and hepatocellular carcinoma (HCC). Although anti-virus therapy has reduced greatly the risk of cirrhosis and HCC, some positive hepatitis B surface antigen (HBsAg) carriers with less HBV-DNA may eventually develop cirrhosis and HCC[1,2]. Liver biopsy is not common. Patients with more than a 2-fold increase in aminotransferase levels were regarded as having severe liver necroinflammation. However, liver fibrosis was difficult to evaluate by noninvasive methods.
Liver fibrosis is known as the major problem causing morbidity and mortality in chronic HBV-infected patients. Once diagnosed, fibrosis should be treated as early as possible by appropriate methods. Although liver biopsy is being used as the gold standard in diagnosing the degree of fibrosis, not many patients are willing to undergo a liver biopsy because of its invasiveness and there might be inter- and intra-observer variability in the evaluation of specimens obtained[3], and a risk of complications (even if it is low) causing discomfort[4,5].
Some noninvasive methods are expected and used in patients with hepatitis C or B virus infection. The best results were obtained with liver stiffness measurement by means of transient elastography (TE) (FibroScan), or with FibroTest-ActiTest (Biopredictive, Labcorp)[4] and Fibrospect II (Prometheus)[5]. However, all these noninvasive methods are expensive and/or require equipments not widely used. Therefore, it is necessary to screen for simpler and cheaper methods for the examination of hepatic fibrosis. Poynard et al[6-10] investigated the correlations between the serum aminotransferases level, age, hyaluronic acid level, collagen level, platelet count and different fibrosis stages (FS), but did not draw clear conclusions. Several scoring systems like age-platelet count (PLT) ratio (AP index), aspartate aminotransferase (AST)-platelet ratio (APRI), age-AST model, fibrosis index (FI), FIB-4 and fibrosis-cirrhosis index (FCI), using different thresholds, have been proposed to detect presence or absence of fibrosis or cirrhosis in patients infected with hepatitis C virus (HCV) or HBV[11-18]. HBV-infected patients are prone to fibrosis; however, there have been few studies on the relationship between noninvasive fibrosis biomarker and liver biopsy. For this purpose, in this study we developed a new noninvasive serum model by assessing several clinicopathological features. We also compared and evaluated the diagnostic accuracy of this noninvasive model consisting of the variables of FI, APRI, FIB-4, FCI, age-AST model and AP index.
MATERIALS AND METHODS
Patients
This is a retrospective cross-sectional study and was carried out from March 2008 to March 2011. Screening of patients was conducted at the Department of Liver Disease (Ruikang Hospital of Guangxi Traditional Chinese Medical University, Nanning, China) and Department of Infectious Disease (The First Affiliated Hospital of Guangxi Medical University, Nanning, China). A total of 114 patients were enrolled to the study (male/female 91/23; mean age 38.32 ± 11.36 years, range 15-67 years. Diagnosis of chronic HBV-infected patients was established based on the presence of positive results of surface antigen (> 0.5 ng/mL) and/or e antigen (> 0.05 NCU/mL) lasting more than six months. Clinical, biochemical and hematologic data were recorded from each patient at the time of liver biopsy. Patients with the following conditions were excluded: presence of other causes of liver disease such as hepatitis A/C/E virus infection, HCC, prior nucleoside medication, prior interferon therapy, fatty liver disease, alcohol intake > 30 g/wk (female) and 60 g/wk (male), insufficient liver tissue for staging of fibrosis, clinical or ultrasonographic evidence of cirrhosis.
Histological staging
Ultrasonographic-guided liver biopsy was performed according to a standardized protocol. The liver biopsy procedure, its advantages and possible adverse effects were explained to the patients. Informed consent was obtained from the patients about the possible transmission of HBV infection. Specimens of 15-20 mm liver tissues were fixed, paraffin-embedded, stained with hematoxylin-eosin and Masson’s trichrome. A minimum of six portal tracts was required for diagnosis. Liver biopsy was evaluated with or without knowing the history of the patients. Five fibrosis degrees of histological staging were defined according to “The program of prevention and cure for viral hepatitis”, generally used in hospitals of China[19], as F0 (no fibrosis), F1 (mild fibrosis without septa), F2 (moderate fibrosis with few septa), F3 (severe fibrosis with numerous septa without cirrhosis) and F4 (cirrhosis). In our study, we simplified the FS as F0-F1 (minimal fibrosis), F2-F3 (advanced fibrosis) and F4 (cirrhosis).
Blood tests
All tests were performed in the patients after a fasting period of 12 h. The routine liver function tests included alanine aminotransferase (ALT), AST, total bilirubin (T.bil), direct bilirubin (D.bil), albumin, globulin (GLOB), glutamyltransferase (GGT), alkaline phosphatase (ALP), PLT, HBV-DNA and prothrombin time (PT). Serum tests were performed using an automatic biochemistry analyzer. The viral load was measured by real-time polymerase chain reaction, with a detection limit of 1000 copies/mL. All biochemical tests and their scores were evaluated without knowledge of liver biopsy results. Thirteen clinical, biochemical and hematological variables were used for the analysis: sex, age, PLT, ALT, AST, albumin, GLOB, T.bil, D.bil, GGT, ALP, HBV DNA and PT.
