Abstract
Over the past decade, rapid signal exchange between astroglia and neurons across the interstitial space emerged as an essential element of synaptic circuit functioning in the brain. How and where exactly this exchange occurs in various physiological scenarios and the underlying cellular cascades remain a subject of intense study. The excitatory neurotransmitter glutamate and the inhibitory neurotransmitter γ-aminobutyric acid are thought to be the primary signal carriers that are regularly dispatched by active synapses to engage target receptors and transporters on the surface of astrocytes. New evidence identifies another ubiquitous messenger, extracellular calcium ions (Ca2+), which can report neural network activity to astroglia. Astrocytes in the hippocampus can respond to activity-induced partial Ca2+ depletion in the extracellular space by generating prominent intracellular Ca2+ waves. The underlying Ca2+ sensing mechanism is proposed to involve the opening of the hemichannel connexin 43 in astrocytes, which in turn triggers the release of adenosine triphosphate to boost the activity of inhibitory interneurons, thus potentially providing negative feedback to tame excessive excitatory activity of neural circuits.
Transfer of electrical and chemical signals through neural networks involves rapid Ca2+ influx into neurons, mainly through voltage-gated Ca2+ channels and Ca2+ permeable receptors [notably of the N-methyl-d-aspartate (NMDA) type]. Because the extracellular space fraction represents only ~20% of the brain-tissue volume (1), intense activity could partially deplete Ca2+ in the extracellular space surrounding active synapses (2-4). Torres et al. (5) mimicked this depletion in the neuropil of acute hippocampal slices with a photolytically activated extracellular Ca2+ buffer (diazo-2), two-photon uncaging of extracellular glutamate, or high-frequency afferent stimulation while monitoring external Ca2+ with Ca2+-sensitive electrodes. They found that these stimuli triggered prominent oscillatory waves of intracellular increases in Ca2+ concentrations, which developed and propagated across the local population of astrocytes on the time scale of 10 to 100 s. The latent (15 to 20 s poststimulus) component of these Ca2+ waves did not require activation of metabotropic glutamate receptors, the common and best-documented signaling pathway for neuron-glia communication (6-8).
Torres et al. next used genetic deletion, genetic enhancement, and pharmacological blockade of connexin 43 (Cx43) to suggest that these hemichannels, by opening in response to extracellular Ca2+ depletion, are the main trigger for the oscillatory Ca2+ increases detected in the activated astrocytes. Classically, Cx43 proteins have been associated with gap junctions, which are relatively small (up to hundreds of nanometers wide) and narrow (~2 nm across) ion-permeable contacts between the opposing membranes of nerve or glial cells. More recently, however, Cx43 has also been detected and characterized as a single-membrane channel that is permeable in its activated state to relatively large molecules (9-11)—in particular, adenosine triphosphate (ATP) (12)—although in cultured astrocytes, ATP release is associated mainly with pannexins rather than Cx43 (13). How many Cx43 hemichannels in astroglia actually occur outside gap junctions and whether their function can always be distinguished from that of pannexins remain debatable issues (14). Cx43 hemichannels increase their permeability in response to reduced external Ca2+ concentrations [reviewed in (11)] and are a good candidate to be a major sensor of extracellular Ca2+ in astrocytes. However, the mechanism and precise conditions of Ca2+-dependent (or voltage-dependent) opening of Cx43 remain poorly understood (10, 11, 14, 15). Torres et al. found that a single-point mutation in the Cx43 gene, which increases the activity of the ATP-releasing channels [Cx43G138R mutant (16)], enhanced Ca2+ waves in astroglia after extracellular Ca2+ depletion. It would be important to know whether the mutation had also lowered the threshold for the physiological stimuli to trigger such Ca2+ waves: If so, this might provide proof-of-principle evidence that the abundance of Cx43 could dynamically regulate the responsiveness of the astroglial network to Ca2+ depletion in the extracellular space.
