Abstract
Our previous studies have shown that high-mobility group box 1 (HMGB1) could physically associate with the retinoblastoma (RB) protein via an LXCXE (leucine-X-cysteine-X-glutamic; X=any amino acid) motif. An identical LXCXE motif is present in the HMGB1–3 protein sequences, whereas a near-consensus LXCXD (leucine-X-cysteine-X-asparagine; X=any amino acid) motif is found in the HMGB4 protein. In this study, we have demonstrated that like HMGB1, HMGB2–3 also associated with the RB in vitro and in vivo, as evidenced by glutathione-s-transferase capture and immunoprecipitation–Western blot assays. A point mutation of the LXCXE or LXCXD motif led to disruption of RB:HMGB1–4 interactions. Enforced expression of HMGB1–3 or HMGB4 by adenoviral-vector-mediated gene transfer resulted in significant inhibition of breast cancer cell proliferation through an LXCXE- or LXCXD-dependent mechanism and an increased radiosensitivity through an LXCXE- or LXCXD-independent mechanism. These results suggest an important role of the LXCXE/D motif in RB:HMGB1–4 association and modulation of cancer cell growth, but not radiosensitivity.
Key words: breast cancer, biotherapy, cancer, gene therapy, irradiation
Introduction
The human high-mobility group box (HMGB) family comprises four highly conserved members: HMGB1, HMGB2, HMGB3, and HMGB4. In general, these HMGBs are structured into three domains: two highly conserved DNA-binding domains (termed the HMG-boxes A and B), and a highly acidic C-terminal tail.1 All HMGB1–3 proteins are expressed in early embryos, but HMGB2 and HMGB3 are downregulated during subsequent embryonic development. HMGB1–2 proteins play an important role in gene transcription, DNA recombination and repair, cell replication, and autophagy via interacting with a number of key cellular proteins, including transcriptional factors, site-specific recombination, and DNA repair proteins.2–5 Moreover, there is increasing evidence that HMGB1 and HMGB2 are novel prognostic markers and potential therapeutic targets for different types of cancers, including breast carcinoma, hepatocellular carcinoma, and squamous-cell carcinoma.6–10 Consistently, enforced expression of HMGB1 alters the sensitivity of cancer cells to DNA-damaging agents.11–14
Our previous studies have revealed a novel role for the HMGB1 protein in the interaction with the retinoblastoma (RB) protein, an important regulator of the cell cycle and cell proliferation. On one hand, a point mutation in the 104LFCSE108 motif of the HMGB1 protein resulted in loss of binding to RB, supporting an essential role for the LXCXE (leucine-X-cysteine-X-glutamic; X=any amino acid) motif in an RB:HMGB1 interaction.13 On the other hand, overexpression of HMGB1 in breast cancer cells led to an inhibition of cell proliferation in an RB-dependent manner, but a parallel elevation of cellular radiosensitivity in an RB-independent fashion.13 Sequence analysis (Fig. 1A) has revealed that, like human HMGB1, the HMGB2 and HMGB3 proteins contain a virtually identical LXCXE site, that is, 104LFCSE108 (HMGB2) and 102LFCSE106 (HMGB3). Similarly, a near-consensus RB-binding motif, 102LFCQD106, is present in the HMGB4 protein. In the current study, we have determined whether all HMGB family members could similarly bind to RB in an LXCXE- or LXCXD (leucine-X-cysteine-X-asparagine; X=any amino acid)-dependent mechanism.
FIG. 1.
(A) Comparison of amino acid sequences of different HMGBs. (B) Schematic diagrams of HMGB1–4 expression vectors utilized in this study. HMGB1, high-mobility group box 1.
Materials and Methods
Cell culture and irradiation
Human breast cancer cell lines MCF-7, T-47D, and MDA-MB-468 were maintained in the Dulbecco's modified Eagle's medium (D-MEM) supplemented with 5% fetal bovine serum, 100 unit/mL penicillin, 100 μg/mL streptomycin, and a mixture of nonessential amino acids (Sigma-Aldrich) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Irradiation was performed with γ-radiation (J. L. Shepherd Mark I Radiator) with a 137Cs source emitting at a fixed dose rate of 3.5 Gy/min.
