Abstract
Covertly attending to a stimulus location increases spatial acuity. Is such increased spatial acuity coupled with a decreased acuity at unattended locations? We measured the effects of exogenous (transient, involuntary) and endogenous (sustained, voluntary) attention on observers’ acuity thresholds for a Landolt gap resolution task at both attended and unattended locations. Both types of attention increased acuity at the attended and decreased it at unattended locations relative to a neutral baseline condition. These trade-off findings support the idea that limited processing resources affect early vision, even when the display is impoverished and there is no location uncertainty. There was no benefit without a cost.
Keywords: spatial covert attention, exogenous attention, endogenous attention, spatial resolution, Landolt acuity, limited resources, cost and benefit
Introduction
1.1 Processing limits and attentional trade-offs
Our lives take place in an overwhelmingly rich visual world. We are confronted with far more information than our visual system can effectively process. The processing capacity of the visual system is limited by the high metabolic cost of cortical computations. Given these limits, we need mechanisms to optimally allocate processing resources according to task demands (Lennie, 2003). Spatial attention is one such mechanism that helps regulate the expenditure of cortical computation (Pestilli & Carrasco, 2005). Covert attention selectively grants priority in processing to information at the attended location, at the expense of information at unattended locations, in the absence of eye movements (Lu & Dosher 1998; Luck, Hillyard, Mouloua, Woldorff, Clark, & Hawkins, 1994; Pestilli & Carrasco, 2005). By trading off processing resources between attended and unattended locations in the visual field and by biasing the competition for processing in favor of selected stimuli, attention allows us to optimize performance in visual tasks while overcoming the visual system’s limited capacity.
These attentional trade-offs manifest themselves in early visual dimensions, i.e. human contrast sensitivity. Attention increases contrast sensitivity at the attended location, while decreasing it at other locations, as compared to a neutral condition. By changing contrast sensitivity across attended and unattended locations of the visual field, attention effectively affects our window of visibility (Ling & Carrasco, 2006a; Pestilli & Carrasco, 2005; Pestilli, Viera & Carrasco 2007). But how general are these trade-off mechanisms? Are they specific to contrast sensitivity or do they generalize to other basic visual dimensions? In this study we investigated whether there are attentional trade-offs for spatial resolution: Does attention affect our ability to discriminate small details in visual stimuli at both attended and unattended locations?
1.2 Exogenous and endogenous attention
There are two types of covert attention, which have been isolated experimentally: exogenous (a transient component) and endogenous (a sustained component). Exogenous attention is a stimulus-driven component that is automatically activated by the sudden onset of a stimulus in the visual field. Its effect on perception is fast and transient, peaking at ~100–120 ms and decaying again quickly by ~250 ms. Endogenous attention is a conceptually-driven component that requires conscious effort and is activated more slowly. It can be voluntarily allocated to a location in the visual field within ~300–500 ms and may be sustained for several seconds. These two types of attention have been effectively manipulated via spatial cues, which induce observers to deploy their attention to a given location of the visual field before stimulus presentation. Peripheral cues, presented briefly next to the stimulus location, automatically draw the observer’s exogenous attention to that location. Central cues, presented at fixation, indicate to the observer where to voluntarily allocate their attention (e.g., Carrasco, Ling & Read, 2004; Cheal & Lyon, 1991; Jonides, 1981; Ling & Carrasco, 2006a,b; Müller & Findlay, 1988; Müller & Rabbitt, 1989; Nakayama & Mackeben, 1989; Posner, 1980; Remington, Johnston & Yantis, 1992).
Although neuroimaging studies indicate that both endogenous and exogenous attention affect early visual processing (e.g., Brefczynski & DeYoe, 1999; Gandhi, Heeger & Boynton, 1999; Liu, Pestilli & Carrasco, 2005; Martinez et al., 1999; Pinsk, Doniger & Kastner, 2004), some studies suggest that whereas endogenous attention is cortical in nature, exogenous attention is mediated by both cortical and subcortical networks (Corbetta & Shulman, 2002; Kastner & Ungerleider, 2000). Moreover, the two types of attention can have differential effects on visual performance, as revealed in visual search (Briand, 1998; Briand & Klein, 1987), contrast sensitivity (e.g., Dosher & Lu, 2000a, 2000b; Ling & Carrasco, 2006b; Lu & Dosher, 1998, 2000), temporal order judgment (Hein, Rolke & Ulrich, 2006), and texture segmentation (Yeshurun, Montagna & Carrasco, 2008) tasks.
Attentional trade-offs for both endogenous and exogenous attention have been described in the literature for a variety of visual tasks. In a typical paradigm, observers are either cued to the target location (valid or cued trial) or away from the target location (invalid or uncued trial) and the benefit and costs of these manipulations of attention are evaluated against a baseline or neutral condition. It is typically considered that on valid trials attention is focused on the target location, on invalid trials it is focused onto a location different than the target location, and that on neutral trials, attention is likely to be distributed across all possible target locations. In most studies attentional trade-offs have been demonstrated for reaction times: Observers are faster at detecting or discriminating visual stimuli at cued locations (benefit) and slower at uncued locations (cost), as compared to a neutral condition (e.g., Eriksen & Hoffman, 1972; Jonides & Mack, 1984; Posner, 1980). Unfortunately, reaction time measures do not allow us to differentiate between attentional effects on decisional criteria or discriminability rather than on the speed of information processing (Dosher, 1979; McElree & Dosher, 1989; Ratcliff, 1978; Reed, 1973; Wickelgren, 1977). Thus, they are not directly informative as to how attention changes the signal quality.
Attentional trade-offs in performance accuracy have been more difficult to be demonstrated unequivocally (e.g., Grindley & Townsend, 1968; Luck, Hillyard, Mouloula & Hawkins, 1996; Shiu & Pashler, 1994, 1995). Furthermore, few studies have focused on attentional trade-offs with regard to early visual dimensions such as contrast sensitivity and spatial resolution (e.g., Pestilli & Carrasco, 2005; Pestilli, Viera & Carrasco 2007; Shiu & Pashler, 1995). For spatial resolution, whereas some studies show differences in processing between valid and invalid cues (e.g., Golla, Ignashchenkova, Haarmeier & Thier, 2004; Shalev & Tsal, 2002), attentional trade-offs, identified as both benefits and costs relative to a baseline or neutral condition have rarely been reported (Shiu & Pashler, 1995). Here we use a baseline condition and show attentional trade-offs in spatial acuity thresholds.
Previous studies have evaluated the effects of exogenous and endogenous attention on contrast sensitivity when attention is directed to the target location as well as when attention is directed elsewhere. For exogenous attention, two studies have systematically characterized how peripheral cues increase contrast sensitivity at cued and decrease it at uncued locations as compared to a neutral baseline condition. The benefit and cost are best described by a response gain mechanism (Pestilli & Carrasco, 2005; Pestilli, Viera & Carrasco 2007). For endogenous attention, in a study investigating the interaction of attention and adaptation, a central cue has shown to increase contrast sensitivity at the attended location and to decrease it at the unattended locations (Ling & Carrasco, 2006a). These findings indicate that attention trade off contrast sensitivity between attended and unattended locations of the visual field. Is this trade-off manifested in spatial resolution as well? Whereas several studies have shown that covert attention enhances spatial resolution at the attended location (e.g., Carrasco, Williams, & Yeshurun, 2002; Golla et al. 2004; Yeshurun & Carrasco, 1999), less is known about whether and how spatial resolution, acuity, changes at a given location of the visual field when covert attention is directed elsewhere. Whereas these studies indicate that both exogenous and endogenous attention affect performance in spatial acuity tasks, their attentional trade-offs, reflected at both validly cued and invalidly cued locations, have not been compared. Moreover, whereas hyperacuity trade-offs have been shown for exogenous attention (e.g., Shiu & Pashler, 1995), the possibility that increased acuity at cued locations is coupled with a cost at uncued locations, has only been inferred for endogenous attention (Golla et al., 2004)1.
1.3 Effects of attention on spatial resolution
In the present study, we explored how attention affects spatial resolution, which is our ability to resolve small details in a visual stimulus, using a standardized Landolt acuity test that allowed us to measure gap resolution thresholds. Specifically, we investigated whether spatial acuity trades off between attended (validly cued) and unattended (invalidly cued) locations as compared to a neutral attentional condition (neutral cue). We asked whether the reported spatial acuity benefit at attended locations (e.g., Carrasco, Williams & Yeshurun 2002; Golla et al., 2004; Yeshurun & Carrasco, 1999) is accompanied by a cost in acuity at unattended locations. We investigated this possibility for both exogenous and endogenous attention and compared the pattern of their trade-offs by maintaining task and stimuli identical while selectively engaging either type of attention (Experiment 1). The fact that the attentional effect was evaluated against a neutral baseline condition for each type of attention, allowed us to establish whether it represented a benefit, a cost, or both. Given that endogenous attention has revealed itself to be more flexible than exogenous attention both in terms of its spatial allocation (e.g., Jonides, 1981; Kinchla, 1980; Müller & Rabbit, 1989), its effect on visual performance (Yeshurun, Montagna & Carrasco, 2008), and its costs and benefits (Giordano, McElree & Carrasco, 2004), it is plausible that this type of attention may be able to better manage the limited resources across attended and unattended locations, as compared to exogenous attention, which may result in a larger benefit and a smaller or no cost. In a control experiment, we established that the cueing effects on acuity are not mediated by a general attentional effect on contrast.
2. Experiment 1
2.1 Methods
Observers, apparatus, stimuli and procedure were identical for the exogenous and endogenous attention conditions except for the few aspects indicated below. The experimental paradigm designed to explore attentional trade-offs for spatial acuity is an extension of the one used by Pestilli and Carrasco (2005). Exogenous and endogenous attention were directed to a stimulus location via different spatial cues (uninformative for exogenous attention, informative for endogenous attention).