Statistical analysis
The data was analyzed using statistical package SPSS version 13.5 for Windows. A P value of 0.05 was considered statistically significant. All data was presented as mean values or number of patients. Spearman’s rank correlation was used to assess the significant correlation between variables and liver FS. The Student t test or variance analysis was used to compare arithmetic means and parameters, while χ2 test was used to compare categorical data.
A predictive model, named Globulin/platelet (GP) model, was constructed by modeling the values of the independent variables and their coefficient of regression. The diagnostic value of the model was assessed by calculating the areas under the receiver operating characteristic (ROC) curves. An area under the curve (AUC) of 1.0 representes an ideal test, whereas 0.5 indicates a test of no diagnostic value. The diagnostic accuracy was calculated by sensitivity, specificity, positive predictive values (PPV) and negative predictive values (NPV).
The best cutoff points were selected according to the Youden index from the ROC curve to identify the presence and absence of minimal fibrosis or cirrhosis. For this purpose, we selected cutoff points with a 95% certainty, thus assuming a 5% false negative result which is acceptable clinically.
Comparison of available noninvasive biomarkers to evaluate patient’s liver biopsy data
Serum samples and data of the characteristics of the patients and liver specimens were collected from each patient for further biochemical analysis. All patients were evaluated for FI, APRI, FIB-4, FCI, age-AST model and AP index. Superiority of GP model was compared with the other selected fibrosis models, and ROC curves and correlation analysis were employed to predict minimal fibrosis or cirrhosis, and deduce the diagnostic accuracy.
RESULTS
Patient data
The demographic and clinical outcomes of the 114 patients with HBV infection are shown in Table 1. The evaluation of inflammatory grade yielded mild chronic hepatitis in 44 patients, moderate chronic hepatitis in 64 patients and severe chronic hepatitis in 6 patients. And 22 patients had liver fibrosis at stage F0, 34 at F1, 28 at F2, 18 at F3, and 12 at F4. Among the patients with HBV DNA < 1000 copies/mL, there were 20 cases of mild chronic hepatitis, 24 moderate chronic hepatitis, and none had severe chronic hepatitis, while among those with HBV DNA > 1000 copies/mL, there were 24 cases of mild chronic hepatitis, 40 moderate chronic hepatitis, and six severe chronic hepatitis. The latter showed a higher inflammatory grade than the former (χ2 = 37.487, P < 0.001). Among the E antigen positive (e+) patients, 12 at stage F0, 15 at F1, 11 at F2, 7 at F3, and 7 at F4. Among E antigen negative (e-) patients, 10 at stage F0, 19 at F1, 17 at F2, 11 at F3 and 5 at F4. There was no significant difference between e+ and e- patients in FS (χ2 = 2.301, P = 0.681).
Table 1.
Characteristics of the study population
| Features | mean ± SD | Minimum | Maximum |
| Gender (male/female) | 91/23 | ||
| HBV DNA (copies/mL) | |||
| (< 1000/≥ 1000) | 44/70 | ||
| (< 103/103-105/≥ 105) | 44/32/38 | ||
| E antigen (+/-) | 52/62 | ||
| Age (yr) | 38.32 ± 11.36 | 15 | 67 |
| PLT( × 109/L) | 174.44 ± 62.68 | 50.6 | 334 |
| T.Bil (g/dL) | 0.88 ± 0.49 | 0.27 | 3.14 |
| D.Bil (g/dL) | 0.29 ± 0.23 | 0.08 | 1.39 |
| ALT (IU/L) | 69.32 ± 116.40 | 10.00 | 983 |
| AST (IU/L) | 48.60 ± 64.56 | 13 | 594 |
| ALB (g/dL) | 4.15 ± 0.43 | 2.85 | 5.26 |
| GLOB (g/dL) | 2.98 ± 0.52 | 1.75 | 4.47 |
| ALP (IU/L) | 80.10 ± 27.38 | 24 | 154 |
| GGT (IU/L) | 59.46 ± 66.92 | 5 | 430 |
| PT (s) | 12.01 ± 1.58 | 9.3 | 18.1 |
HBV: Hepatitis B virus; GLOB: Globulin; PLT: Platelet count; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; T.bil: Total bilirubin; D.bil: Direct bilirubin; ALB: Albumin; GGT: Glutamyltransferase; ALP: Alkaline phosphatase; PT: Prothrombin time.
Correlation between clinical findings and three FS
Three levels of liver fibrosis (F0-F1, F2-F3 and F4) were analyzed. Thirteen demographical, hematological, and biochemical variables were studied. HBV DNA was divided into two groups (≥ 1000 copies/mL and < 1000 copies/mL) or three groups (< 1000 copies/mL, ≥ 1000 copies/mL and < 105 copies/mL, ≥ 105 copies/mL) and gender was described as categorical data. Of the 13 variables studied by Bivariate Spearman’s rank correlation analysis, 6 variables (age, PLT, T.bil, D.bil, GLOB and PT) were correlated significantly with the three FS (correlation coefficients were 0.23, -0.412, 0.208, 0.220, 0.314 and 0.212; P value 0.014, < 0.001, 0.026, 0.018, 0.001 and 0.024).