HMGB and RB vectors
The wild-type (wt) HMGB1–4 expression plasmids (wtHMGB1–4) were generated by subcloning the full-length HMGB1–4 cDNAs into a mammalian expression vector pcDNA3 (Invitrogen). HMGB1–3 mutLXCXE and HMGB4 mutLXCXE expression vectors were created by replacing the cysteine (C) of the LXCXE and LXCXD motif with phenylalanine (F) using site-directed mutagenesis kits (Stratagene). pGEX5X-RB and pGEX5X-IκBα expression plasmids were generated as described elsewhere.15
Transient transfection
Subconfluent proliferating cells in 100-mm Petri tissue culture dishes were transfected overnight with 10 μg of recombinant plasmid DNA using LipofectAMINE 2000 (Invitrogen) according to the manufacturer's instructions. After extensive washing to remove the LipofectAMINE 2000 and the excess plasmid DNA, cell cultures were subjected to various assays.
Generation of recombinant HMGB1–4 adenoviruses
A recombinant adenovirus (pAd/CMV/V5-DEST; Invitrogen) containing a DNA fragment, encoding the open-reading frames of human HMGB1–4 (Ad5-HMGB1–4) or HMGB1–4 mutLXCXE, between the CMV promoter (P cmv) and the polyadenylation signal (TK pA) was prepared as previously described.13 To control for the biological effect of the virus per se, the vector Ad5.CMV.Null, expressing no transgene (Ad5), was constructed in a similar manner.
In vitro cell growth kinetics
Cells were inoculated into six-well dishes at 3×104 cells per well in 5.0 mL of a growth medium containing Ad5-HMGB1–4 viruses encoding wtHMGB1–4 proteins or HMGB1–4 mutants in the LXCXE or LXCXD motif, or Ad5-lacZ (as a negative control) at a 100 pfu/cell on day 0. Duplicate wells were counted with a hemocytometer on days 1–8.
Immunoprecipitation and Western blot assays
An immunoprecipitation–Western blot (IP-WB) assay was carried out as previously described.13,15 Briefly, ∼500 μg nuclear protein extracts were immunoprecipitated with 6 μg of specific antibody or antibody combinations. The immunoprecipitated proteins were collected by protein G chromatography, eluted in the boiling Laemmli sample buffer, and subjected to the WB analysis. The RB IP antibody was a monoclonal anti-RB antibody (C-2, sc-74562; Santa Cruz Biotech.). The control IP antibody was a normal mouse IgG (Santa Cruz Biotech.). The following were the primary antibodies used for WB: HMGB1 (anti-HMGB1 antisera),16 HMGB2 (ab11973, rabbit polyclonal, 1:200 dilution; Abcam), HMGB3 (EPR2838, rabbit monoclonal, 1:500 dilution; Gene Tex), HMGB4 (NBP1-26398, goat polyclonal, 1:200 dilution; Novus Biologicals), and α-actin (I-19, goat polyclonal IgG, 1:500 dilution; Santa Cruz Biotech.).
Glutathione-S-transferase capture assays
Glutathione-S-transferase (GST) capture assays were performed essentially as described previously.13,15 Briefly, 35S-methionine-labeled proteins were prepared by in vitro transcription and translation using the T7 promoter of the pcDNA3 vector. The RB-GST fusion proteins were generated by subcloning an RB cDNA into the GST vector (pGEX), expressed in Escherichia coli, and purified by affinity chromatography. In vitro translation-labeled proteins were incubated with either GST alone or RB GST fusion proteins for 4 hours at 42°C, recovered using GSH agarose beads, eluted in the boiling Laemmli sample buffer, and analyzed by SDS-PAGE autoradiography.
Assays of transcriptional activity
Briefly, proliferating cells in 24-well dishes were incubated overnight with 0.5 μg of each vector in a serum-free D-MEM containing LipofectAMINE 2000 (Invitrogen) according to the supplier's protocol. The total transfected DNA was kept constant by addition of the pcDNA3 vector. To control transfection efficiency, plasmid pRSV-β-gal was cotransfected to allow normalization of luciferase values to β-galactosidase activity in the same sample. The results were shown as means±SEM of three independent experiments in duplicates (n=6).
MTT assay of cell viability
Cell viability was assessed by MTT assays as previously described,13,15 and expressed as the amount of dye reduction relative to that of unirradiated control cells. Ten replicate wells were tested per assay condition, and each experiment was repeated at least three times. SEMs from 10 wells in three independent experiments were less than 10%.