2.1.1 Observers
Seven observers participated in the experiment. All had normal or corrected-to-normal vision and had previously participated in psychophysical experiments. All except two authors (Observers 1 and 4) were naïve as to the purpose of the study. The New York University Committee on Activities involving Human Subjects approved the experimental protocol.
2.1.2 Apparatus
Stimuli were presented on a 21-in CRT monitor (Graphics Series G220fb, ViewSonic, Richmond Hill, Ontario) controlled by a PowerMac G4 computer (Apple Inc., Cupertino, California). Stimulus generation, presentation, data collection and analysis were performed using Matlab 7.3.0 (The MathWorks, Inc., Natick, MA) with an OpenGL psychophysical extension mgl (http://justingardner.net/mgl). Monitor resolution was set to 1600 × 1200 pixels at a frame rate of 85 Hz. A chin rest stabilized head position and maintained viewing distance at 114 cm. Observers viewed the stimuli binocularly. For the endogenous attention condition, eye position was monitored using an infrared camera (iSCAN, Burlington, MA) pointed at the observers’ right eyes. The experiment was conducted in a dark and quiet room.
2.1.3 Stimuli
Stimuli were presented on a black background (1.5 cd/m2). Observers fixated a cross (0.5 × 0.5° of visual angle) presented at the center of the screen throughout the experiment. To alert observers of trial onset, the color of the fixation cross was set to green (43 cd/m2) during trials and to white (57 cd/m2) during inter-trial-intervals (Figure 1). On each trial, two Landolt squares (side length: 1°; line width: 0.05°, luminance: 57 cd/m2) were presented, one in the right and one in the left hemifield, along either one of the monitor’s two main diagonals (±24.3° from horizontal). Landolt squares were centered at 9.375° eccentricity. Each square had a small gap either on its top or bottom side.
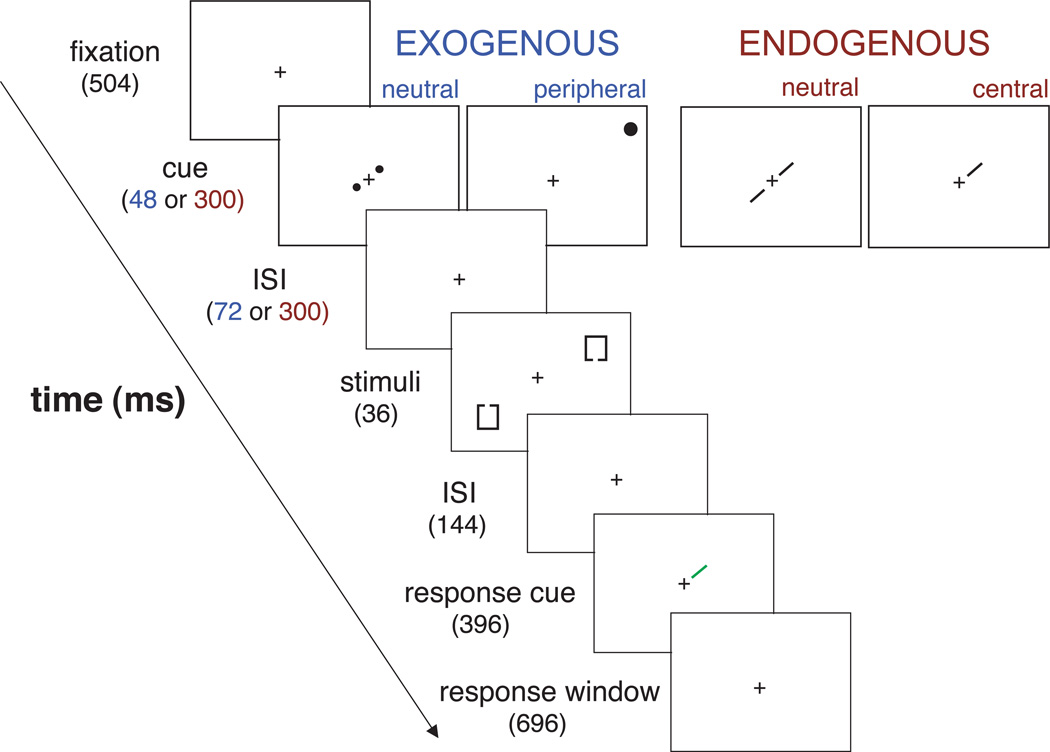
Figure 1.
Trial sequence. The trial sequence was identical for the exogenous and endogenous attention conditions except for the spatiotemporal characteristics of the cues, which are indicated in the figure in blue (peripheral cue) and red (central cue).
Exogenous attention
On peripherally cued trials (attended and unattended trials), a white dot (0.35° in diameter, luminance: 57 cd/m2) was presented at 0.5° from the outer vertical edge of one of the two Landolt squares. On neutral trials, a pair of smaller dots (diameter: 0.175°, luminance: 57 cd/m2) were presented at 0.375° eccentricity, one to the right and the other to the left of fixation, falling on the monitor’s diagonal on which the Landolt squares of that trial were to be presented.
Endogenous attention
On centrally cued trials (attended and unattended trials), a white line (length: 0.625°, width: 0.025°, luminance: 57 cd/m2), extending from fixation and pointing at one of the two upcoming Landolt squares’ location, indicated to the observer where to voluntarily sustain their covert attention, pointing toward the most likely (70% probability) target location. On neutral trials, two white lines (each of length: 0.625°, 57 cd/m2), extending from fixation, pointed at both upcoming Landolt squares’ locations.
A response cue was composed of a green line (length: 0.625°, width: 0.025°) extending from fixation and pointing at one of the two Landolt squares’ locations, always indicating the target Landolt square, for which the observer was to report the gap side.
2.1.4 Procedure and design
Spatial covert attention was manipulated via cues preceding stimulus presentation. On each trial, a precue either indicated a specific stimulus location (cued trials) or indicated both stimulus locations (neutral trials). Different types of cues selectively engaged either exogenous (peripheral uninformative cue) or endogenous (central informative cue) attention. Observers reported the location of a gap (top or bottom side) in the target Landolt-square indicated by a response cue following stimuli offset. The two attentional conditions, exogenous and endogenous, were blocked per session and each had its corresponding neutral cue baseline condition to quantify the magnitude of the attentional effects. There was one session per day, in which cued and neutral trials were randomly interleaved within each block or trials.
Figure 1 depicts a trial sequence. Each trial began with a 504-ms green fixation cross at the center of the screen. Observers were instructed to fixate the cross throughout the entire duration of a block of trials. Next, a precue was presented. The spatiotemporal parameters of the peripheral and central cues were explicitly manipulated to maximize the effects of exogenous and endogenous attention, respectively (e.g., Cheal & Lyon, 1991; Jonides, 1981; Müller & Rabbitt, 1989; Nakayama & Mackeben, 1989; Posner, 1980; Remington, Johnston & Yantis, 1992). Exogenous attention is activated quickly and is transient, such that its effect is maximal at ~100–120 ms from cue onset and then decays rapidly (Cheal & Lyon, 1991; Nakayama & Mackeben, 1991). The effect of exogenous attention is completely extinguished at ~500 ms from cue onset (Carrasco, Ling & Read, 2004; Fuller, Rodriguez & Carrasco, 2008; Liu, Fuller & Carrasco, 2006; Turatto, Vescovi & Valsecchi, 2007). Endogenous attention is activated more slowly, such that its maximum effect is not reached until after ~300 ms, and the magnitude of the effect is maintained for ~1s (Cheal & Lyon, 1991; Ling & Carrasco, 2006a; Liu, Stevens & Carrasco, 2007; Müller & Findlay, 1988). Based on these previous studies we presented our stimuli 120 ms after cue onset for exogenous and 600 ms after cue onset for endogenous attention. To directly compare the effects of these two types of attention, it is important to ensure that their individual effects are maximized (e.g., Hein, Rolke & Ulrich, 2006; Ling & Carrasco, 2006b; Yeshurun, Montagna & Carrasco, 2008).
Exogenous attention
On 2/3 of the trials (cued trials), a 48-ms white dot (peripheral cue) flashed briefly next to one of the upcoming Landolt squares’ location, automatically drawing observers’ exogenous attention to that location. Observers were informed that this cue was uninformative, that is, it was not predictive of target location or gap side (Pestilli & Carrasco, 2005; Pestilli, Viera & Carrasco, 2007). On the remaining 1/3 of the trials (neutral trials) a pair of white dots (neutral cue) was presented to the right and left of fixation on the same diagonal as the stimuli. As in the cued trials, this precue indicated stimulus onset and the diagonal along which the stimuli were about to appear. However, it did not draw observers’ exogenous attention to either of the stimulus locations. After an inter-stimulus-interval (ISI) of 72 ms, the stimulus display comprising the two Landolt squares was presented for 36 ms. Given that about 250 ms are necessary to execute goal-directed saccades (Leigh & Zee, 1991; Mayfrank, Kimmig & Fischer, 1987) no eye movements could occur between the onset of the precue and the offset of the stimulus display (156 ms).
Endogenous attention
On 2/3 of the trials (cued trials), a 300-ms white line extending from fixation (central cue) indicated one of the upcoming Landolt squares’ locations. Observers were informed that this cue would indicate the target location on 70% of the central-cue trials and were instructed to voluntarily allocate their endogenous attention to the cued location. On the remaining 1/3 of the trials (neutral trials), a pair of white lines extending from fixation toward both upcoming stimulus locations (neutral cue) distributed attention across both stimulus locations. The central and neutral cues both indicated the stimulus onset and diagonal. However, only the central cue indicated a specific stimulus location for endogenous attention to be allocated to. After an inter-stimulus-interval (ISI) of 300 ms, the stimulus display, comprising the two Landolt squares, was presented for 36 ms. Given that the time interval between the onset of the precue and the offset of the stimulus display (363 ms) exceeded the saccadic latency (Leigh & Zee, 1991; Mayfrank, Kimmig & Fischer, 1987), eye position was monitored to ensure that observers effectively maintained fixation.
