Abstract
Adolescence is a highly conserved period during which mammals undergo a number of hormonal, biological and behavioral changes (Spear, 2000). Ethical constraints limit the research that can be done in human adolescents. Rodents provide a useful model of at least some of the features of adolescence, including increases in body growth, differences in sleep/wake and eating patterns, as well as differences in risk-taking, novelty seeking and exploratory behaviors. Much of the available developmental research has utilized rats; however the use of inbred mouse strains provides a unique means to assess the genetic factors involved in behavioral differences during adolescence. We assessed differences between adults and adolescents in anxiety-like, locomotor, and consummatory behaviors using two commonly used inbred strains of mice, the DBA/2J and C57BL/6J strains. Age and genotype dependent differences were found in all three behaviors measured, suggesting both factors are important determinants of behavior in mice.
Keywords: C57BL/6J, DBA/2J, mouse, development, adolescent, elevated plus maze, anxiety, locomotor activity, consumption
Adolescence is a critical time of development during which many neurobiological, hormonal and behavioral changes occur (for review, see Spear, 2000). Although human adolescence has neither a definitive start nor end age, typically the period is assumed to take place between the ages of 12 and 18 years (Spear, 2000). A number of transformations occur during this period, including physical changes, growth spurts, and puberty. Additionally, human adolescents display a number of behavioral alterations. For example, often human adolescents show hyperphagia (although this tends to be observed more often in males, because females are showing an increased tendency to diet) along with increased rates of metabolism (Ganji & Betts, 1995; Post & Kemper, 1993). Human adolescents also sleep less (Levy, Gray-Donald, Leech, Zvagulis, & Pless, 1986); and while sleeping, display less slow-wave sleep (Dahl, 1996). Additionally, adolescents have a propensity to go to bed and wake up later, suggesting a phase-delay (Carskadon, Vieira, & Acebo, 1993). Social interactions also change during adolescence. Compared to adults, human adolescents exhibit a greater propensity to interact with their peers (Csikszentmibalyi, Larson, & Prescott, 1977) and display more conflicts with their parents compared to other age groups (Steinberg, 1989). Additionally, human adolescents display higher rates of reckless behavior, sensation seeking, and risk taking behavior compared to adults (Trimpop, Kerr, & Kirkcaldy, 1999) which unfortunately leads to an increased mortality rate during this time period (Irwin, 1993; Irwin & Millstein, 1992). However, despite the dangers associated with these “risky” behaviors, adolescent risk-takers report that following such activities they feel more accepted by their peers, and consider this risk-taking reinforcing (Maggs, Almeida, & Galambos, 1995). With this increase in and enjoyment of risk-taking also comes a higher rate of drug and alcohol use; this risk-taking behavior is a strong predictor of substance use in humans (Andrucci, Archer, Pancoast, & Gordon, 1989; Baumrind, 1987; Wills, Vaccaro, & McNamara, 1994). Interestingly, some of these same changes in adolescent behavior expand beyond just humans, and can be seen even in rodents (Spear, 2000; Nance, 1983, Alfóldi, Tobler, & Borbély, 1990; Primus & Kellogg, 1989; Darmani, Shaddy, & Gerdes, 1996; Spear, Shalaby, & Brick, 1980; Adriani, Chiarotti, & Laviola, 1998; Lancaster, Brown, Coker, Elliott, & Wren, 1996), making rodent models useful in the investigations of developmental behavioral changes associated with adolescence.
The adolescent period in rodents has been defined as the time just after weaning (post natal day [PND] 21) to the beginning of adulthood (PND 60). This rather large time period has also been divided into early (PND 21–34), middle (PND 34–46) and late (PND 46–59; Laviola, 2003). A more conservative definition can help to avoid some of the gray areas between the adolescent time period and the juvenile and adult periods. Spear (2000) has set this conservative definition to be within PND 28–42.
Thus far, much of the developmental research using rodent models is based largely on rats. Very few developmental studies of adolescence have used mice, and even fewer have compared inbred strains of mice. Inbred mouse strains provide a unique means to assess genetic factors related to development, as they provide rigorous genetic control allowing for correlations of various phenotypes across inbred strain genotype (Crabbe, Belknap, & Buck, 1994). Two common inbred strains used in research are the C57BL/6J (B6) and the DBA/2J (D2) strains. The behavioral phenotypes of these two strains have been well characterized in adulthood. For example, compared to DBA/2J mice, C57BL/6J mice perform better on grip tests (van Mooij-vam Malsen et al., 2009), spend more time in the center of an open field (Moy et al., 2007), have shorter latencies to find a hidden platform in the Morris Water Maze task (Moy et al., 2007), and consume greater amounts of ethanol in various intake procedures (McClearn & Rodgers, 1959; Rhodes et al., 2005; Lê et al., 1994). DBA/2J mice, on-the-other-hand, spend more time with a stranger in a social behavior test, exhibit better acquisition learning on a T-Maze (Moy et al., 2007) and display greater anxiety-like behavior on an elevated plus maze (Yilmazer-Hanke et al., 2003; Moy et al., 2007). However, very few studies have utilized these strains during adolescence, and only two studies to date have compared these two strains during adolescence (Linsenbardt et al., 2009; Moore et al., 2010).
Understanding the basal differences between these two strains during development is essential if we are to understand more complicated behaviors, such as drug and alcohol consumption and sensitivity during adolescence. Therefore, in the current investigation we compared adolescent and adult B6 and D2 mice in a variety of behaviors, including anxiety-like behavior on the elevated plus maze, spontaneous locomotor activity, and food and water consumption over a 24 h period. Based on the available rat and human literature, we predicted that adolescent mice would show decreased anxiety-like behavior in the plus maze, increased spontaneous locomotion, and greater food and water consumption compared to adults. Additionally, we hypothesized that adolescents would show a phase-delay in circadian rhythm that could be reflected in differences between adults and adolescents in their peak food/water consumption. Finally, we predicted that genotype would have an important influence on the magnitude of behavioral differences between the ages.
Materials and Methods
Animals
Male and female C57BL/6J (B6) and DBA/2J (D2) inbred mice were used in the present work. For Experiments 1, 2 and 4 male and female mice were bred in house at Binghamton University. For Experiments 3 (male) and 6 (male and female) mice bred at the animal facilities at Indiana University-Purdue University, Indianapolis were used. For the elevated plus maze and locomotor activity tests, mice were group housed 2–4 per cage, in standard mouse cages with same-sex littermates. When monitoring animals for their consummatory behavior, animals were singly-housed in standard mouse cages. Over the course of behavioral testing adolescent mice were 28–46 days old, and adults were 65–95 days old. Lighting was maintained on a 12 h light-dark cycle with lights off at 7 PM. The temperature of the colony room was maintained at 21 ± 1 degrees Celsius. Animals had free access to food and water at all times except during elevated plus maze and locomotor activity tests. All procedures were approved by the Binghamton University Institutional Animal Care and Use Committee or the Indiana University-Purdue University, Indianapolis School of Science Institutional Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (The National Academies Press, 2003).
Experiment 1: Consummatory Behavior
Experimentally naïve adolescent and adult mice were used to measure food (LabDiet 5001 oval pellets, Richmond, IN) and water consumption over 3 separate 24 h intervals spaced 7 days apart. At the start of the experiment, adolescents were aged 30±2 and the adults were aged 75±2, whereas at the conclusion of the experiment adolescents were aged 44±2 and adults were aged 89±2. Starting at 7PM animals were weighed, replaced in their home cage, and then food and water weight were measured (starting at 7PM) every 2 hours throughout a 26 hour period, with the last food/water measurement recorded at 9PM the next day (for a total of 13 measurements). The animals were then left undisturbed until the next recording interval started 7 days later at 7PM. A final recording interval started 14 days following the first recording interval, again at 7PM.