Selection of variables and construction of a model for predicting FS
Univariate analysis revealed that only PLT and GLOB of the 13 variables were independent predictive factors and significantly different in FS (PLT: F = 11.772, P < 0.001, GLOB: F = 6.612, P = 0.002). There was no difference among the other variables (P all > 0.05). These biochemical markers can also be helpful in staging the liver fibrosis. Figure 1 shows the box plots of the two markers with liver histological stages. It is clear from Figure 1 that as the fibrosis progressed, GLOB level increased, while PLT gradually decreased with fibrosis progression. However, it was interesting to note that GLOB and PLT both were in the normal limits, making the diagnosis of fibrosis difficult by using single biochemical markers.
Figure 1.
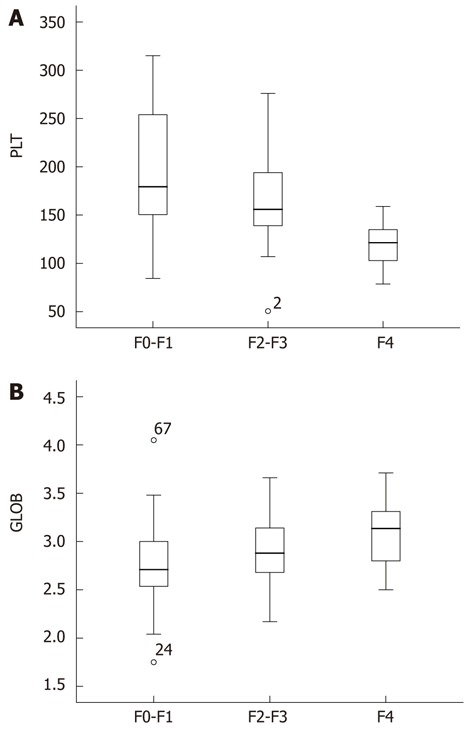
Scores of platelet count (A) and globulin (B) in three fibrosis stages (F0-F1, F2-F3 and F4). The top and bottom of each box are the 25% and the 75% centiles. The line through the box is the median, and the error bars are the 5th and 95th centiles. GLOB: Globulin; PLT: Platelet count.
To amplify the opposite relationship between the stage of fibrosis and the two markers, GLOB and PLT, based on their significance not only by the rank correlation analysis but also by univariate analysis, we developed a new fibrosis model named GP model in HBV infection for predicting cirrhosis and minimal fibrosis. It can be represented as GP model = GLOB (g/dL) × 100/PLT (× 109/L).
The GP model distribution for the patients in the respective FS is represented in Figure 2. The median values for GP model in F0-F1, F2-F3 and F4 patients were 1.461, 1.720 and 2.634, respectively. GP model was correlated significantly with the liver FS (r = 0.466, P < 0.001). The diagnostic values of GP model to predict F0-F1 and F4 patients were evaluated by the AUC (Figure 3).
Figure 2.
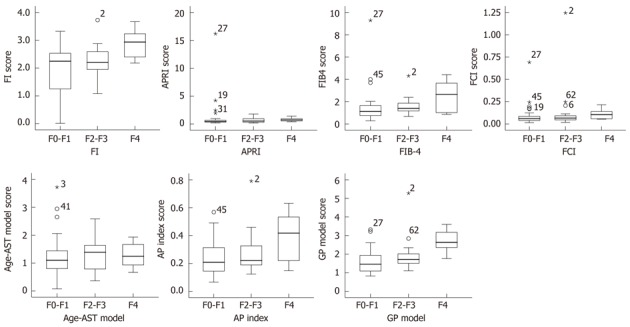
Box plot of fibrosis index, aspartate aminotransferase-platelet ratio, FIB-4, fibrosis-cirrhosis index, age-aspartate aminotransferase model, age-platelet count ratio and globulin/platelet model in relation to F0-F1, F2-F3 and F4 fibrosis stage. The box represents the interquartile range. The whiskers indicate the highest and lowest values, and the asterisks represent outliers. The line across the box indicates the median value. FI: Fibrosis index; GP: Globulin/platelet; FCI: Fibrosis-cirrhosis index; AST: Aspartate aminotransferase; APRI: AST-platelet ratio; AP index: Age-platelet count ratio.
Figure 3.
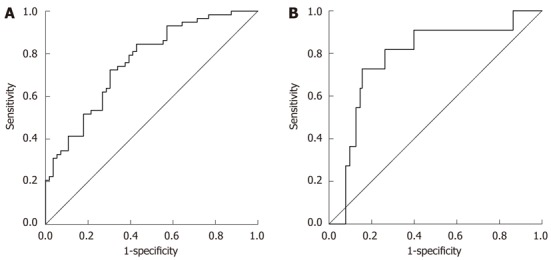
Receiver operating characteristic curves generated by globulin/platelet model for prediction of F0-F1 (A) and F4 (B). The area under the curve of GP model for the discrimination between minimal fibrosis (F0-F1) and significant fibrosis (F2, F3, F4), and between non-cirrhosis (F0, F1, F2, F3) and cirrhosis (F4) was 0.732 [confidence interval (CI): 0.642-0.823] and 0.738 (CI: 0.612-0.864). Using a cutoff value of < 1.68, GP model had a sensitivity of 72.4%, a positive predictive value (PPV) of 71.2%, a specificity of 69.6%, and a negative predictive value (NPV) of 70.8% for the prediction of F0-F1. For the prediction of cirrhosis (F4) at a cutoff value of > 2.53, GP model had a sensitivity of 72.7%, a PPV of 33.4%, a specificity of 84.5%, and a NPV of 96.7%.
