Abstract
Lithium is a mood stabilizer shown to have neuroprotective effects against several chronic and acute neuronal injuries, including stroke. However, it is unknown whether lithium treatment protects against brain injury post-stroke in a rat model of permanent distal middle cerebral artery occlusion (MCAo) combined with transient bilateral common carotid artery occlusion (CCAo), a model that mimics human stroke with partial reperfusion. In addition, whether lithium treatment alters Akt activity as measured by the kinase activity assay has not been reported, although it is known to inhibit GSK3β activity. After stroke, Akt activity contributes to neuronal survival while GSK3β activity causes neuronal death. We report that a bolus of lithium injection at stroke onset robustly reduced infarct size measured by 2,3,5-triphenyltetrazolium chloride (TTC) staining at 48 h post-stroke and inhibited cell death in the ischemic penumbra, but not in the ischemic core, as shown by TUNEL staining performed 24 h post-stroke. However, lithium treatment did not alter the reduction in Akt activity as measured by Akt kinase assay. We further showed that lithium did not alter phosphorylated GSK3β protein levels, or the degradation of β-catenin, a substrate of GSK3β, which is consistent with previous findings that long-term treatment is required for lithium to alter GSK3β phosphorylation. In summary, we show innovative data that lithium protects against stroke in a focal ischemia model with partial reperfusion, however, our results dispute the importance of Akt activity in the protective effects of lithium.
Keywords: Lithium, Akt, Cerebral focal ischemia, GSK3β, β-catenin
Lithium has been clinically used for more than 60 years [1] and it is still one of the primary drugs used to treat bipolar mood disorder [2]. In addition, lithium treatment has neuroprotective effects against diverse diseases, including Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease in both animal models and human patients [3, 4]. Lithium treatment also decreases ischemic damage in both focal and global cerebral ischemia in adult animal models and in ischemia/hypoxia models of neonatal animals [4–10]. Beneficial effects of post-ischemic administration of lithium have also been reported [8, 11]. In these studies either permanent focal ischemia or ischemia with total reperfusion was induced. Previous studies, however, have reported that a large portion of stroke patients experience focal ischemia with only partial reperfusion, that most spontaneous recanalization after stroke results in partial reperfusion [12] and that t-PA treatment leads to partial reperfusion in most stroke patients [13]. Therefore, the mechanisms of brain injury in focal ischemia with partial reperfusion versus ischemia with permanent occlusion or with total reperfusion may differ. Whether lithium treatment also reduces brain injury in focal ischemia with partial reperfusion remains to be clarified.
In addition, the underlying protective mechanisms of lithium treatment are not well understood. The Akt pathway has a critical role in neuronal survival after stroke. Activated Akt blocks apoptosis by phosphorylating its substrates, including GSK3β. Non-phosphorylated, thus activated GSK3β phosphorylates β-catenin, which leads to its proteasomal degradation and apoptosis [14]. The protective effects of lithium converge on dual inhibitory functions—it directly inhibits GSK3β activity as a competitive inhibitor of Mg2+ or indirectly inhibits GSK3β activity through promotion of GSK3β phosphorylation [15]. However, few reports have examined the protective effects of lithium on Akt activity in focal ischemia. Furthermore, most studies only examined protein levels of phosphorylated Akt [14], but a series of our and others’ studies have shown that Akt phosphorylation does not represent its true activity [16–19]; an in vitro Akt kinase assay should therefore be performed. Here, we studied the effects of acute treatment of lithium in a distal MCAo model of stroke with partial reperfusion and examined the neuroprotective mechanisms involving the Akt pathway.