Experiment 2: Anxiety-Like Behavior on the Standard Mouse Elevated Plus Maze
Adolescent (32±2 days) and adult (65–75 days) experimentally naïve mice were tested for anxiety-like behavior using a mouse elevated plus maze (Med Associates, Inc., St. Albans, Vermont). The maze comprised two opposing open arms and two opposing closed arms set at 90 degree angles from each other and elevated 74.5 cm above the ground. The end of one arm to the end of the opposing arm measured 76 cm. Whereas the two opposing closed arms had walls extending 20.5 cm vertically from the elevated floor, the two opposing open arms did not. Animals were placed in the center of the plus maze, and the 5 min test was immediately started. Time spent, as well as entries into the open and closed arms, vertical activity, stretches and head dips were recorded. Animals were considered to have entered an arm when all 4 paws were deemed to have been placed in that arm. Animals exhibited vertical activity when they stood on their hind paws and placed their front two paws on the closed arms. Stretches were scored when the animal kept their hind two paws in one arm (or the center of the maze) while placing their front two paws into another arm (or the center). Head dips were rated when the animal placed their head below the level of the open arm. At the conclusion of each test, animals were returned to their home cages and the plus maze was cleaned with a 10% ethanol solution.
Experiment 3: Anxiety-Like Behavior on the Elevated Plus Maze Adjusted for Mouse Size
Male adolescent (32±2 days) and adult (70–82 days) experimentally naïve mice were tested for anxiety-like behavior using a mouse elevated plus maze that was adjusted to consider the size of the mouse (Med Associates, Inc., St. Albans, Vermont). A standard mouse elevated plus maze (please refer to the description in Experiment 2) was used to test the adults, whereas an elevated plus maze that had been reduced in size by 25% (while maintaining the same elevation off the floor) was used to test adolescents. Therefore, the adjusted elevated plus maze also was comprised of two opposing open arms and two opposing closed arms set at 90 degree angles from each other and elevated 74.5 cm above the ground. The end of one arm to the end of the opposing arm measured 57 cm. The two opposing closed arms had walls extending 20.5 cm vertically from the elevated floor, while the two opposing open arms lacked this wall.
The size reduction was chosen based on the body weights of the male adolescent mice compared to male adult mice used in Experiment 3 (please refer to Table 2). The male adolescents were on average approximately 75% of the body weight of the male adult mice.
Table 2.
Average weights (g)
| Weight 1 | Weight 7 | Weight 14 | |||
|---|---|---|---|---|---|
| Mean SEM | Mean SEM | Mean SEM | |||
| B6 | adol | M | 16.2 ± 0.7 | 18.8 ± 0.5 | 20.8 ± 0.4 |
| F | 14.3 ± 0.7 | 16.2 ± 0.5 | 17.8 ± 0.3 | ||
| adult | M | 24.5 ± 0.5 | 24.7 ± 0.4 | 24.7 ± 0.8 | |
| F | 20.5 ± 0.3 | 20.5 ± 0.3 | 21.6 ± 0.4 | ||
| D2 | adol | M | 12.9 ± 0.4 | 16.0 ± 0.7 | 18.5 ± 0.8 |
| F | 11.8 ± 0.4 | 14.4 ± 0.5 | 16.4 ± 0.4 | ||
| adult | M | 22.9 ± 0.8 | 22.7 ± 0.8 | 23.1 ± 0.8 | |
| F | 18.4 ± 0.5 | 18.5 ± 0.4 | 19.5 ± 0.4 |
Note. Animal weights were taken at 7PM (the start) of each 24 h food/water intake measurement period.
Adult animals were placed in the center of the larger (standard) plus maze, whereas the adolescent animals were placed in the center of the smaller (adjusted) plus maze. The 5 min test was immediately started upon placement of the animal. The same scores and criteria were used as described in Experiment 2. At the conclusion of each test, animals were returned to their home cages and the plus maze was cleaned with a 10% ethanol solution.
Experiment 4: Spontaneous Locomotor Activity in Standard Activity Chambers
The same adolescent (33±2 days) and adult (66–76 days) animals used in Experiment 2 were also used to examine differences in locomotor activity (the following day). A VersaMax Animal Activity Monitoring System (Accuscan Instruments, Columbus, OH, USA) was used to monitor locomotor activity over a 2 h period, in 30-min time bins. Movement was detected by eight pairs of intersecting photocell beams (2 cm above the chamber floor) evenly spaced along the walls of the 40×40-cm test chamber. Sound-attenuating box chambers (inside dimensions, 53 cm across × 58 cm deep × 43 cm high) equipped with a house light and fan for ventilation and background noise encased the test chamber. The locomotor activity testing equipment was interfaced with a Dell computer. Animals were placed in the chambers and total distance (cm) was measured every 30 min during the 2 h test. Animals were returned to their home cages following the test, and activity chambers were cleaned with 10% ethanol solution.
Experiment 5: Spontaneous Locomotor Activity in Standard Activity Chambers
Experimentally naïve adolescent (33±2 days) and adult (80–92 days) B6 and D2 mice were tested for differences in locomotor activity using activity chambers scaled for size. The VersaMax Animal Activity Monitoring System (Accuscan Instruments, Columbus, OH, USA) described previously was again used to monitor locomotor activity over a 2 h period, in 30-min time bins; however in this experiment adolescent mice were tested in 30×30-cm test chambers (which was placed in the front left quadrant of the photocell beam box), while adult mice were tested in the standard 40×40-cm test chambers (centered within the photocell beam box). The same testing/measurement parameters described previously apply to this experiment.
Statistics
Due to a malfunction in the activity chamber, 7 animals had to be removed from Experiment 4 (4 B6, and 3 D2 adult mice). Data were analyzed using Statistica release 7 (StatSoft Inc.). To reduce the complexity of the design in Experiment 1, an a priori decision was made to analyze food and water consumption data over each of the three 24 h periods separately. Each 24 h interval was analyzed by four-way mixed measure ANOVAs with time as the within subjects factor, and sex, developmental period and genotype as the between subjects factors. Weight data for each 24 h period was analyzed by a four-way mixed measure ANOVA, with time as the within subjects factor, and sex, developmental period and genotype as the between subjects factors. In Experiment 2 percent open time as well at total entries, percent center time, percent closed time, vertical activity, stretches and head dips on the elevated plus maze were each analyzed using a three-way between subjects analysis of variance (ANOVA), with sex, developmental period, and genotype as the between subjects factors. In Experiment 3 only males were used and therefore the data was analyzed using a two-way between subjects ANOVA, with developmental period and genotype as the between subjects factors. Further, adult male anxiety-like behavior on the elevated plus maze from Experiments 2 and 3 were analyzed by a between subjects ANOVA to determine the influence of location on this behavioral measure. In Experiments 4 & 5, locomotor activity was analyzed with a four-way mixed measures ANOVA, with time as the within subjects factor, and sex, developmental period and genotype as the between subjects factors. Experiments 4 & 5 were not compared to assess differences in the behavior due to location of the test, because in Experiment 4 mice were not naïve (they had been tested the day prior on the elevated plus maze, whereas naïve animals were used in Experiment 5). With the exception of the weight data and the locomotor activity data from Experiment 5, no significant interactions of sex were detected in Experiments 1, 2 or 4. Therefore analyses were collapsed over sex to reduce their complexity. Newman-Keuls post-hoc tests were carried out where appropriate. Results were considered significant at p<0.05.
Results
Experiment 1: Consummatory Behavior
An a priori decision was made to analyze each day of the food and water consumption data separately, in order to reduce the complexity of the design. Thus, each data set was analyzed by a three-way repeated measures ANOVA, with time as the within subjects factor and genotype and developmental period as the between subjects factors, for days 1, 7 and 14 separately.
