Abstract
To determine the effect of a 12-week high intensity intermittent exercise (HIIE) intervention on total body, abdominal, trunk, visceral fat mass, and fat free mass of young overweight males. Participants were randomly assigned to either exercise or control group. The intervention group received HIIE three times per week, 20 min per session, for 12 weeks. Aerobic power improved significantly (P < 0.001) by 15% for the exercising group. Exercisers compared to controls experienced significant weight loss of 1.5 kg (P < 0.005) and a significant reduction in total fat mass of 2 kg (P < 0.001). Abdominal and trunk adiposity was also significantly reduced in the exercising group by 0.1 kg (P < 0.05) and 1.5 kg (P < 0.001). Also the exercise group had a significant (P < 0.01) 17% reduction in visceral fat after 12 weeks of HIIE, whereas waist circumference was significantly decreased by week six (P < 0.001). Fat free mass was significantly increased (P < 0.05) in the exercising group by 0.4 kg for the leg and 0.7 kg for the trunk. No significant change (P > 0.05) occurred in levels of insulin, HOMA-IR, and blood lipids. Twelve weeks of HIIE resulted in significant reductions in total, abdominal, trunk, and visceral fat and significant increases in fat free mass and aerobic power.
1. Introduction
Obesity levels continue to increase in both developed and developing countries [1]. As being overweight is associated with numerous health problems, effective fat loss strategies are required [2]. Although dieting has been the major fat loss method, aerobic exercise programs have been shown to increase cardiorespiratory fitness [3] and preserve fat-free mass [4]. Most aerobic exercise interventions have consisted of moderate-intensity steady-state exercise, for about 30 to 40 min for 3 to 4 days per week, over a four- to six-month period. Disappointingly, these kinds of exercise programs have resulted in minimal fat loss [5, 6].
In contrast, high-intensity intermittent exercise (HIIE) has been shown to result in greater fat loss [7]. For example, Trapp et al. [8] conducted a HIIE program in young women for 15 weeks with three 20 min sessions per week. HIIE consisted of an 8 s sprint followed by 12 s of low intensity cycling, repeated for 20 min. Another group of women carried out an aerobic cycling protocol for 40 min each session. Results showed that women in the HIIE group lost 2.5 kg of subcutaneous fat, whereas no change occurred with steady state aerobic exercise. Fat loss accruing through 15 weeks of HIIE was attained with 50% less exercise time commitment and a similar energy expenditure to that of steady-state exercise. Importantly, the women in this study also showed a significant 0.6 kg increase in fat-free mass (FFM) after HIIE, whereas FFM of the steady state exercise group was unchanged. The lack of increase in FFM accompanying steady-state exercise is in agreement with prior research in this area [9].
With regard to abdominal fat, 15 weeks of HIIE led to a 0.15 kg reduction of fat in previously untrained young women [8]. As women in this study possessed moderate levels of abdominal fat it is feasible that the greater abdominal, trunk, and visceral fat of men may show greater reductions after exposure to HIIE. For example, Boudou et al. [10] studied older type 2 diabetic males and found that after 8 weeks of HIIE, abdominal adiposity was decreased by 44%. Whether regular HIIE will also reduce the abdominal and visceral fat of young nondiabetic but overweight males is undetermined.
Therefore, the purpose of this study was to examine the effects of 20 min bouts of HIIE, repeated three times weekly for 12 weeks, on body composition of overweight males. It was hypothesized that HIIE would result in significant reductions in total abdominal, trunk, and visceral fat and a significant increase in fat-free mass and aerobic power.
2. Subjects and Methods
2.1. Subjects
Forty-six inactive, overweight men were recruited from a university population and randomly allocated into either exercise (n = 25) or control groups (n = 21). The exercisers and controls were similar in terms of age (24.7 ± 4.8 and 25.1 ± 3.9 years) and body mass index (BMI: 28.4 ± 0.5 and 29 ± 0.9 kg m−2). The study received approval from a University Research Ethics Committee. Forty-six subjects underwent initial testing, however, for various reasons five withdrew from the exercise group and three from the control group. There was no significant difference for any variable between the nonadherents and those males who completed the study.