Statistical analysis
Data were represented as means and standard deviations, and statistical comparisons of the experimental results between groups were made using the two-tailed Student's t-test. All statistical tests were performed by SPSS version 17.0. A p-value of ≤0.05 between groups was considered significant.
Results
RB interacts with HMGB1–4
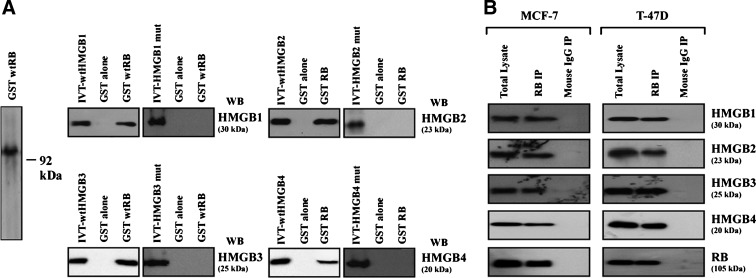
As indicated in the amino acid sequences of all four human HMGB proteins (Fig. 1A), an identical LXCXE motif was present in the HMGB1–3 proteins, whereas a near-consensus LXCXD motif was found in the HMGB4 protein. To investigate whether the HMGB2–4 proteins, like HMGB1,13 interact with RB, we first performed the GST capture-binding assays. In vitro translation 35S-labeled HMGB-4 was incubated with beads coated with a bacterially produced GST-wtRB fusion protein. Beads coated with GST were used as a control. The HMGB1–4 cDNAs examined in this study are illustrated in Figure 1B. Consistent with our previous reports,13 the 35S-labeled HMGB1 bound to the beads coated with GST-RB, but not to those coated with GST alone (Fig. 2A). Like HMGB1, HMGB2 and HMGB3 tightly bound to GST-RB, whereas the HMGB4 protein bearing an LXCXD motif bound to RB to a less extent. The binding specificity was also demonstrated by experiments showing no binding of IVT-HMGBs to the GST-IκBα protein (data not shown).
FIG. 2.
HMGBs bind to RB. (A) In vitro HMGB:RB protein interaction. The GST capture assay was performed as described previously.13,15 wtHMGB1–4 wt proteins, but not HMGB1–3 mutLXCXE or HMGB4 mutLXCXD mutants, were pulled-down by 35S-methionine-labeled GST-wtRB proteins. The input lanes showed 10% of IVT wtHMGB1–4 or 10% of IVT HMGB1–3 mutLXCXEs or HMGB4 mutLXCXD products used in the assay. (B) Intracellular association of HMGBs and RB. HMGB1–4 were detected in RB IPs from nuclear extracts of MCF-7 and T-47D cells by WB. Representative results were shown from three independent experiments. Rb, retinoblastoma; WB, Western blot; IP, immunoprecipitation; GST, glutathione-S-transferase. LXCXE, leucine-X-cysteine-X-glutamic (X=any amino acid); LXCXD, leucine-X-cysteine-X-asparagine (X=any amino acid); wt, wild-type.
To further determine the specificity of this interaction, we generated a C-to-F point mutation in the LXCXE motif of HMGB1–3 proteins or the LXCXD site of HMGB4 using site-directed mutagenesis kits. As shown in Figure 2A, HMGB1–3 proteins bearing the mutated LXFXE motif lost their capacity in RB binding; HMGB4 with LXFXD mutation similarly failed to interact with RB. Taken together, these data suggest that the LXCXE/D motifs are required for the binding of HMGB1–3/HMGB4 to RB; moreover, the cysteine within the LXCXE or LXCXD is necessary and essential for RB binding.
Next, we examined the possible in vivo RB:HMGB interaction by IP-WB assays. As shown in Figure 2B, incubation of nuclear extract of wtRB-expressing MCF-7 cells with an antibody to RB led to co-IP of RB with the HMGB1–4 proteins. Similarly, the HMGB1–4 proteins were also detected in the RB IPs of another wtRB cell line, T-47D. In a sharp contrast, the HMGB1–4 proteins were not immunoprecipitated by irrelevant control murine IgGs, indicating that endogenous HMGB1–4 physically associate with RB in these tumor cells.