Food Consumption (Day 1)
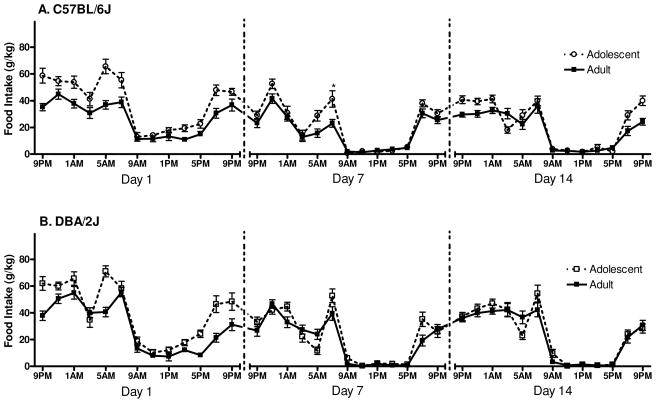
The pattern of food consumption over each of the 24 h periods can be seen for B6 mice in Figure 1A, and D2 mice in Figure 1B. Day 1 ANOVA for food consumption revealed a significant main effect of developmental period [F(1, 61)=84.3, p<0.001] indicating that on average, adolescents consumed more food than adults (per gram body weight, see Table 1). A significant main effect of time was also revealed [F(12, 732)=88.2, p<0.001], with post hoc tests indicating that the greatest food consumption occurred between 9PM and 7AM (the dark cycle) and 5PM-7PM (just before the beginning of the next dark cycle) of the 26 h observation period, compared to consumption during the hours of 9AM–3PM (during the light cycle, p’s<0.001). The ANOVA also revealed a significant time x genotype interaction [F(12, 732)=2.6, p<0.01], however post hoc tests did not reveal any specific differences between the genotypes at any of the 13 time points. Finally, the ANOVA revealed a significant time x developmental period interaction [F(12, 732)=5.3, p<0.001], with post hoc tests revealing that adolescent mice consumed more food (per gram body weight) than did adults at 9PM, 5AM, and 7PM (p’s<0.001).
Figure 1.
Daily food intake: lights on at 7AM, lights off at 7PM (n=16–18). A. The pattern of food intake for B6 mice over days 1, 7 and 14 of the experiment. (Asterisk denotes significant difference between B6 adults and adolescents [* indicates p<0.05]). B. The pattern of food intake for D2 mice over days 1, 7 and 14 of the experiment.
Table 1.
Total Food Intake (g/kg)
| Intake 1 | Intake 7 | Intake 14 | ||
|---|---|---|---|---|
| Mean SEM | Mean SEM | Mean SEM | ||
| B6 | adol | 510.2 ± 19.6 | 277.6 ± 10.9 | 292.0 ± 10.4 |
| adult | 353.5 ± 15.1 | 214.2 ± 10.6 | 236.6 ± 13.2 | |
| D2 | adol | 529.4 ± 16.4 | 278.8 ± 10.3 | 312.1 ± 12.3 |
| adult | 379.3 ± 15.4 | 249.3 ± 12.6 | 294.9 ± 16.1 |
Note. Food intake represents the total amount of food consumed in 26h over 3 intervals separated by 7 days.
Food Consumption (Day 7)
The Day 7 ANOVA for food consumption revealed a main effect of developmental period, showing that adolescents consumed significantly more food than did adults (per gram body weight, see Table 1) [F(1, 61)=17.4, p<0.001]. The ANOVA also revealed a main effect of time [F(12, 732)=109.9, p<0.001]; post hoc tests showed that the most food consumption occurred between 9PM and 7AM (during the dark cycle) and between 7 and 9PM (p’s<0.05) compared to consumption between 7AM and 3PM (during the light cycle). A significant time x genotype interaction was also detected [F(12, 732)=4.6, p<0.001], with post hoc tests showing that D2 mice consumed more food than B6 mice at 1–3AM and 7AM (p’s<0.05). The ANOVA also revealed a significant time x developmental period interaction [F(12, 732)=3.0, p<0.001]. Post hoc tests indicated that adolescent mice consumed more food (per gram body weight) than did adult mice at 7AM and 7PM, p’s<0.001). Finally, a significant time x genotype x developmental period interaction was detected [F(12, 732)=2.5, p<0.01]. Newman-Keuls post hoc tests revealed that B6 adolescents consumed more food (per gram body weight) than did B6 adults at 7AM (p<0.05); and they consumed more food than did D2 adolescents at 5AM (p<0.01). Additionally, D2 adults consumed more food than did B6 adults at7AM (p<0.05).
Food Consumption (Day 14)
The Day 14 ANOVA for food consumption revealed a significant main effect of genotype [F(1, 61)=9.4, p<0.01], indicating that D2 mice consumed more food than B6 mice. A significant main effect of developmental period was also detected [F(1,61)=7.1, p<0.01], revealing that adolescent mice consumed more food than adults (per gram body weight, see Table 1). The ANOVA also revealed a significant main effect of time [F(12, 732)=128.3, p<0.001]. Post hoc tests showed that the most food consumption occurred during 9PM and 7AM and during 7 and 9PM, compared to 9AM through 5PM (the light cycle, p’s<0.001). A significant time x genotype interaction was also detected [F(12, 732)=4.4, p<0.001]. Newman-Keuls post hoc tests revealed that D2 mice consumed more food than B6 mice at 1AM (p<0.001) and 5AM (p<0.05). The ANOVA also revealed a significant time x developmental period interaction [F(12, 732)=2.4, p<0.01], although post hoc tests did not reveal any specific differences between late-adolescents and adults at any of the time points measured. Finally, a significant time x genotype x developmental period interaction was detected [F(12, 732)=2.6, p<0.01]. Newman-Keuls post hoc tests revealed that at 1AM D2 adolescents consumed more food than did B6 adolescents (p<0.001).
Water Consumption (Day 1)
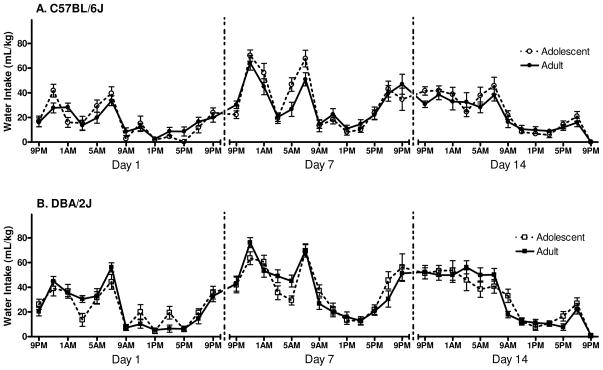
The pattern of water consumption over each of the 3 time periods can be seen in Figure 2A–B. The Day 1 ANOVA for water consumption revealed a significant main effect of genotype [F(1, 61)=15.5, p<0.001], indicating that D2 mice consumed more water than did B6 mice. A significant main effect of time was also detected [F(12, 732)=43.0, p<0.001]. Newman-Keuls post hoc tests indicated that mice consumed the least amount of water between 9AM and 5PM (during the light cycle, p’s <0.05), and the peak point of water consumption occurred at 9PM and 5AM (p’s<0.01). The ANOVA also revealed a significant time x genotype x developmental period interaction [F(12, 732)=2.7, p<0.01]. However, post hoc tests did not reveal any important differences between B6 and D2 early-adolescents and adults at any time point.
Figure 2.
Daily water intake: lights on at 7AM, lights off at 7PM (n=16–18). A. The pattern of water intake for B6 mice over days 1, 7 and 14 of the experiment. B. The pattern of water intake for D2 mice over days 1, 7 and 14 of the experiment.
Water Consumption (Day 7)
The Day 7 ANOVA for water consumption revealed a significant main effect of genotype [F(1, 61)=7.2, p<0.01], demonstrating that D2 mice consumed significantly more water than B6 mice. A significant main effect of time was also detected [F(12, 732)=58.6, p<0.001], with post hoc tests showing that the lowest amount of water consumption occurred between 9AM and 5PM (the light cycle, p’s<0.01), and the peak periods of water consumption occurred at 9PM and 5AM (p’s<0.001). The ANOVA also revealed a significant time x genotype interaction [F(12, 732)=2.9, p<0.001], however Newman-Keuls post hoc tests did not reveal any important interactions between B6 and D2 mice at any time. Finally, although a significant time x genotype x developmental period interaction was detected [F(12, 732)=2.4, p<0.01], Newman-Keuls post hoc tests did not reveal any important differences between B6 and D2 adolescent and adult mice at any time point.
