Abstract
Well differentiated liposarcoma (WDLS) and dedifferentiated liposarcoma (DDLS) represent the most common biological group of liposarcoma, and there is a pressing need to develop targeted therapies for patients with advanced disease. To identify potential therapeutic targets, we sought to identify differences in the adipogenic pathways between DDLS, WDLS, and normal adipose tissue. In a microarray analysis of DDLS (n=84), WDLS (n=79), and normal fat (n=23), C/EBPα, a transcription factor involved in cell cycle regulation and differentiation, was underexpressed in DDLS compared to both WDLS and normal fat (15.2 fold and 27.8 fold, respectively). In normal adipose-derived stem cells, C/EBPα expression was strongly induced when cells were cultured in differentiation media, but in three DDLS cell lines, this induction was nearly absent. We restored C/EBPα expression in one of the cell lines (DDLS8817) by transfection of an inducible C/EBα expression vector. Inducing C/EBPα expression reduced proliferation and caused cells to accumulate in G2/M. Under differentiation conditions, the cell proliferation was reduced further, and 66% of the DDLS cells containing the inducible C/EBPα expression vector underwent apoptosis as demonstrated by annexin V staining. These cells in differentiation conditions expressed early adipocyte-specific mRNAs such as LPL and FABP4, but they failed to accumulate intracellular lipid droplets, a characteristic of mature adipocytes. These results demonstrate that loss of C/EBPα is an important factor in suppressing apoptosis and maintaining the dedifferentiated state in DDLS. Restoring C/EBPα may be a useful therapeutic approach for dedifferentiated liposarcomas.
Keywords: Liposarcoma, adipogenesis, differentiation, CCAAT/enhancer binding proteins, PPARγ, apoptosis
Introduction
Liposarcoma, which accounts for 20% of all soft tissue sarcomas, is the most common soft tissue sarcoma in adults. Liposarcoma is generally subdivided into three biological groups encompassing five subtypes: well-differentiated (WDLS), dedifferentiated (DDLS), myxoid, round cell, and pleomorphic (Dalal et al., 2006; Singer et al., 2003), which have different biology and patterns of behavior. WDLS and DDLS account for 46% and 18% of liposarcomas, respectively, and are commonly found in retroperitoneal locations (Dalal et al., 2008). The main treatment option for liposarcoma is surgical resection. Even after complete resection of these tumors, more than 30% of WDLS and 80% of DDLS will eventually develop unresectable recurrence (Park et al., 2009; Singer et al., 2003). Unfortunately, WDLS and DDLS show only occasional partial responses to current chemotherapy regimens. Therefore, new therapeutic approaches are urgently needed for patients with advanced WDLS/DDLS.
To define the molecular abnormalities in liposarcoma, our lab has profiled the copy number alterations in 50 DDLS samples (Barretina et al., 2010). Along with 12q amplification, which was found in approximately 90% of DDLS, we also detected significant amplifications of 1p, 1q, 5p, 6q, and 20q. In 24% of cases, the proto-oncogene JUN (1p32) was both amplified and overexpressed. While oncogenic JUN plays a role in controlling adipocyte dedifferentiation through repression of C/EBPβ (Mariani et al., 2007), the 24% frequency of amplification in this series cannot explain the adipogenesis block in all tumors. However, C/EBPβ is also negatively regulated by other genes, including DDIT3 (Fawcett et al., 1996).
DDIT3 is on 12p13.3 near CDK4 and was also amplified and overexpressed in about 30% of DDLS (Barretina et al., 2010). DDIT3 alterations were mutually exclusive with those affecting JUN. It is therefore tempting to speculate that DDIT3 serves as an alternative effector of deregulated adipogenesis in tumors without amplification of JUN. Our lab has also performed gene expression profiling of WDLS and DDLS to uncover the signaling pathways driving liposarcoma progression (Singer et al., 2007). This analysis demonstrated that WDLS and DDLS, compared with normal fat, overexpress a network of genes that regulate the cell cycle and checkpoints, revealing several potential therapeutic targets. In the present study, we focus on genes in the adipocytic differentiation pathway, because dysregulation of genes involved in differentiation is a key component in the development of cancer.
One transcription factor involved in differentiation, as well as in cell cycle arrest, is CCAAT/enhancer binding protein α (C/EBPα). C/EBPα belongs to a family of basic region leucine zipper transcription factors intimately involved in regulating terminal differentiation of many cell types. It is expressed at high levels in certain tissues and cell types, such as adipose tissue, liver, myeloid cells, and airway epithelial cells. Several cancers, including acute myeloid leukemia, lung cancer, and breast cancer, commonly have low functional levels of C/EBPα, due to either mutation or reduced expression (Costa et al., 2006; Gery et al., 2005; Halmos et al., 2002). A role for C/EBPα as a tumor suppressor has been proposed in lung and squamous cell skin cancer (Bennett et al., 2007; Loomis et al., 2007); however, its role in liposarcomagenesis has never been examined.
In immortalized preadipocyte cell lines (3T3-L1, F-442A), C/EBPα collaborates with another transcription factor, peroxisome proliferator-activated receptor gamma (PPARγ), to switch uncommitted cells from proliferation to terminal differentiation (Cowherd et al., 1999; Rosen et al., 2002; Wu et al., 1999). C/EBPα and PPARγ are capable of inducing each other’s expression in a positive feedback loop to ensure full terminal differentiation, which is characterized by cell cycle arrest and accumulation of intracellular lipid droplets. In addition, C/EBPα directly induces many adipogenic genes important in energy metabolism, such as lipid binding protein (FABP4, also known as aP2), glucose transporter IV (SLC2A4, also known as GLUT4), fatty acid synthetase (FASN), lipoprotein lipase (LPL), and adipsin (CFD, also known as DF) (Cowherd et al., 1999). Cebpa knockout mice display, among other abnormalities, an inability of adipocytes to accumulate lipids, and consequently newborn Cebpa knockout mice lack white adipose tissue (Johnson, 2005; Wang et al., 1995). Cells from knockout mice proliferate rapidly, accumulate chromosomal abnormalities, and, when injected into nude mice, are capable of forming nodules (Soriano et al., 1998). Mice with knockout of Cebpa in the epidermis display susceptibility to skin tumors involving Ras (Loomis et al., 2007).
In this paper, we show that WDLS and DDLS have decreased expression of C/EBPα. Based on this finding, we investigate the functional significance of C/EBPα underexpression for the cell cycle, cell proliferation, apoptosis, and differentiation in DDLS cells.