Effects of HMGBs on RB-mediated transcription repression
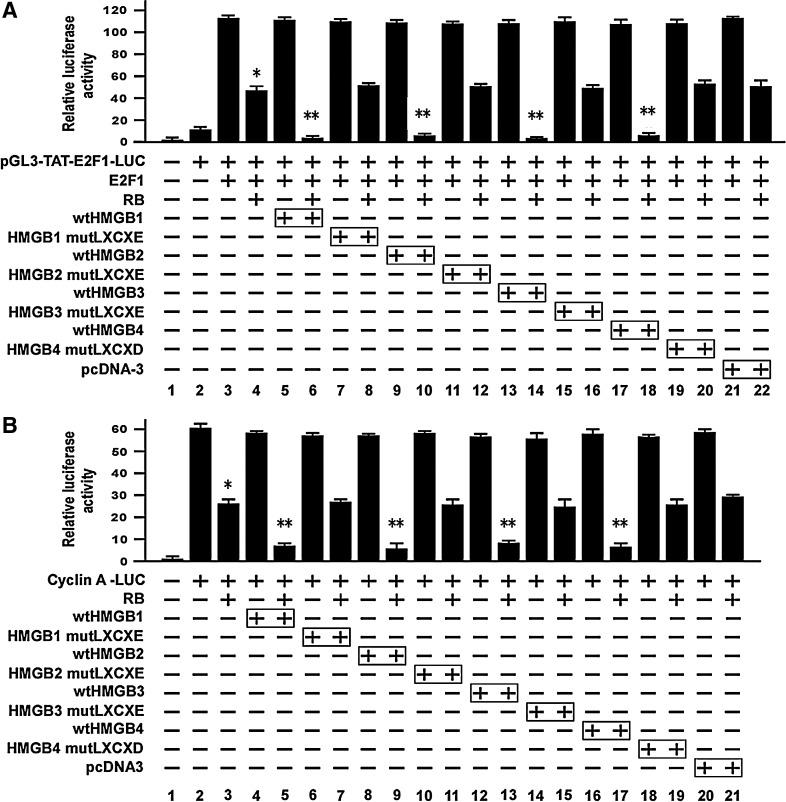
The tumor suppression function of RB is dependent, at least in part, on interactions with the E2F family of DNA-binding transcription factors (E2F).17,18 To understand the importance of the RB/HMGB interaction, we examined whether HMGBs exhibited potential effects on RB-meditated E2F or cyclin A transcription repression using a luciferase reporter assay. As shown in Figure 3A, transfection of RB into RB-negative MDA-MB-468 cells led to >50% repression of the E2F1 activities (lane 4, p<0.05), which was significantly enhanced by the cotransfection of HMGB1 (lane 6, 95% reduction, p<0.01), Student's t-test), HMGB2 (lane 10), HMGB3 (lane 14), or HMGB4 (lane 18) (p<0.01). The RB-mediated repression of E2F1 activity was not affected by cotransfection of HMGB1–3 LXFXE (lanes 8, 12, and 16) or HMGB4 LXFXD mutants (lane 20), or the empty control pcDNA3 vector (lane 22). The wtHMGB-mediated alteration of E2F1 transcription activities was undetectable in the absence of RB expression (lanes 5, 9, 13, and 17). Similarly, cotransfection of HMGB1–4 wt proteins, but not HMGB1–3 mutLXCXEs or HMGB4 mutLXCXD, significantly decreased RB repression of cyclin A-mediated transcription activities (Fig. 3B). The enhancement of RB-mediated E2F1 and cyclin A transcription activities by HMGB1–4 was also observed in another RB-negative cell line, SAOA-2 (data not shown). Together, these data suggest HMGB1–4 as important cofactors for RB-mediated repression of E2F1 and cyclin A transcription activities.
FIG. 3.
HMGBs enhance RB-mediated transcription repression. (A) HMGBs enhance RB-mediated E2F transcription repression. (B) HMGBs enhance RB-mediated cyclin A transcription repression. RB-negative MDA-MB-468 cells were cotransfected with indicated vectors (0.5 μg/well of each vector in 24-well tissue culture dishes) in the serum-free Dulbecco's modified Eagle's medium containing LipofectAMINE 2000 for 24 hours and then assayed for luciferase activities. Total transfected DNA was kept constant by adding a pcDNA3 control vector. A pSV-βGal plasmid was cotransfected as an internal standard for normalization of luciferase values. Luciferase values are means±SEMs from three independent experiments. The statistical significances were analyzed by the two-tail Student's t-test. *p<0.05; **p<0.01.