2.2. Procedures
Subjects were advised to avoid strenuous activity and caffeine consumption for 24 hours prior to testing, and attended the laboratory after a 10-hour overnight fast. Tests for all subjects in control and exercise groups were completed at the same time of day. The Physical Activity Readiness Questionnaire [11] was filled out and information on subjects' personal and familial medical history obtained. Fasting blood (300 mL) was drawn at baseline, and at weeks 3, 6, and 12 from an antecubital vein in EDTA vacutainers. An automated enzymatic method (Cholestech LDX, USA) was applied to quantify blood lipid profiles and glucose concentrations from whole blood. The remaining whole blood in EDTA tubes was spun immediately in a chilled centrifuge (Model Megafuge 1.0R, Heraeus, Germany) at 4°C and frozen at −86°C for later analysis. Aerobic power was assessed using a TrueMax 2400 Metabolic Cart (ParvoMedics Inc, USA) and an electronically braked cycle ergometer, Monark 869 (Monark, Sweden). For subjects who could not achieve the criteria for O2max, due to the strenuous nature of the exercise session O2peak was used as an indicant of aerobic power.
2.3. Resting Metabolic Rate (RMR)
Fasted subjects relaxed in a reclined position for 30 minutes. Resting heart rate, resting energy expenditure (REE), O2, and CO2 were assessed using a metabolic cart (TrueMax 2400 Metabolic Cart, ParvoMedics Inc, USA).
O2 represents the rate of oxygen utilised by subjects during exercise, whereas CO2 represents the rate of carbon dioxide exhaled. Subjects were advised not to sleep and breathe naturally during testing. The first 10 minutes of data collection were excluded from analysis to allow for subject stabilization.
2.4. Diet
Subjects in both exercise and control groups were advised to maintain their normal eating habits during the study. On their first and last visit to the laboratory subjects provided a 3-day diet inventory which was analyzed using diet analysis software (SERVE Nutrition Management Systems, Professional Edition, version 5, Australia).
2.5. Body Composition
A Dual Energy X-Ray Absorptiometry (DEXA) scan with a Lunar Prodigy scanner (software version 7.51, GE Corporation, USA) was used to measure body mass and percentage body fat. Fat mass (FM) along with FFM in kg was measured for the whole body. DEXA also provided information on abdominal and trunk fat, as indicators of central adiposity. Computerised tomography (CT) scans (Philips Gemini GXL 16, the Netherlands) were also used to measure abdominal and visceral fat distribution. Axial slices (3 × 10 mm) were performed through the abdomen at L2/L3 and L4/L5. Fat density of 0.9 mg/L was assumed [12], and it was automatically selected at any tissue between 150 to 50 Hounsfield Units (HU). Gemini software (GXL Host system) was used to analyse the CT images. Abdominal, visceral, and subcutaneous fat were determined at the levels of L2/L3 and L4/L5. BMI was calculated by dividing weight by height squared (kg m−2).
2.6. High-Intensity Intermittent Exercise Training
Subjects in the exercise group completed supervised exercise (8 s sprint, 12 s recovery) continuously throughout each 20-min session. The HIIE workload was set at 80–90% of each subject's heart rate (HR) peak at a cadence between 120 and 130 r.p.m and recovery was set at the same amount of resistance but at a cadence of 40 r.p.m. Subjects were instructed to keep their exercise intensity at a level necessary to produce a HR between 80–90% of HR peak. As subjects adapted to HIIE training, workload was increased so HR stayed at the appropriate 80–90% HR peak level. HIIE was coordinated with a prerecorded compact disc counting down each sprint in a 3-2-1 manner. Subjects performed a 5-min warm-up and cool-down on the bike prior to and after each exercise session. All training cycling data included continuous recording of HR and r.p.m, whereas rating of perceived exertion [13] (RPE) was assessed at 5-min intervals.
2.7. Assays
Insulin was measured using commercially available ELISA immunoassay kits. The degree of enzymatic turnover of the substrate was determined by dual wavelength absorbance measurement at 450 and 620 nm (Dako K6219, Denmark). HOMA-IR, an insulin resistance index [14], was calculated as follows:
| (1) |
2.8. Statistical Analysis
Data were analysed with the Statistical Package for Social Science for Windows software (SPSS 18, USA). To examine changes after the intervention, an analysis of covariance (ANCOVA) was used to evaluate differences between the two groups for variables that did not violate ANCOVA assumptions. Preintervention values were used as covariates. Where assumptions were violated, an independent t-test was conducted on the difference scores. The statistical analysis was considered significant when the probability level was less than 0.05.
3. Results
There was no significant difference between the two groups for body mass, BMI (Table 1), and age prior to the training program.