Step-wise multiple regression analysis, which was used to formulate a suitable multivariable model for the correlation between 11 count variables and the three FS, F0-F1, F2-F3 and F4, revealed that PLT and GLOB were also independently correlated with histological FS, R2 = 0.237. The contribution of PLT and GLOB to R2 was 0.197 and 0.04, respectively (data not shown). The final multiple regression model incorporating both PLT and GLOB was: FS = 1.683 - 0.008 × PLT (× 109/L) + 0.485 × GLOB (g/dL). We simplified it as: FS = 1.7 - 0.01 × PLT (× 109/L + 0.5 × GLOB (g/dL).
The median values for FS model in F0-F1, F2-F3 and F4 patients were 1.218, 1.480 and 2.085, respectively. FS model significantly correlated with the liver FS (Spearman’s rank correlation coefficient, r = 0.47, P < 0.001, data not shown). The diagnostic values of FS to differentiate F0-F1 and F4 patients were assessed using the ROC. Interestingly, AUC was very close between FS model and GP model in F0-F1 (0.760 vs 0.762) and F4 (0.781 vs 0.783), and in complete coincident sensitivity and specificity for the detection of minimal fibrosis and cirrhosis (data not shown). GP model was simpler than FS model in term of calculation. Therefore, we used GP model to express our findings.
Comparison of GP model with FI, APRI, FIB-4, FCI, age-AST model and AP index
The scores of six indexes (FI, APRI, FIB-4, FCI, age-AST model and AP index) were calculated by the formula shown in Table 2. The relationship between the histological severity of fibrosis (F0-F1, F2-F3 and F4) and six models is shown in Figure 2 and Table 3. There was a significant correlation between F0-F1, F2-F3 and F4 and serum indexes of FIB-4, AP index and GP model (P < 0.05), but not in FI, APRI, FCI, age-PLT model (P > 0.05). A gradual increase at the level of AP index, FIB-4, GP model was observed in different FS. GP model showed the strongest correlation with the three FS (r = 0.441, P < 0.001).
Table 2.
Noninvasive simple fibrosis models composed of routine clinical and laboratory parameters
| Fibrosis test | Calculation |
| FI | 8.0-0.01 × PLT (× 109/L)-ALB (g/dL) |
| APRI | AST (IU/L) × 100/PLT (× 109/L) |
| FIB-4 | Age (yr) × AST (IU/L)/PLT (× 109/L) × ALT (IU/L) 1/2 |
| FCI | ALP (IU/L) × T.bil (g/dL)/ALB (g/dL)/ PLT (× 109/L) |
| Age-AST model | Age (yr)/AST (IU/L) |
| AP index | Age (yr)/PLT (× 109/L) |
PLT: Platelet count ; ALB: Albumin; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; T.bil: Total bilirubin; AP index: Age-PLT ratio; AST: Aspartate aminotransferase; APRI: AST-platelet ratio; FI: Fibrosis index; FCI: Fibrosis-cirrhosis index.
Table 3.
Correlation analysis of seven models and different fibrosis stages (F0-F1, F2-F3 and F4)
| F0-F1, F2-F3 and F4 fibrosis stages | Bivariate Spearman’s rank correlation coefficient | P value |
| FI | 0.231 | 0.06 |
| APRI | 0.146 | 0.237 |
| FIB-4 | 0.307 | 0.012 |
| FCI | 0.159 | 0.20 |
| Age-AST model | 0.132 | 0.286 |
| AP index | 0.246 | 0.045 |
| GP model | 0.441 | 0.000 |
AP index: Age-platelet count ratio; AST: Aspartate aminotransferase; APRI: AST-platelet ratio; FI: Fibrosis index; GP: Globulin/platelet; FCI: Fibrosis-cirrhosis index.
The predictive value of the seven noninvasive models for predicting minimal fibrosis (F0-F1) is summarized in Table 4. APRI, FIB-4 and FCI had a high PPV, specificity, but the sensitivity and NPV were low. AP index had a high sensitivity and NPV, but specificity and PPV were low. GP model had not only high PPV and specificity, but also high NPV and sensitivity. AUC of age-AST model was close to 0.5, showing little value for prediction of minimal fibrosis. We compared the AUC of GP model with the other five noninvasive models using the 95% confidence interval (CI) and the standard error (SE) of the mean, and found that although the differences were not statistically significant, the GP model seemed to have a best predictive value (largest AUC) (P all > 0.05) (Table 4).
Table 4.
Validity of age-platelet count ratio, aspartate aminotransferase-platelet ratio, age-aspartate aminotransferase model, fibrosis index, FIB-4 and fibrosis-cirrhosis index for prediction of minimal fibrosis (F0-F1) and comparison with globulin/platelet model
| Fibrosis test | Cutoff value | Specificity % | Sensitivity % | PPV % | NPV % | AUC (95% CI) | Standard error | P value(vs GP model) |
| FI | 1.58 | 41.1 | 89.7 | 61.2 | 79.4 | 0.695 (0.600-0.791) | 0.049 | > 0.05 |
| APRI | 0.85 | 87.5 | 41.4 | 77.4 | 59.0 | 0.635 (0.533-0.738) | 0.052 | < 0.05 |
| FIB-4 | 1.7 | 85.7 | 51.7 | 78.9 | 63.1 | 0.720 (0.627-0.813) | 0.047 | > 0.05 |
| FCI | 0.17 | 80.4 | 55.2 | 74.5 | 63.4 | 0.692 (0.594-0.790) | 0.050 | > 0.05 |
| Age-AST model | - | 19.6 | 94.8 | 55.0 | 78.4 | 0.524 (0.417-0.632) | 0.055 | < 0.001 |
| AP index | 0.17 | 46.4 | 89.7 | 63.4 | 81.3 | 0.726 (0.634-0.818) | 0.047 | > 0.0 |
| GP model | 1.68 | 69.6 | 72.4 | 71.2 | 70.8 | 0.762 (0.676-0.848) | 0.044 |
AP index: Age-platelet count ratio; AST: Aspartate aminotransferase; APRI: AST-platelet ratio; FI: Fibrosis index; GP: Globulin/platelet; FCI: Fibrosis-cirrhosis index; PPV: Positive predictive values; NPV: Negative predictive values; AUC: Area under the curve; CI: Confidence interval.