MATERIALS AND METHODS
Focal Cerebral Ischemia and lithium treatment
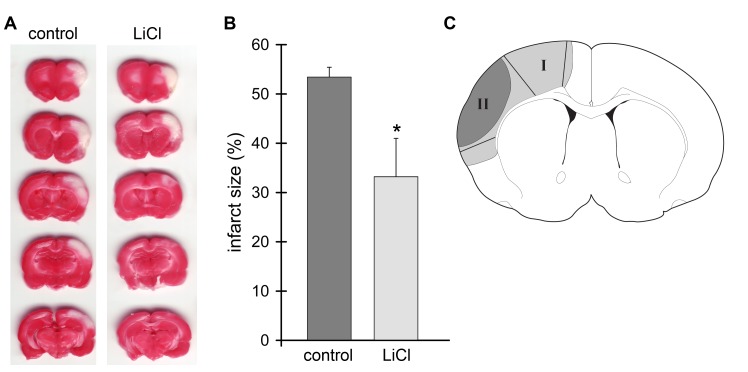
All experimental protocols performed on animals were approved by the Stanford University Administrative Panel on Laboratory Animal Care according to the NIH guide lines for the care and use of laboratory animals. Focal cerebral ischemia was generated as described previously [16, 17, 20–22]. Male Sprague-Dawley rats weighing 270–350 g (Charles River, Wilmington, MA, USA) were used. After rats were anesthetized with 2–3% isoflurane, the bilateral common carotid arteries (CCAs) were isolated and occluded by micro clips, and after 2 min the distal portion of MCA was permanently cauterized. CCAs remained occluded for 30 min and the clips were removed. Lithium chloride (LiCl, 3 mg/kg i.p.) or normal saline was administered when stroke was induced. The surgery for stroke was performed as a blinded procedure by a surgeon who did not know the treatment condition. Rats were sacrificed at 1, 5, 24 h after stroke (Fig.1A), and tissues corresponding to the ischemic core and penumbra were dissected and prepared for Western blot analysis (Fig.1B). The ischemic region spared by lithium treatment is defined as the ischemic penumbra (region I) and the area of infarction not spared by lithium treatment is defined as ischemic core (region II). Samples from sham-operated rats without stroke were also prepared as control.
Fig. 1.
Administration of lithium reduced infarct size. A. Representative cerebral infarcts stained by a 2% TTC solution. Ischemic brains were harvested 48 h after stroke, sectioned into 5 blocks, and stained with TTC solution. Control, the group with control ischemia; LiCl, the group receiving both ischemia and lithium treatment. B. Infarct size of the ischemic cortex was measured, normalized to the contralateral cortex and expressed as a percentage according to the following formula: [(area of non-ischemic cortex - area of remaining ischemic cortex)/area of non-ischemic cortex] x 100. A mean value from the 5 blocks is presented. * P<0.05, vs. control. N=7/each group. C. Definition of ischemic core and penumbra. Tissue corresponding to the ischemic penumbra and core was dissected for the Akt kinase and Western blot assays. The gray region (I), including the black region (II), represents ischemic injury in a control rat with ischemia alone; the black region (II) represents infarction in a rat receiving ischemia plus lithium. The region spared by lithium is defined as the penumbra (region I) and the black area is defined as the ischemic core (region II). These regions were dissected for Western blot analysis. The corresponding non-ischemic cortex from a sham animal without ischemia was dissected for comparison.
Infarct size measurement
Infarct size of the ischemic cortex was measured as described previously [16, 17, 20–22]. In brief, 5 blocks of 2-mm coronal sections were prepared from rat brains 48 h after stroke, stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) for 10 min and fixed with 4% paraformaldehyde. Infarct size was normalized to the contralateral cortex and expressed as a percentage according to the following formula: [(area of non-ischemic cortex - area of remaining ischemic cortex)/area of non-ischemic cortex] x 100.
Tissue preparation and Western blot Analysis
Protein solutions for Western blot analysis were prepared as previously described [16, 17, 23, 24]. Brain tissues were homogenized in cell lysis buffer (Cell Signaling Technology, MA) with protease inhibitor cocktail and phosphatase inhibitor cocktail I and II (Sigma, St Louis, MO). The lysates were centrifuged at 14,000 x g for 10 min and the supernatant was removed for immunoblotting. A total of 12 μg of protein was subjected to SDS-PAGE using 4–15% Ready Gel Tris-HCl gel (Bio-Rad, Hercules, CA). Protein bands were transferred from the gel to Hybond-LFP PVDF membranes (GE Healthcare Bioscience, Arlington Heights, IL). After transfer, the membranes were incubated with antibodies to GSK3β (1:1000,Cell Signaling Technology #9315, p-GSK3β(Ser9) (1:1000,Cell Signaling Technology #9336) and β-catenin (1:1000,Upstate #05–482) overnight at 4°C followed by a horse-radish peroxidase-conjugated secondary anti-rabbit antibody (1:2000; Cell Signaling Technology, Beverly, MA) for 1 hr. Membranes were developed in ECL Plus reagent (GE Healthcare Bioscience, Arlington Heights, IL). The membranes were also incubated with an anti-β-actin antibody (Sigma, St Louis, MO) followed by Alexa Fluor 647 donkey anti-mouse IgG (Invitrogen, Carlsbad, CA) to confirm equal loading of protein. Signals were detected by scanning with Typhoon Trio (GE Healthcare Bioscience, Arlington Heights, IL). Densities of protein bands were analyzed with ImageQuant software (Molecular Dynamics).