Table 1.
Change in body composition, aerobic power, resting heart rate, RQ, resting energy expenditure, carbohydrate, and fat oxidation for the high-intensity intermittent exercise and no exercise control group (N = 38; mean and standard error).
| Exercise | Control | |||
|---|---|---|---|---|
| Pre* | Post | Pre* | Post | |
| Weight (kg) | 87.8 ± 2.7 | 86.3 ± 2.7** | 89 ± 2.9 | 89.4 ± 3.1 |
| BMI (kg m−2) | 28.4 ± 0.5 | 27.9 ± 0.5** | 29 ± 0.9 | 29.1 ± 0.9 |
| Waist circumference (cm) | 93.3 ± 1.4 | 89.8 ± 1.4** | 93.7 ± 1.9 | 95.1 ± 1.9 |
| Fat mass (kg) | 29.8 ± 1.6 | 27.8 ± 1.5** | 31.7 ± 2.2 | 31.8 ± 2.3 |
| % Fat mass | 34.8 ± 1.1 | 32.8 ± 1.1** | 36.3 ± 1.4 | 36.0 ± 1.5 |
| Fat-free mass (kg) | 54.3 ± 1.5 | 55.5 ± 1.4** | 53.8 ± 1.3 | 54.2 ± 1.3 |
| O2peak (l min−1) | 3.0 ± 0.1 | 3.4 ± 0.1** | 2.6 ± 0.1 | 2.7 ± 0.1 |
| O2peak (mL kg−1 min−1) | 34.2 ± 1.0 | 39.4 ± 0.8** | 29.1 ± 1.3 | 30.6 ± 1.4 |
| Work output (watts) | 246.3 ± 8.1 | 289.8 ± 8.0** | 224.4 ± 7.3 | 225.9 ± 6.3 |
| HR (bpm) | 62.2 ± 2.5 | 57.9 ± 1.8** | 62.7 ± 2.0 | 63.7 ± 1.7 |
| RQ | 0.85 ± 0.01 | 0.83 ± 0.01** | 0.82 ± 0.02 | 0.86 ± 0.01 |
| REE (Kcal/day) | 1793 ± 54 | 1841 ± 56 | 1788 ± 58 | 1794 ± 53 |
| Carbohydrate oxidation (g/day) | 232.6 ± 14.3 | 201.5 ± 13.1** | 186.7 ± 22.3 | 252.1 ± 21.2 |
| Fat oxidation (g/day) | 93.8 ± 6.6 | 106.1 ± 6.5** | 110.2 ± 10.0 | 82.0 ± 10.9 |
*Pre vales were used as covariates for ANCOVA.
**P < 0.05, change in exercise group significantly greater compared to that of control group. BMI: body mass index; REE: resting energy expenditure; HR: heart rate; RQ: respiratory quotient; REE: resting energy expenditure.
3.1. Exercise Heart Rates, RPE, and Work Load
The average HR during the HIIE training sessions for the exercise group was 160 ± 9 beats min−1 which corresponded to 88% of HR peak and the average RPE was 13.6 ± 0.5. Maximal work load significantly increased in the exercise group (P < 0.001) by 43.5 watts (Table 1).
3.2. Response in Aerobic Power following the Intervention
HIIE resulted in a significant increase in both absolute and relative O2peak (P < 0.005) with absolute O2peak being increased by 13% and relative O2peak by 15% (Table 1).
3.3. Total Body Mass and Body Fat Assessed by DEXA
Total body mass significantly decreased (P < 0.005) in the exercise group (Table 1) by 1.5 kg (2%), whereas total FM significantly decreased (P < 0.005) by 2.0 kg (6.7%; Figure 1). The FM of controls was unchanged after 12 weeks (Table 1). Percent body fat in exercisers at pretest was not correlated to changes in percent body fat after the intervention (r = 0.17, P > 0.05).
Figure 1.