Results
Inhibition of C/EBPα–PPARγ signaling in WDLS/DDLS
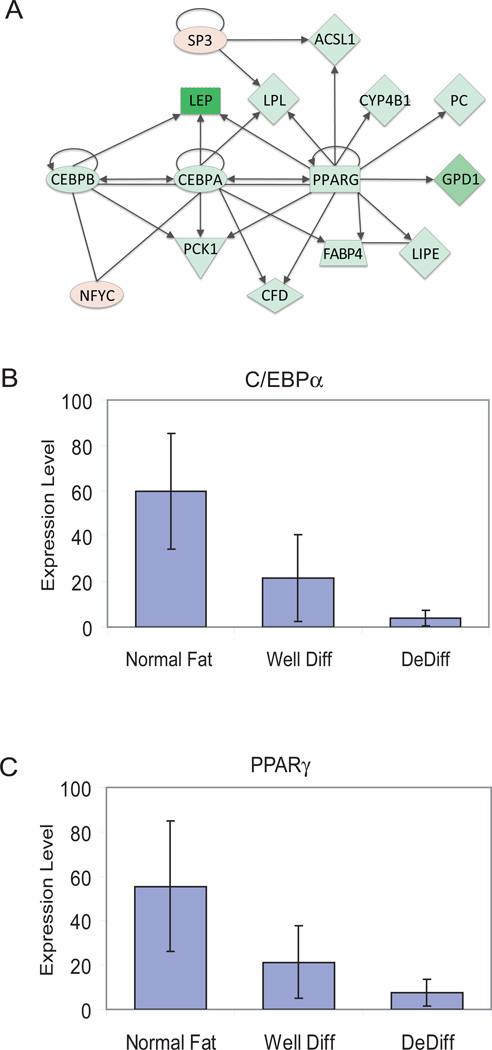
Microarray data generated by the Affymetrix U133A Gene Chip were analyzed using the Ingenuity Pathway Analysis application (http://ingenuity.com), a web-based bioinformatics tool that maps gene lists (ranked by FDR or fold change) to a database of fully annotated biological interactions between genes. This application was used to determine the gene interaction networks significantly affected by WDLS and DDLS based on genes that were overexpressed or underexpressed at least 2 fold in WDLS or DDLS compared to normal fat. One of the interaction networks that was most significant (Ingenuity network score 44, corresponding to a P-value of 10−44) and informative contained 35 genes; a subsection of this network consists of genes that function in adipogenic signaling (Figure 1A). Eleven of the genes in this subset are transcriptional targets of PPARγ and C/EBPα, and all 11 were significantly underexpressed in DDLS compared to normal fat (Table 1). In addition, this subset includes three of the transcription factors important for normal adipocyte differentiation: C/EBPα, C/EBPβ, and PPARγ. All three were underexpressed in DDLS tissue samples compared to normal fat, C/EBPα by 28 fold, C/EBPβ by 3 fold, and PPARγ by 24 fold. C/EBPα and PPARγ were both underexpressed in DDLS compared to WDLS. Several other genes in the interaction network that are not direct C/EBPα–PPARγ targets were also differentially expressed in WDLS/DDLS compared with normal fat (Table 1). For example, both WDLS and DDLS samples had overexpression of NFYC, a transcription factor that regulates the G2/M transition of the cell cycle and interacts with C/EBPα.
Figure 1.
Altered adipogenic gene signaling in well-differentiated and dedifferentiated liposarcoma compared to normal fat. A: Interaction network of C/EBPα and PPARγ derived from Ingenuity pathway analysis. Underexpression in WDLS/DDLS tissue samples is indicated by green and overexpression by red, with the intensity of color proportional to the fold change in expression. The shapes indicate classes of the gene products, e.g. ovals for transcription factors, triangles for kinases, and diamonds for various enzymes. Arrows indicate a protein acting on another protein or gene; plain lines indicate binding. C/EBPα, C/EBPβ, and PPARγ interact with each other to regulate fat-specific genes such as LEP (leptin), FABP4 (fatty acid binding protein 4), LPL (lipoprotein lipase), GPD1 (glycerol-3-phosphate dehydrogenase), LIPE (hormone-sensitive lipase), FACL2 (=ACSL1, fatty acid co-enzyme A ligase long-chain 2). B, C: Quantitative real-time RT-PCR RNA analysis of 6 normal, 6 DDLS, and 8 WDLS tissue samples. The graphs show RNA levels of C/EBPα (B) and PPARγ (C).
Table 1.
Differential expression of adipocyte differentiation genes in microarray analysis of WDLS (n=79), DDLS (n=84), and normal fat (n=23), and cultured adipocytes.
| Fold change in expression | ||||||
|---|---|---|---|---|---|---|
| Gene Symbol |
Gene Description | PA vs MA |
WD vs NF |
DD vs WD |
DD vs NF |
Ref. CEBPα/PPARγ target |
| Transcriptional Targets of C/EBPα–PPARγ | ||||||
| CEBPA | CCAAT/enhancer binding protein alpha | −17 | NS | −15.2 | −27.8 | (Tang et al., 2004) |
| PPARG | Peroxisome proliferative activated receptor, gamma | −3.0 | NS | −12.2 | −24.2 | (Tang et al., 2004) |
| CEBPB | CCAAT/enhancer binding protein, beta | NS | −1.6 | −1.8 | −2.9 | (Tang et al., 2003) |
| LEP | Leptin | −40 | −8.5 | −69.9 | −596.4 | (Francis et al., 2003; Miller et al., 1996; Willson et al., 2000) |
| LPL | Lipoprotein lipase | −134 | NS | −115.3 | −282.3 | (Francis et al., 2003; Hu et al., 1995) |
| PC | Pyruvate carboxylase | −7.8 | −2.1 | −4.6 | −9.8 | (Yu et al., 2003) |
| CYP4B1 | Cytochrome P450, family 4, subfamily 4B, polypeptide 1 | −4.3 | −4.5 | −9.4 | −42.1 | (Yu et al., 2003) |
| PCK1 | phosphoenolpyruvate carboxykinase 1 | −54 | NS | −117.5 | −264.3 | (Hu et al., 1995) |
| LIPE | Lipase, hormone-sensitive | −8.6 | −2.8 | −11.6 | −32.4 | (Yu et al., 2003) |
| FABP4 | Fatty acid binding protein 4, adipocyte | −513 | NS | −36.5 | −61.7 | (Liu and Farmer, 2004; Schadinger et al., 2005) |
| ACSL1 | Fatty-acid-coenzyme A ligase, long-chain 2 | −9.2 | −2.5 | −10.8 | −26.6 | (Francis et al., 2003) |
| Other Genes | ||||||
| CFD | D component of complement (adipsin) | −6.8 | NS | −10.6 | −18.5 | NA |
| GPD1 | Glycerol-3-phosphate dehydrogenase 1 | −33 | −7.8 | −83.1 | −335.3 | NA |
| NFYC | Nuclear transcription factor Y, gamma | NS | +1.3 | 1.2 | +1.5 | NA |
| SP3 | Sp3 transcription factor | NS | +1.4 | 1.4 | +2.0 | NA |
DD, dedifferentiated liposarcoma; MA, mature adipocytes; NA, not applicable; NF, normal fat; NS, no significant change in expression; PA, preadipocytes; WD, well-differentiated liposarcoma.
The microarray expression data for C/EBPα and PPARγ were validated using quantitative real-time RT-PCR (qRT-PCR) to compare 6 normal subcutaneous fat, 6 DDLS, and 8 WDLS tissue samples (Figure 1B, C). Compared to normal fat, both WDLS and DDLS underexpressed C/EBPα (WD P= 0.023; DD P=0.007) and PPARγ (WD P=0.058; DD P=0.022). Comparing DD to WD, only C/EBPα showed a significant further decrease in expression (P=0.035).
C/EBPα and PPARγ levels in dedifferentiated liposarcoma cell lines
To study the effects of differentiation on C/EBPα and PPARγ expression, we used normal and differentiating media to grow normal adipose-derived stem cells (ASCs) and three dedifferentiated liposarcoma cell lines (DDLS8817, RDD2213, and LPS141) isolated from patient DDLS samples. ASCs cultured in regular medium continued to resemble preadipocytes, but when cultured in differentiation media containing a potent PPARγ ligand (GI262570) for 8 days, ASCs differentiated into mature adipocytes. This was associated with a 65-fold and 26-fold increase in mRNA levels of C/EBPα and PPARγ, respectively, as determined by qRT-PCR (Figure 2). In regular medium, C/EBPα expression varied among the DDLS cell lines (Figure 2A). In all these cell lines, however, C/EBPα mRNA expression showed little or no increase in differentiation media. PPARγ baseline transcript levels in the DDLS cell lines were higher than those in ASCs (Figure 2B), and PPARγ transcript levels increased in differentiation medium, although the levels in the DDLS cell lines were still substantially lower than the level in differentiated ASCs (day 8). The increased expression of PPARγ in DDLS cell lines was detectable by day 2, but this increase was short lived, with levels falling back nearly to baseline by day 4 (Figure 2C, D). In addition, the small amount of PPARγ that was induced was phosphorylated at S82, which inhibits its transcriptional activity (Chan et al., 2001; Hu et al., 1996). This suggests that although the baseline C/EBPα transcript levels in DDLS cells can vary significantly, a major deficiency in the C/EBPα and PPARγ signaling pathways is a failure to significantly upregulate C/EBPα transcript levels in response to differentiation media containing a PPARγ ligand.