Akt kinase assay
The Akt Kinase Assay Kit (Cell Signaling Technology, MA) was used to analyze Akt kinase activity. Cell lysates were prepared similarly to those for Western blotting. Cell lysates were incubated with beads conjugated with Phospho-Akt (Ser473) (D9E)Rabbit monoclonal antibody (IgG) for co-immunoprecipitation with Akt, and the beads were then incubated with peptide GSK3 substrate. Akt activity was determined by the amount of GSK3 phosphorylated by Akt as detected by Western blot and described previously [16, 17].
Terminal deoxynucleotidyl transferase-mediated uridine 5’-triphosphate-biotin nick end labeling (TUNEL) staining
Brains 48 h after MCAo were post-fixed with 4% PFA and 20% sucrose for 24 h, and cut into four 3-mm coronal blocks. A cryostat was used to prepare 40 μm sections. TUNEL staining was performed as described previously [22].
Statistics
All results were presented as mean ± S.E.M. Infarct sizes between two groups were compared using the t-test. Comparisons for more than 3 groups of Western blot results were performed using ANOVA followed by the Student-Newman-Keuls post hoc test.
RESULTS
Administration of lithium reduced brain injury in focal ischemia with partial reperfusion
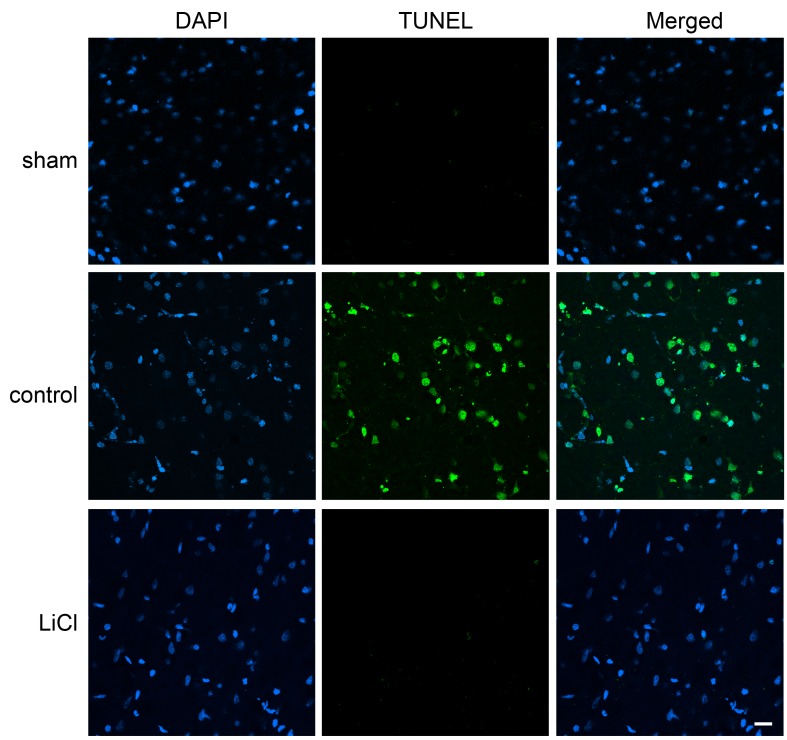
Stroke induced by distal MCAo plus bilateral CCAo generated ischemic infarction in the cortex (Fig.1A) as we described previously [17, 22]. When the bilateral CCAs are released while the distal MCA remains occluded, partial reperfusion occurs in the ischemic brain. Our results showed that lithium injected at the onset of stroke significantly reduced infarct size compared to vehicle control measured at 48 h after stroke (Fig.1B). In addition, TUNEL staining in brain tissues harvested 24 h after ischemia confirmed that lithium treatment reduced cell death. The staining regions of ischemic core and penumbra are shown in Fig. 1C; the ischemic region that developed into the infarction is defined as ischemic core and the ischemic region that was spared by lithium is defined as the ischemic penumbra. TUNEL-positive staining was found in both the ischemic core (data not shown) and penumbra of brains treated with vehicle (Fig.2); however, it was found in the core but not in the penumbra in lithium-treated ischemic brains (Fig.2). No positive staining was detected in sham rats without ischemia. Taken together, lithium treatment reduced brain injury in the stroke model with partial reperfusion.