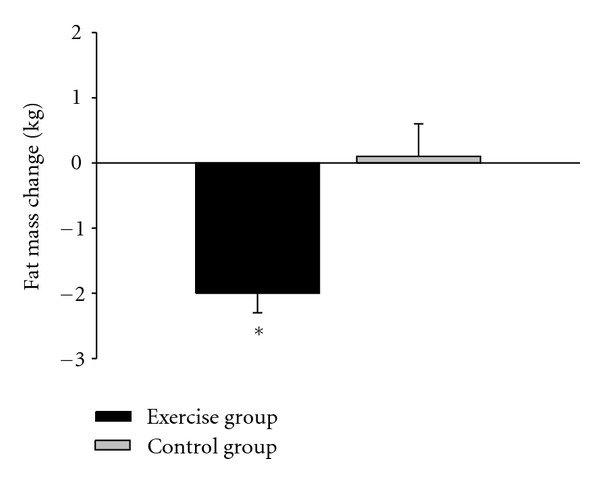
Total fat change for the high-intensity intermittent exercise and no exercise control groups (N = 38, mean and standard error). *Significantly different from control group (P < 0.05).
3.4. Abdominal and Trunk Fat Assessed by DEXA
There was a significant decrease in abdominal fat by 0.14 kg (6.6%) for the exercise group (P < 0.05) with no change for the control group (Table 2). The exercise group also significantly decreased (P < 0.001) trunk fat by 1.4 kg (8.4%), whereas trunk fat was slightly increased in controls (Table 2).
Table 2.
Regional changes in body composition for the high-intensity intermittent exercise and no exercise control groups (N = 38; mean and standard error).
| Region of fat mass | Exercise | Control | ||
|---|---|---|---|---|
| Pre* | Post | Pre* | Post | |
| Leg fat (kg) | 9.6 ± 0.8 | 9.0 ± 0.7 | 9.9 ± 0.7 | 9.8 ± 0.7 |
| Leg lean (kg) | 18.6 ± 0.6 | 19.0 ± 0.6** | 18.5 ± 0.6 | 18.5 ± 0.5 |
| Arm fat (kg) | 2.7 ± 0.2 | 2.5 ± 0.2** | 2.6 ± 0.1 | 2.7 ± 0.2 |
| Arm lean (kg) | 6.7 ± 0.2 | 6.7 ± 0.2 | 6.4 ± 0.4 | 6.6 ± 0.3 |
| Abdominal fat (kg) | 2.3 ± 0.1 | 2.2 ± 0.1** | 2.4 ± 0.2 | 2.4 ± 0.2 |
| Abdominal lean (kg) | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.5 ± 0.1 | 3.5 ± 0.1 |
| Trunk fat (kg) | 17.0 ± 0.9 | 15.5 ± 0.9** | 17.2 ± 1.2 | 17.3 ± 1.3 |
| Trunk lean (kg) | 24.9 ± 0.7 | 25.6 ± 0.7** | 24.0 ± 0.8 | 23.9 ± 0.7 |
*Prevalues were used as covariates for ANCOVA.
**P < 0.05, change significantly greater compared to that of control group.
3.5. Regional Body Composition Assessed by DEXA
There was no significant difference between groups in absolute FM loss in the leg (P > 0.05), whereas arm FM loss was greater for exercisers (P < 0.01; Table 2).
Total, leg, and trunk FFM (P < 0.05) were significantly increased after 12 weeks of HIIE in the exercise group compared to the control group, whereas arm FFM (P > 0.05) showed no significant change after the intervention (Figure 2).
Figure 2.
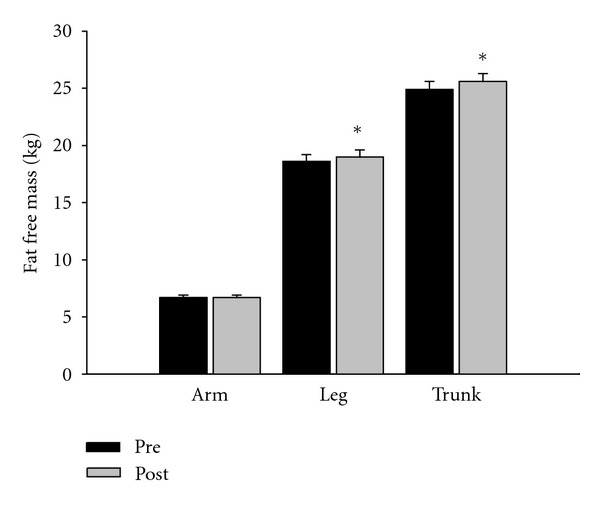
Fat-free mass change for the high-intensity intermittent exercise groups (N = 38; mean and standard error). *Significant difference between pre- and posttesting (P < 0.05).