Figure 2.
Expression analysis of C/EBPα and PPARγ. (A, B) qRT-PCR analysis of RNA levels of C/EBPα (A) and PPARγ (B) in three DDLS cell lines (DDLS8817, RDD8107, and LS141) and in primary normal human preadipocytes (ASC) under normal growth and differentiation conditions. (C) Western blot of PPARγ and phosphorylated PPARγ at various times after DDLS8817 cells were transferred to differentiation medium. (D) qRT-PCR analysis of PPARγ (upper panel) and C/EBPα (lower panel) at various times after DDLS8817 cells were transferred to differentiation medium.
Forced C/EBPα Expression Restores PPARγ Expression
Stimulation of PPARγ receptor with a PPARγ ligand in some cancer cell types is known to promote G1 cell cycle arrest, differentiation, and apoptosis (Han and Roman, 2007). However, PPARγ ligand therapy alone is insufficient to initiate differentiation or growth arrest in DDLS, which we postulated results from a failure to induce C/EBPα expression. We hypothesized that restoration of C/EBPα expression might induce DDLS cells to differentiate. We therefore restored C/EBPα expression in the DDLS8817 cell line by transfecting an expression vector bearing full-length human CEBPA cDNA under the control of a zinc-inducible metallothionein promoter (pMT). Cells transfected with the C/EBPα expression vector (DDLS-pMTα) and cells transfected with empty vector (DDLS-pMT) were grown in the presence of 100 µM zinc. After 48 hours of induction, C/EBPα mRNA from various clones was measured using qRT-PCR (Figure 3A). DDLS-pMTα clone #14 expressed C/EBPα mRNA levels 20-fold higher than DDLS-pMT (P=3·10−6) or 2-fold higher than normal human ASCs (P=9·10−5) (Figure 3A). Nevertheless, this level should be nontoxic since it was still only 4% of the level in differentiated ASCs (day 8). In conjunction with the expression of C/EBPα, PPARγ levels in DDLS-pMTα rose by 8 fold as compared to DDLS-pMT (Figure 3B), indicating the transfected C/EBPα is functional and capable of inducing PPARγ.
Figure 3.
Expression of C/EBPα and PPARγ after transfection with a zinc-inducible C/EBPα expression vector (pMTα) or empty vector (pMT). Expression of C/EBPα and PPARγ was analyzed 48 hours after induction with 0 µM, 50 µM, and 100 µM of ZnSO4. Normal human pre-adipocytes and adipocytes (cultured without supplemental zinc) were also analyzed. qRT-PCR was used to analyze RNA levels of A: C/EBPα and B: PPARγ. Levels are shown relative to pre-adipocytes. C: Western blot. Both the 42 kDa and 30 kDa isoforms of C/EBPα can be seen.
The increased expression of C/EBPα and PPARγ was confirmed on a protein level by Western blot (Figure 3C). Both forms of C/EBPα were produced, with the more prominent being the 42 kDa form, which is the functional form (Koschmieder et al., 2009). The empty vector in the presence of zinc caused a slight increase in PPARγ protein levels without a correlated increase in mRNA. Zinc is known to increase PPARγ levels (Meerarani et al., 2003; Shen et al., 2008; Xinqin et al., 2009). Despite the zinc-induced increase, the PPARγ level in DDLS-pMT was still much lower than that in DDLS-pMTα. Immunofluorescence demonstrated that DDLS-pMTα cells cultured with zinc expressed C/EBPα in differentiating as well as regular conditions (Supplemental Figure 1).
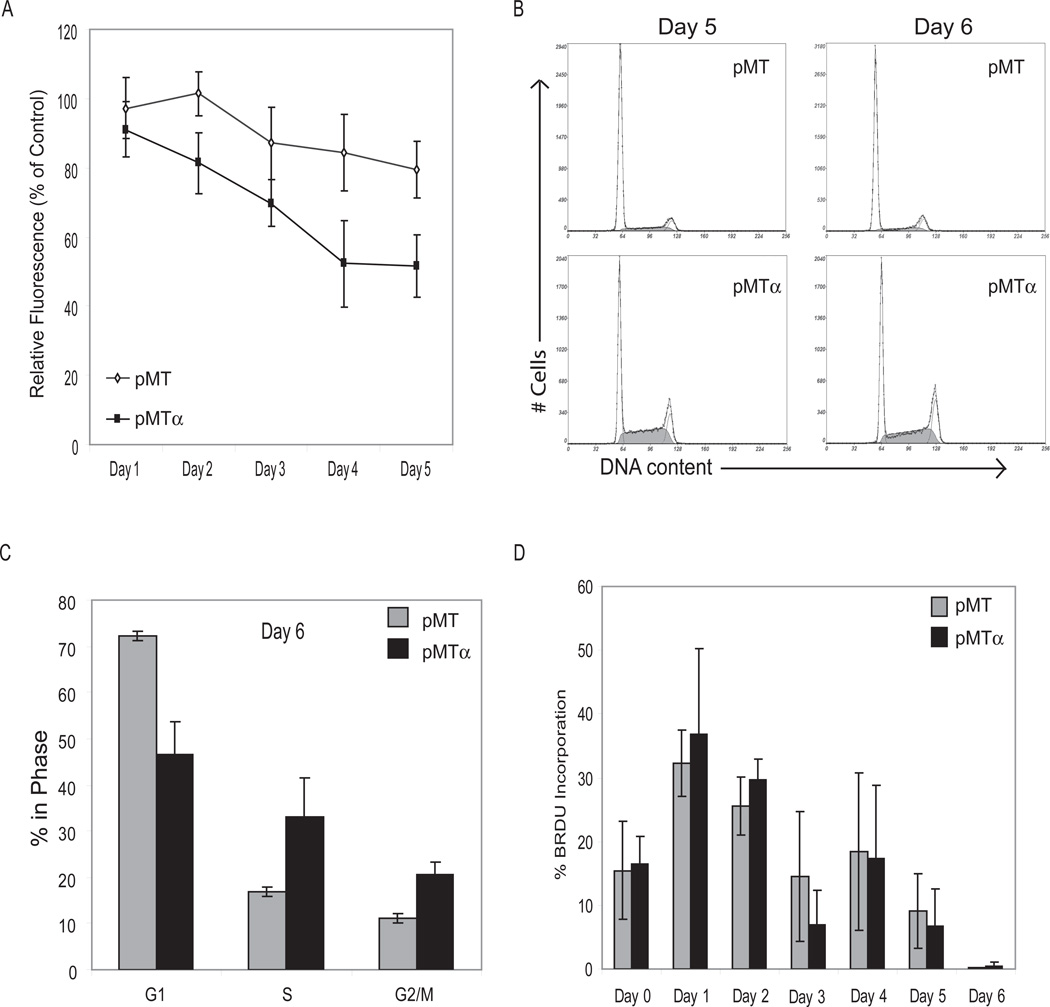
C/EBPα expression induces proliferation arrest in G2/M phase
One of the earliest characteristics of terminal differentiation is growth arrest. C/EBPα plays an integral role in growth arrest during the initiation of differentiation. To assess C/EBPα’s ability to arrest DDLS cells, we generated proliferation curves by quantifying the DNA of attached (i.e. live) cells. As shown in Figure 4A, proliferation on day 5 of DDLS-pMT cultured in regular medium plus zinc was 20% lower than that of the same cells grown without zinc. This decrease in proliferation of DDLS-pMT may be due to zinc’s effect of increasing PPARγ levels; PPARγ alone can cause some growth inhibition (Altiok et al., 1997; Fajas et al., 2003; Theocharis et al., 2004). In DDLS-pMTα, however, zinc caused a significantly greater drop in proliferation from day 2 to day 5 (P<10−6 for DDLS-pMTα vs DDLS-pMT). In cell cycle analysis (Figure 4B, C), DDLS-pMTα, compared to DDLS-pMT, showed a greater proportion of cells in S and G2/M phases. We then assessed entry into S phase using bromodeoxyuridine (BrdU) incorporation. Both cell lines incorporated BrdU, albeit little on day 3 (when cells needed to be fed) and day 6 (when cells reached confluence). The two cell lines incorporated similar amounts of BrdU, indicating that the proportion of cells entering S phase was similar. Therefore, the differences in the cell cycle profiles are best explained by DDLS-pMTα cells lagging in S phase and eventually accumulating in G2/M.