Each Landolt square in the stimulus display contained a small gap in either the top or the bottom side. The side of the gap was randomized across trials and independently for each Landolt square in a pair. To prevent systematic afterimages in correspondence of the gap locations, each gap’s center was jittered by 0, 6, 12, or 16 pixels around the center of the square’s side. Briefly after (144 ms) the stimulus display, a response cue pointing at one of the two stimuli’s locations for 396 ms, indicated to the observer for which of the two squares to report the gap side (e.g., Downing, 1988; Hawkins et al., 1990; Lu & Dosher, 2004; Luck, Hillyard, Mouloua, Woldorff, Clark, & Hawkins, 1994; Pestilli & Carrasco, 2005; Pestilli, Viera, & Carrasco, 2007). The spatiotemporal characteristics of the response-cue were identical across the three cueing conditions (valid, invalid, and neutral). Observers were allotted 1100 ms from the onset of the response cue to respond by pressing one of two possible keys. Observers were given auditory feedback for both correct and incorrect responses.
Response cue and precue validity
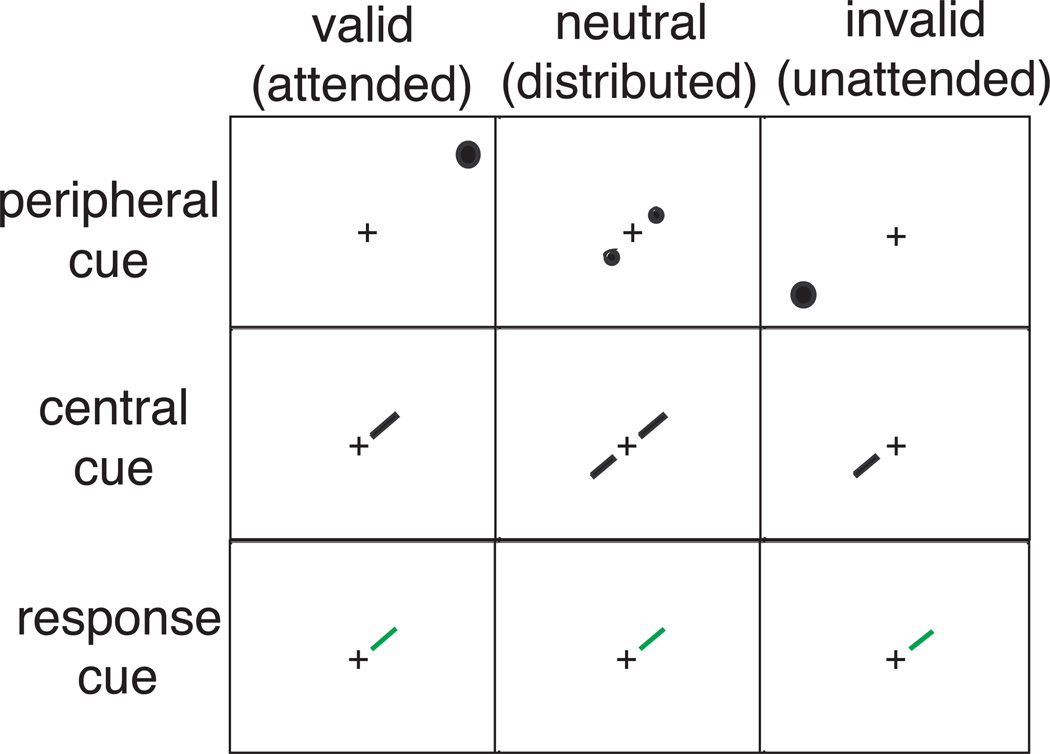
The validity of the precue was determined by the relation between the location indicated by the precue and that indicated by the response cue, which appeared after the stimulus offset (as in Pestilli & Carrasco, 2005). This relation is represented schematically in Figure 2 for both peripheral (first row) and central (second row) cues. When the location indicated by the precue and the response cue matched, observers reported the gap side of the precued (directly attended) stimulus and the trial was valid; when they mismatched, observers reported the gap side of the uncued (unattended) stimulus and the trial was invalid. On neutral trials neither of the two stimulus locations was precued.
Figure 2.
Cue validity was determined for both peripheral and central cues, by the relationship between the location indicated by the precue and the one indicated by the response cue.
Acuity measurement
To assess acuity, gap-size thresholds (75% localization accuracy) were measured for each cue type (valid, neutral, invalid) using an adaptive staircase procedure, QUEST (Watson & Pelli, 1983). QUEST staircases (3 for endogenous and 6 for exogenous attention) for the different trial types (valid, invalid, neutral) started at different times into the block of trials and were randomly interleaved within each block of trials to ensure that observers could not adopt different criteria across the three cueing conditions.
For exogenous attention, each threshold was derived from 40 trials. About 15 thresholds per cueing condition (valid, neutral, invalid) were collected for each observer for a total of ~3600 experimental trials per observer. For endogenous attention, given that cue validity was manipulated (as described above), thresholds were derived from the following number of trials in the three cueing conditions: valid: 96, neutral: 68, invalid: 40. About 15 thresholds per cueing condition were collected for each observer for a total of ~3060 experimental trials per observer. Each observer participated in both attention conditions; four performed the exogenous condition first.
2.2 Results
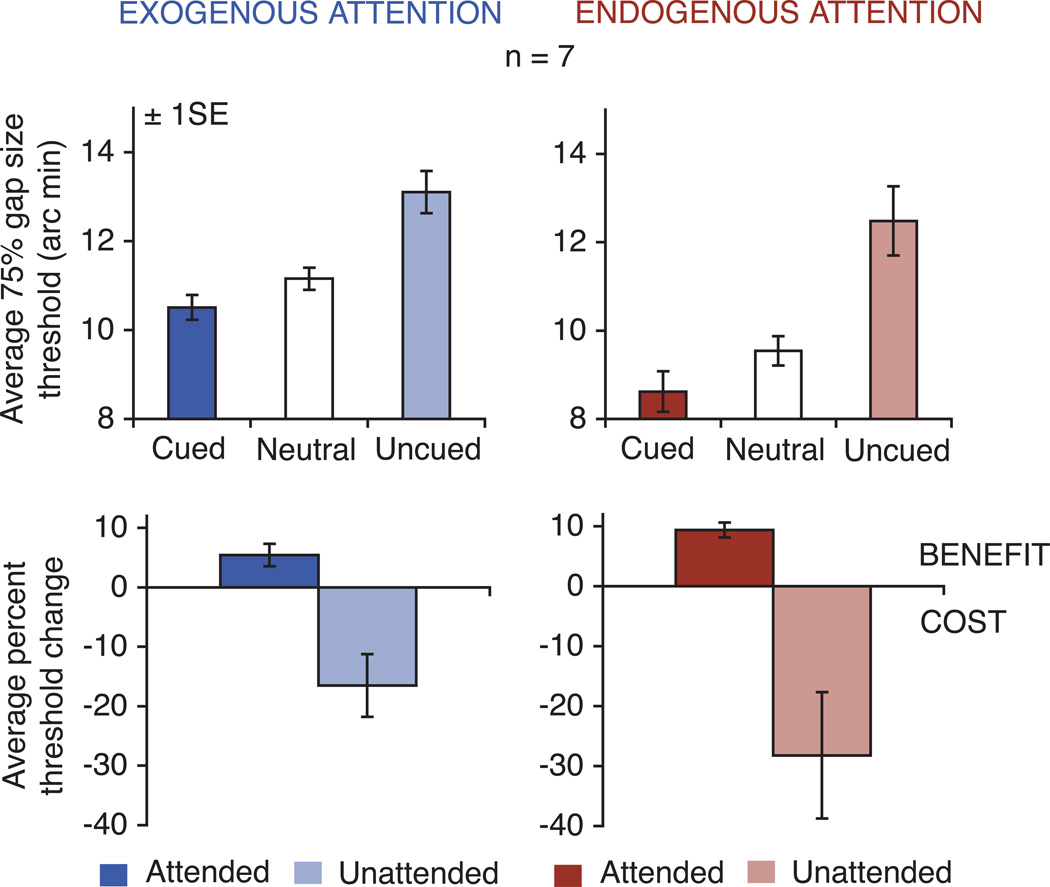
Gap-size thresholds (75% localization accuracy) were measured for each attention condition (exogenous, endogenous) and each cueing condition (cued, neutral, uncued)2. The median acuity threshold was calculated for each observer, and cue condition. Figure 3 depicts average data across observers for both exogenous (upper left panel) and endogenous (upper right panel) attention for the cued, neutral, and uncued conditions. Error bars indicate ± 1 SE appropriate for a within-subjects design (Cousineau, 2005).
Figure 3.
Attention and spatial acuity. Average gap-size thresholds (75% localization accuracy) for both exogenous (upper left panel) and endogenous (upper right panel) attention for the cued, neutral, and uncued conditions. The lower panels depict the average percent change in acuity thresholds at cued and uncued locations as compared to the neutral condition for exogenous (left) and endogenous (right) attention. Values below zero indicate a cost in acuity, whereas values above zero indicate a benefit. Error bars show ± 1 SE.