The authors reported that the reduction in the extracellular Ca2+ concentration evoked by either electrical or photolysis-dependent stimuli lasted for only several seconds, whereas the late, glutamate-receptor–independent Ca2+ wave started ~20 s after the stimulus. At the same time, inter-neuron excitation attributed to the action of ATP released from astrocytes was evident before this late Ca2+ wave. Taken together, these data suggest that ATP release occurs before the late Ca2+ wave. A better understanding of the events occurring in the astrocytes during the period between the stimulus that triggers Ca2+ depletion and the late Ca2+ wave may therefore hold the key to uncovering the important cascades triggered downstream of Cx43 opening. Because a substantial proportion of astrocytic Ca2+ activity takes place locally, with-in microscopic or even nanoscopic (17) domains of thin astrocytic processes (18), it is reasonable to think that more sensitive Ca2+ detection methods will be able to determine whether local Ca2+-dependent molecular cascades may be associated with the Ca2+-dependent opening of Cx43.
Release of ATP by astrocytes is a well-documented phenomenon, and in many cases its physiological consequences are associated with suppression of excitatory transmission through activation of presynaptic adenosine A1 receptors (19) [reviewed in (8)]. The work by Torres et al., however, shows that astroglia-released ATP activated purinergic P2Y1 receptors in interneurons, thereby enhancing inhibitory neurotransmission. Although the net physiological outcome of either mechanism seems consistent with a negative-feedback signal sent by astroglia to synaptic networks, it would be important to understand the exact relationship between ATP-modulated activities of principal neurons and interneurons in this case. An inhibitory action through presynaptic adenosine A1 receptors could arise through local Ca2+ depletion and thus remain contained within the local presynaptic environment. In contrast, an excitatory influence on an interneuron through purinergic P2Y1 receptors will, by definition, impinge on multiple synapses in the network. Torres et al. detected no changes in field potential after ATP release: This might indicate, for instance, that a decrease in the excitability of principal neurons takes place mainly through an increase in the overall membrane conductance (shunting) rather than through neuronal hyperpolarization en masse.
Many intriguing questions are raised by the exciting findings of Torres et al. Two basic and somewhat counteracting mechanisms relate extracellular Ca2+ homeostasis to the condition of the brain extracellular space. On one hand, rapid changes in the extracellular space volume [which could, in some cases, be associated with marked changes in neural activity (1)] would produce immediate concomitant changes in the volume-averaged Ca2+ concentration. Conversely, a larger local extracellular volume would mean that more Ca2+ is available for influx before depletion occurs, and vice versa. How these relationships contribute to the response of astroglia to external Ca2+ fluctuations remains to be seen. Similarly, it seems important to establish whether the newly discovered Ca2+-sensing mechanism and the Cx43 hemichannels in astrocytes also contribute to the release of glutamate and d-serine from astroglia (20, 21). Another potentially important aspect of glial sensitivity to extracellular Ca2+ is its role in regulating signal integration in the local neural network, such as astrocyte-dependent synchronous initiation of heightened excitability (“UP state”) in multiple cortical neurons (22). Again, if the Ca2+ sensors in astroglia (presumably Cx43) are located predominantly near the main Ca2+ sinks (such as voltage-gated Ca2+ channels or NMDA receptors) in the vicinity of active synapses, then negative feedback through glial ATP will tend to moderate flow of information through these individual connections. If, however, Ca2+ sinks at multiple synapses or cells must be summed spatiotemporally to reach and activate these glial Ca2+ sensors, then the mechanism in question can be thought of as an integrator that moderates global network activity. Alternatively, a local Ca2+ depletion signal followed by ATP release could initiate a spreading self-sustained wave of ATP release from astroglia initiated by intracellular Ca2+ waves (23-25) (Fig. 1). Whether either, all, or none of these suggestions turn out to be correct, the discovery by Torres et al. will undoubtedly encourage investigators to look at the astroglia-neuron exchange from a conceptually different angle.