Water Consumption (Day 14)
The Day 14 ANOVA for water consumption revealed a significant main effect of genotype [F(1, 61)=12.7, p<0.001], indicating that D2 mice consumed more water than B6 mice. A significant main effect of time was also detected [F(12, 732)=68.7, p<0.001], and Newman-Keuls post hoc tests revealed that the greatest levels of water consumption occurred during 9PM and 7AM (the dark cycle, p’s<0.001). Finally, the ANOVA also revealed a significant time x genotype interaction [F(12, 732)=3.1, p<0.001]. However, post hoc tests did not reveal any important differences between B6 and D2 mice at any of the time points.
Weight
Weight data over each of the 24 h periods is represented in Table 2. The ANOVA for weight data during each 24 h interval revealed a significant main effect of genotype [F(1, 57)=34.8, p<0.001], indicating that B6 mice weighed more than D2 mice. Significant main effects of developmental period [F(1, 57)=242.6, p<0.001], and sex [F(1, 57)=68.7, p<0.001], were also detected, revealing that adult mice weighed more than adolescent mice, and male mice weighed more than female mice, respectively. A significant developmental period x sex interaction was also detected [F(1, 57)=6.9, p=0.01], indicating that the difference in weight between adolescent and adult males was greater than the difference in weight between adolescent and adult females. A significant main effect of time was detected [F(2, 114)=152.3, p<0.001], with post hoc tests indicating that weights increased over time (p’s<0.001). A significant time x developmental period interaction was detected [F(2, 114)=90.0, p<0.001] and Newman-Keuls post hoc tests revealed that the adolescent weights increased over time at a greater rate than did adult weights (p<0.001). Finally, a significant time x developmental period x sex interaction was detected [F(2, 114)=5.7, p<0.001]. Newman-Keuls post hoc tests revealed that although adult male weights did not differ over the 3 time points, adult female weights increased over time, with adult female weight at time 3 significantly greater than their weight at time 1 (p<0.001); male and female adolescent weights also increased over each time period (p’s<0.001).
Experiment 2: Anxiety-Like Behavior on the Standard Elevated Plus Maze
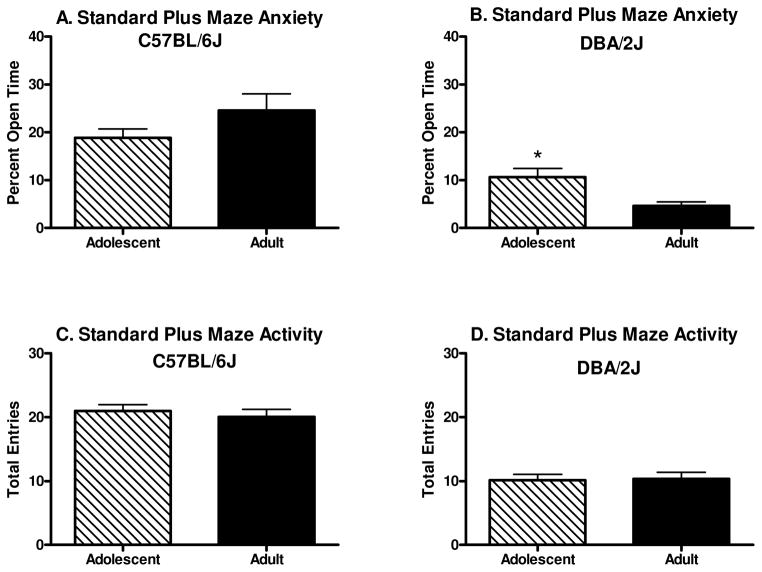
Percent time spent on the open arms of the standard elevated plus maze can be seen in Figure 3A–B. The two-way between subjects ANOVA revealed a main effect of genotype [F(1, 102)=45.6, p<0.001] and a genotype x developmental period interaction [F(1, 102)=7.9, p<0.01]. Newman-Keuls post hoc tests revealed that B6 adults and adolescents showed less anxiety-like behavior than D2 adults and adolescents, respectively (p’s<0.01); and B6 adolescents showed less anxiety-like behavior than D2 adolescents (p<0.01). Although D2 adolescents showed less anxiety-like behavior than D2 adults (p<0.05), the difference between B6 adults and adolescents did not quite reach significance (p=0.06).
Figure 3.
Anxiety-like behavior on the standard elevated plus maze (n=24–28). A–B. Percent of time spent on the open arm of the plus maze. D2 adult mice spent significantly less time in the open arms than did B6 adult mice, and D2 adults spent significantly less time than D2 adolescents in the open arms. C–D. Total entries into the open and closed arms of the plus maze. D2 mice had significantly fewer arm entries than did B6 mice. (Asterisk depicts significant differences between D2 adolescent and D2 adult, [*indicates p<0.05]).
Total arm entries on the standard elevated plus maze can be seen in Figure 3C–D. The two-way between subjects ANOVA revealed a main effect of genotype [F(1,102)=100.3, p<0.001] revealing that B6 mice showed greater activity in the elevated plus maze than did the D2 mice. No other significant main effects or interactions were detected.
Percent time spent in the center of the standard elevated plus maze, as well as frequency of vertical activity, stretches and head dips can be seen in Table 3. The two-way ANOVA of percent time spent in the center of the plus maze revealed a main effect of genotype [F(1,102)=75.6, p<0.001], and a genotype x developmental period interaction [F(1,102)=9.9, p<0.01]. Subsequent Newman-Keuls post-hoc tests revealed that both the B6 adults and adolescents spent more time in the center of the maze than did D2 adults and adolescents, respectively (p’s<0.01). Additionally, B6 adults spent more time in the center of the maze than did B6 adolescents (p<0.01), however there was no difference between D2 adults and adolescents in center time.
Table 3.
Standard Elevated Plus Maze
| % Center Time | Vertical Activity | Stretches | Head Dips | ||
|---|---|---|---|---|---|
| Mean SEM | Mean SEM | Mean SEM | Mean SEM | ||
| B6 | adol | 20.0 ± 1.6 | 12.0 ± 1.0 | 6.6 ± 0.7 | 4.8 ± 0.4 |
| adult | 25.6 ± 1.6 | 11.3 ± 1.3 | 7.5 ± 1.1 | 7.5 ± 0.8 | |
| D2 | adol | 12.3 ± 1.3 | 8.9 ± 0.9 | 5.2 ± 0.3 | 1.9 ± 0.5 |
| adult | 9.2 ± 1.1 | 11.3 ± 1.2 | 8.6 ± 0.8 | 1.1 ± 0.3 |
The two-way ANOVA of vertical activity found no significant main effects or interactions. However, the two-way ANOVA of stretches revealed a main effect of developmental period [F(1,102)=8.3, p<0.01], indicating that on average adults had a greater frequency of stretches than did the adolescents. The two-way ANOVA of head dips indicated a main effect of genotype [F(1,102)=83.5, p<0.001], a trend towards a main effect of developmental period [F(1,102)=3.4, p=0.07], and a genotype x developmental period interaction [F(1,102)=11.9, p<0.001]. Subsequent Newman-Keuls post-hoc tests found that B6 adults displayed more head dips than B6 adolescents (p<0.001), and B6 adults and adolescents exhibited more head dips than did D2 adults and adolescents, respectively (p’s<0.001).