For both exogenous and endogenous attention, acuity thresholds were lower in the cued and higher in the uncued condition compared to the neutral baseline condition. The relative magnitude of the cueing effect is shown as benefit and cost in acuity in the two lower panels of Figure 3. These graphs represent the percent change in threshold, averaged across observers, at cued (attended) and uncued (unattended) locations relative to the neutral baseline. For both exogenous and endogenous attention, all observers show the same pattern of results (Figure 4) – acuity increased at the cued location and decreased at the uncued location (except one observer who showed a benefit at the cued location and a slight benefit at the uncued location for endogenous attention).
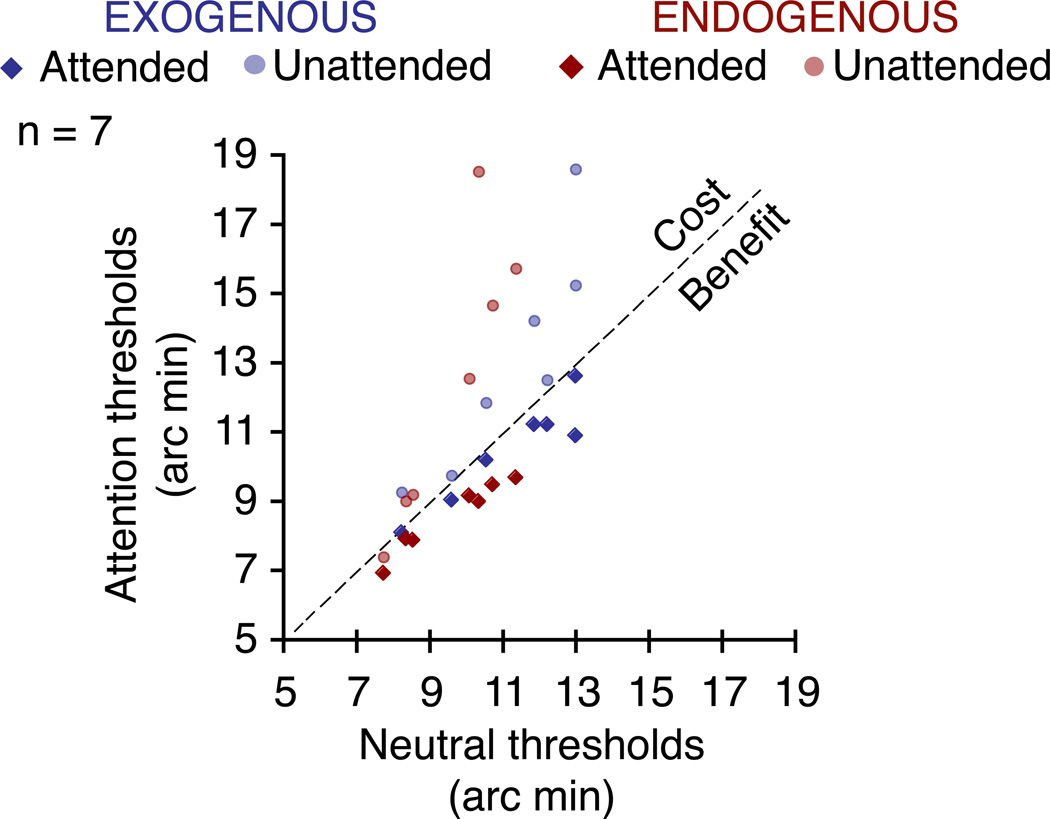
Figure 4.
Individual acuity thresholds. Scatterplot showing individual median gap-size thresholds (75% localization accuracy) for the attentional conditions (exogenous – blue – and endogenous – red) as a function of thresholds for the neutral condition. Thresholds for the valid cue conditions are represented as diamonds, those for the invalid cue conditions as circles. No attentional effect would result in all data points falling on the unit line. Data points below the unit line represent an acuity benefit, those above the line represent a cost. Notice how all data points for the valid cue condition (diamonds) fall underneath the unit line, whereas data points for the invalid cue condition (circles) fall above. This is the case for both endogenous and exogenous attention (except one observer’s threshold for endogenous attention).
A within-subjects two-way analysis of variance (attention condition: exogenous, endogenous; cueing condition: valid, neutral, invalid) was performed on the individual median acuity thresholds. Multivariate tests3 indicate a significant main effect of cueing (Wilks’ Λ = 0.208, F(2,5) = 9.541, p < 0.05, ηp2 = 0.792), but no significant effect of attention or interaction (p > 0.1). The lack of a significant interaction indicates that the cueing effect was similar for both types of attention. Pair-wise comparisons performed for exogenous and endogenous attention confirmed that acuity thresholds were lower in the cued (t(6) = 2.6, p < 0.05, d = 0.36; t(6) = 5.69, p = 0.001, d = 0.71, respectively) and higher in the uncued (t(6) = 2.77, p < 0.05, d = 0.68; t(6) = 2.64, p < 0.05, d = 0.89, respectively) condition as compared with the neutral condition, indicating a benefit in acuity at cued and a cost at uncued locations compared to the neutral baseline condition. For endogenous attention, the magnitudes of the cost and benefit (expressed as percent change relative to the neutral condition) were positively correlated (rS = 0.714, p < 0.05). In general, observers who showed larger benefits at the attended location also suffered from larger costs at the unattended location.
We tested for the presence of any systematic speed-accuracy trade-offs. For each observer, we first computed average reaction time (RT) across all the trials of a staircase run (i.e., for each threshold); next, we computed the median RT across thresholds in each attention condition. A within-subjects two-way analysis of variance (attention condition: exogenous, endogenous; cueing condition: valid, neutral, invalid) was performed on the individual median RTs. Multivariate tests indicate the main effect of cueing was significant (Wilks’ Λ = 0.284, F(2,5) = 6.309, p < 0.05, ηp2 = 0.716), but there was no significant effect of attention or interaction (p > 0.1). The lack of a significant interaction indicates that RTs were similar for the two types of attention. Pair-wise comparisons for both exogenous and endogenous attention revealed no significant difference in RTs between the cued and neutral conditions (p = 0.71; p = 0.27, respectively) RTs in the uncued trials were significantly longer than those in the neutral trials (t(6) = 2.96, p < 0.05, d = 0.5; t(6) = 3.3, p < 0.05, d = 0.79, respectively), indicating that when observers’ thresholds were higher and acuity was therefore worse, RTs were also slower. Thus, no systematic speed-accuracy trade-offs acuity differences between cued and uncued locations.
In sum, for both exogenous and endogenous attention, acuity increased at cued and decreased at uncued locations as compared to the neutral condition, indicating the presence of an acuity trade-off between attended and unattended locations. Could these changes in acuity have been mediated by attentional effects on perceived contrast rather than representing attentional effects on acuity? This possibility was directly assessed in a control Experiment.
3. Control Experiment
Spatial acuity increases as a function of contrast (e.g., Ikeda, Noda & Yamaguchi, 1983; Johnson & Casson, 1995; Pointer, 2001) and spatial covert attention has been shown to affect performance on tasks mediated by contrast. Both exogenous and endogenous attention increase contrast sensitivity at attended and decrease it at unattended locations (Ling & Carrasco, 2006a; Pestilli & Carrasco, 2005; Pestilli, Viera & Carrasco 2007). Moreover, both have also been shown to increase perceived contrast at the attended location (Abrams, Liu & Carrasco, 2008; Carrasco, Ling & Read, 2004; Carrasco, Fuller & Ling, 2008). Could the found increase in acuity at cued and decrease at uncued locations have been mediated by an increase and decrease in perceived contrast?
Landolt acuity increases sharply for contrast levels up to 10 or 20%, but only gradually for higher contrasts (Johnson & Casson, 1995; Pointer, 2001). Although exogenous and endogenous attention increase perceived contrast by ~5%, the size of the effect is much smaller than the changes in contrast for which differences in Landolt acuity have been measured (~50%, Johnson & Casson, 1995; ~55%, Pointer, 2001). Given that we used high-contrast stimuli (94% Michelson contrast) it is unlikely that changes in perceived contrast underlie the found attentional effect on acuity. Furthermore, given that for high stimulus contrasts, sensitivity is increased more by exogenous than by endogenous attention (Ling & Carrasco, 2006b), were the effects of attention on acuity mediated by the attentional effects on contrast, we would have expected a greater attentional effect with exogenous than with endogenous attention. However, we explicitly assessed the possible role of perceived contrast in the attentional effect on acuity.
Were the effects of attention on acuity to be mediated by the effects of attention on contrast perception, acuity accuracy would have to increase systematically with contrast around the contrast level used in Experiment 1. To test this, we asked observers to perform an acuity task identical to the neutral trials of the main Experiment (the neutral cue appeared 120 ms before stimulus onset), but varied the contrast of the Landolt square. The contrast of the Landolt square varied from 84% to 96%; i.e., it encompassed contrast differences equivalent to the magnitude of the attentional effect on perceived contrast (Carrasco, Ling & Read, 2004; Ling & Carrasco, 2007). Two observers participated in this control experiment; one had participated in the main experiment4.
3.1 Methods
The stimuli, apparatus, and procedure of the control Experiment were identical to the exogenous attention condition of Experiment 1, except for the following: 1) the neutral cue was presented on every trial; 2) the Michelson contrast of the Landolt square was set to 84, 87, 90, 93 and 96%. To vary contrast, the luminance of the Landolt squares was changed while keeping the background constant at 1.5 cd/m2.