3.6. Abdominal, Visceral, and Subcutaneous Fat Mass Assessed by CT
Total, abdominal, visceral, and subcutaneous FM at levels of L2/L3 and L4/L5 were significantly reduced (P < 0.05) after 12 weeks of HIIE compared to the control group (Table 3). Abdominal fat decreased by 8.5% at L2/L3 and 6.6% at L4/L5. Visceral fat was significantly decreased (P < 0.05) by 17% at level L2/L3 and 10% at level L4/L5 (Table 3; Figure 3).
Table 3.
Changes in computed tomography scan variables for the high-intensity intermittent exercise and no exercise control groups (N = 38; mean and standard error).
| Exercise | Control | |||
|---|---|---|---|---|
| Pre* | Post | Pre* | Post | |
| L2/L3 total fat mass (g) | 564.0 ± 22.3 | 538.0 ± 21.4** | 587.1 ± 26.3 | 591.0 ± 30.6 |
| L2/L3 abdominal fat (g) | 280.0 ± 21.4 | 256.1 ± 19.6** | 304.8 ± 26.5 | 311.9 ± 30.6 |
| L2/L3 visceral fat (g) | 94.6 ± 10.6 | 84.8 ± 9.9** | 102.1 ± 11.5 | 101.5 ± 11.4 |
| L2/L3 subcutaneous (g) | 177.3 ± 16.3 | 161.7 ± 14.7** | 194.2 ± 20.2 | 200.4 ± 23.4 |
| L4/L5 total fat mass (g) | 576.9 ± 24.3 | 555.7 ± 21.7** | 595.7 ± 28.4 | 602.2 ± 32.7 |
| L4/L5 abdominal fat (g) | 327.8 ± 23.0 | 306.3 ± 20.9** | 346.5 ± 27.3 | 355.5 ± 31.0 |
| L4/L5 visceral fat (g) | 62.6 ± 6.2 | 51.8 ± 5.1** | 69.7 ± 9.7 | 67.3 ± 8.4 |
| L4/L5 subcutaneous (g) | 259.7 ± 22.1 | 247.8 ± 20.0** | 271.7 ± 26.1 | 281.7 ± 27.7 |
*Prevalues were used as covariates for ANCOVA.
**P < 0.05, change significantly greater compared to that of control.
Figure 3.
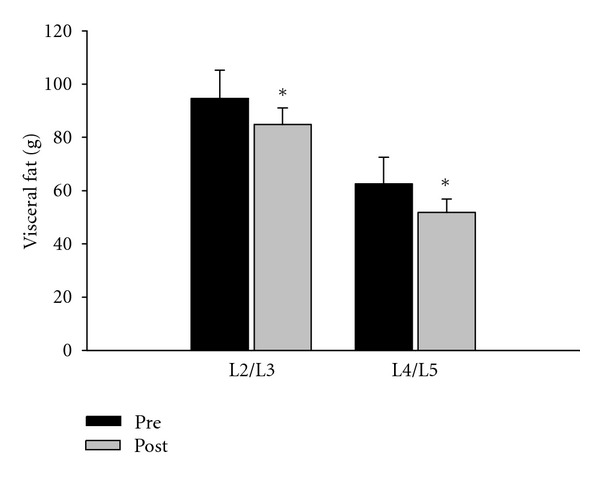
Visceral fat change for the high-intensity intermittent exercise and no exercise control groups (N = 38; mean and standard error). *Significantly different from control group (P < 0.05).
3.7. Change in Body Composition after 3, 6, and 9 Weeks
At weeks 3 and 6 there were no change in body mass and BMI, however, after 9 weeks body mass (P < 0.005) and BMI (P < 0.005) were significantly decreased. At the end of the trial, body mass and BMI were significantly decreased (P < 0.001; Table 1), yet, at week 6, WC was significantly lower than that at baseline, from 93.3 to 90.7 cm (P < 0.001). There was a further WC reduction from week 6 (90.7 cm) to week 12, (89.8 cm), which was not significant (P > 0.05). Also the major reduction in visceral fat brought about by HIIE appears to have occurred within the first six weeks as reduction in waist circumference was significantly correlated (r = 0.57, P < 0.05) with reduction in visceral fat (L4/L5) at week six.
3.8. Response in Resting Metabolic Rate following the Intervention
After the intervention exercise subjects had significantly lower resting HR (P < 0.01) and respiratory quotient (RQ; P < 0.01) compared to subjects in the control group. There was no significant change in resting metabolic rate after the intervention (P > 0.05), however, subjects in the exercise group had significantly higher (13%) fat oxidation (P < 0.001) and lower carbohydrate oxidation (P < 0.001) after the intervention compared to the control group (Table 1).