Mutant LXCXE or LXCXD disrupts HMGB inhibition of cell proliferation
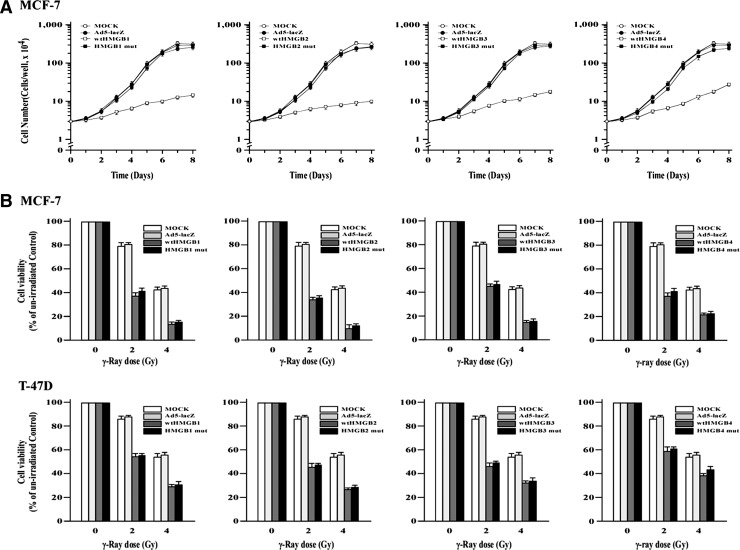
Alteration of HMGB1 expression levels affects growth, invasion, and metastasis of cancer cells, implicating HMGB1 as a potential target for cancer therapy.5–9 To determine the impacts of HMGBs on proliferation of breast cancer cells, we infected MCF-7 cells with recombinant adenovirus that expressed either wtHMGB1–4 (Ad5-HMGB1–4), or HMGB1–4 with C to F mutation in the LXCXE or LXCXD (Ad5-HMGB1–3 mutLXCXE or Ad5-HMGB4 mutLXCXD). As expected, expression levels of HMGB1–4 or mutant proteins were significantly elevated after infection of MCF-7 cells with recombinant adenovirus encoding Ad5-HMGB1–4, Ad5-HMGB1–3 mutLXCXE, or Ad5-HMGB4 mutLXCXD at an MOI of 100 p.f.u./cell (Fig. 4A). Furthermore, enhanced expression of HMGB1–4 proteins resulted in reduction of MCF-7 cell proliferation, with a maximal effect in Ad5-HMGB2-infected cells. In contrast, no cell growth alteration was observed in the cells infected with Ad5-HMGB1–3 mutLXCXE or Ad5-HMGB4 mutLXCXD. Similar results were observed in other RB-positive cell lines, such as T-47D and BT-549 (data not shown). Collectively, these results indicate that enforced expression of HMGB1–4 resulted in inhibition of breast cancer cell proliferation potentially by facilitating RB:HMGB interactions.
FIG. 4.
(A) HMGBs inhibit cell proliferation. MCF-7 cells (3×104 cells/well in six-well tissue culture dishes) were cultured in the medium containing Ad5-wtHMGB1–3, Ad5-HMGB1–3 mutLXCXE, Ad5-HMGB4 mutLXCXD, or Ad5-lacZ (a control) at an MOI of 100 p.f.u./cell. At the indicated time points, the cells were counted with a hemocytometer. SEMs from 10 wells in three independent experiments were less than 10%. (B) HMGBs increase radiosensitivity. Exponentially growing MCF-7 and T-47D cells were infected with Ad5-wtHMGB1–4, Ad5-HMGB1–3 mutLXCXE, Ad5-HMGB4 mutLXCXD, or Ad5-lacZ (a control) at an MOI of 100 p.f.u./cell for 24 hours and then exposed to γ-ray irradiation at indicated doses. MTT survival assays were conducted for cell viability 24 hours after irradiation.