3.2 Results
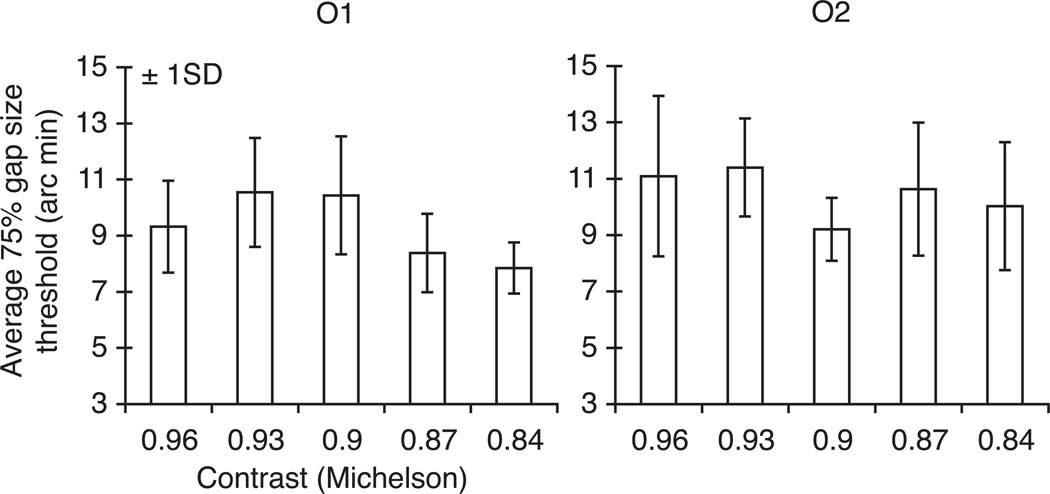
We measured ~15 acuity thresholds (75% localization accuracy) per observer at each contrast level. Figure 5 depicts median thresholds for each observer as a function of the Landolt Squares’ contrast (note that to simplify the direct comparison with the graphs for attention in Figure 3, the contrasts decrease along the abscissa). Stimulus contrast affected acuity thresholds (O1: F(4,78) = 10.014, p < 0.001; O2: F(4,67) = 3.132, p < 0.05), but they did not progressively increase as contrast decreased, as would be required for the attentional effect of acuity to be mediated by the attentional effect on contrast.
Figure 5.
Contrast and spatial acuity. Median gap-size thresholds (75% localization accuracy) as a function of the Landolt squares’ contrast for Observer 1 (left panel) and Observer 2 (right panel). Contrasts decrease along the abscissa. Error bars indicate ± 1 SD.
We can safely conclude that the attentional trade-offs in acuity are not mediated by a general effect of attention on contrast. A general effect of attention on contrast would affect spatial-frequency-selective mechanisms of many scales encoding the whole stimulus spectrum, i.e. it would be a broadband effect. It may instead be possible that, when the task at hand is mediated by filters selective to higher spatial frequencies (as it is the case for acuity), attention may selectively affect the specific mechanisms mediating the task (i.e., spatial filters selective to high spatial frequencies).
4. Discussion
In the present study we measured attentional trade-offs in spatial acuity. We evaluated exogenous and endogenous attention separately while maintaining task and stimuli identical; this allowed for the direct comparison of the pattern of effects between the two types of attention. The present results indicate that with very simple, non-cluttered displays – two Landolt squares – both exogenous and endogenous attention increased acuity at attended and decreased it at unattended locations, as compared to a neutral condition. These findings indicate that both exogenous and endogenous attention trade-off visual acuity, such that when covert attention is directed to a given location, our ability to discriminate details in an image increases at that location but worsens elsewhere. The relative magnitude of cost and benefits were similar for exogenous and endogenous attention. For endogenous attention, the magnitudes of benefits and costs were positively correlated, indicating that larger benefits at the cued location were associated with larger costs at unattended locations.
4.1 The automaticity/flexibility of covert attention
Notwithstanding the different temporal dynamics of exogenous and endogenous attention, it could be argued that when an exogenous precue always correctly predicts the target location some of its effect could be attributed to a conceptually-driven component of attention. In the present study, to guarantee the automaticity of the precue and that only the stimulus-driven component of attention is selectively engaged, the exogenous precue’s validity was set to be 50%: half the time the precue appeared next to the target square (valid cue), whereas the other half it appeared next to the non-target square (invalid cue). Thus, even when the precue was uninformative with respect to both target location and gap side, we show that exogenous attention automatically enhanced acuity at the attended location and decreased it at an unattended location5.
Contrary to exogenous attention, whose effect is indeed reflexive and automatic, endogenous attention is a more flexible mechanism. Endogenous attention is under the observer’s conscious control, it can adjust its operation to benefit performance depending on task demands (Hein, Rolke & Ulrich, 2006; Yeshurun, Montagna & Carrasco, 2008), and it has been shown to better manage benefits and costs as a function of attentional allocation as compared to exogenous attention (Giordano et al., 2004). For example, a study employing a speed-accuracy trade-off (SAT) procedure, which enables conjoint measures of discriminability and temporal dynamics, showed that with central cues, the observed attentional benefits increased with cue validity while costs remained relatively constant. However, with peripheral cues, the valid-cue benefits and the invalid-cue costs in both discriminability and processing speed were comparable across the range of cue validities. These results provide compelling time-course evidence that exogenous attention is automatic, but sustained attention can be flexibly allocated in a manner that increases the benefit of the valid cue and decreases the cost of the invalid cue (Giordano, McElree & Carrasco, 2004).
Given the evidence of such flexibility, we had hypothesized that endogenous attention may be able to better manage the limited resources between attended and unattended locations, which may result in a larger benefit and a smaller cost as compared to exogenous attention. This was not the case; the magnitude of costs and benefits was similar for exogenous and endogenous attention. Moreover, for endogenous attention, those participants who showed larger benefits at the cued location, in general also showed larger costs at the unattended location. Contrary to our expectations based on the evidence that endogenous attention can be more flexible and adaptable than exogenous attention (e.g., Giordano, McElree & Carrasco, 2004; Jonides, 1981; Kinchla, 1980; Müller & Rabbit, 1989; Yeshurun, Montagna & Carrasco, 2008), the voluntary allocation of attention implied a cost at unattended locations that increased as the benefit increased. This correlation further supports the idea that attention helps manage metabolic consumption in the brain: the more metabolic processing resources are allocated at the attended location, the less resources are allocated at the unattended location.
4.2 The ‘resolution hypothesis’: Psychophysical and neurophysiological evidence
The attentional benefit for acuity at cued locations is consistent with the ‘resolution hypothesis,’ which states that spatial covert attention can enhance spatial resolution, allowing us to resolve finer details in a stimulus at the attended location. For instance, for visual search, directing exogenous attention to the target location reduces the performance decrement typically found as target eccentricity increases. This finding suggests that exogenous attention reduces differences in spatial resolution between the fovea and the periphery (Carrasco & Yeshurun, 1998), much like cortical magnification (Carrasco & Frieder, 1997). Moreover, in a texture segmentation task that is limited by spatial resolution, attention enhances performance where resolution is too low for the task (peripheral locations) but actually impairs performance where resolution is already too high (fovea). This finding indicates that exogenous attention increases spatial resolution (e.g., Carrasco, Loula & Ho, 2006; Yeshurun & Carrasco, 1998, 2000, 2008; Yeshurun, Montagna & Carrasco, 2008).
The resolution hypothesis is in line with Shalev and Tsal’s (2002) finding that attention improves line-gaps localization. They proposed that attention increases resolution thereby increasing the perceived distance between the lines. Similarly, in a Landolt square, the ends of the lines creating the gap may be perceived to be farther from each other, increasing the perceived gap size (Gobell & Carrasco, 2005) and improving gap localization performance as we have found here.
The hypothesis that attention enhances resolution is consistent with neurophysiological studies on endogenous attention, demonstrating that a neuron’s response to its preferred stimulus is greatly reduced when the preferred stimulus is not attended, and an attended, non-preferred stimulus is also presented within the neuron’s receptive field. These findings suggest that attention contracts the cell’s receptive field around the attended stimulus (e.g., Desimone & Duncan, 1995; Luck, Chelazzi, Hillyard & Desimone, 1997; Moran & Desimone, 1985; Reynolds & Desimone, 1999; Womelsdorf, Anton-Erxleben, Piepe & Treue, 2006).
4.3 Covert attention and spatial acuity
Spatial covert attention enhances performance at the attended location in standard visual tasks specifically designed to assess spatial resolution, such as acuity (e.g., Landolt acuity or gap resolution) and hyperacuity (positional or Vernier resolution) tasks. Performance in hyperacuity tasks improves when exogenous attention is directed to the target location via spatial precues (Mackeben & Nakayama, 1993; Nakayama & Mackeben, 1989; Shiu & Pashler, 1995). In these studies the target was presented among several distracters, making it difficult to establish whether attention actually enhanced spatial resolution at the cued location or whether it reduced the detrimental influence of the distracters on the processing of the target. Later studies have shown that both acuity and hyperacuity increase at the cued location, even when all additional sources of external noise (e.g., low visibility of the target, presence of distracters or local post-masks) are not present in the display (Carrasco, Williams & Yeshurun, 2002; Yeshurun & Carrasco, 1999). This increase in spatial resolution at the attended location brought about by exogenous attention is accompanied by changes in the stimuli’s appearance: Both the perceived spatial frequency and the perceived size of a gap in the attended stimulus increase correspondingly (Gobell & Carrasco, 2005).
In a comparative study, Golla et al. (2004) evaluated the effects of spatial covert attention on Landolt acuity as a function of different stimulus-onset-asynchronies (SOAs) for human and non-human primates. They found that acuity was enhanced when the target location was precued as compared to a no-cue condition (i.e., when there was no temporal or spatial indication for both trial onset and target location). Although the authors do not distinguish between exogenous and endogenous attention theoretically or methodologically, the spatiotemporal characteristics of the employed precues suggest the contribution of both exogenous and endogenous attention to the benefit found at short stimulus-onset-asynchronies (SOAs), whereas endogenous attention seems to underlie the benefit found at longer SOAs. Interestingly, when the cue timing was chosen to activate both exogenous and endogenous attention the effect was roughly double the effect of brief cues (presumably mostly driving exogenous attention) and the effect of longer cues (presumably mostly activating endogenous attention). This suggests that the effects of endogenous and exogenous attention may have been additive. In the present study, we extended their findings using spatiotemporal characteristics for the cues that guarantee that either exogenous or endogenous attention is selectively engaged.