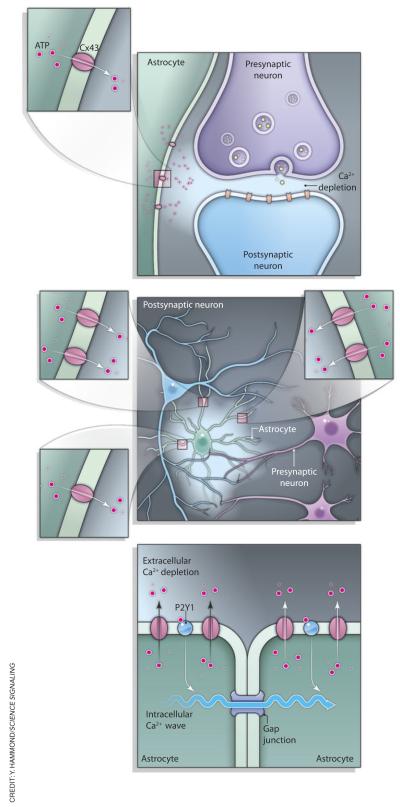
Fig. 1.
Possible scenarios relating extracellular Ca2+ depletion to CX43-dependent release of ATP from astroglia. Activation of individual synapses could be sufficient to deplete extracellular Ca2+ in the local environment and thus trigger Cx43-dependent release of ATP from a local astroglial process (top). Otherwise, synchronous activity of multiple synapses could be required to trigger the spatially and temporally integrated depletion of extracellular Ca2+ to an extent sufficient to initiate ATP release from astrocytes on a global scale (middle). Alternatively, local Ca2+ depletion–triggered activation of Cx43 and release of ATP (bottom) could initiate a spreading, self-sustaining wave of Ca2+-dependent ATP release from astroglia. The underlying regenerative mechanism may involve Ca2+ store–generated long-range Ca2+ oscillations propagating inside individual astrocytes and across interastrocyte gap junctions or, alternatively, activation of purinergic “autoreceptors” in astroglia (24), or both.
Acknowledgments
Funding: This work was supported by the Wellcome Trust (UK), the Medical Research Council (UK), and the Biotechnology and Biological Sciences Research Council (UK).
References and Notes
- 1.Syková E, Nicholson C. Diffusion in brain extracellular space. Physiol. Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egelman DM, Montague PR. Calcium dynamics in the extracellular space of mammalian neural tissue. Biophys. J. 1999;76:1856–1867. doi: 10.1016/s0006-3495(99)77345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusakov DA. The role of perisynaptic glial sheaths in glutamate spillover and extracellular Ca2+ depletion. Biophys. J. 2001;81:1947–1959. doi: 10.1016/S0006-3495(01)75846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusakov DA, Fine A. Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron. 2003;37:287–297. doi: 10.1016/s0896-6273(03)00025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, Takano T, Nedergaard M. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci. Signal. 2012;5:ra8. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haydon PG. GLIA: Listening and talking to the synapse. Nat. Rev. Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 7.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat. Rev. Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat. Rev. Neurosci. 2010;11:227–238. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- 9.Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 10.Stout C, Goodenough DA, Paul DL. Connexins: Functions without junctions. Curr. Opin. Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: A critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 12.Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: The molecular substrate of astrocyte “hemichannels. J. Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scemes E. Nature of plasmalemmal functional “hemichannels. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbamem.2011.06.005. 10.1016/j.bbamem.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras JE, Sáez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dobrowolski R, Sasse P, Schrickel JW, Watkins M, Kim JS, Rackauskas M, Troatz C, Ghanem A, Tiemann K, Degen J, Bukauskas FF, Civitelli R, Lewalter T, Fleischmann BK, Willecke K. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum. Mol. Genet. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergersen LH, Morland C, Ormel L, Rinholm JE, Larsson M, Wold JFH, Røe ÅT, Stranna A, Santello M, Bouvier D, Ottersen OP, Volterra A, Gundersen V. Immunogold detection of l-glutamate and d-serine in small synaptic-like microvesicles in adult hippocampal astrocytes. Cereb. Cortex. 2011 doi: 10.1093/cercor/bhr254. 10.1093/cercor/bhr254. [DOI] [PubMed] [Google Scholar]
- 18.Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- 19.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul J-Y, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 20.Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 21.Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc. Natl. Acad. Sci. U.S.A. 2011;108:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11023–11028. doi: 10.1073/pnas.1834448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowser DN, Khakh BS. Vesicular ATP is the pre-dominant cause of intercellular calcium waves in astrocytes. J. Gen. Physiol. 2007;129:485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]