Figure 4.
Proliferation and cell cycle analysis of DDLS-pMT and DDLS-pMTα in regular medium with 100 µM zinc to induce C/EBPα expression. A: Proliferation of DDLS-pMT and DDLS-pMTα with zinc induction relative to the same cell lines grown without zinc. B: Representative flow cytometry cell cycle curves on days 5 and 6 after addition of zinc for DDLS-pMT (top panels) and DDLS-pMTα (bottom panels). The smooth curves show the cells gated into G1, S, and G2/M phases. C: Proportions of cells in each phase of the cell cycle, derived from 3 separate experiments on DDLS-pMT and DDLS-pMTα on day 6 after zinc induction. D: Percentage of DDLS-pMT and DDLS-pMTα cells with BrdU incorporation on days 0–6 after zinc induction.
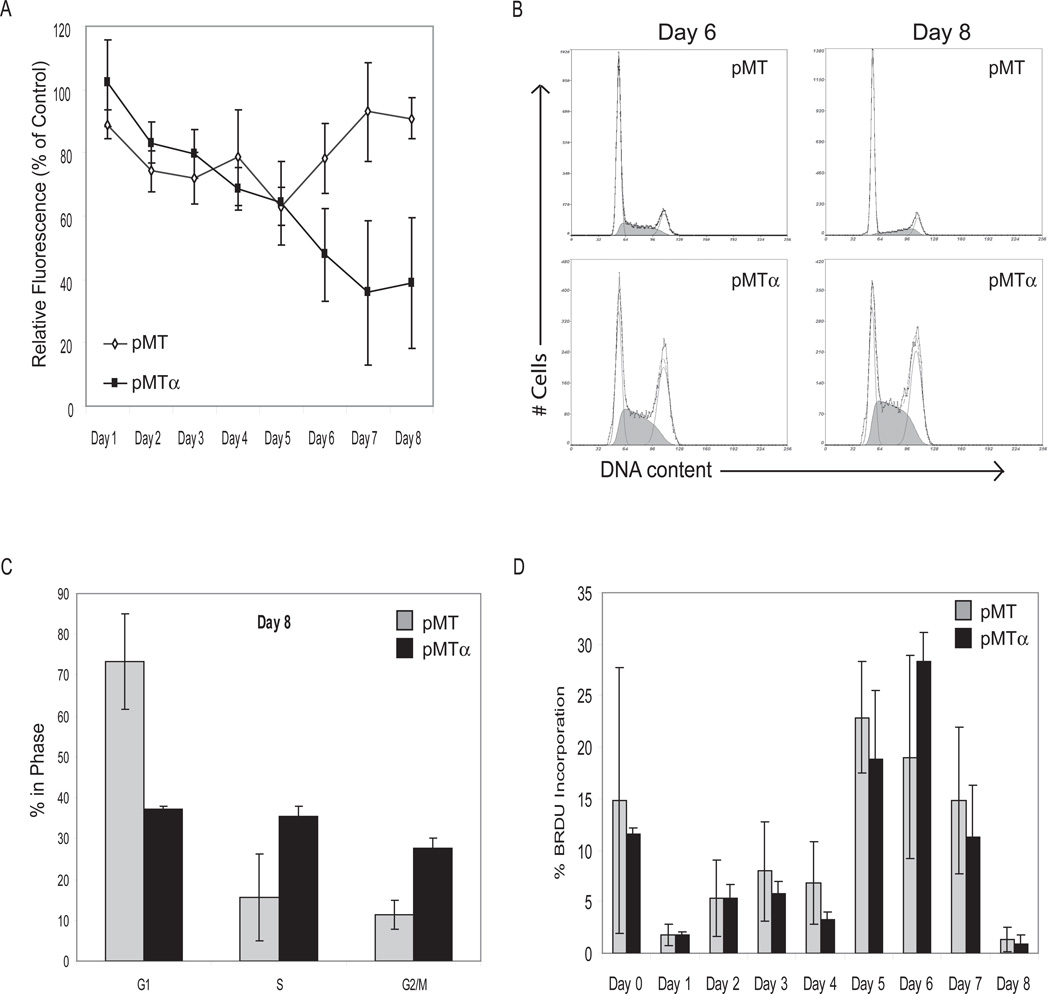
We next investigated proliferation of DDLS-pMT and DDLS-pMTα under adipocyte differentiation conditions. During the first 4 days, when cells were in differentiation initiating medium, DDLS-pMT and DDLS-pMTα had equal decreases in proliferation in the presence of zinc relative to the zinc-free controls (Figure 5A). After the medium was changed to differentiation maintenance medium on day 4, DDLS-pMT regained its growth potential and by day 8 had 90% the number of cells of DDLS-pMT in maintenance medium without zinc. DDLS-pMTα, on the other hand, did not regain its growth potential and on day 8 had 40% the number of cells of DDLS-pMTα in medium without zinc.
Figure 5.
Proliferation of DDLS-pMT and DDLS-pMTα in differentiation media with 100 µM zinc to induce expression of C/EBPα. Cells were grown in initiating medium on days 1–4 and in maintenance medium on days 5–8. A: Proliferation of DDLS-pMT and DDLS-pMTα with zinc induction relative to the same cell lines grown without zinc. B: Representative flow cytometry cell cycle curves of DDLS-pMT (top panels) and DDLS-pMTα (bottom panels) on days 6 and 8 after zinc induction. The smooth curves show the cells gated into G1, S, and G2/M phases. C: Proportions of cells in each phase of the cell cycle, derived from 3 separate experiments on DDLS-pMT and DDLS-pMTα on day 8. D: Percentage of DDLS-pMT and DDLS-pMTα cells with BrdU incorporation on days 0–8 after zinc induction.
Cell cycle analysis and BrdU incorporation in differentiation conditions are shown in Figure 5B–D. During the 4 days in differentiation initiation medium, cells replicated very little as indicated by BrdU incorporation (Figure 5D). After the cells were placed in differentiation maintenance medium, DDLS-pMT started proliferating and returned to a normal cell cycle distribution (Figure 5B and C). DDLS-pMTα also started to proliferate but did not return to a normal cell cycle distribution, instead showing a lag in S phase followed by G2/M arrest.
In summary, forced C/EBPα expression resulted in a lag in S phase with eventual accumulation of cells in G2/M. These effects were seen under both regular and differentiation conditions, but were more pronounced in differentiation conditions.