HMGB1–4-mediated radiosensitivity does not require an intact LXCXE or LXCXD
Previously, we and others reported that HMGB1 alters the sensitivity of tumor cells to DNA damaging agents (e.g., ionizing radiation).11–14 To determine the effects of HMGBs on the radiosensitivity of breast cancer cells, RB-positive MCF-7 and T-47D cells were exposed to 2 or 4 Gy of γ-rays at 24 hours postinfection with Ad5-HMGB1–4, Ad5-HMGB1–3 mutLXCXE, Ad5-HMGB4 mutLXCXD, or Ad5-lacZ (a negative control) adenovirus. Radiation caused a greater reduction of cell viability in MCF-7 cells infected with Ad5-HMGB1–4 than those uninfected (MOCK) or infected with Ad5-lacZ (Fig. 4B). For example, in MCF-7 cells, γ-ray irradiation at 4 Gy reduced cell viability by ∼40% in uninfected or Ad5-lacZ-infected control cells, but decreased cell viability by >80%–90% in cells infected with Ad5-HMGB1–4 (p<0.05, Student's t-test). Surprisingly, a significant, greater reduction of cell viability was also found in cells infected with Ad5-HMGB1–3 mutLXCXE or Ad5-HMGB4 mutLXCXD. Again, essentially similar results were obtained in another RB-positive cell line, T-47D, although T-47D cells were less sensitive to γ-ray irradiation than MCF-7 cells were. These findings indicate that an increase in HMGB1–4 expression elevates the radiosensitivity of human breast cancer cells possibly through an LXCXE/D-independent mechanism.
Discussion
The RB gene encodes a 928-amino-acid phosphoprotein that occupies an important role in the regulation of cell proliferation, cell cycle progression, apoptosis, and telomerase activity. However, it is often lost or functionally inactivated in many types of tumors, including breast cancer.18,19 One target of RB is the E2F family of cell cycle transcription factors, the binding of which by RB blocks E2F-mediated transcriptional activation.17 RB contains at least four distinct protein-binding domains, including the large A/B pocket (amino acid 395–876), corresponding to the binding site for E2F, the A/B pocket, the C-pocket, and the N-terminal domain.18,19 The inhibitory activity of the cell cycle and the cell growth of RB is regulated via the A/B pocket interactions with the LXCXE (X=any amino acid) motif of target proteins, such as cell cycle regulatory proteins (G1/S cyclins and CDKs) and several DNA tumor virus oncoproteins (E1A from adenovirus, E7 from HPV, and T antigen from SV40).20 Mutation of the LXCXE sequence in proteins of these DNA tumor viruses prevents their inhibitory effects on RB, thereby impairing their abilities to transform cells.21
At present, four members of the human HMGB family, HMGB1–4, have been identified. These HMGBs are structured into three domains—two basic HMG boxes (HMG domains A and B) and a highly acidic C-terminal tail.3,4 Via these HMG boxes, HMGBs can interact with various proteins ranging from nuclear cellular proteins to viral proteins. Our group proposed a novel role for the HMGB1 protein in the cell growth and radiosensitivity through RB-interaction-dependent and -independent mechanisms.13 In the present study, we demonstrated that like HMGB1, other HMGB family members, HMGB2–4, similarly form a complex with RB in vitro and in vivo, as judged by GST pull-down and IP-WB assays. Furthermore, we showed that the effective interaction requires the presence of a consensus RB-binding LXCXE motif on HMGB1–3, because even a point mutation in LXCXE completely disrupted the HMGB1–3:RB interactions. These findings suggest that the HMGB1–3:RB interaction may resemble a typical LXCXE:RB pocket domain interaction. LXCXE is essential and necessary for a number of cellular target proteins and several DNA tumor virus oncoproteins to bind to RB.20 In addition, although HMGB4 contains a slightly variant LXCXE motif, that is, LXCXD, we did find an association of HMGB4 with RB that similarly required the presence of the intact LXCXD motif on the RB-target protein. In fact, it has been reported that an LXCXD motif is critical for the interaction of the human cytomegalovirus pp71 protein with RB.22
Here we provide evidence to support a possible role for the RB:HMGB interaction in the regulation of cell proliferation and transcription of RB-regulating target genes (e.g., E2F1 and Cyclin A). As regulatory intermediates for cell growth and cell cycle progression, E2F1 and Cyclin A are expressed abundantly in proliferating tumor cells. We found that all four HMGB family members functionally enhanced the capacity of RB in repressing E2F1 and cyclin A promoter transcription activities. Overexpression of the HMGB1–4 gene by transfecting a replication-deficient adenovirus expressing wt, but not the mutated HMGB1–4 proteins, led to inhibition of proliferation of RB-expressing breast cancer cell lines. Taken together with our prior results that HMGB1 did not influence cell growth in RB-negative cells,13 current results indicate that elevated expression of HMGBs could result in inhibition of tumor cell proliferation possibly through LXCXE/D-dependent RB:HMGBs interactions.