The predictive value of GP model for cirrhosis (F4) is shown in Table 5. All of the models had very good NPV (> 96%), also high sensitivity (> 70%). The AUC of age-AST model was less than 0.5, which cannot be used to predict cirrhosis. We compared the AUC of GP model with the other five noninvasive models as well using the 95% CI and the SE, and found that AUC of GP model was not significantly high (P all > 0.05) (Table 5).
Table 5.
Validity of age-platelet count ratio, aspartate aminotransferase-platelet ratio, age-aspartate aminotransferase model, fibrosis index, FIB-4, and fibrosis-cirrhosis index for prediction of cirrhosis (F4) and comparison with globulin/platelet model
| Fibrosis test | Cutoff value | Specificity % | Sensitivity % | PPV % | NPV % | AUC (95% CI) | Standard error | P value(vs GP model) |
| FI | 2.16 | 53.4 | 81.8 | 15.8 | 96.4 | 0.717 (0.569-0.865) | 0.075 | > 0.05 |
| APRI | 0.77 | 72.8 | 72.7 | 22.2 | 96.1 | 0.703 (0.584-0.822) | 0.061 | > 0.05 |
| FIB-4 | 2.29 | 89.3 | 72.7 | 42.0 | 96.8 | 0.768 (0.050-0.931) | 0.083 | > 0.05 |
| FCI | 0.10 | 68.0 | 81.8 | 21.4 | 97.2 | 0.738 (0.612-0.864) | 0.064 | > 0.05 |
| Age-AST model | - | - | - | - | 0.486 (0.365-0.608) | 0.062 | - | |
| AP index | 0.32 | 74.8 | 72.7 | 23.6 | 96.2 | 0.735 (0.575-0.895) | 0.082 | > 0.05 |
| GP model | 2.53 | 84.5 | 72.7 | 33.4 | 96.7 | 0.781 (0.638-0.924) | 0.073 |
AP index: Age-platelet count ratio; AST: Aspartate aminotransferase; APRI: AST-platelet ratio; FI: Fibrosis index; GP: Globulin/platelet; FCI: Fibrosis-cirrhosis index; PPV: Positive predictive values; NPV: Negative predictive values; AUC: Area under the curve; CI: Confidence interval.
DISCUSSION
Although only a small number of patients with chronic inactive HBV infection develop advanced liver disease[20-22], the risk of HCC is higher for HBV infected patients than for those without HBV infection[23]. The cirrhosis in HBsAg carriers often progress insidiously. Chu et al[24] concluded that the so-called inactive carrier state cannot be considered generally as an innocuous, persistent condition with good prognosis, suggesting that regular follow-up is necessary. Active HBV infection more often caused significant fibrosis and cirrhosis than inactive HBV infection. We attempted to find a simple and common index to evaluate their fibrosis in active and inactive HBV infections. We retrospectively studied the pathological changes about fibrosis in patients with HBV infection from two liver disease centers. We found that the HBV DNA with a higher inflammation grade, was not significantly different among different FS. We also found no significant difference between e+ and e- populations in FS. A study[25] recruited patients with HBV DNA < 104 copies/mL, and found the mean liver stiffness in inactive HBsAg carriers was 5.6 ± 2.1 kPa, significantly higher than in normal subjects. In 16.4% (23) of inactive carriers, liver stiffness exceeded 7 kPa (the cutoff for fibrosis F2-F4); and in patients with undetectable viral loads (with a detection limit of 51 copies/mL for HBV DNA), the mean liver stiffness was significantly lower than in those with detectable DNA (< 104 copies/mL). Assessing the noninvasive liver stiffness in inactive HBsAg carriers by transient elastography, Fattovich et al[26] reported that HBeAg-negative chronic hepatitis patients had a higher risk for progression to cirrhosis (8-10 per 100 person years) than HBeAg-positive chronic hepatitis patients. This may reflect the duration of infection, and a late phase in the natural history of the disease, as opposed to de novo infection with a variant not producing HBeAg. Another study found in chronic HBV carriers with clinical normal liver function tests, inflammation grade and FS had no correlation with the level of HBV DNA or the state of HBeAg positivity[27]. Our study with a detection limit of 1000 copies HBV DNA/mL, enrolled all of HBV-infected patients, including some patients with active inflammation. This may be the reason for the inconsistency with other studies[28].