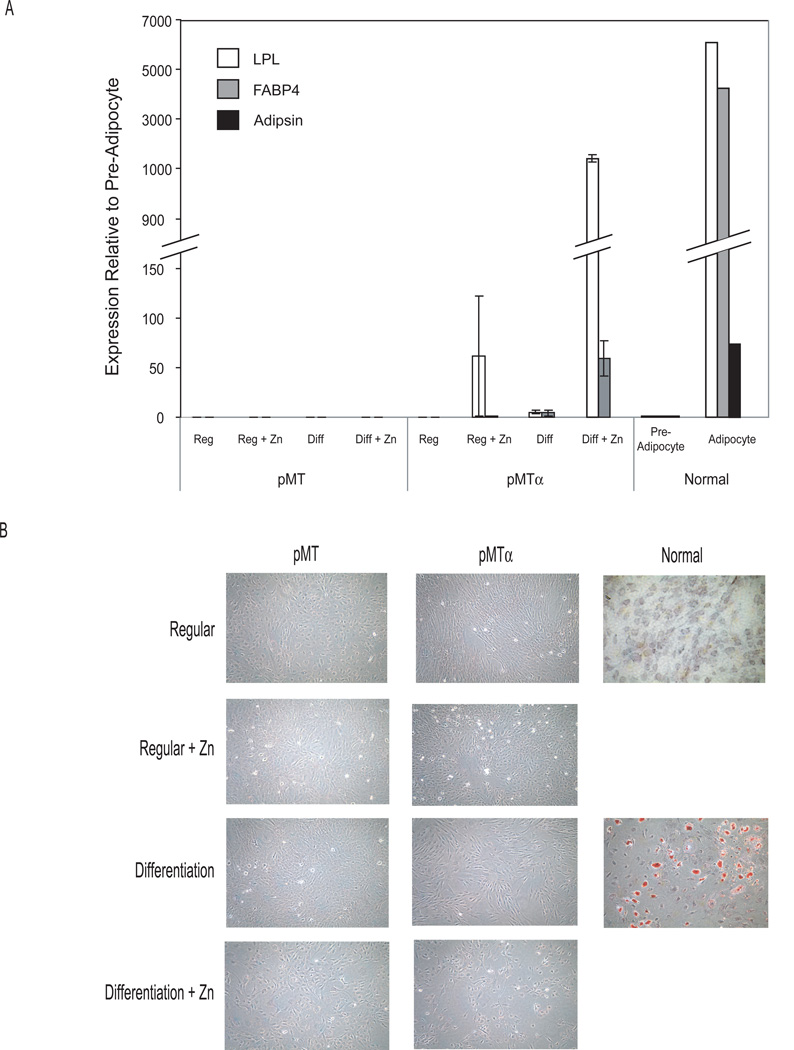
Restoring C/EBPα under differentiating conditions allows expression of early adipogenesis genes but not the accumulation of lipid droplets
Analysis of adipogenesis markers by qRT-PCR (Figure 6A) indicates that forced expression of C/EBPα induced initiation of the adipogenesis pathway in DDLS-pMTα cells. In regular conditions, inducing C/EBPα expression with zinc resulted in a 63-fold increase in mRNA of the earliest adipogenesis marker, LPL. In differentiating conditions, the increase in LPL mRNA was greater (1100 fold), and FABP4 expression also increased (60 fold). Both these increases were, however, less than those in mature adipocytes (LPL, 6000 fold; FABP4, 4000 fold). Adipsin, a late adipogenesis marker, remained absent in the DDLS cells. In addition, DDLS-pMTα in all four conditions failed to accumulate intracellular lipid droplets, as assessed by Oil Red O staining (Figure 6B). Normal human preadipocytes in differentiation conditions, but not regular conditions, accumulated intracellular lipids (characteristic of mature adipocytes).
Figure 6.
Analysis of differentiation. A: qRT-PCR analysis of adipogenesis markers in DDLS-pMT and DDLS-pMTα cells grown in various media (regular and differentiating media with and without zinc) on day 8. B: Intracellular lipid accumulation as visualized by Oil Red O staining of DDLS-pMT, DDLS-pMTα, and normal adipocyte-derived stem cells grown in the same media as above.
Restoring C/EBPα under differentiating conditions induces apoptosis in DDLS cell lines
Our proliferation assay detects only viable cells, as non-viable cells are washed off and not analyzed. One possible reason for the proliferation decline of DDLS-pMTα cells grown with zinc is that forced C/EBPα expression may make these cells more prone to apoptosis. To quantify apoptotic cells under different conditions, we used annexin V staining and 7-AAD counter-staining.
Six days after zinc was added (Figure 7A, black bars), DDLS-pMTα in regular medium plus zinc had 16% of the cells stained with annexin V, compared to 5.6% for DDLS-pMT under the same conditions (P=0.019) (Figure 7A). The results were similar on day 8 (P=0.0026 for DDLS-pMTα vs DDLS-pMT). In differentiation medium plus zinc, DDLS-pMTα had even higher apoptosis levels: 26% on day 6 and 66% on day 8, compared to 6%–7% for DDLS-pMT under the same conditions (P=0.0005 and 0.024, respectively). In the absence of zinc, DDLS-pMT and DDLS-pMTα had similar baseline apoptotic rates of 5–10%.
Figure 7.
Apoptosis induced by C/EBPα expression. A: Annexin V quantification of apoptosis of DDLS-pMT and DDLS-pMTα on days 6 and 8 grown in regular and differentiating media, with and without zinc to induce C/EBPα expression. B: Western blot of PARP cleavage, an apoptosis marker, under the different conditions.
The apoptosis results seen with annexin V stain were confirmed by Western analysis of PARP cleavage, an apoptosis marker (Figure 7B). Both floating and attached cells were collected. Little PARP cleavage was detected in DDLS-pMT in any of the four conditions. Only in DDLS-pMTα cells in the differentiation medium plus zinc did we see strong cleavage of PARP. The slight decrease in PARP band intensity on day 8 for DDLS-pMTα in differentiating medium plus zinc was due to breakdown of cellular debris.
Discussion
For patients with liposarcoma, the most important determinant of clinical outcome is histologic subtype, a measure of the extent of differentiation. DDLS have a higher metastatic potential and are more locally aggressive compared to WDLS. The molecular mechanisms driving progression of WDLS to DDLS remain to be discovered. We therefore examined whether dysregulation of genes involved in normal adipocyte differentiation contributes to the dedifferentiated state of DDLS. We found that C/EBPα and its partner PPARγ were underexpressed in DDLS and, to a lesser extent, in WDLS. DDLS cell lines grown in differentiating conditions lacked the normal induction of C/EBPα expression, although they partially induced PPARγ. Restoration of C/EBPα expression restored PPARγ expression, decreased cell proliferation, and, especially under adipocyte differentiation conditions, induced expression of early differentiation markers, accumulation of cells in G2/M, and apoptosis. These findings provide strong evidence that underexpression of C/EBPα contributes to the phenotypes of DDLS, and suggest that dysregulation of C/EBPα expression, along with other alterations, may provide a mechanism in which WDLS progresses to DDLS.
During normal adipogenesis, hormonal induction factors cause a transient spike in C/EBPβ and δ levels, which then induce PPARγ (Saladin et al., 1999). C/EBPβ binds to the C/EBPα promoter in association with histone deacetylase 1 (HDAC1). When ligand-bound PPARγ is present, HDAC1 dissociates from the promoter, allowing C/EBPα to be expressed (Zuo et al., 2006). Once induced, PPARγ and C/EBPα promote each other’s expression in a positive feedback loop to maintain high levels of the mRNAs and to maintain the differentiated state.
What disrupts C/EBPα expression in DDLS? The primary defect is unlikely to be in the expression of PPARγ or in proteins upstream of PPARγ induction, given that PPARγ expression could be induced somewhat under differentiation conditions and that this increase was not accompanied by increased C/EBPα expression. However, several mechanisms are possible. Two strong possibilities are a defect in the promoter of the C/EBPα gene (CEBPA) or a defect in the release of HDAC1 after PPARγ has docked. Supporting these possibilities, our lab recently discovered CEBPA methylation in 24% of DDLS and somatic mutations in HDAC1 in 8.3% of DDLS (Taylor et al., 2011). Moreover, treatment of DDLS cells with demethylating agents and histone deacetylase inhibitor SAHA increased C/EBPα expression 19 fold (Taylor et al., 2011). Another strong possibility is a defect in PPARγ activity. Supporting this is our observation that, in DDLS cells in differentiation conditions, the small amount of PPARγ that was induced was phosphorylated, a modification that inhibits its transcriptional activity (Chan et al., 2001; Hu et al., 1996). We have also found phosphorylation of the PPARγ induced after forced expression of C/EBPα (Okada T et al, unpublished data). Further studies with kinase inhibitors will be required to determine whether the phosphorylation state or the total amount of PPARγ is more important. We also note the possibility that phosphorylation of PPARγ may contribute to dedifferentiation in a manner independent of any effect on C/EBPα expression. A final factor that may contribute to underexpression of C/EBPα is deletion of the gene. In a comparative genomic hybridization (CGH) study, Thirty four percent of DDLS had copy number loss of 19q13 (location of CEBPA), and this loss was associated with the dedifferentiated subtype, more aggressive disease, and poorer prognosis (Crago AM, manuscript submitted to Cancer Research). The specific mechanism of C/EBPα underexpression may differ among DDLS tumors, but taken together, these results suggest that alterations in CEBPA or HDAC1, perhaps acting in concert with PPARγ phosphorylation, reduce C/EBPα transcription and interfere with the normal PPARγ–C/EBPα positive feedback loop in DDLS.