3.9. Response in Blood Variables following the Intervention
Fasting glucose, plasma insulin, glucose, HOMA-IR, and lipid levels were unchanged in the exercise compared to the control group (P > 0.05). For exercisers and controls preintervention levels of insulin, glucose, HOMA-IR, total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein (Table 4) were within the normal range for males of this age [15].
Table 4.
Glucose, insulin, HOMA-IR, and lipid change for the high-intensity intermittent exercise and no exercise control groups (N = 38; mean and standard error).
| Exercise | Control | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Glucose (mmoL·l−1) | 4.86 ± 0.14 | 4.97 ± 0.10 | 4.91 ± 0.14 | 4.91 ± 0.14 |
| Insulin (μU·mL−1) | 6.98 ± 0.66 | 6.72 ± 0.63 | 8.67 ± 0.90 | 8.29 ± 0.67 |
| HOMA-IR | 1.51 ± 0.15 | 1.47 ± 0.14 | 1.90 ± 0.24 | 1.82 ± 0.17 |
| Total cholesterol (mmoL·l−1) | 4.18 ± 0.25 | 3.97 ± 0.24 | 4.59 ± 0.21 | 4.36 ± 0.18 |
| Triglycerides (mmoL·l−1) | 1.20 ± 0.27 | 1.18 ± 0.36 | 1.52 ± 0.21 | 1.31 ± 0.18 |
| High-density lipoprotein (mmoL·l−1) | 1.31 ± 0.09 | 1.35 ± 0.09 | 1.08 ± 0.09 | 1.03 ± 0.08 |
| Low-density lipoprotein (mmoL·l−1) | 2.51 ± 0.16 | 2.35 ± 0.18 | 2.82 ± 0.18 | 2.81 ± 0.16 |
| Very low-density lipoprotein (mmoL·l−1) | 0.48 ± 0.07 | 0.43 ± 0.10 | 0.66 ± 0.09 | 0.56 ± 0.09 |
| TC: HDL ratio | 3.51 ± 0.32 | 2.99 ± 0.20 | 4.62 ± 0.31 | 4.52 ± 0.27 |
3.10. Diet
There was no significant change in macro- or micronutrient content before or after the intervention for the 3-day diet diary of the exercise or control group. Macronutrient levels before and after the 12-week intervention are shown in Table 5.
Table 5.
Macronutrient levels before and after the 12-week-intervention (N = 38; mean and standard error).
| Exercise | Control | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Kilojoules | 8102 ± 428 | 8142 ± 414 | 8569 ± 343 | 8642 ± 343 |
| % carbohydrate | 43.6 ± 1.8 | 42.9 ± 1.5 | 46.2 ± 1.8 | 46.8 ± 1.9 |
| % protein | 19.8 ± 1.1 | 19.5 ± 1.1 | 18.2 ± 1.1 | 18.3 ± 1.5 |
| % fat | 36.4 ± 1.7 | 37.5 ± 1.4 | 35.7 ± 1.7 | 34.9 ± 1.4 |
| % saturated fat | 13.2 ± 0.8 | 13.0 ± 0.7 | 12.4 ± 0.7 | 11.3 ± 0.6 |
| % monounsaturated fat | 13.6 ± 0.7 | 14.2 ± 0.7 | 13.6 ± 0.7 | 13.8 ± 0.7 |
| % polyunsaturated fat | 6.3 ± 0.6 | 7.0 ± 0.7 | 6.3 ± 0.6 | 6.5 ± 0.7 |
| Cholesterol (mg) | 397.3 ± 37.5 | 394.7 ± 41.9 | 304.8 ± 41.6 | 381.8 ± 57.0 |
| Fibre (g) | 19.5 ± 2.5 | 19.5 ± 2.4 | 20.3 ± 2.4 | 21.4 ± 2.8 |
| Sodium (mg) | 2564 ± 320 | 2709 ± 335 | 2390 ± 345 | 2547 ± 335 |
4. Discussion
The major findings of this study were that HIIE significantly increased O2peak and significantly reduced total, abdominal, trunk, and visceral fat of young, overweight males. Also trunk and leg fat-free mass was significantly increased after HIIE.