Many studies on prediction of significant fibrosis (F2-F4) and cirrhosis (F4) in patients with HBV infection have been published in the past few years, such as Fibroscan and FibroTest-ActiTest, galactose and methacetin breath tests, TE, fibrotest, cirrhosis discriminant score, AST/ALT ratio, APRI, FIB-4 and AP index[11,29-33]. However, some of the tests need expensive instruments and some are somewhat difficult to use in clinical practice, since these assay utilizes less common biochemical markers such as α2-macroglobulin, haptoglobin, and apolipoprotein A1, and also requires use of a special computer program to perform calculations.
In our study, we attempted to develop a single model using routinely available laboratory test results to predict minimal fibrosis and cirrhosis in treatment-naive patients with HBV infection. We found by Step-wise multiple regression analysis and correlation analysis, that PLT and GLOB were significantly correlated with different FS. Other variables such as T.bil, D.bil, PT, or GGT may play a role in the discrimination function and have been found useful in patients with minimal fibrosis or cirrhosis. However, compared with PLT and GLOB, other variables were correlated with the histological FS at a much smaller coefficient of determination. Wai et al[16] also suggested that two variables can be practically used as a prediction index, and in our model we used PLT and GLOB because of their convenience of application in general practice. Nevertheless, the value of these two parameters was proposed to evaluate minimal fibrosis and cirrhosis. The concept in prediction of minimal fibrosis by a ratio of two important variables is not new. These findings echoed results from many previous studies. The value of PLT as a marker of liver fibrosis has already been reported[6,16,34-37]. Some studies showed that GLOB was correlated with FS and was a predictor of either significant fibrosis or cirrhosis[28,38].
GP model was simple to use and accurate in predicting both minimal fibrosis and cirrhosis in HBV-infected patients (Figure 3 and Tables 3, 4, 5). To evaluate its predictive value, we compared GP model with the other available models, because the variables used in these models were also involved in our collected data. GP model showed the highest correlation coefficient with FS. Comparison of GP model with the other six models in AUC, GP model showed the highest value than other models, although there was no significant difference. Using values below the lower cut-off level (1.68), a presence of minimal fibrosis could be predicted in 71% of patients. Similarly, using values below the higher cut-off level (2.53), a prediction of non-cirrhosis could be made in 96% of patients.
There exist some limitations in our study. Our study is based on the data from liver biopsy, which is considered as the gold standard for assessing hepatic fibrosis, but sampling error as well as intra- and inter-observer variability can complicate the correlations between histology and noninvasive markers of hepatic fibrosis. Arase et al[39] found that some patients with a nodular liver surface at laparoscopy were not diagnosed as having liver cirrhosis when only histological samples were used; HBV-positive patients with a nodular liver surface have a tendency of sampling error compared with the HCV-positive patients. On the other hand, there was overlap among patients with different stages of fibrosis. Thus, the value of GP model for the prediction of fibrosis in individual patients with HBV infection must be confirmed in prospective studies. However, we are not yet able to get enough patient data for verification of GP model in a new cohort. The data of patients were derived from two different hospitals, and the sample size is still small. Whether the model can reflect the change of FS in treatment process awaits further studies.
In conclusion, We established a simple model using available laboratory variables. Minimal fibrosis and cirrhosis can be diagnosed accurately using this model, thus reducing the need for liver biopsy in chronic HBV-infected patients.
COMMENTS
Background
Liver fibrosis is known as the major condition causing morbidity and mortality in chronic hepatitis B virus (HBV)-infection patients. Liver biopsy (LB) as an invasive method is used as the gold standard in diagnosing the degree of fibrosis, but because there is a risk of complications causing discomfort, not many patients are willing to undergo a LB. HBV-infected patients are prone to fibrosis, but there have been few studies about the relationship between noninvasive fibrosis biomarker and liver LB among these patients.
Research frontiers
Some noninvasive methods were expected and used in patients with hepatitis C or B virus infection. Simpler and cheaper methods for the prediction of hepatic fibrosis are being studied. Several scoring systems have been proposed, such as age-platelet count (PLT) ratio (AP index), aspartate aminotransferase (AST)-platelet ratio, age-AST model, fibrosis index, FIB-4, and fibrosis-cirrhosis index, using different thresholds to predict presence or absence of fibrosis or cirrhosis in patients infected with hepatitis C virus or HBV.
Innovations and breakthroughs
This study developed a new noninvasive serum model named Globulin/platelet (GP) model, including globulin and PLT, by assessing several clinicopathological features. Comparing with six available models, GP model showed highest correlation coefficient. The sensitivity and positive predictive value at a cutoff value < 1.68 for predicting minimal fibrosis F0-F1 were 72.4% and 71.2%. The specialty and negative predictive value at a cutoff value < 2.53 for the prediction of cirrhosis were 84.5% and 96.7%. The area under the curve of GP model for predicting minimal fibrosis and cirrhosis were 0.762 and 0.781, respectively, which is the largest among the seven models.
Applications
The simple model developed in this study using readily available laboratory results can identify chronic HBV-infected patients with minimal fibrosis and cirrhosis with a high degree of accuracy, and it seems more efficient than frequently used models. This model may decrease the need for liver biopsy in HBV-infected patients.
Peer review
This study might be interesting and useful for the readers regarding the daily management of patients with viral hepatitis. Application of this model may decrease the need for liver biopsy in HBV infection cases.