Adipogenesis, which involves growth inhibition, activation of adipocyte-specific genes, and terminal differentiation, requires both C/EBPα and PPARγ (Cowherd et al., 1999; Rosen et al., 2002; Wu et al., 1999). Previous attempts at differentiating liposarcoma with PPARγ ligand (pioglitazone or troglitazone) alone succeeded in WDLS and myxoid/round cell subtypes but not in DDLS (Demetri et al., 1999; Tontonoz et al., 1997). In this paper we show no significant induction of C/EBPα expression in our panel of DDLS cell lines when cultured under differentiating conditions with a PPARγ ligand. This, taken together with the clinical results with PPARγ ligand noted above, leads one to hypothesize that WDLS, but not DDLS, expresses enough C/EBPα to start and sustain adipogenesis. We therefore examined whether forced expression of C/EBPα would allow DDLS cells to differentiate. Restoration of C/EBPα in a DDLS cell line with low baseline C/EBPα levels had the desired effect. In regular medium (which contains no PPARγ ligand), PPARγ levels increased appropriately with the increase in C/EBPα, suggesting that the underexpression of PPARγ in DDLS is the consequence, not the cause, of C/EBPα underexpression. Restoration of C/EBPα also slowed cell proliferation, caused cells to lag in S phase and accumulate in G2/M phase, and slightly increased apoptosis. C/EBPα is believed to promote growth inhibition by repressing E2F-mediated transcription (Porse et al., 2001; Slomiany et al., 2000). In liver cells, for example, C/EBPα regulates binding of E2F to RBL1 (p107) (Timchenko et al., 1999), interacts directly with CDKN1A, CDK2, and CDK4 (Harris et al., 2001; Wang et al., 2001), and acts as a transcription factor to induce expression of CDKN1A (p21) and CDKN1B (p27) (Cram et al., 1998; Timchenko et al., 1996; Wang et al., 2006). We note, however, that the mechanism of growth inhibition by C/EBPα may be tissue specific. In DDLS cells, restoration of C/EBPα had more pronounced effects in differentiating conditions, in which over 60% of cells eventually became apoptotic. These results may indicate that in differentiating conditions the cells remained arrested in G2/M, which in many cells eventually resulted in apoptosis, while in regular medium some cells finished mitosis and returned to G1. The proliferation rates were also presumably affected by the number of cells lost to apoptosis, which increased more dramatically in differentiation conditions.
Restoration of C/EBPα expression also allowed DDLS cells in differentiation conditions to express early markers of adipogenesis, although they did not express adipsin or accumulate intra-cellular lipid droplets. This effect of C/EBPα on differentiation is consistent with our CGH results, in which the loss of 19q13 (location of C/EBPα and adipsin) was associated with the dedifferentiated subtype (Crago AM, manuscript submitted to Cancer Research). Previous studies have also demonstrated C/EBPα’s ability to regulate differentiation and act as a tumor suppressor in acute myeloid leukemia, lung cancer, breast cancer, head and neck squamous cancer, and skin cancer (Bennett et al., 2007; Gery et al., 2005; Halmos et al., 2002; Loomis et al., 2007). Forced expression of C/EBPα in acute myeloid leukemia and lung cancer cell lines causes these cells to differentiate (Halmos et al., 2002; Tavor et al., 2003).
We postulate that increasing C/EBPα expression may offer a way to make liposarcoma cells enter a quiescent state, undergo regulated terminal differentiation, and respond appropriately to DNA damage and oncologic stress. Increasing C/EBPα levels may be possible with HDAC inhibitors. Numerous studies have shown that inhibition of HDAC or down-regulation of its expression results in adipocyte differentiation (Fajas et al., 2002; Fu et al., 2005; Yoo et al., 2006). Another possible strategy, at least for those tumors that have CEBPA methylation, is demethylating agents. Taylor and colleagues recently showed that treatment of DDLS cells with demethylating agents and the histone deacetylase inhibitor SAHA increased expression of C/EBPα, induced expression of differentiation markers, and had anti-proliferative and pro-apoptotic effects (Taylor et al., 2011). Therefore, HDAC inhibition and methylation inhibition are promising concepts for differentiation-based therapy for patients with liposarcoma.
Methods and Materials
Patient sample acquisition, cell culture and RNA isolation
Informed consent was obtained from all patients, and the project was approved by the local review board. Following histological review by a pathologist (C.A.), tissue samples were macro-dissected from cryomolds to ensure subtype uniformity and to remove necrotic/normal tissue.
DDLS8817, LPS141, and RDD8107 liposarcoma cell lines were established from DDLS samples obtained from patients who signed informed consent. The dedifferentiated (DDLS8817, RDD8107 and LPS141) cell lines were confirmed by cytogenetic analysis and by DNA copy number arrays (Agilent 244K) to harbor 12q amplification. All three cell lines show MDM2, CDK4 and HMGA2 amplification. LPS141 has both JUN and DDIT3 amplification. DDLS8817 and RDD8107 are not amplified for JUN or DDIT3. Primary human adipose-derived stem cells (ASCs) were isolated as described (Gimble and Guilak, 2003) using subcutaneous fat tissue samples from consenting patients. Cell lines were maintained in regular medium: DMEM HG:F12 supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen). All cultures were Mycoplasma free.
Tissues samples were lysed using QIAzol lysis reagent as previously described (Singer et al., 2007). RNA was isolated from frozen tissue using RNeasy Lipid Tissue Mini Kit and from cell lines using the RNeasy Kit (Qiagen, Valencia, CA).
Microarray analyses
The HG U133A microarray analysis was performed on 79 WDLS, 84 DDLS, and 23 normal fat patient tissue samples as previously described (Singer et al., 2007). The genes selected for further analysis were the 998 genes that showed at least 2-fold differential expression compared to the normal fat with an FDR <0.01 for WDLS or <0.0001 for DDLS. All these genes were entered into the Ingenuity Pathway Analysis application (http://www.ingenuity.com), a web-based bioinformatics tool that maps gene lists (ranked by FDR or fold change) to biological interactions between genes to determine the significant gene interaction networks based on WDLS and DDLS expression profiles.
U133A microarrays were used to generate triplicate gene expression profiles of ASCs at baseline (day 0) and 8 days following treatment with differentiation media.
Drug preparation
GI262570 (GlaxoSmithKline Pharmaceuticals, Collegeville, PA) stock solution was prepared in DMSO. Zinc sulfate heptahydrate (Sigma, St. Louis, MO) stock solutions were prepared in sterile water.
Plasmids and transfection
The C/EBPα expression vector (pMTα) (Tavor et al., 2003) was a generous gift from Dr. H.P. Koeffler at Cedars-Sinai Medical Center, Los Angeles, CA. The corresponding empty vector CB6+ (pMT) was a generous gift from F.J. Rauscher III at The Wistar Institute, Philadelphia, PA. DDLS8817 cells were transfected with either pMTα or pMT using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. At 24 hours after transfection, G418 (600 µg/ml) was added to select for stably transfected cells. Multiple monoclonal and polyclonal cultures were screened for C/EBPα expression after 48 hrs of 100 µM zinc induction by qRT-PCR and Western blot analysis.
Quantitative real-time reverse transcription PCR (qRT-PCR)
Reverse transcription was performed using random hexamer priming and TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). Quantitative real-time PCR (qPCR) using TaqMan Gene Expression Assays (Applied Biosystems) was done on the ABI Prism 7900HT Sequence Detection System and analyzed using SDS version 2.1 software (Applied Biosystems). Relative gene expression was calculated by normalizing expression of genes of interest to an endogenous control, 18S rRNA.