Fig.2.
Cell death detection using TUNEL staining in the ischemic penumbra. Ischemic brains harvested 24 h after stroke were post-fixed with 4% PFA and 20% sucrose for 24 h, and sectioned for TUNEL and DAPI staining. No TUNEL-positive staining was detected in sham animals, but positive cells were abundant in ischemic brains with control ischemia. Lithium treatment blocked TUNEL-positive staining in the ischemic penumbra but not in the ischemic core. N=3/each group. Scale bar, 20 μm.
Lithium treatment did not change Akt kinase activity after stroke, arguing against the importance of Akt
We performed an in vitro Akt kinase assay to determine whether lithium affects Akt activity. Brain tissues were dissected from ischemic penumbra 1, 5 and 24 h after ischemia. Akt kinase activity was significantly decreased at 1 and 24 h after ischemia (Fig.3). It was also decreased at 5 h post-stroke, although no significant difference was detected when compared to the sham control. Even though lithium reduced infarct size, it did not significantly alter Akt activity (Fig.3).
Fig.3.
Lithium treatment did not change Akt kinase activity after stroke. Akt kinase activity at 1, 5 and 24 h after ischemia was measured. Akt activity was determined by the amount of GSK3 substrate phosphorylated by Akt as detected by Western blot. Representative relative optical densities of protein bands from the ischemic penumbra are shown in the bar graphs. Optical densities were normalized to those of sham samples, which were set as 100%. n=4–5 per group. *P<0.05 vs. sham.
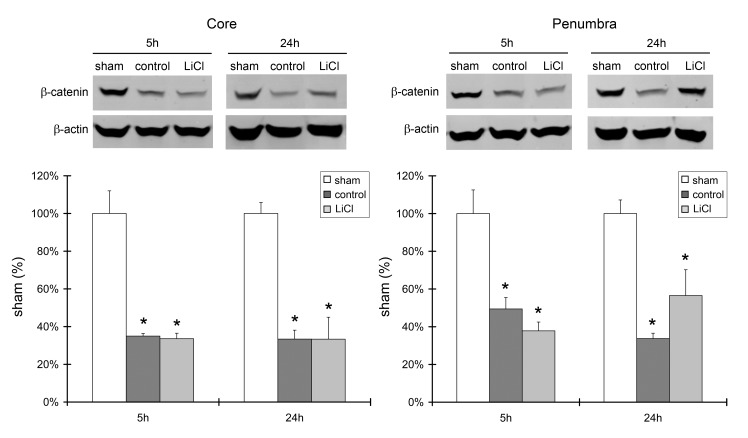
Lithium had no effect on GSK3β phosphorylation or β-catenin protein levels
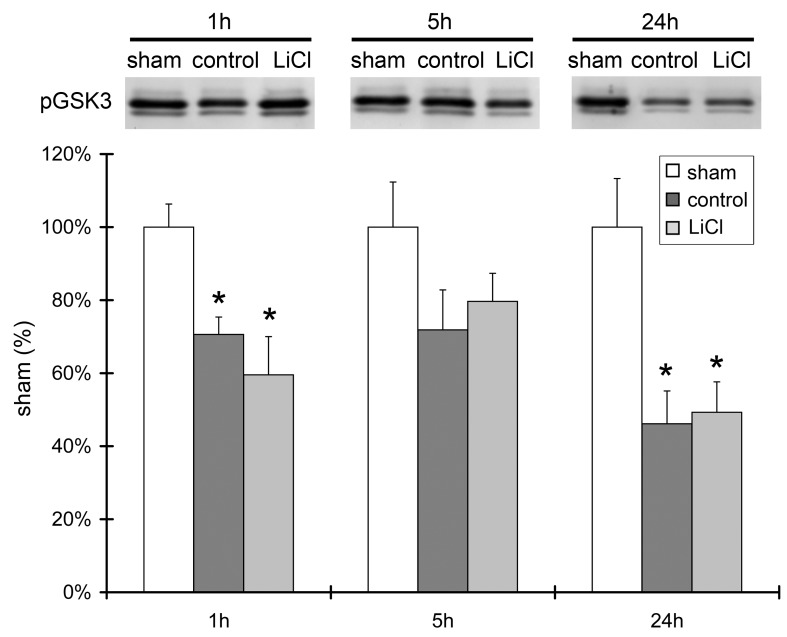
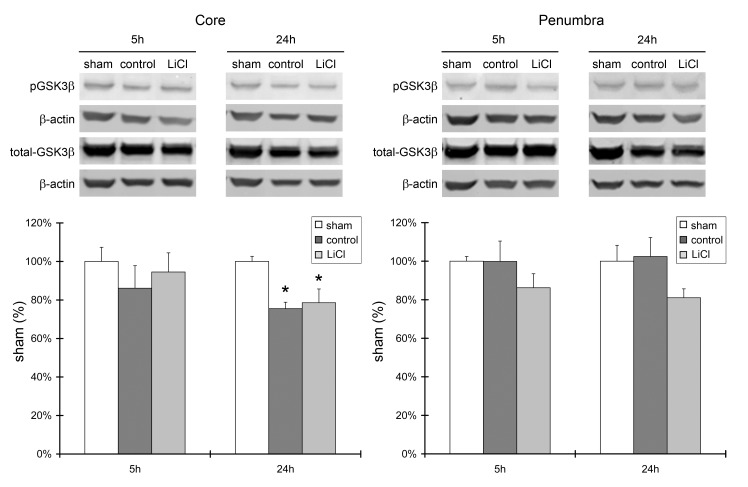
Lithium treatment did not affect Akt activity; therefore, we further investigated whether it affects downstream signaling molecules in the Akt pathway, including GSK3β and β-catenin. Western blot results showed a significantly reduced level of p-GSK3β protein in the ischemic core at 24 h after stroke, but not in the ischemic penumbra. Lithium treatment had no effect on its expression level (Fig.4). In addition, the level of β-catenin protein expression was robustly reduced after stroke in both the ischemic core and penumbra, at both 5 and 24 h. Lithium treatment did not alter its level of expression (Fig. 5).
Fig.4.
A. Lithium treatment did not affect protein levels of phosphorylated GSK3β and total GSK3β. Representative protein bands of p-GSK3β, total GSK3β, and β-actin are shown. The bar graphs show the quantitation results of the protein bands. Brain samples harvested 5 and 24 h after ischemia were investigated, and ischemic core and penumbra were compared. n=5 per group. *P<0.05 vs. sham.