Experiment 3: Anxiety-Like Behavior on the Elevated Plus Maze Adjusted for Size
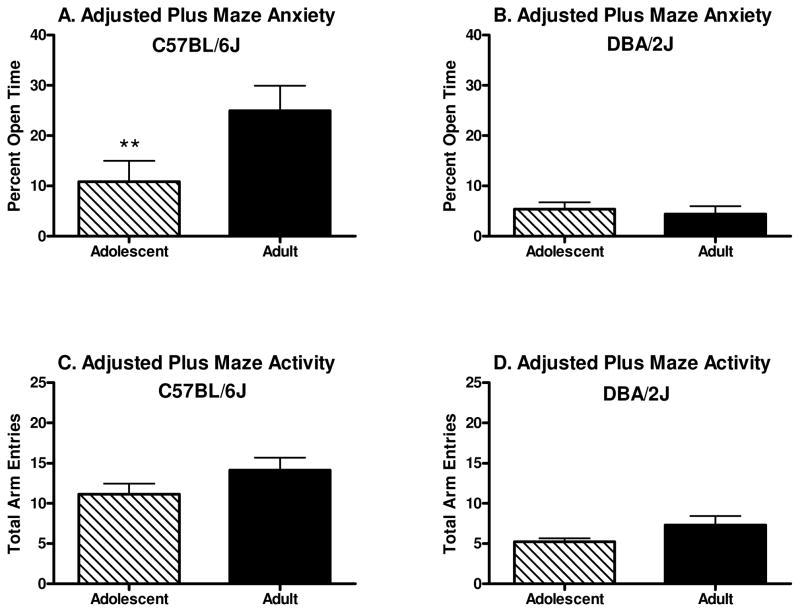
Percent time on the open arms of the adjusted elevated plus maze can be seen in Figure 4A–B. The two-way between subjects ANOVA revealed a main effect of genotype [F(1, 52)=14.4 p<0.001], a trend toward a main effect of developmental period [F(1, 52)=3.6 p=0.06], and a genotype x developmental period interaction [F(1, 52)=4.9 p<0.05]. Newman-Keuls post hoc tests revealed that B6 adults showed less anxiety-like behavior than either the B6 adolescents (p<0.01) or the D2 adults (p<0.001). No significant differences were observed between D2 adults and adolescents.
Figure 4.
Anxiety-like behavior on the elevated plus maze adjusted for size (n=14). A–B. Percent of time spent in the open arm of the plus maze. B6 adolescent mice spent significantly less time in the open arms than did B6 adult mice, no differences were observed among D2 adolescent and adult mice. C–D. Total entries into the open and closed arms of the plus maze. D2 mice had significantly fewer arm entries than did B6 mice and adolescents had significantly fewer arm entries than did adults. (Asterisk depicts significant differences between B6 adolescent and B6 adult [** indicates p<0.01]).
Total arm entries on the adjusted elevated plus maze can be seen in Figure 4C–D. The two-way between subjects ANOVA revealed a main effect of genotype [F(1,52)=29.1, p<0.001] and a main effect of developmental period [F(1, 52)=4.6 p<0.05]. These results suggest that on average B6 mice display greater activity than did the D2 mice, and adults exhibited greater activity than did adolescents.
Percent time spent in the center of the adjusted elevated plus maze, as well as frequency of vertical activity, stretches and head dips can be seen in Table 4. The two-way ANOVA of percent time spent in the center of the plus maze did not find any significant main effects or interactions.
Table 4.
Adusted Elevated Plus Maze
| % Center Time | Vertical Activity | Stretches | Head Dips | ||
|---|---|---|---|---|---|
| Mean SEM | Mean SEM | Mean SEM | Mean SEM | ||
| B6 | adol | 16.1 ± 4.5 | 15.6 ± 1.7 | 6.3 ± 0.5 | 3.2 ± 0.8 |
| adult | 13.0 ± 1.5 | 13.8 ± 1.8 | 6.6 ± 1.1 | 6.1 ± 1.2 | |
| D2 | adol | 13.7 ± 4.6 | 11.4 ± 1.6 | 7.2 ± 0.7 | 0.5 ± 0.2 |
| adult | 14.4 ± 5.4 | 9.6 ± 1.6 | 7.5 ± 0.8 | 0.4 ± 0.2 |
The two-way ANOVA of vertical activity found a main effect of genotype [F(1, 52)=6.3 p<0.05], indicating that B6 mice displayed more vertical activity than did D2 mice. The two-way ANOVA of stretches revealed no significant main effects or interactions. The two-way ANOVA of head dips indicated a main effect of genotype [F(1, 52)=31.8 p<0.001], as well as a trend toward a main effect of developmental period [F(1,52)=3.5, p=0.07], and a genotype x developmental period interaction [F(1,52)=3.9, p=0.05]. Subsequent Newman-Keuls post-hoc tests found that B6 adults had more head dips than B6 adolescents (p<0.01), and B6 adults and adolescents had more head dips than did D2 adults and adolescents, respectively (p’s<0.05).
Statistical Analysis of Differences in Anxiety-Like Behavior due to Location of the Test
Experiment 2 was conducted at Binghamton University (BU) and Experiment 3 was conducted at Indiana University-Purdue University, Indianapolis (IUPUI); which presented the opportunity to analyze differences in behavior based upon location. Male adult behavioral data from each of those two experiments were therefore analyzed separately to determine if location of the test had any effect on anxiety-like behaviors. The same experimenter administered the behavioral tests and the same elevated plus maze (adult sized) apparatus was used in both locations.
The percent of time spent in the open arms of the elevated plus maze at BU for B6 mice is 23.0±4.7, and for D2 mice is 4.0±1.0; and at IUPUI for B6 mice is 24.9±5.0, and for D2 mice is 4.4±1.6. A two-way ANOVA of percent open time with genotype and location as the between subjects factors revealed no significant differences due to location of the test and no location x genotype interaction, however there was a significant main effect of genotype [F(1,51)=33.3, p<0.001].
The total entries on the elevated plus maze at BU for B6 mice is 19.6±2.1, and for D2 mice is 10.1±1.4; and at IUPUI for B6 mice is 14.1±1.5, and for D2 mice is 7.3±1.2. The two-way ANOVA of total entries revealed a main effect of location [F(1,51)=7.2, p<0.01] and a main effect of genotype [F(1,51)=28.5, p<0.001], but no interaction was found. Indicating that the mice tested at BU exhibited significantly greater activity levels on the elevated plus maze.
The percent of time spent in the center of the elevated plus maze at BU for B6 mice is 26.9±2.7, and for D2 mice is 9.2±1.6; and at IUPUI for B6 mice is 13.0±1.5, and for D2 mice is 14.4±5.4. The two-way ANOVA for percent center time revealed a main effect of genotype [F(1,51)=6.3, p<0.05] and an interaction of location and genotype [F(1,51)=8.7, p<0.01]. Newman Keuls post hoc tests indicated that B6 mice tested at BU spent more time in the center of the plus maze compared to B6 mice tested at IUPUI (p<0.05) and D2 mice tested at either location (p’s<0.01).
The frequency of vertical activity on the elevated plus maze at BU for B6 mice is 10.3±2.2, and for D2 mice is 10.5±1.4; and at IUPUI for B6 mice is 13.8±1.8, and for D2 mice is 9.6±1.6. When analyzed by two-way ANOVA no significant differences based upon location tested, genotype, or interaction of these factors was observed. The frequency of stretches on the elevated plus maze at BU for B6 mice is 9.9±1.8, and for D2 mice is 9.1±0.8; and at IUPUI for B6 mice is 6.6±1.1, and for D2 mice is 7.5±0.8. The two-way ANOVA for stretches revealed a main effect of location [F(1,51)=4.8, p<0.05] but no significant differences among the genotypes or interactions, indicating that mice tested at BU tended to exhibit more stretching behaviors than mice tested at IUPUI. The frequency of head dips on the elevated plus maze at BU for B6 mice is 6.7±1.0, and for D2 mice is 0.5±0.2; and at IUPUI for B6 mice is 6.1±1.2, and for D2 mice is 0.4±0.2. The two-way ANOVA for head dips revealed a main effect of genotype [F(1,51)=56.9, p<0.001], but no significant effect of location or interaction.