Proliferation assays
DNA content in fixed LS cell lines was estimated using the CyQuant Cell Proliferation Kit (Molecular Probes, Eugene, OR) and the Spectramax M2 fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) at 480/520 nm excitation/emission, per the manufacturer’s instructions.
Cell cycle analysis
DDLS-pMT and DDLS-pMTα cells (3 ×105) were plated in 10-cm culture plates, synchronized by serum starvation for 3 days, and then treated with media containing 100 µM zinc for days 0–6. Incorporation of BrdU (Sigma) was measured in cells incubated with 10 µM BrdU for 1 hr. BrdU content was analyzed as described (Schutte et al., 1987) using FITC-conjugated monoclonal anti-BrdU (BD Biosciences, San Diego, CA). Labeled cells were washed and resuspended in propidium iodide (PI) (20 µg/ml) (Calbiochem, San Diego, CA) with DNase-free RNAse (Qiagen, Valencia, CA) for 30 minutes at room temperature before fluorescence-activated cell sorting (FACS) analysis.
Induction of differentiation
Preadipocytes, DDLS-pMT, and DDLS-pMTα were plated at 70%–80% confluence 24 hours before differentiation. After reaching confluence, cells switched to initiating medium, consisting of regular medium plus 1 µM insulin, 1 µM dexamethasone, 250 µM 3-isobutyl-1-methylxanthine (IBMX), 33 µM biotin, 17 µM pantothenic acid, 200 µM indomethacin (Sigma Chemical Co, St. Louis, MO), and 100 nM of the PPARγ ligand GI262570. After 4 days, media were changed to maintenance medium, which is the same as initiating medium except that it lacks IBMX and indomethacin. Cells were fed with maintenance medium every 4 days thereafter.
Oil Red O staining
On day 8 of differentiation, cells were washed twice with PBS and fixed with 100% propylene glycol for 2 minutes. Once fixed, the cells were stained with a filtered solution of 0.5% (weight/volume) Oil Red O in propylene glycol) for one hour at room temperature. Cells were then fixed in 85% propylene glycol for 1 minute and washed twice with distilled water. Harris’ hematoxylin stain was used as a counter-stain. After the cells were washed again with distilled water, they were visualized and photographed (Nikon Eclipse TE300, RT Slider Diagnostic Instruments Inc).
Apoptosis assay
Apoptosis was evaluated using the Guava PCA (Guava Technologies, Hayward, CA) on cells stained with Annexin V: PE Apoptosis Kit (BD Biosciences) per the manufacturer’s instructions. Cells positive for Annexin V were counted using the Guava Personal Cytometer and analyzed using Guava software to determine the percentage of cells undergoing early apoptosis and late apoptosis.
Western blot
Protein from total cell lysates was resolved by SDS-PAGE, then transferred onto Immun-Blot polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Immunoblots were variously probed with antibodies for C/EBPα, PPARγ, GADPH (Santa Cruz Biotechnology, Santa Cruz, CA), or poly(ADP-ribose) polymerase (PARP) (BD PharMingen, San Diego, CA) or phosphorylated PPARγ (Millipore, Billerica, MA) or beta-actin (Cell signaling, Danvers, MA). Detection of the secondary antibodies was accomplished using enhanced chemiluminescence (ECL) reagents (Amersham Biosciences, Piscataway, NJ).
Acknowledgments
This work was supported in part by the Soft Tissue Sarcoma Program Project (P01 CA047179, S.S. and C.A.), the Starr Foundation Cancer Consortium, and by a donation from Richard Hanlon and Mortimer B. Zuckerman.
Footnotes
Conflict of interest
We have no conflicts of interest
References
- Altiok S, Xu M, Spiegelman BM. PPARgamma induces cell cycle withdrawal: inhibition of E2F/DP DNA-binding activity via down-regulation of PP2A. Genes Dev. 1997;11(15):1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, Decarolis PL, Shah K, Socci ND, Weir BA, Ho A, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42(8):715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett KL, Hackanson B, Smith LT, Morrison CD, Lang JC, Schuller DE, Weber F, Eng C, Plass C. Tumor suppressor activity of CCAAT/enhancer binding protein alpha is epigenetically down-regulated in head and neck squamous cell carcinoma. Cancer Res. 2007;67(10):4657–4664. doi: 10.1158/0008-5472.CAN-06-4793. [DOI] [PubMed] [Google Scholar]
- Chan GK, Deckelbaum RA, Bolivar I, Goltzman D, Karaplis AC. PTHrP inhibits adipocyte differentiation by down-regulating PPAR gamma activity via a MAPK-dependent pathway. Endocrinology. 2001;142(11):4900–4909. doi: 10.1210/endo.142.11.8515. [DOI] [PubMed] [Google Scholar]
- Costa DB, Dayaram T, D'Alo F, Wouters BJ, Tenen DG, Meyerson M, Tsao M-S, Halmos B. C/EBP[alpha] mutations in lung cancer. Lung Cancer. 2006;53(2):253–254. doi: 10.1016/j.lungcan.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Cowherd RM, Lyle RE, McGehee RE., Jr Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol. 1999;10(1):3–10. doi: 10.1006/scdb.1998.0276. [DOI] [PubMed] [Google Scholar]
- Cram EJ, Ramos RA, Wang EC, Cha HH, Nishio Y, Firestone GL. Role of the CCAAT/enhancer binding protein-alpha transcription factor in the glucocorticoid stimulation of p21waf1/cip1 gene promoter activity in growth-arrested rat hepatoma cells. J Biol Chem. 1998;273(4):2008–2014. doi: 10.1074/jbc.273.4.2008. [DOI] [PubMed] [Google Scholar]
- Dalal K, Antonescu C, Singer S. Diagnosis and management of lipomatous tumors. J Surg Oncol. 2008;97(4):298–313. doi: 10.1002/jso.20975. [DOI] [PubMed] [Google Scholar]
- Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri GD, Fletcher CDM, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci USA. 1999;96(7):3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril M-B, Miard S, Auwerx J. The retinoblastoma-histone deacetylase 3 complex inhibits PPAR[gamma] and adipocyte differentiation. Dev Cell. 2002;3(6):903–910. doi: 10.1016/s1534-5807(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Fajas L, Egler V, Reiter R, Miard S, Lefebvre A-M, Auwerx J. PPAR[gamma] controls cell proliferation and apoptosis in an RB-dependent manner. Oncogene. 2003;22(27):4186–4193. doi: 10.1038/sj.onc.1206530. [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Eastman HB, Martindale JL, Holbrook NJ. Physical and functional association between GADD153 and CCAAT/enhancer-binding protein beta during cellular stress. J Biol Chem. 1996;271(24):14285–14289. doi: 10.1074/jbc.271.24.14285. [DOI] [PubMed] [Google Scholar]
- Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- Fu M, Rao M, Bouras T, Wang C, Wu K, Zhang X, Li Z, Yao T-P, Pestell RG. Cyclin D1 inhibits peroxisome proliferator-activated receptor gamma-mediated adipogenesis through histone deacetylase recruitment. J Biol Chem. 2005;280(17):16934–16941. doi: 10.1074/jbc.M500403200. [DOI] [PubMed] [Google Scholar]
- Gery S, Tanosaki S, Bose S, Bose N, Vadgama J, Koeffler HP. Down-regulation and growth inhibitory role of C/EBPalpha in breast cancer. Clin Cancer Res. 2005;11(9):3184–3190. doi: 10.1158/1078-0432.CCR-04-2625. [DOI] [PubMed] [Google Scholar]
- Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5(5):362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- Halmos B, Huettner CS, Kocher O, Ferenczi K, Karp DD, Tenen DG. Down-regulation and antiproliferative role of C/EBPalpha in lung cancer. Cancer Res. 2002;62(2):528–534. [PubMed] [Google Scholar]
- Han S, Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs. 2007;18(3):237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- Harris TE, Albrecht JH, Nakanishi M, Darlington GJ. CCAAT/enhancer-binding protein-alpha cooperates with p21 to inhibit cyclin-dependent kinase-2 activity and induces growth arrest independent of DNA binding. J Biol Chem. 2001;276(31):29200–29209. doi: 10.1074/jbc.M011587200. [DOI] [PubMed] [Google Scholar]
- Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274(5295):2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci U S A. 1995;92(21):9856–9860. doi: 10.1073/pnas.92.21.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PF. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci. 2005;118(12):2545–2555. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- Koschmieder S, Halmos B, Levantini E, Tenen DG. Dysregulation of the C/EBPalpha differentiation pathway in human cancer. J Clin Oncol. 2009;27(4):619–628. doi: 10.1200/JCO.2008.17.9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279(43):45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- Loomis KD, Zhu S, Yoon K, Johnson PF, Smart RC. Genetic ablation of CCAAT/enhancer binding protein alpha in epidermis reveals its role in suppression of epithelial tumorigenesis. Cancer Res. 2007;67(14):6768–6776. doi: 10.1158/0008-5472.CAN-07-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani O, Brennetot C, Coindre JM, Gruel N, Ganem C, Delattre O, Stern MH, Aurias A. JUN oncogene amplification and overexpression block adipocytic differentiation in highly aggressive sarcomas. Cancer Cell. 2007;11(4):361–374. doi: 10.1016/j.ccr.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Meerarani P, Reiterer G, Toborek M, Hennig B. Zinc modulates PPARgamma signaling and activation of porcine endothelial cells. J Nutr. 2003;133(10):3058–3064. doi: 10.1093/jn/133.10.3058. [DOI] [PubMed] [Google Scholar]
- Miller SG, De Vos P, Guerre-Millo M, Wong K, Hermann T, Staels B, Briggs MR, Auwerx J. The adipocyte specific transcription factor C/EBPalpha modulates human ob gene expression. Proc Natl Acad Sci U S A. 1996;93(11):5507–5511. doi: 10.1073/pnas.93.11.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JO, Qin LX, Prete FP, Antonescu C, Brennan MF, Singer S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Ann Surg. 2009;250(6):977–982. doi: 10.1097/sla.0b013e3181b2468b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porse BT, Pedersen TÅ, Xu X, Lindberg B, Wewer UM, Friis-Hansen L, Nerlov C. E2F repression by C/EBP[alpha] is required for adipogenesis and granulopoiesis in vivo. Cell. 2001;107(2):247–258. doi: 10.1016/s0092-8674(01)00516-5. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Hsu C-H, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16(1):22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin R, Fajas L, Dana S, Halvorsen YD, Auwerx J, Briggs M. Differential regulation of peroxisome proliferator activated receptor gamma1 (PPARgamma1) and PPARgamma2 messenger RNA expression in the early stages of adipogenesis. Cell Growth Differ. 1999;10(1):43–48. [PubMed] [Google Scholar]
- Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005;288(6):E1195–E1205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- Schutte B, Reynders MM, van Assche CL, Hupperets PS, Bosman FT, Blijham GH. An improved method for the immunocytochemical detection of bromodeoxyuridine labeled nuclei using flow cytometry. Cytometry. 1987;8(4):372–376. doi: 10.1002/cyto.990080405. [DOI] [PubMed] [Google Scholar]
- Shen H, Oesterling E, Stromberg A, Toborek M, MacDonald R, Hennig B. Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-kappaB and PPAR signaling. J Am Coll Nutr. 2008;27(5):577–587. doi: 10.1080/07315724.2008.10719741. [DOI] [PubMed] [Google Scholar]
- Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238(3):358–371. doi: 10.1097/01.sla.0000086542.11899.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S, Socci ND, Ambrosini G, Sambol E, Decarolis P, Wu Y, O'Connor R, Maki R, Viale A, Sander C, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res. 2007;67(14):6626–6636. doi: 10.1158/0008-5472.CAN-07-0584. [DOI] [PubMed] [Google Scholar]
- Slomiany BA, D'Arigo KL, Kelly MM, Kurtz DT. C/EBPalpha inhibits cell growth via direct repression of E2F-DP-mediated transcription. Mol Cell Biol. 2000;20(16):5986–5997. doi: 10.1128/mcb.20.16.5986-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano HE, Kang DC, Finegold MJ, Hicks MJ, Wang ND, Harrison W, Darlington GJ. Lack of C/EBP alpha gene expression results in increased DNA synthesis and an increased frequency of immortalization of freshly isolated mice [correction of rat] hepatocytes. Hepatology. 1998;27(2):392–401. doi: 10.1002/hep.510270212. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci U S A. 2003;100(3):850–855. doi: 10.1073/pnas.0337434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Zhang JW, Daniel Lane M. Sequential gene promoter interactions by C/EBPbeta, C/EBPalpha, and PPARgamma during adipogenesis. Biochem Biophys Res Commun. 2004;318(1):213–218. doi: 10.1016/j.bbrc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Tavor S, Park DJ, Gery S, Vuong PT, Gombart AF, Koeffler HP. Restoration of C/EBPalpha expression in a BCR-ABL+ cell line induces terminal granulocytic differentiation. J Biol Chem. 2003;278(52):52651–52659. doi: 10.1074/jbc.M307077200. [DOI] [PubMed] [Google Scholar]
- Taylor BS, DeCarolis P, Angeles CV, Brenet F, Schultz N, Antonescu CR, Scandura JM, Sander C, Viale AJ, Socci ND, et al. Frequent alterations and epigenetic silencing of differentiation pathway genes in structurally rearranged liposarcomas. Cancer Discovery. 2011 doi: 10.1158/2159-8290.CD-11-0181. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharis S, Margeli A, Vielh P, Kouraklis G. Peroxisome proliferator-activated receptor-[gamma] ligands as cell-cycle modulators. Cancer Treat Rev. 2004;30(6):545–554. doi: 10.1016/j.ctrv.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Timchenko NA, Wilde M, Darlington GJ. C/EBPalpha regulates formation of S-phase-specific E2F-p107 complexes in livers of newborn mice. Mol Cell Biol. 1999;19(4):2936–2945. doi: 10.1128/mcb.19.4.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ. CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10(7):804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Singer S, Forman BM, Sarraf P, Fletcher JA, Fletcher CD, Brun RP, Mueller E, Altiok S, Oppenheim H, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proc Natl Acad Sci USA. 1997;94(1):237–241. doi: 10.1073/pnas.94.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G-L, Shi X, Salisbury E, Sun Y, Albrecht JH, Smith RG, Timchenko NA. Cyclin D3 maintains growth-inhibitory activity of C/EBPalpha by stabilizing C/EBPalpha-cdk2 and C/EBPalpha-Brm complexes. Mol Cell Biol. 2006;26(7):2570–2582. doi: 10.1128/MCB.26.7.2570-2582.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Iakova P, Wilde M, Welm A, Goode T, Roesler WJ, Timchenko NA. C/EBP[alpha] arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell. 2001;8(4):817–828. doi: 10.1016/s1097-2765(01)00366-5. [DOI] [PubMed] [Google Scholar]
- Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269(5227):1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43(4):527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3(2):151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- Xinqin K, Wei Z, Jie L, Zhenyuan S, Craig JM, Kang YJ, Zhanxiang Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50(4):1241–1250. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo EJ, Chung J-J, Choe SS, Kim KH, Kim JB. Down-regulation of histone deacetylases stimulates adipocyte differentiation. J Biol Chem. 2006;281(10):6608–6615. doi: 10.1074/jbc.M508982200. [DOI] [PubMed] [Google Scholar]
- Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278(1):498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- Zuo Y, Qiang L, Farmer SR. Activation of CCAAT/enhancer-binding protein (C/EBP) alpha expression by C/EBP beta during adipogenesis requires a peroxisome proliferator-activated receptor-gamma-associated repression of HDAC1 at the C/ebp alpha gene promoter. J Biol Chem. 2006;281(12):7960–7967. doi: 10.1074/jbc.M510682200. [DOI] [PubMed] [Google Scholar]