Our previous studies indicated that overexpression of HMGB1 or HMGB1 LXCXE-mutant rendered breast cancer cells more sensitive to radiation therapy.13 Here we show that like Ad5-wtHMGB1, elevation of expression levels of wtHMGB2–4 or HMGB2–4 mutants, and HMGB1–3 mutLXCXEs similarly increased tumor cell radiosensitivity. These results have suggested an essential role for the LXCXE/D motifs in the RB:HMGBs interaction, but not necessary for HMGB-mediated regulation of radiosensitivities. This conclusion is supported by the observation that elevated HMGB1 expression increased radiosensitivity of an RB-negative cell line, BT-549. A follow-up study is being carried out to determine whether HMGB1–4 affect tumor cell radiosensitivity via interaction with other as-yet-undefined cellular regulators that are involved in DNA strand break and repair. This possibility has been supported by the findings that (1) HMGB1 may affect DNA interstrand crosslink repair by enhancing the interactions between nucleotide excision repair (NER) factors23; (2) HMGB1 binds preferentially to damaged nucleosomes containing linker DNA12; (3) HMGB1 alters chromatin reorganization after DNA damage24; (4) HMGB1 physically interacts with MutSalpha and is required at a step before the excision of mispaired nucleotide in mismatch repair25; and (5) HMGB1 specifically inhibits repair of the 1,2-intrastrand crosslink by the human excision nuclease.26
Several studies have implicated HMGB1 in the regulation of chemosensitivity, apoptosis, and autophagy.4,11–14 It will be interesting to investigate whether HMGB2–4, like HMGB1, are also involved in regulation of chemosensitivity, apoptosis, and autophagy through LXCXE/D-binding-dependent mechanisms. These studies will improve our understanding of the biological functions of the HMGB family, and shed light on the development of novel therapies that specifically target RB-signaling pathways.
In conclusion, we have demonstrated that, like HMGB1, the other three members of the human HMGB family could similarly interact with RB via the LXCXE or LXCXD motif and thereby modulate the cell growth. Thus, the LXCXE motif of HMGB1–3 or the LXCXD motif of HMGB4 is functionally important in mediating some activities of HMGB1–4, including cell proliferation.
Acknowledgments
This work was supported by grants from the PCSIRT (IRT0849), the NNSFC (81071906 and 81172127), the Doctoral Fund of Ministry of Education of China (20103201120016), the PAPD, and the Major Program for the Natural Science Fundamental Research of the Higher Education Institutions of Jiangsu Province (SZ16933). This work was also supported in part by the U.S. NIH-NIGMS (R01GM063075) and the NCCAM (R01AT05076).
Disclosure Statement
There are no existing financial conflicts.