Fig.5.
Lithium did not block β-catenin degradation after stroke. Western blot analysis of protein levels of β-catenin in the ischemic core and penumbra harvested at 5 and 24 h post-stroke. Representative protein bands are shown in the upper panels, and the bar graphs representing protein levels are shown in the bottom panels. n=5 per group. *P<0.05 vs. sham.
DISCUSSION
We demonstrated that a bolus of lithium administration robustly reduced infarct size in a model of distal MCAo with transient bilateral CCAo. This result suggests that lithium treatment can reduce brain injury induced by focal ischemia with partial reperfusion, which is a common phenomenon in stroke patients. Therefore, our study provides novel data that supports the potential of lithium as a treatment for patients who experience stroke with partial reperfusion. The protective effect of lithium was further confirmed when lithium treatment was shown to abolish TUNEL-positive staining in the ischemic penumbra, but not in the ischemic core. However, although the Akt pathway has been shown to be critical for neuronal survival, lithium did not increase Akt activity in our study. This suggests that the neuroprotective effects of lithium following stroke do not necessarily involve Akt activity.
The Akt pathway is known to support neuronal survival after stroke [14], implying that Akt activity is necessary for neuroprotective effects. Previous studies, including our own, have demonstrated that ischemia/reperfusion results in transient increases in Akt phosphorylation at ser473. Most of these studies used the quantity of p-Akt as a marker for Akt activity. However, we found increased p-Akt protein levels early after stroke onset yet decreased Akt activity at the same time point. The quantity of p-Akt, therefore, does not represent true Akt activity [17]. We repeated this finding in hypothermic, ischemic preconditioning and ischemic postconditioning models [16, 17, 25]. Whether lithium alters Akt kinase activity has not been previously reported. In this study we measured Akt activity using an Akt kinase assay rather than the level of p-Akt. We found decreased Akt activity after stroke and lithium did not increase Akt activity. This differs from our previous studies where all neuroprotectants, hypothermia, preconditioning, and postconditioning increased Akt activity independently of p-Akt levels [16, 17, 25]. Our current study suggests that Akt activity is not always involved in how certain neuroprotectants exert their effects.