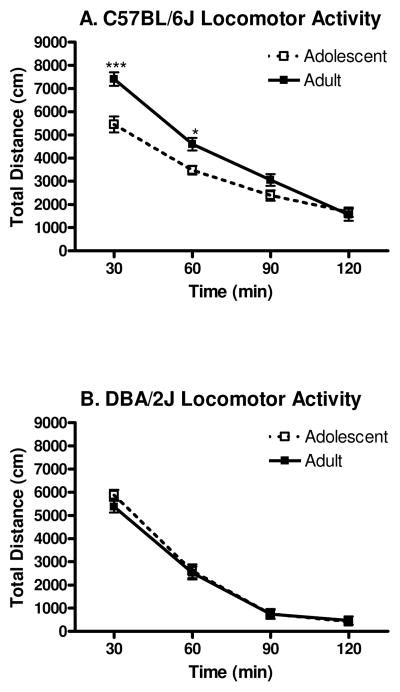
Experiment 4: Spontaneous Locomotor Activity in Standard Activity Chambers
Spontaneous locomotor activity can be seen in Figure 5A–B. The three way ANOVA revealed a significant main effect of genotype [F(1,95)=62.7, p<0.001], indicating that B6 mice were more active than D2 mice. A significant main effect of developmental period [F(1, 95)=5.2, p<0.05] was also found, revealing that on average, adult mice were more active than adolescent mice. A significant main effect of time [F(3,285)=476.8, p<0.001] was also revealed, showing that over time, locomotor activity decreased. Additionally, a significant genotype x developmental period interaction [F(1, 95)=8.0, p<0.01] was revealed. Neuman-Keuls post hoc tests indicated that B6 adults were more active than D2 adults (p<0.001), and more active than adolescents of both genotypes (p’s<0.001); additionally, B6 adolescents were more active than both D2 adults (p<0.001) and D2 adolescents (p<0.01). The three-way ANOVA also revealed a time x genotype interaction [F(3, 285)=6.0, p<0.001]. Newman-Keuls post hoc tests indicated that although there was no significant differences between the genotypes at the first 30 min time point, by the second 30 min time point differences between the genotypes emerged, with B6 mice demonstrating more locomotor activity than D2 mice (p<0.001), and this effect continued for both the third 30 min time point (p<0.001), and the fourth 30 min time point (p<0.01). The ANOVA also revealed a significant time x developmental period interaction [F(3, 285)=3.1, p<0.05], but Newman-Keuls post hoc tests did not detect any specific differences between adults and adolescents at any of the time points tested. Finally, a significant time x genotype x developmental period interaction [F(3, 285)=7.4, p<0.001] was also detected. Newman-Keuls post hoc tests indicated that B6 adults showed significantly greater locomotor activity in the first (p<0.001) and second (p<0.05) 30 min compared to B6 adolescents, however this difference did not continue into the third or fourth 30 min time points. There were no significant differences between D2 adults and adolescents at any of the time points measured.
Figure 5.
Locomotor activity in standard chambers (n=20–28). A. B6 adults showed the greatest amount of locomotor activity, compared to either B6 adolescents or D2 mice. B. There was no difference between D2 adult and adolescent mice in their distance traveled. (Asterisks depict significant differences between B6 adults and B6 adolescents [* indicates p<0.05, *** indicates p<0.001]).
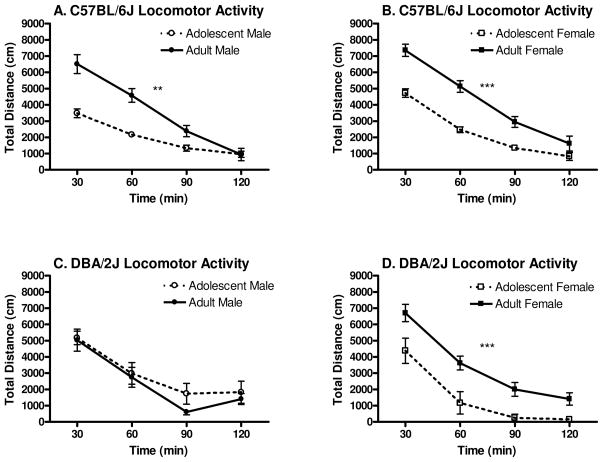
Experiment 5: Spontaneous Locomotor Activity in Adjusted Activity Chambers
Spontaneous locomotor activity can be seen in Figure 6A–B. The four way ANOVA revealed a significant main effect of genotype [F(1,57)=5.1, p<0.05], indicating that overall B6 mice were more active than D2 mice. A significant main effect of developmental period [F(1, 57)=35.5 p<0.001] was also found, revealing that on average, adult mice were more active than adolescent mice. A significant main effect of time [F(3,171)=254.6, p<0.001] was also revealed, showing that over time, locomotor activity decreased. Additionally, a marginally significant genotype x sex interaction was detected [F(1,57)=3.1, p=0.08]. Newman-Keuls post hoc tests did not reveal any specific differences. A significant genotype x developmental period interaction [F(1, 57)=6.1, p<0.05] was revealed. Neuman-Keuls post hoc tests indicated that B6 adults were more active than B6 adolescents (p<0.001) and D2 adults (p<0.001). Additionally, D2 adults were more active than D2 adolescents (p<0.01). A significant sex x developmental period interaction was also detected [F(1,57)=10.6, p<0.001]. Post hoc tests indicated that male adults were more active than both male and female adolescents (p’s<0.05), but less active than female adults (p<0.01). Female adults were also more active than male or female adolescents (p<0.001). No differences were detected among male and female adolescents. A genotype x sex x developmental period interaction was detected [F(1,57)=6.4, p<0.05]. Newman Keuls post hoc tests revealed a marginally significant difference among the D2 male and female adults (p=0.06), with D2 adult females displaying greater locomotor activity than males. A statistically significant difference among the D2 male and female adolescents was also detected (p<0.01), with D2 adolescent males displaying greater locomotor activity than females. Further, D2 female adults were more active than their adolescent counterparts (p<0.001), whereas no difference was detected between D2 male adults and adolescents. Although no differences were detected among B6 male and female adults, they both displayed greater locomotor activity than their adolescent counterparts of either sex (p’s<0.01).
Figure 6.
Locomotor activity in adjusted chambers (n=5–13). A–B. B6 adult mice display significantly greater locomotor activity than their adolescent counterparts. C. There is no significant difference between D2 male adolescent and adult locomotor activity. D. D2 female adults display significantly greater locomotor activity than do D2 female adolescents. (Asterisks depict significant differences among adults and adolescents in the 2h time period [** indicates p<0.01, *** indicates p<0.001]).
The four-way ANOVA also revealed a time x genotype interaction [F(3, 171)=4.5, p<0.01]. Newman-Keuls post hoc tests indicated that although there was no significant differences between the genotypes at the first 30 min time point, by the second 30 min time point a marginally significant difference between the genotypes emerged, with B6 mice demonstrating greater locomotor activity than D2 mice (p=0.06), and this effect continued for the third 30 min time point (p=0.06), but the effect diminished by the fourth 30 min time point. The ANOVA also revealed a significant time x sex interaction [F(3,171)=3.2, p<0.05]. Post hoc tests revealed a marginally significant difference among males and females at the first 30min time point (p=0.09), with females displaying slightly greater activity than males. No other differences were detected among males and females at any other time point. A time x developmental period interaction was also detected [F(3, 171)=3.2, p<0.001]. Newman-Keuls post hoc tests revealed that adults displayed significantly greater activity than adolescents at both the first and second 30min time points (p’s<0.001) and marginally greater activity at the third 30min time point (p=0.08); however there was no differences between the ages at the fourth 30min time point.
Finally, a marginally significant time x genotype x developmental period interaction [F(3, 171)=2.5, p=0.06] was also detected. Newman-Keuls post hoc tests detected marginally significant differences among D2 adults and adolescents at the first and second 30min time points (p=0.07 and p=0.08, respectively), with D2 adults displaying greater activity than adolescents at both time points. Significant differences were also detected among B6 adults and adolescents at the first and second 30min time points (p’s<0.001), with B6 adults displaying greater activity than adolescents at both time points. Additionally, a marginally significant differences among D2 and B6 adults at the first 30min time point was detected (p=0.08), and a statistically significant difference was detected at the second 30min time point was found (p<0.05), with B6 adults displaying greater locomotor activity than D2 adults at both time points; however no difference between B6 and D2 adolescents was detected at any time point.
Discussion
The goal of the current work was to investigate the basal differences in consummatory, anxiety-like, and locomotor behaviors of two commonly used inbred mouse strains (B6 and D2) during adolescence and adulthood. Differences in age and genotype were observed in all three behaviors tested; however often these differences were dependent upon the behavioral apparatus used, prior experience of the animal, and/or location of testing.