When directly comparing acuity at validly and invalidly cued locations, Golla et al. (2004) found that acuity was better at cued than uncued locations. Because the cueing effect was larger when acuity was compared across cued and uncued trials than when it was compared across cued and no-cue trials, the authors speculate that the latter may represent a benefit, whereas the former may have arisen from the sum of benefits and costs. Similarly, Shalev and Tsal (2002) investigated the effects of exogenous attention on a gap resolution task by directly comparing performance at peripherally cued and uncued locations, and found that acuity was higher at the attended than at the unattended location. In both studies, the lack of a baseline condition against which to evaluate the attentional effects makes it impossible to establish whether the found cueing effect reflects a benefit, a cost, or possibly both. The presence of a neutral baseline condition in the present study allows us to interpret the results in terms of costs and benefits for acuity.
Investigating the effects of exogenous attention on a vernier acuity task at both validly and invalidly cued locations as compared to a neutral baseline condition, Shiu and Pashler (1995) reported both costs and benefits. Given that in this study a local mask immediately followed the target display, and given that attention speeds information accrual at the cued location (e.g., Carrasco & McElree, 2001; Carrasco, Giordano & McElree, 2004), attention may have allowed for more information to be accrued before processing was interrupted by the mask (Smith, 2000). Similarly, a cost in performance at the uncued location could have resulted from slower accrual before the mask. Indeed, in a feature search task in addition to affecting discriminability, both exogenous and endogenous attention also speed information accrual at the attended location but slow it down at the unattended location as compared to a neutral baseline condition (Giordano, McElree, & Carrasco, 2004). Note that in the present study no post-masks were presented, thus it is most likely that changes in discriminability, rather than in speed of processing, at cued and uncued locations underlie the effects on acuity. This interpretation is consistent with the finding that the attentional benefits on acuity are the same regardless of the presence or absence of the mask (Carrasco, Williams & Yeshurun, 2002).
4.4 Attentional trade-offs and limited resources
The idea of attentional trade-offs is consistent with the idea that visual stimuli compete for limited resources (e.g., Broadbent, 1958; Neisser, 1967; Kinchla, 1980) and that attention biases this competition in favor of the attended stimuli. According to this biased competition hypothesis, stimuli in the visual field activate populations of neurons that engage in competitive interactions, possibly at the intracortical level. When observers attend to visual stimulation at a given location, the competition is biased in favor of the neurons encoding information at the attended area. Thus, neurons at that location remain active or become more active, while suppressing neural activity corresponding to ignored or unattended stimuli in the visual field (for a review, see Desimone & Duncan, 1995). Both single–cell (e.g., Martinez-Trujillo & Treue, 2002) and fMRI studies (Pinsk, et al., 2004) have found evidence for such competitive interactions when only two stimuli are presented either close together or far from one another.
In terms of resource allocation the attention costs and benefits reported here could reflect the following: on neutral trials, when attention is most likely distributed across both stimulus locations, the limited processing resources are deployed to both target and distractor; on valid trials, when attention is focused on the target location, more resources are allocated to target processing; on invalid trials, attention is directed away from the target location and processing resources are allocated preferentially to the distractor. This interpretation is consistent with classic interpretations of attentional trade-offs in terms of allocation of limited resources in behavioral studies (e.g., Kinchla, 1980, 1992). Recently, fMRI experiments reporting a retinotopically-specific signal-enhancement at the focus of attention have also reported a signal reduction outside the attended area when attention is allocated elsewhere (Slotnick, Schwarzbach, & Yantis, 2003; Somers, Dale, Seiffert, & Tootell, 1999; Tootell, Hadjikhani, Hall, Marrett, Vanduffel, Vaughan, & Dale, 1998). Similarly, directing attention to a specific location leads to widespread baseline-activity reduction throughout the remaining visual field (Smith, Singh, & Greenlee, 2000). These results are consistent with the idea that more resources are allocated to the attended location, at the cost of available resources at the unattended location. Moreover, as attention is distributed across a larger region, the extent of activated retinotopic visual cortex increases but the level of neural activity in a given subregion decreases (Müller, Bartelt, Donner, Villringer, & Brandt, 2003), suggesting that as the attended region increases, resources available at any subregion decreases. Accordingly, behavioral studies have shown that when attention is distributed over a larger region of the visual field rather than being focused on one location, processing efficiency for any subregion declines (e.g., Erliksen & Yeh, 1985; Castiello & Umiltà, 1992).
The fact that acuity trade-offs emerge for very simple, non-cluttered displays, in which only two stimuli are competing for processing challenges the idea that perceptual processes are of unlimited capacity (e.g., Palmer, Verghese, & Pavel, 2000), or that attentional selection is required only once the perceptual load exceeds the capacity limit of the system (e.g., Lavie, 1995). On the contrary, it suggests that trade-offs are a mandatory and basic characteristic of attentional allocation and that such a mechanism has a general effect across different stimulus and task conditions.
What mechanism may underlie the acuity trade-off? When Pestilli and Carrasco (2005) found a cost and benefit in contrast sensitivity at cued and ucued locations, they related this effect to the idea that the visual system is limited by the availability of bioenergetic resources (e.g., Lennie, 2003) and that attention helps manage these resources across locations of the visual field. This idea is consistent with neurophysiological findings indicating that neural response to contrast and BOLD fMRI signal increase at attended locations and a decrease at unattended locations (e.g., Pinsk et al., 2004; Reynolds et al., 2000). But whereas increasing contrast directly increases neural response (Albrecht & Hamilton, 1982; Boynton, Demb, Glover & Heeger, 1999), it is not clear why an increase in acuity should be related to increased neural activity and the corresponding energetic demands.
Beyond the optical and retinal limits, spatial acuity is mostly limited by the tuning properties of the spatial-frequency and orientation selective filters involved in the task at hand. Increases and decreases in spatial resolution/acuity may arise from changes in the relative contribution of spatial-frequency selective filters to perception (Davis & Graham, 1981). Following the resolution hypothesis, it may be possible that in a task requiring high spatial resolution, attention may increase and decrease the gain of smaller filters at the attended and unattended locations respectively. This would affect acuity performance by changing the normalized population response involving filters of all scales. Increasing the gain of smaller filters at attended locations would result in a sensitivity shift toward higher spatial frequencies (Carrasco, Loula & Ho, 2006), and in a more crisp representation of the edges of the gap and better acuity (Gobell & Carrasco, 2005). Correspondingly, decreasing the gain of smaller filters at unattended locations would result in a more blurred representation of the gap’s edges and worse acuity. This idea is consistent with the evidence from the control experiment that attentional effects on acuity were not mediated by a general change in contrast, which would imply changes in gain across spatial-frequency selective filters of many different scales. Rather, it may be possible that when a task is mediated by high spatial resolution (such as the Landolt acuity task used here), the attentional effect may be specific to smaller, high-spatial-frequency selective filters.
4.5 Attentional mechanisms: External noise reduction, signal enhancement, and location uncertainty reduction
The following mechanisms have been hypothesized to underlie the effects of spatial covert attention on visual processing: external noise reduction, signal enhancement, and location uncertainty reduction. According to the external noise reduction idea, attention diminishes the impact of stimuli that are outside its focus (such as masks or distracters) and the spatial and temporal uncertainty regarding both the target and distracters (e.g., Dosher & Lu, 2000a; Eckstein, 1998; Lu & Dosher, 1998, 2000; Palmer 1994; Shiu & Pashler 1994). The signal enhancement hypothesis proposes that attention directly enhances the quality of the signal at the attended location, for instance by enhancing its contrast or spatial resolution (e.g., Lu & Dosher, 1998; Cameron, Tai & Carrasco, 2002; Carrasco, Penpeci & Eckstein, 2000; Carrasco, Williams & Yeshurun, 2002). Given that we used suprathreshold stimuli, no local post-masks, and impoverished displays in which only two stimuli (one target, one distracter) appearing at known locations were competing for processing resources, this attentional trade-off suggests the concomitant attentional effect of an enhanced signal at the attended location and a weaker signal at the unattended location as compared to the signals in the neutral condition.
Several authors have also argued that informative spatial precues may reduce location uncertainty at the decisional level, a mechanism that explains attentional effects without implying any capacity limitations or resource allocation (e.g., Eckstein, Shimozaki & Abbey, 2002; Luck, Hillyard, Mouloula & Hawkins, 1996; Palmer, 1994; Shaw, 1984; Sperling & Dosher, 1986). According to this view, when a target is presented among several distracters and at several possible locations, given that the cue allows observers to prioritize information gathered at the relevant location only, it reduces the statistical uncertainty about which sources of information are relevant for the decision to be made in order to respond. On validly cued trials, observers can minimize errors by reporting information only at the cued location, thus excluding noise at the uncued locations from the decision, and thereby perform at a higher accuracy. On invalidly cued trials, information would be primarily gathered from an irrelevant location while the relevant information at the target location would be excluded as noise. In the present study, for the exogenous attention condition the precue was completely uninformative as to the target location; in the endogenous attention condition, the cue was informative and its validity was higher (70%). Observers were explicitly told the cue validity of both conditions. Were uncertainty reduction the mechanism mediating the observed cueing effects, they should have been larger with central informative cues than with peripheral uninformative cues. This was not the case.