Footnotes
Peer reviewers: Yasuji Arase, MD, Department of Gastroenterology, Toranomon Hospital, 2-2-2Toranomonminato-ku, Tokyo 105-8470, Japan; Ming-Lung Yu, MD, PhD, Professor, Division of Hepatology, Department of Medicine, Kaohsiung Medical University Hospital, 100 Tzyou 1st Rd, Kaohsiung 807, Taiwan, China
S- Editor Lv S L- Editor Ma JY E- Editor Zhang DN
References
- 1.Chon CY, Han KH, Lee KS, Moon YM, Kang JK, Park IS, Park C. Peritoneoscopic liver biopsy findings in asymptomatic chronic HBsAg carriers with normal liver function tests and no hepatomegaly. Yonsei Med J. 1996;37:295–301. doi: 10.3349/ymj.1996.37.5.295. [DOI] [PubMed] [Google Scholar]
- 2.Gaia S, Marzano A, Olivero A, Abate M, Rizzetto M, Smedile A. Inactive hepatitis B virus carriers: a favourable clinical condition. Eur J Gastroenterol Hepatol. 2005;17:1435–1436. doi: 10.1097/00042737-200512000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SL. Preface. Hepatic fibrosis: pathogenesis, diagnosis, and emerging therapies. Clin Liver Dis. 2008;12:xiii–xxiv. doi: 10.1016/j.cld.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 5.Patel K, Nelson DR, Rockey DC, Afdhal NH, Smith KM, Oh E, Hettinger K, Vallée M, Dev A, Smith-Riggs M, et al. Correlation of FIBROSpect II with histologic and morphometric evaluation of liver fibrosis in chronic hepatitis C. Clin Gastroenterol Hepatol. 2008;6:242–247. doi: 10.1016/j.cgh.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat. 1997;4:199–208. doi: 10.1046/j.1365-2893.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 7.Geramizadeh B, Janfeshan K, Saberfiroozi M. Serum hyaluronic acid as a noninvasive marker of hepatic fibrosis in chronic hepatitis B. Saudi J Gastroenterol. 2008;14:174–177. doi: 10.4103/1319-3767.43274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu GG, Luo CY, Wu SM, Wang CL. The relationship between staging of hepatic fibrosis and the levels of serum biochemistry. Hepatobiliary Pancreat Dis Int. 2002;1:246–248. [PubMed] [Google Scholar]
- 9.Parsian H, Rahimipour A, Nouri M, Somi MH, Qujeq D. Assessment of liver fibrosis development in chronic hepatitis B patients by serum hyaluronic acid and laminin levels. Acta Clin Croat. 2010;49:257–265. [PubMed] [Google Scholar]
- 10.Xie SB, Yao JL, Zheng RQ, Peng XM, Gao ZL. Serum hyaluronic acid, procollagen type III and IV in histological diagnosis of liver fibrosis. Hepatobiliary Pancreat Dis Int. 2003;2:69–72. [PubMed] [Google Scholar]
- 11.Kim SM, Sohn JH, Kim TY, Roh YW, Eun CS, Jeon YC, Han DS, Oh YH. [Comparison of various noninvasive serum markers of liver fibrosis in chronic viral liver disease] Korean J Hepatol. 2009;15:454–463. doi: 10.3350/kjhep.2009.15.4.454. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad W, Ijaz B, Javed FT, Gull S, Kausar H, Sarwar MT, Asad S, Shahid I, Sumrin A, Khaliq S, et al. A comparison of four fibrosis indexes in chronic HCV: development of new fibrosis-cirrhosis index (FCI) BMC Gastroenterol. 2011;11:44. doi: 10.1186/1471-230X-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342:1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 14.Parise ER, Oliveira AC, Figueiredo-Mendes C, Lanzoni V, Martins J, Nader H, Ferraz ML. Noninvasive serum markers in the diagnosis of structural liver damage in chronic hepatitis C virus infection. Liver Int. 2006;26:1095–1099. doi: 10.1111/j.1478-3231.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 15.Castera L, Pinzani M. Non-invasive assessment of liver fibrosis: are we ready? Lancet. 2010;375:1419–1420. doi: 10.1016/S0140-6736(09)62195-4. [DOI] [PubMed] [Google Scholar]
- 16.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 17.Ohta T, Sakaguchi K, Fujiwara A, Fujioka S, Iwasaki Y, Makino Y, Araki Y, Shiratori Y. Simple surrogate index of the fibrosis stage in chronic hepatitis C patients using platelet count and serum albumin level. Acta Med Okayama. 2006;60:77–84. doi: 10.18926/AMO/30729. [DOI] [PubMed] [Google Scholar]
- 18.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 19.Association Society of Infectious Diseases and Parasitology and Chinese Society of Hepatology of Chinese Medical Association. The programme of prevention and cure for viral hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324–329. [Google Scholar]
- 20.Popper H, Shafritz DA, Hoofnagle JH. Relation of the hepatitis B virus carrier state to hepatocellular carcinoma. Hepatology. 1987;7:764–772. doi: 10.1002/hep.1840070425. [DOI] [PubMed] [Google Scholar]
- 21.Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Hepatology. 1988;8:493–496. doi: 10.1002/hep.1840080310. [DOI] [PubMed] [Google Scholar]