We were interested in whether adolescent B6 and/or D2 mice showed a phase-delay in their sleep/wake patterns. We chose to assess this by looking at changes in food/water consumption over 24 h periods across adolescence. These measures also allowed us to determine if adolescent animals showed increases in their daily food/water intake as compared to adults. Previous reports have indicated that adolescent rats display hyperphagic-behaviors, consuming more calories per gram body weight than do other aged rats (Nance, 1983), and display alterations in sleep patterns, staying awake longer into their light cycle (the predominant period of sleeping for these nocturnal animals; Alfóldi et al., 1990). Data indicated that there was no phase-delay in peak consummatory behaviors for our adolescent animals at any of the time points measured in either of the genotypes. However, adolescent animals did show significantly greater amounts of food intake (relative to body weight) than did adults at all three time points. This eating pattern is not surprising considering the increased rate of growth during adolescence. Indeed, body weight data indicated that adolescent mice were gaining much more weight than their adult counterparts, and that this weight gain was dependent upon sex and genotype.
Fluid intake in adolescent and adult animals was also examined, though no differences in water intake among the ages were observed. However, a significant difference between the genotypes was found: D2 mice consistently consumed more water than did B6 mice (relative to body size). Interestingly, Mittleman, Van Brunt, & Matthews (2003) have reported that D2 adult mice consume more 0.1% sucrose solution compared to B6 mice in a schedule-induced polydipsia procedure, however they failed to see any difference between the genotypes in water consumption using the same procedure. It isn’t immediately clear why our findings differ, however it is possible that by removing water bottles every 2 h we may have inadvertently interfered with the D2’s normal drinking pattern.
An important consideration regarding the food and water intake data is that our animals were singly housed. We chose to singly-house our mice to allow for precise measurement of food/water consumption. However, isolate-housing could be stressful for the animals, and these stress effects may differ ontogenetically or across genotype. Indeed, housing (social versus isolate) condition dependent differences in the rewarding properties of social interactions have been observed in adolescent and adult rats (Douglas, Varlinskaya & Spear, 2004); however it is unknown if housing conditions may differentially affect consummatory behavior. Therefore, a potential confound in the present data is that stress may have affected the adolescent animals to a greater degree than the adult animals, and it is possible that it could have affected the pattern or overall amount of food/water intake. Investigations into behavioral differences due to alterations in housing conditions (isolate versus social) may be an important next step in future ontogenetic examinations.
We were also interested in any difference in basal anxiety levels between the ages and genotypes. Anxiety-like behavior was measured on the elevated plus maze (EPM). Initially, both ages were tested on a standard mouse EPM and in this test, D2 mice displayed significantly greater levels of anxiety-like behavior as measured by percent of time spent in the open arms. This is not surprising, as previous research has previously reported that D2 mice spent the least amount of absolute time in the open arms of an EPM compared to 6 other inbred strains of mice (Yilmazer-Hanke, Roskoden, Zilles & Schwegler, 2003). Although no developmental differences in anxiety-like behavior among B6 mice were found, D2 adolescents displayed significantly less anxiety-like behavior on the EPM than did D2 adults. However, adolescent mice are smaller than their adult counterparts (not surprisingly) and therefore it was of interest to determine whether adjusting the size of the EPM to reflect this difference in size between the ages would influence the results. Therefore, in a separate experiment adult mice were tested on the standard EPM while adolescent mice were tested on an EPM that was reduced in size by 25% (adjusted). Scaling adolescent behavioral apparatuses to account for the size discrepancy among the ages is routinely done in the rat literature (see Doremus, Brunnel, Varlinskaya, & Spear, 2003; Doremus, Varlinskaya, & Spear, 2004; 2006; Varlinskaya & Spear 2002; 2006; 2007). Using the EPM adjusted for mouse size we replicated the finding that B6 mice display less anxiety-like behavior than D2 mice. Yet, on the adjusted EPM we did not see any significant differences in anxiety-like behavior among D2 adults and adolescents. On the adjusted EPM, B6 adolescents displayed increased anxiety-like behavior as compared to adults; this effect was not observed when both ages were tested on the standard EPM (although the general pattern was seen in the data on the standard plus maze, see Figure 3A, the effects did not reach significance). To our knowledge, this is the first reported instance of genetically based developmental differences in anxiety-like behavior in mice.
In addition to measuring anxiety-like behavior, total arm entries were also examined as a measure of overall activity on the EPM. When both ages were tested on the standard EPM, no differences between adolescents and adults were detected. However there was a difference in EPM activity between the genotypes; with B6 mice showing significantly more activity than D2 mice. The results from the elevated plus maze adjusted for mouse size, replicated the higher activity levels observed in B6 mice compared to D2 mice. Additionally, a difference between the ages was detected: on average adult mice were more active than adolescent mice (this age effect was also observed in the spontaneous locomotor activity data).
An important caveat when comparing the data obtained from the adjusted and standard elevated plus maze experiments is that these two studies were conducted in different locations. The standard elevated plus maze data was collected at Binghamton University (BU), while the adjusted elevated plus maze experiments were collected at Indiana University-Purdue University, Indianapolis (IUPUI). In order to determine the extent to which location may have affected our results, we compared the data collected from adult male mice at BU to that of the adult male mice collected at IUPUI. This allowed us to effectively assess possible differences in behavior due to the location of the test, with most other factors controlled for, except for time (the same experimenter administered the test using the same apparatus). Although percent open time and the frequency of vertical activity as well as head dips on the elevated plus maze seemed to be unaffected by the location of the test, the percent of time spent in the center of the maze, the frequency of stretches and activity as measured by total entries all were affected by location. Previously, Wahlsten et al. (2006) demonstrated that although some behaviors are consistent across laboratories and time, anxiety-like behavior on the elevated plus maze seems to be particularly sensitive to these changes. We do not believe this effect alters the credibility of the current data in any way. In fact, we believe that we were provided a unique and intriguing opportunity to evaluate possible influences of physical location and facilities on anxiety-like behavior. The results of our comparisons are a valuable reminder of the sensitivity of this apparatus and highlight the importance of establishing within-laboratory baseline behavioral measures.
Previously Yilmazer-Hanke et al. (2003) reported elevated plus maze activity results in which they observed little difference in total arm entries between B6 and D2 mice, with a tendency for the D2 mice to exhibit more total arm entries than the B6 mice. Although the anxiety-like behavior (as measured by open arm time on the EPM) reported by Yilmazer-Hanke and colleagues (2003) and that reported in the current report are consistent, there are some minor discrepancies regarding the activity results. It is possible that the differences between the data reported by Yilmazer-Hanke et al. (2003) and the current report are due to differences in location (as described previously). However, another possibility is that these differences are due to alterations in the behavioral apparatus used. While the plus maze in the current work had black open and closed arms, the plus maze used in the investigation by Yilmazer-Hanke and colleagues (2003) had white arms, with a dim light shining into the closed arms. The plus maze is a very sensitive behavioral measure (Wahlsten et al., 2006), and subtle differences such as these may account for the differences in behavior observed between experiments. It is nevertheless worth noting that using the same standard apparatus, we were able to replicate our activity data in two different laboratory environments.