Furthermore, location uncertainty reduction plays a more critical role when there is a high level of uncertainty regarding the target location; i.e. when the target appears with a large number of distracters, because the noise they introduce can be confused with the target signal (e.g., Eckstein, 1998; Foley & Schwartz, 1998; Palmer, 1994); when performance level is low, and when stimuli are of low visibility (Pelli, 1985). The stimuli that we used were at 94% contrast; there is no uncertainty for such suprathreshold stimuli. Moreover, the impoverished nature of the displays implies that the uncertainty as to the location from which to extract the relevant information implied a decision between two known stimulus locations only; on any given trial the cue, regardless of its type, indicated the diagonal along which the two upcoming stimuli would appear.
In addition, it has been suggested that precueing effects can reflect decisional factors, i.e. the fact that observers are encouraged to adopt a more liberal criterion or to assign more weight to visual information at the cued location (Kinchla, 1980; Kinchla, Chen & Evert, 1995; Palmer, 1994; Shaw, 1984). In the present study the exogenous cue did not predict the target location or the gap side, its presence was indeed not a relevant factor in decision-making, because it did not convey any information about the correct response. Similarly, the endogenous cue, although indicating the more likely target location, did not predict the gap side; thus, observers could not rely on its presence to respond accurately.
5. Conclusions
We demonstrate an attentional trade-off for spatial resolution: Our ability to resolve small details in a stimulus increases at the attended location, while decreasing elsewhere for both exogenous and endogenous attention. This trade-off was measured for spatial acuity thresholds and was found even in impoverished, non-cluttered, displays in which only two stimuli (one target and one distracter) appear at known locations to compete for processing resources. This finding suggests that the cost in acuity at unattended locations may be a mandatory consequence of the attentional allocation of resources to the attended location. Together with the effects of covert attention on contrast sensitivity (Ling & Carrasco, 2006a; Pestilli & Carrasco, 2005; Pestilli, Viera & Carrasco, 2007), this study suggests that visual processing trade-offs are a general mechanism of attentional allocation, whose perceptual consequences affect several basic visual dimensions, and supports the idea that spatial covert attention helps regulate the expenditure of cortical computation.
Acknowledgments
We would like to thank Jared Abrams, David Carmel, Emma Ferneyhough, and Stuart Fuller, for their helpful comments on previous versions of this manuscript.
Support: NIH 1 R01 EY016200-01A2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Golla et al. (2004) inferred a cost at uncued locations because the performance difference between valid and no-cue trials (Exp. 2) was smaller than the difference between valid and invalid trials (Exp. 1). Unfortunately, this comparison is limited because valid, invalid and no-cue trials were not randomly interleaved within a given block of trials which would allow for a better control of differences in criteria across trial type.
For both the exogenous and endogenous attention conditions, outlier thresholds were excluded from the analysis (criteria for exclusion: thresholds > (3rd quartile + 1.5 * inter-quartile range) and thresholds < (1st quartile - 1.5 * inter-quartile range)). Given that eye movements only occurred on average ~3% of the endogenous attention trials, all the data – except for the outliers – were analyzed.
We used multivariate tests that do not require the sphericity assumption to be met because such an assumption was not met.
The observer who showed a benefit for acuity with endogenous attention at both cued and uncued locations was not part of the control experiment.
This methodology was identical to the one used by Pestilli and Carrasco (2005). In both studies, observers were advised of the uninformative nature of the cue.
References
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: Contrast response function. Journal of Neurophysiology. 1982;48(11):217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Demb JB, Glover GH, Heeger DJ. Neuronal basis of contrast discrimination. Vision Research. 1999;39:257–269. doi: 10.1016/s0042-6989(98)00113-8. [DOI] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA. A physiological correlate of the spotlight of visual attention. Nature Neuroscience. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- Briand KA. Feature integration and spatial attention: more evidence of a dissociation between endogenous and exogenous orienting. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1243–1256. [Google Scholar]
- Briand KA, Klein RM. Is Posner's “beam” the same as Treisman's “glue”? On the relation between visual orienting and feature integration theory. Journal of Experimental Psychology: Human Perception and Performance. 1987;13:228–241. doi: 10.1037//0096-1523.13.2.228. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Perception and Communication. London: Pergamon Press; 1958. [Google Scholar]
- Cameron EL, Tai JC, Carrasco M. Covert attention affects the psychometric function of contrast sensitivity. Vision Research. 2002;42:949–967. doi: 10.1016/s0042-6989(02)00039-1. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Frieder KS. Cortical magnification neutralizes the eccentricity effect in visual search. Vision Research. 1997;37(1):63–82. doi: 10.1016/s0042-6989(96)00102-2. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Fuller S, Ling S. Transient attention does increase perceived contrast of suprathreshold stimuli: A reply to Prinzmetal, Long and Leonhardt (2008) Perception & Psychophysics. 2008;70:1151–1164. doi: 10.3758/pp.70.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Loula F, Ho Y-X. How attention enhances spatial resolution: Evidence from selective adaptation to spatial frequency. Perception & Psychophysics. 2006;68:1004–1012. doi: 10.3758/bf03193361. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Penpeci-Talgar C, Eckstein M. Spatial attention increases contrast sensitivity across the CSF: Support for signal enhancement. Vision Research. 2000;40:1203–1215. doi: 10.1016/s0042-6989(00)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M, Williams PE, Yeshurun Y. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. Journal of Vision. 2002;2:467–479. doi: 10.1167/2.6.4. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Yeshurun Y. The contribution of covert attention to the set-size and eccentricity effects in visual search. Journal of Experimental Psychology: Human Perception & Performance. 1998;24:673–692. doi: 10.1037//0096-1523.24.2.673. [DOI] [PubMed] [Google Scholar]
- Castiello U, Umiltà C. Splitting focal attention. Journal of Experimental Psychology: Human Perception & Performance. 1992;18:837–848. doi: 10.1037//0096-1523.18.3.837. [DOI] [PubMed] [Google Scholar]
- Cheal M, Lyon D. Central and peripheral precuing of forced-choice discrimination. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 1991;43A:859–880. doi: 10.1080/14640749108400960. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cousineau D. Confidence Intervals in within-subject designs: A simpler solution to Loftus and Masson’s method. Tutorial in Quantitative Methods for Psychology. 2005;1(1):42–45. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- DeValois RL, DeValois KK. Spatial Vision. New York: Oxford University; 1988. [Google Scholar]
- Dosher BA. Empirical approaches to information processing: Speed-accuracy tradeoff functions or reaction time. Acta Psychologica. 1979;43:347–359. [Google Scholar]
- Dosher BA, Lu ZL. Noise exclusion in spatial attention. Psychological Science. 2000a;11(2):139–146. doi: 10.1111/1467-9280.00229. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Mechanisms of perceptual attention in precuing of location. Vision Research. 2000b;40(10–12):1269–1292. doi: 10.1016/s0042-6989(00)00019-5. [DOI] [PubMed] [Google Scholar]
- Downing CJ. Expectancy and visual-spatial attention: Effects on perceptual quality. Journal of Experimental Psychology: Human Perception and Performance. 1988;14(2):188–202. doi: 10.1037//0096-1523.14.2.188. [DOI] [PubMed] [Google Scholar]
- Eckstein MP. The lower efficiency for conjunctions is due to noise and not serial attentional processing. Psychological Science. 1998;9:111–118. [Google Scholar]
- Eckstein MP, Shimozaki SS, Abbey CK. The footprints of visual attention in the Posner cueing paradigm revealed by classification images. Journal of Vision. 2002;2:25–45. doi: 10.1167/2.1.3. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Hoffman JE. Temporal and spatial characteristics of selective encoding from visual displays. Perception & Psychophysics. 1972;12:201–204. [Google Scholar]
- Eriksen CW, Yeh YY. Allocation of attention in the visual field. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:583–597. doi: 10.1037//0096-1523.11.5.583. [DOI] [PubMed] [Google Scholar]
- Fahle M, Henke-Fahle S. Interobserver variance in perceptual performance and learning. Investigative Ophtalmology and Visual Science. 1996;37(5):869–877. [PubMed] [Google Scholar]
- Foley JM, Schwarz W. Spatial attention: Effect of position uncertainty and number of distractor patterns on the threshold-versus-contrast function for contrast discrimination. Journal of Optical Society of America. 1998;A 15(5):1036–1047. [Google Scholar]
- Fuller S, Rodriguez RZ, Carrasco M. Apparent contrast differs across the vertical meridian: Visual and attentional factors. Journal of Vision. 2008;8(1):1–16. doi: 10.1167/8.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano AM, McElree B, Carrasco M. On the automaticity and flexibility of covert attention [Abstract] Journal of Vision. 2004;4627(8):627a. doi: 10.1167/9.3.30. http://journalofvision.org/4/8/627/, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobell J, Carrasco M. Attention alters the appearance of spatial frequency and gap size. Psychological Science. 2005;16:644–651. doi: 10.1111/j.1467-9280.2005.01588.x. [DOI] [PubMed] [Google Scholar]
- Golla H, Ignashchenkova A, Haarmeier T, Thier P. Improvement of visual acuity by spatial cueing: a comparative study in human and non-human primates. Vision Research. 2004;44(13):1589–1600. doi: 10.1016/j.visres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Grindley GC, Townsend V. Voluntary attention in peripheral vision and its effects on acuity and differential thresholds. Quarterly Journal of Experimental Psychology. 1968;20:11–19. doi: 10.1080/14640746808400123. [DOI] [PubMed] [Google Scholar]
- Hawkins HL, Hillyard SA, Luck SJ, Mouloua M, Downing CJ, Woodward DP. Visual attention modulates signal detectability. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:802–811. doi: 10.1037//0096-1523.16.4.802. [DOI] [PubMed] [Google Scholar]