Lithium may not alter Akt activity because it is not a direct Akt activator. Rather, it may inhibit neuronal death by inhibiting GSK3β activity, a protein downstream of Akt, as reported in previous studies [15]. Although our results showed that lithium had no effect on p-GSK3β or β-catenin protein levels, we cannot exclude the possibility that lithium did inhibit GSK3β activity. Previous studies have shown that lithium directly inhibits GSK3β activity as a competitive inhibitor of Mg2+, since GSK-3β catalyzes the phosphorylation of many protein substrates in the presence of Mg2+–ATP[15]. Lithium may also indirectly inhibit GSK3β activity by promoting GSK3β phosphorylation, however, chronic lithium treatment is required to promote GSK3β phosphorylation and alter mRNA levels of β-catenin [15, 26]. A single bolus of lithium injection in our study may not be able to improve GSK3β phosphorylation or β-catenin protein levels.
Our current study has some limitations. First, although we showed that lithium did not alter Akt activity, disputing the importance of Akt in the protective effects of lithium against stroke, we do not know which cell signaling pathways were involved in its protective effects. Second, one dose of lithium was used in the current study, and the therapeutic time windows were not explored. Third, only acute effects of lithium as early as 48 h after stroke were studied. Other studies have shown that lithium inhibits caspase-mediated apoptosis [9], blocks p53 activity [5], promotes Bcl-2 proteins [5], recovers heat shock proteins (HSP) 70 and 90β [11, 27, 28], and attenuates inflammatory factors, such as c-jun and c-fos [9]. Thus, lithium injection may reduce infarct size through cell-signaling pathways other than the Akt pathway. To fully understand the protective effects and mechanisms of lithium, future experiments should include a series of experiments with multiple pharmacological and genetic approaches using in vivo and in vitro models. In addition, long term protective effects as well as neurological scores should be evaluated.
In summary, lithium treatment provided strong protection against focal cerebral ischemia with partial reperfusion in a model that mimics a large portion of human stroke cases. Our data offer novel evidence for the potential clinical translation of lithium for the treatment of stroke, and that upregulation of Akt activity. However, whether lithium truly inhibits GSK3β activity in this setting requires further study.
Acknowledgments
The authors thank Elizabeth Hoyte for preparing the figures and Ms. Cindy H. Samos for manuscript assistance. This study was supported by NIH R01 NS27292-03 (GKS/HZ), and AHA grant in aid, NIH grants 1R21NS057750-01A2 (HZ) and 1R01NS 064136-01 (HZ).
References
- [1].Schou M, Juel-Nielsen N, Stromgren E, Voldby H. The treatment of manic psychoses by the administration of lithium salts. J Neurol Neurosurg Psychiatry. 1954;17:250–260. doi: 10.1136/jnnp.17.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Altamura AC, Lietti L, Dobrea C, Benatti B, Arici C, Dell'Osso B. Mood stabilizers for patients with bipolar disorder: the state of the art. Expert Rev Neurother. 2011;11:85–99. doi: 10.1586/ern.10.181. [DOI] [PubMed] [Google Scholar]
- [3].Manji HK, Moore GJ, Chen G. Lithium up-regulates the cytoprotective protein Bcl-2 in the CNS in vivo: a role for neurotrophic and neuroprotective effects in manic depressive illness. J Clin Psychiatry. 2000;61(Suppl 9):82–96. [PubMed] [Google Scholar]
- [4].Wada A, Yokoo H, Yanagita T, Kobayashi H. Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sci. 2005;99:307–321. doi: 10.1254/jphs.crj05009x. [DOI] [PubMed] [Google Scholar]
- [5].Bian Q, Shi T, Chuang DM, Qian Y. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007;1184:270–276. doi: 10.1016/j.brainres.2007.09.054. [DOI] [PubMed] [Google Scholar]
- [6].Li H, Li Q, Du X, Sun Y, Wang X, Kroemer G, Blomgren K, Zhu C. Lithium-mediated long-term neuroprotection in neonatal rat hypoxia-ischemia is associated with antiinflammatory effects and enhanced proliferation and survival of neural stem/progenitor cells. J Cereb Blood Flow Metab. 2011. [DOI] [PMC free article] [PubMed]