- 22.Yu MW, Hsu FC, Sheen IS, Chu CM, Lin DY, Chen CJ, Liaw YF. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol. 1997;145:1039–1047. doi: 10.1093/oxfordjournals.aje.a009060. [DOI] [PubMed] [Google Scholar]
- 23.Yu MW, Chen CJ. Hepatitis B and C viruses in the development of hepatocellular carcinoma. Crit Rev Oncol Hematol. 1994;17:71–91. doi: 10.1016/1040-8428(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 24.Chu CM, Liaw YF. Incidence and risk factors of progression to cirrhosis in inactive carriers of hepatitis B virus. Am J Gastroenterol. 2009;104:1693–1699. doi: 10.1038/ajg.2009.187. [DOI] [PubMed] [Google Scholar]
- 25.Sporea I, Nicolita D, Sirli R, Deleanu A, Tudora A, Bota S. Assessment of noninvasive liver stiffness in inactive HBsAg carriers by transient elastography: Fibroscan in inactive HBsAg carriers. Hepat Mon. 2011;11:182–185. [PMC free article] [PubMed] [Google Scholar]
- 26.Fattovich G, Brollo L, Alberti A, Pontisso P, Giustina G, Realdi G. Long-term follow-up of anti-HBe-positive chronic active hepatitis B. Hepatology. 1988;8:1651–1654. doi: 10.1002/hep.1840080630. [DOI] [PubMed] [Google Scholar]
- 27.Wei N, Yang D, Yang F, Wang Y, Zhao B, Lü DG. [A study on the hepatic histological changes and clinical manifestations in chronic HBV carriers] Zhonghua Ganzangbing Zazhi. 2007;15:330–333. [PubMed] [Google Scholar]
- 28.Schmilovitz-Weiss H, Tovar A, Halpern M, Sulkes J, Braun M, Rotman Y, Tur-Kaspa R, Ben-Ari Z. Predictive value of serum globulin levels for the extent of hepatic fibrosis in patients with chronic hepatitis B infection. J Viral Hepat. 2006;13:671–677. doi: 10.1111/j.1365-2893.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee IC, Chan CC, Huang YH, Huo TI, Chu CJ, Lai CR, Lee PC, Su CW, Hung HH, Wu JC, et al. Comparative analysis of noninvasive models to predict early liver fibrosis in hepatitis B e Antigen-negative Chronic Hepatitis B. J Clin Gastroenterol. 2011;45:278–285. doi: 10.1097/MCG.0b013e3181dd5357. [DOI] [PubMed] [Google Scholar]
- 30.Tong MJ, Hsu L, Hsien C, Kao JH, Durazo FA, Saab S, Blatt LM. A comparison of hepatitis B viral markers of patients in different clinical stages of chronic infection. Hepatol Int. 2010;4:516–522. doi: 10.1007/s12072-010-9179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SH, Kim CH, Kim DJ, Cheong JY, Cho SW, Hwang SG, Lee YJ, Cho M, Yang JM, Kim YB. Development and validation of a model to predict advanced fibrosis in chronic hepatitis B virus-infected patients with high viral load and normal or minimally raised ALT. Dig Dis Sci. 2011;56:1828–1834. doi: 10.1007/s10620-010-1477-x. [DOI] [PubMed] [Google Scholar]
- 32.Poynard T, Ngo Y, Marcellin P, Hadziyannis S, Ratziu V, Benhamou Y. Impact of adefovir dipivoxil on liver fibrosis and activity assessed with biochemical markers (FibroTest-ActiTest) in patients infected by hepatitis B virus. J Viral Hepat. 2009;16:203–213. doi: 10.1111/j.1365-2893.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 33.Stibbe KJ, Verveer C, Francke J, Hansen BE, Zondervan PE, Kuipers EJ, de Knegt RJ, van Vuuren AJ. Comparison of non-invasive assessment to diagnose liver fibrosis in chronic hepatitis B and C patients. Scand J Gastroenterol. 2011;46:962–972. doi: 10.3109/00365521.2011.574725. [DOI] [PubMed] [Google Scholar]
- 34.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92:1302–1304. [PubMed] [Google Scholar]
- 35.Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142–3146. doi: 10.1111/j.1572-0241.2001.05268.x. [DOI] [PubMed] [Google Scholar]
- 36.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 37.Kaul V, Friedenberg FK, Braitman LE, Anis U, Zaeri N, Fazili J, Herrine SK, Rothstein KD. Development and validation of a model to diagnose cirrhosis in patients with hepatitis C. Am J Gastroenterol. 2002;97:2623–2628. doi: 10.1111/j.1572-0241.2002.06040.x. [DOI] [PubMed] [Google Scholar]
- 38.Schmilovitz-Weiss H, Cohen M, Pappo O, Sulkes J, Braun M, Tur-Kaspa R, Ben-Ari Z. Serum globulin levels in predicting the extent of hepatic fibrosis in patients with recurrent post-transplant hepatitis C infection. Clin Transplant. 2007;21:391–397. doi: 10.1111/j.1399-0012.2007.00657.x. [DOI] [PubMed] [Google Scholar]
- 39.Arase Y, Suzuki F, Suzuki Y, Akuta N, Sezaki H, Kobayashi M, Kawamura Y, Yatsuji H, Hosaka T, Saito S, et al. Potential of laparoscopy in chronic liver disease with hepatitis B and C viruses. Hepatol Res. 2008;38:877–885. doi: 10.1111/j.1872-034X.2008.00343.x. [DOI] [PubMed] [Google Scholar]