Locomotor activity in novel environment was also examined in the current investigation. Previous research using rats and mice has indicated that adolescents are often hyperactive in novel environments (Darmani et al., 1996; Spear et al., 1980). Additionally, adolescent mice have been shown to exhibit increased rates of novelty seeking compared to adults (Adriani et al., 1998), while adolescent rats have been observed to be hyper-reactive to novel stimuli (Spear 2000). When all mice were tested in the standard locomotor activity chambers, B6 mice were found to be generally more active than D2 mice. Although there were no significant differences detected between D2 adults and adolescents, B6 adults showed significantly more locomotor activity than did B6 adolescents (who showed activity similar to the D2 animals). We also assessed locomotor activity differences using a scaled locomotor activity chamber. Again, we found that B6 mice were generally more active than D2 mice. We also replicated the finding that B6 adults are more active than B6 adolescents, however using the scaled locomotor activity chambers, we observed greater activity in D2 adults as compared to D2 adolescents (an effect that was not seen when testing all mice in the standard chambers). Further, interactions of sex with other factors were detected that had not been observed previously, likely driven by difference among D2 female adults and adolescents that was not present in D2 males. There are two possible explanations for the newly observed D2 sex and age-related effects in Experiment 6: 1) use of the scaled chamber was able to detect age- and/or sex-related effects that could not be detected in the standard chambers; 2) use of mice in Experiment 5 with prior experimental exposure to the EPM (ie – not experimentally naïve) resulted in altered behavior when tested in the locomotor activity chambers. We chose to always test the animals on the elevated plus maze before testing them for locomotor activity because previous research in rats have reported that pretest conditions have a large effect on outcome of behavior on the elevated plus maze (Doremus et al., 2004). The age and sex differences observed among D2 adolescent and adult mice in Experiment 6 (but not in Experiment 5) is likely due to the differences in scaled vs non-scaled activity chambers. Other work from both our own lab and others (using standard chambers to assess age-related differences in locomotor activity) are consistent with the results obtained with the standard activity chambers in Experiment 5. For example, Melón et al. (submitted) found that in a 15min test of locomotor activity, B6 adults were more active than B6 adolescents, and found no difference between D2 adults and adolescents, regardless of sex. Similarly, Hefner & Holmes (2007) reported that B6 adult male mice displayed greater locomotor activity compared to early-adolescent male mice during a portion of their 12 min activity test; and Stevenson, Besheer, & Hodge (2008) reported previously that there was no difference between D2 male adolescents and adults in their basal locomotor activity. Therefore, it appears as though scaling the locomotor activity chambers to consider the size of the mouse allows for the detection of age and sex-related differences in the D2 mouse that cannot be found without this scaling.
In conclusion, the current work demonstrates that there are indeed differences in behavior between adult and adolescent mice. Further, this work shows that these age-related behavioral differences in mice are also dependent upon genotype and upon testing conditions (location of the test, the use of scaled apparatuses to control for size of the animal, etc). To our knowledge, this is the first time basal behavioral differences have been compared in B6 and D2 mice during adolescence. The current work sets the stage for further characterization of ontogenetic differences between adult and adolescent B6 and D2 mice. Such work will assess differences in ethanol-related phenotypes across age in multiple inbred strains of mice; this will allow for the generation of genetic correlations to assess the impact of genotype on ethanol-related phenotypes during adolescence and adulthood.
Acknowledgments
This work was supported in part by the Center for Development and Behavioral Neuroscience at Binghamton University and NIAAA grants AAA015434, AA016789, and AA018910. We would like to thank Regina Antoni, Cherie Walsh, Sina Zomorrodian, John Mariani, Carly Gross, Austin Cavelli, and Frances Kigel for their aid and support.
References
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112(5):1152–66. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Alfóldi P, Tobler I, Borbély AA. Sleep regulation in rats during early development. Am J Physiol. 1990;258(3 pt 2):R634–44. doi: 10.1152/ajpregu.1990.258.3.R634. [DOI] [PubMed] [Google Scholar]
- Andrucci GL, Archer RP, Pancoast DL, Gordon RA. The relationship of MMPI and sensation seeking scales to adolescent drug use. J Pers Assess. 1989;53(2):253–66. doi: 10.1207/s15327752jpa5302_4. [DOI] [PubMed] [Google Scholar]
- Baumrind D. A developmental perspective on adolescent risk taking in contemporary America. In: Irwin CE Jr, editor. Adolescent social behavior and health. San Francisco, CA: Jossey-Bass; 1987. pp. 93–125. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Buck KJ. Genetic animal models of alcohol and drug abuse. Science. 1994;264(5166):1715–23. doi: 10.1126/science.8209252. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, Prescott S. The ecology of adolescent activity and experience. J Youth Adolesc. 1977;6(3):281–94. doi: 10.1007/BF02138940. [DOI] [PubMed] [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Dev Psychopathol. 1996;8(1):3–27. [Google Scholar]
- Darmani NA, Shaddy J, Gerdes CF. Differential ontogenesis of three DOI-induced behaviors in mice. Physiol Behav. 1996;60(6):1495–500. doi: 10.1016/s0031-9384(96)00323-x. [DOI] [PubMed] [Google Scholar]
- de Mooij-van Malsen JG, Yu KL, Veldman H, Oppelaar H, van den Berg LH, Olivier B, Kas MJ. Variations in ventral root axon morphology and locomotor behavior components across different inbred strains of mice. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.09.008. in press. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev Psychobio. 2004;45(3):153–62. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2003;75(2):411–8. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Age-related differences in elevated plus maze behavior between adolescent and adult rats. Ann N Y Acad Sci. 2004;1021:427–30. doi: 10.1196/annals.1308.057. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83(4):570–7. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmocology (Berl) 2007;191(2):311–22. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Ganji V, Betts N. Fat, cholesterol, fiber and sodium intakes of US population: evaluation of diets reported in 1987–88 Nationwide Food Consumption Survey. Eur J Clin Nutr. 1995;49(12):915–20. [PubMed] [Google Scholar]
- Irwin CE., Jr . Adolescence and risk taking: how are they related? In: Bell NJ, Bell RW, editors. Adolescent risk taking. Newbury Park, CA: Sage Publications; 1993. pp. 7–28. [Google Scholar]
- Irwin CE, Jr, Millstein SG. Correlates and predictors of risk-taking behavior during adolescence. In: Lipsitt LP, Mitnick LL, editors. Self-regulatory behavior and risk taking: causes and consequences. Norwood, NJ: Ablex Publishing; 1992. pp. 3–21. [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period in Sprague–Dawley rats. Alcohol Clin Exp Res. 1996;20(6):1043–9. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;1–2:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lê AD, Ko J, Chon S, Quan B. Alcohol consumption by C57BL/6J, BALB/c, and DBA/2J mice in a limited access paradigm. Pharmacol Biochem Behav. 1994;47:375–8. doi: 10.1016/0091-3057(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Levy D, Gray-Donald K, Leech J, Zvagulis I, Pless IB. Sleep patterns and problems in adolescents. J Adolesc Health Care. 1986;7(6):386–9. doi: 10.1016/s0197-0070(86)80239-x. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., II Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33(3):464–76. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggs JL, Almeida DM, Galambos NL. Risky business: The paradoxical meaning of problem behavior for young adolescents. J Early Adolesc. 1995;15(3):344–62. [Google Scholar]
- McClearn G, Rodgers D. Differences in alcohol preference among inbred strains of mice. Q J Stud Alcohol. 1959;20:691–5. [Google Scholar]
- Mittleman G, Van Brunt CL, Matthews DB. Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin Exp Res. 2003;27(6):918–25. doi: 10.1097/01.ALC.0000071930.48632.AE. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance DM. The developmental and neural determinants of the effects of estrogen on feeding behavior in the rat: a theoretical perspective. Neurosci Biobehav Rev. 1983;7(2):189–211. doi: 10.1016/0149-7634(83)90015-5. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press; 2003. [PubMed] [Google Scholar]
- Post GB, Kemper HC. Nutrient intake and biological maturation during adolescence. The Amsterdam growth and health longitudinal study. Eur J Clin Nutr. 1993;47(6):400–8. [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22(6):633–43. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology (Berl) 1980;70(1):47–58. doi: 10.1007/BF00432369. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Pubertal maturation and parent-adolescent distance: An evolutionary perspective. In: Adams GR, Montemayor R, Gullotta TP, editors. Advances in adolescent behavior and development. Newbury Park, CA: Sage Publications; 1989. pp. 71–97. [Google Scholar]
- Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology (Berl) 2008;197(3):361–70. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimpop RM, Kerr JH, Kirkcaldy B. Comparing personality constructs of risk-taking behavior. Pers Individ Diff. 1999;26:237–54. [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: Role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol induced social inhibition in Sprageu-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–44. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;1:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci USA. 2006;103:16364–9. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: An application of Cloninger’s theory. J Subst Abuse. 1994;6(1):1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Roskoden T, Zilles K, Schwegler H. Anxiety-related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behav Brain Res. 2003;145(1–2):145–59. doi: 10.1016/s0166-4328(03)00107-4. [DOI] [PubMed] [Google Scholar]