- [7].Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport. 1998;9:2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- [8].Sasaki T, Han F, Shioda N, Moriguchi S, Kasahara J, Ishiguro K, Fukunaga K. Lithium-induced activation of Akt and CaM kinase II contributes to its neuroprotective action in a rat microsphere embolism model. Brain Res. 2006;1108:98–106. doi: 10.1016/j.brainres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- [9].Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- [10].Yan XB, Wang SS, Hou HL, Ji R, Zhou JN. Lithium improves the behavioral disorder in rats subjected to transient global cerebral ischemia. Behav Brain Res. 2007;177:282–289. doi: 10.1016/j.bbr.2006.11.021. [DOI] [PubMed] [Google Scholar]
- [11].Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci U S A. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Neumann-Haefelin T, du Mesnil de Rochemont R, Fiebach JB, Gass A, Nolte C, Kucinski T, Rother J, Siebler M, Singer OC, Szabo K, Villringer A, Schellinger PD. Effect of incomplete (spontaneous and postthrombolytic) recanalization after middle cerebral artery occlusion: a magnetic resonance imaging study. Stroke. 2004;35:109–114. doi: 10.1161/01.STR.0000106482.31425.D1. [DOI] [PubMed] [Google Scholar]
- [13].Alexandrov AV, Burgin WS, Demchuk AM, El-Mitwalli A, Grotta JC. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation. 2001;103:2897–2902. doi: 10.1161/01.cir.103.24.2897. [DOI] [PubMed] [Google Scholar]
- [14].Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- [15].Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- [16].Gao X, Zhang H, Takahashi T, Hsieh J, Liao J, Steinberg GK, Zhao H. The Akt signaling pathway contributes to postconditioning's protection against stroke; the protection is associated with the MAPK and PKC pathways. J Neurochem. 2008;105:943–955. doi: 10.1111/j.1471-4159.2008.05218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci. 2005;25:9794–9806. doi: 10.1523/JNEUROSCI.3163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gao X, Zhang H, Steinberg G, Zhao H. The Akt pathway is involved in rapid ischemic tolerance in focal ischemia in Rats. Transl Stroke Res. 2010;1:202–209. doi: 10.1007/s12975-010-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, Zukin RS. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008;86:2505–2511. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- [21].Ren C, Gao X, Niu G, Yan Z, Chen X, Zhao H. Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS One. 2008;3:e3851. doi: 10.1371/journal.pone.0003851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- [23].Shimohata T, Zhao H, Sung JH, Sun G, Mochly-Rosen D, Steinberg GK. Suppression of deltaPKC activation after focal cerebral ischemia contributes to the protective effect of hypothermia. J Cereb Blood Flow Metab. 2007;27:1463–1475. doi: 10.1038/sj.jcbfm.9600450. [DOI] [PubMed] [Google Scholar]
- [24].Zhao H, Yenari MA, Cheng D, Barreto-Chang OL, Sapolsky RM, Steinberg GK. Bcl-2 transfection via herpes simplex virus blocks apoptosis-inducing factor translocation after focal ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:681–692. doi: 10.1097/01.WCB.0000127161.89708.A5. [DOI] [PubMed] [Google Scholar]
- [25].Gao X, Zhang H, Steinberg G, Zhao H. The Akt Pathway Is Involved in Rapid Ischemic Tolerance in Focal Ischemia in Rats. Translational Stroke Research. 2010;1:202–209. doi: 10.1007/s12975-010-0017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- [27].Xu XH, Hua YN, Zhang HL, Wu JC, Miao YZ, Han R, Gu ZL, Qin ZH. Greater stress protein expression enhanced by combined prostaglandin A1 and lithium in a rat model of focal ischemia. Acta Pharmacol Sin. 2007;28:1097–1104. doi: 10.1111/j.1745-7254.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- [28].Han R, Gao B, Sheng R, Zhang LS, Zhang HL, Gu ZL, Qin ZH. Synergistic effects of prostaglandin E1 and lithium in a rat model of cerebral ischemia. Acta Pharmacol Sin. 2008;29:1141–1149. doi: 10.1111/j.1745-7254.2008.00873.x. [DOI] [PubMed] [Google Scholar]