References
- 1.Read CM. Cary PD. Crane-Robinson C, et al. Solution structure of a DNA-binding domain from HMG1. Nucleic Acids Res. 1993;21:3427. doi: 10.1093/nar/21.15.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lotze MT. Tracey KJ. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 3.Wang H. Zhu S. Zhou R, et al. Therapeutic potential of HMGB1-targeting agents in sepsis. Expert Rev Mol Med. 2008;10:32. doi: 10.1017/S1462399408000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang R. Livesey KM. Zeh HJ, 3rd, et al. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011;7:1256. doi: 10.4161/auto.7.10.16753. [DOI] [PubMed] [Google Scholar]
- 5.Tang D. Kang R. Zeh HJ, 3rd, et al. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14:1315. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao X. Zhao G. Yang H, et al. Overexpression of high-mobility group box 1 correlates with tumor progression and poor prognosis in human colorectal carcinoma. J Cancer Res Clin Oncol. 2010;136:677. doi: 10.1007/s00432-009-0706-1. [DOI] [PubMed] [Google Scholar]
- 7.Kwon JH. Kim J. Park JY. Overexpression of high-mobility group box 2 is associated with tumor aggressiveness and prognosis of hepatocellular carcinoma. Clin Cancer Res. 2010;16:5511. doi: 10.1158/1078-0432.CCR-10-0825. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y. Xie C. Zhang X, et al. Elevated expression of HMGB1 in squamous-cell carcinoma of the head and neck and its clinical significance. Eur J Cancer. 2010;46:3007. doi: 10.1016/j.ejca.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Kostova N. Zlateva S. Ugrinova I, et al. The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol Cell Biochem. 2010;337:251. doi: 10.1007/s11010-009-0305-0. [DOI] [PubMed] [Google Scholar]
- 10.Ellerman JE. Brown CK. de Vera M, et al. Masquerader: High mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- 11.Nagaki S. Yamamoto M. Yumoto Y, et al. Non-histone chromosomal proteins HMG1 and 2 enhance ligation reaction of DNA double-strand breaks. Biochem Biophys Res Commun. 1998;246:137. doi: 10.1006/bbrc.1998.8589. [DOI] [PubMed] [Google Scholar]
- 12.Ugrinova I. Zlateva S. Pashev IG, et al. Native HMGB1 protein inhibits repair of cisplatin-damaged nucleosomes in vitro. Int J Biochem Cell Biol. 2009;41:1556. doi: 10.1016/j.biocel.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Jiao Y. Wang HC. Fan SJ, et al. Growth suppression and radiosensitivity increase by HMGB1 in breast cancer. Acta Pharmacol Sin. 2007;28:1957. doi: 10.1111/j.1745-7254.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 14.Tang D. Loze MT. Zeh HJ, et al. The redox protein HMGB1 regulates cell death and survival in cancer treatment. Autophagy. 2010;6:1181. doi: 10.4161/auto.6.8.13367. [DOI] [PubMed] [Google Scholar]
- 15.Fan S. Yuan R. Ma YX, et al. Disruption of BRCA1 LXCXE motif alters BRCA1 functional activity and regulation of RB family but not RB protein binding. Oncogene. 2001;20:4827. doi: 10.1038/sj.onc.1204666. [DOI] [PubMed] [Google Scholar]
- 16.Li W. Zhu S. Li J, et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem Pharmacol. 2011;81:1152. doi: 10.1016/j.bcp.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chellappan SP. Hiebert S. Mudryj M, et al. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- 18.Classon M. Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- 19.van Deursen JM. Rb loss causes cancer by driving mitosis mad. Cancer Cell. 2007;11:1. doi: 10.1016/j.ccr.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Chan HM. Smith L. La Thangue NB. Role of LXCXE motif-dependent interactions in the activity of the retinoblastoma protein. Oncogene. 2001;20:6152. doi: 10.1038/sj.onc.1204793. [DOI] [PubMed] [Google Scholar]
- 21.Bourgo RJ. Thangavel C. Ertel A. RB restricts DNA damage-initiated tumorigenesis through an LXCXE-dependent mechanism of transcriptional control. Mol Cell. 2011;43:663. doi: 10.1016/j.molcel.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalejta RF. Shenk T. Proteasome-dependent, ubiquitin-independent degradation of the Rb family of tumor suppressors by the human cytomegalovirus pp71 protein. Proc Natl Acad Sci U S A. 2003;100:3263. doi: 10.1073/pnas.0538058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ALange SS. Reddy MC. Vasquez KM. Human HMGB1 directly facilitates interactions between nucleotide excision repair proteins on triplex-directed psoralen interstrand crosslinks. DNA Repair (Amst) 2009;8:865. doi: 10.1016/j.dnarep.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange SS. Mitchell DL. Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A. 2008;105:10320. doi: 10.1073/pnas.0803181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan F. Gu L. Guo S, et al. Evidence for involvement of HMGB1 protein in human DNA mismatch repair. J Biol Chem. 2004;279:20935. doi: 10.1074/jbc.M401931200. [DOI] [PubMed] [Google Scholar]
- 26.Huang JC. Zamble DB. Reardon JT, et al. HMG-domain proteins specifically inhibit the repair of the major DNA adduct of the anticancer drug cisplatin by human excision nuclease. Proc Natl Acad Sci U S A. 1994;91:10394. doi: 10.1073/pnas.91.22.10394. [DOI] [PMC free article] [PubMed] [Google Scholar]