- Hein E, Rolke B, Ulrich R. Visual attention and temporal discrimination: Differential effects of automatic and voluntary cueing. Visual Cognition. 2006;13(1):20–50. [Google Scholar]
- Ikeda K, Noda K, Yamaguchi S. Visual acuity as a function of luminance contrast of Landolt rings and adaptation luminance. Journal of Light & Visual Environment. 1983;7(1):28–36. [Google Scholar]
- Johnson CA, Casson EJ. Effects of luminance, contrast and blur on visual acuity. Optometry and Vision Science. 1995;72:864–869. doi: 10.1097/00006324-199512000-00004. [DOI] [PubMed] [Google Scholar]
- Jonides J. Voluntary vs. automatic control over the mind's eye's movement. In: Long JB, Baddeley AD, editors. Attention and Performance IX. Hillsdale, NJ: Lawrence Erlbaum Associates; 1981. pp. 187–204. [Google Scholar]
- Jonides J, Mack R. On the cost and benefit of cost and benefit. Psychological Bulletin. 1984;96:29–44. [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kinchla RA. Technical Report, No. 29. Hamilton, Ontario: Dept. Psychol., McMaster Univ.; 1969. An attention operating characteristic in vision. [Google Scholar]
- Kinchla RA. The measurement of attention. In: Nikerson RS, editor. Attention and Performance IIX. Hillsdale, NJ: Lawrence Erlbaum Associates; 1980. pp. 213–238. [Google Scholar]
- Kinchla RA. Attention. Annual Review of Psychology. 1992;43:711–742. doi: 10.1146/annurev.ps.43.020192.003431. [DOI] [PubMed] [Google Scholar]
- Kinchla RA, Chen Z, Evert D. Precue effects in visual search: Data or resource limited? Perception & Psychophysics. 1995;57(4):441–450. doi: 10.3758/bf03213070. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21(3):451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The neurology of eye movements. Philadelphia, PA: F.A. Davis; 1991. [Google Scholar]
- Lennie P. The cost of cortical computation. Current Biology. 2003;13(6):493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Ling S, Carrasco M. When sustained attention impairs perception. Nature Neuroscience. 2006a;9:1243–1245. doi: 10.1038/nn1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Carrasco M. Sustained and transient covert attention enhance the signal via different contrast response functions. Vision Research. 2006b;46:1210–1220. doi: 10.1016/j.visres.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Carrasco M. Transient covert attention does alter appearance: A reply to Schneider (2006) Perception & Psychophysics. 2007;69:1051–1058. doi: 10.3758/bf03193943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Abrams J, Carrasco M. Voluntary attention enhances contrast appearance. Psychological Science. doi: 10.1111/j.1467-9280.2009.02300.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Fuller S, Carrasco M. Attention alters the appearance of motion coherence. Psychonomic Bulletin & Review. 2006;13:1091–1096. doi: 10.3758/bf03213931. [DOI] [PubMed] [Google Scholar]
- Liu T, Pestilli F, Carrasco M. Transient attention enhances perceptual performance and fMRI response in human visual cortex. Neuron. 2005;45:469–477. doi: 10.1016/j.neuron.2004.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Stevens ST, Carrasco M. Comparing the time course and efficacy of spatial and feature-based attention. Vision Research. 2007;47:108–113. doi: 10.1016/j.visres.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Dosher BA. External noise distinguishes attention mechanisms. Vision Research. 1998;38:1183–1198. doi: 10.1016/s0042-6989(97)00273-3. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Dosher BA. Spatial attention: different mechanisms for central and peripheral temporal precues? Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1534–1548. doi: 10.1037//0096-1523.26.5.1534. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Dosher BA. Spatial attention excludes external noise without changing the spatial frequency tuning of the perceptual template. Journal of Vision. 2004;4(10):955–966. doi: 10.1167/4.10.10. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. Journal of Neurophysiology. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Hawkins HL. Mechanisms of visual-spatial attention: Resource allocation or uncertainty reduction? Journal of Experimental Psychology: Human Perception and Performance. 1996;22:725–737. doi: 10.1037//0096-1523.22.3.725. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Mackeben M, Nakayama K. Express attentional shifts. Vision Research. 1993;33:85–90. doi: 10.1016/0042-6989(93)90061-z. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hilliard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nature Neuroscience. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Mayfrank L, Kimmig H, Fischer B. In: Eye Movements: from physiology to cognition. O’Regan JK, Levy-Schoen A, editors. New York: North-Holland; 1987. pp. 37–45. [Google Scholar]
- McElree B, Dosher BA. Serial position and set size in short-term memory: Time course of recognition. Journal of Experimental Psychology: General. 1989;118:346–373. [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Müller NG, Bartelt OA, Donner TH, Villringer A, Brandt SA. A physiological correlate of the "Zoom Lens" of visual attention. Journal of Neuroscience. 2003;23:3561–3565. doi: 10.1523/JNEUROSCI.23-09-03561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HJ, Findlay JM. The effect of visual attention on peripheral discrimination thresholds in single and multiple element displays. Acta Psychologica. 1988;69:129–155. doi: 10.1016/0001-6918(88)90003-0. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Research. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Neisser U. Cognitive Psychology. Englewood Cliffs, NJ: Prentice-Hall Inc.; 1967. [Google Scholar]
- Palmer J. Set-size effects in visual search: The effect of attention is independent of the stimulus for simple tasks. Vision Research. 1994;34:1703–1721. doi: 10.1016/0042-6989(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Pelli DG. Effects of visual noise. England, UK: University of Cambridge; 1981. Unpublished doctoral dissertation. [Google Scholar]
- Pestilli F, Carrasco M. Attention enhances contrast sensitivity at cued and impairs it at uncued locations. Vision Research. 2005;45:1867–1875. doi: 10.1016/j.visres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Pestilli F, Viera G, Carrasco M. How do attention and adaptation affect contrast sensitivity? Journal of Vision. 2007;7(7):1–12. doi: 10.1167/7.7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsk MA, Doniger GM, Kastner S. Push–pull mechanism of selective attention in human extrastriate cortex. Journal of Neurophysiology. 2004;92(1):622–629. doi: 10.1152/jn.00974.2003. [DOI] [PubMed] [Google Scholar]
- Pointer JS. The influence of level and polarity of figure-ground contrast on vision. Acta Ophthalmologica Scandinavica. 2001;79(4):422–425. doi: 10.1034/j.1600-0420.2001.079004422.x. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychological Review. 1978;85:59–108. [Google Scholar]
- Reed AV. Speed-accuracy trade-off in recognition memory. Science. 1973;181:574–576. doi: 10.1126/science.181.4099.574. [DOI] [PubMed] [Google Scholar]
- Remington R, Johnston JC, Yantis S. Attentional capture by abrupt onsets. Perception & Psychophysics. 1992;51:279–290. doi: 10.3758/bf03212254. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annual Review of Neuroscience. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Desimone R. The Role of neural mechanisms of attention in solving the binding problem. Neuron. 1999;24:19–29. doi: 10.1016/s0896-6273(00)80819-3. [DOI] [PubMed] [Google Scholar]
- Shalev L, Tsal Y. Detecting gaps with and without attention: Further evidence for attentional receptive fields. European Journal of Cognitive Psychology. 2002;14:3–26. [Google Scholar]
- Shaw ML. Division of attention among spatial locations: A fundamental difference between detection of letters and detection of luminance increments. In: Bouma H, Bouwhuis DG, editors. Attention and Performance X. Hillsdale, NJ: Erlbaum; 1984. pp. 109–121. [Google Scholar]
- Shiu L-P, Pashler H. Neglible effect of spatial precuing on identification of single digits. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:1037–1054. [Google Scholar]
- Shiu LP, Pashler H. Spatial attention and vernier acuity. Vision Research. 1995;35:337–343. doi: 10.1016/0042-6989(94)00148-f. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. Neuroimage. 2003;19(4):1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Smith PL. Attention and luminance detection: Effects of cues, masks, and pedestals. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1401–1420. doi: 10.1037//0096-1523.26.4.1401. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW. Attentional suppression of activity in the human visual cortex. Neuroreport. 2000;11:271–277. doi: 10.1097/00001756-200002070-00010. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling G, Dosher BA. Strategy and optimization in human information processing. Chapter 2. In: Boff K, Kaufman L, Thomas J, editors. Handbook of perception and human performance: Vol. 1. Sensory processes and perception. New York: Wiley; 1986. pp. 1–65. [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- Turatto M, Vescovi M, Valsecchi M. Attention makes moving objects be perceived to move faster. Vision Research. 2007;47:166–178. doi: 10.1016/j.visres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Perception & Psychophysics. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Wickelgren W. Speed-accuracy tradeoff and information processing dynamics. Acta Psychologica. 1977;41:67–85. [Google Scholar]
- Womelsdorf T, Anton-Erxleben K, Pieper F, Treue S. Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nature Neuroscience. 2006;9:1156–1160. doi: 10.1038/nn1748. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. Attention improves or impairs visual perception by enhancing spatial resolution. Nature. 1998;396:72–75. doi: 10.1038/23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. Spatial attention improves performance in spatial resolution tasks. Vision Research. 1999;39:293–306. doi: 10.1016/s0042-6989(98)00114-x. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. The locus of attentional effects in texture segmentation. Nature Neuroscience. 2000;3:622–627. doi: 10.1038/75804. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Carrasco M. The effects of transient attention on spatial resolution and the size of the attentional cue. Perception & Psychophysics. 2008;70(1):104–113. doi: 10.3758/pp.70.1.104. [DOI] [PubMed] [Google Scholar]
- Yeshurun Y, Montagna B, Carrasco M. On the flexibility of sustained attention and its effects on a texture segmentation task. Vision Research. 2008;48(1):80–95. doi: 10.1016/j.visres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]