The 15% increase in O2max is similar to results of a previous study that used a 8 s/12 s HIIE protocol [8]. Talanian et al. [16] also found that a HIIE program significantly elevated aerobic power. In this paper the oxidative enzyme β-hydroxy-acyl-CoA dehydrogenase was used as a marker of mitochondrial volume and showed that intermittent sprinting enhances mitochondrial capacity. Different forms of HIIE have also been shown to significantly increase aerobic power [17, 18]. Thus, collectively these data show that HIIE results in significant improvements in aerobic fitness. The improvement in cardiorespiratory fitness after HIIE is an attractive feature of this mode of exercise as aerobic fitness has been shown to be an important predictor of positive health [19].
That HIIE resulted in significant subcutaneous fat reduction supports prior research in women using a similar protocol [8]. Results of the present study with males extend these findings showing that HIIE is an effective and efficient way of controlling body composition in both genders. With regard to abdominal fat, it has been found that 15 weeks of HIIE led to significantly reduced abdominal fat (0.15 kg) in untrained young women [8]. The 0.13 kg decrease in abdominal fat and 1.5 kg decrease in trunk fat found in the current study demonstrates that this effect is also present in young males and supports findings by Boudou et al. [10] who showed that 8 weeks of HIIE significantly reduced abdominal adiposity in older diabetic males.
The significant 17% decrease in visceral fat found in the present study extends the findings of Mourier et al. [20] who showed a significant reduction in visceral fat measured by MRI, following an exercise regimen consisting of steady-state exercise and HIIE for 8 weeks. These findings also add to the results of studies that have shown that aerobic training interventions decrease visceral adipose tissue [21]. The present study, however, appears to be the first to examine the effects of 20 min bouts of HIIE on visceral fat of young males. As visceral compared to overall obesity is more strongly associated with cardiovascular disease risk [22] the ability of HIIE to reduce visceral fat may have positive health implications. For example, it was shown that reduction in visceral fat was associated with improvement in glucose and lipid metabolism, [3] whereas Okauchi et al. [23] found that a reduction in visceral fat lowered the risk of atherosclerotic cardiovascular disease. Interestingly, Ohkawara et al. [21] estimated the optimal dose of aerobic exercise necessary to significantly reduce visceral fat and concluded that 3,780 kcal expended per week was needed. As an exercise session (e.g., cycling on a stationary cycle ergometer) lasting around an hour at a moderate exercise intensity expends about 520–550 kcal then to reach an optimal exercise caloric expenditure of 3,780 kcal per week an individual would have to perform approximately seven one-hour exercise sessions per week. In contrast, subjects in the present study exercised for only one hour per week. Also Donnelly et al. [24] conducted 16 months, 5 hours of aerobic exercise per week program with overweight young males and recorded a 23% decrease in visceral fat. Thus, it appears HIIE can bring about significant decreases in visceral fat with programs that are both significantly shorter in length (e.g., 16 months versus 3 months) and have less exercise commitment per week (5 hours versus 1 hour). Also the major decrease in visceral fat brought about by HIIE may have occurred within the first six weeks as reduction in visceral fat was correlated with reduction in waist circumference (r = 0.57, P < 0.05) at week six after which waist circumference did not further decrease.
Although the effect of HIIE on FFM has not been extensively examined, one study using DEXA found that trunk muscle mass was significantly increased in young females by 0.6 kg after 15 weeks of HIIE, [8] whereas another study using MRI showed a significant increase in thigh muscle cross sectional area of older males and females after HIIE [10]. The 1.2 kg increase in total FFM found after HIIE in the present study confirms the ability of this type of exercise to increase FFM. However, the length of this 12-week intervention was 3 weeks less than that conducted by Trapp et al. [8] that used females as subjects. As exercise HRs and relative exercise intensity of the two trials were similar it appears that males responded with a similar decrease in total fat but a greater increase in FFM after HIIE. FFM increase in the trunk after HIIE was 0.7 kg for males and 0.4 kg for females, whereas in the legs was 0.4 kg for males and 0.1 kg for females. Thus, males compared to females recorded greater increases in FFM in the trunk and legs. This characteristic may be important for fat loss programs as it has been shown that muscle mass is typically decreased after dietary restriction [25] and is typically unchanged after aerobic exercise training [9]. The significant increase in leg FFM may also reflect important metabolic adaptations resulting in enhanced insulin sensitivity [26].
Possible mechanisms underlying the HIIE-induced fat loss effect are undetermined but may include enhanced exercise and postexercise fat oxidation and suppressed postexercise appetite [7]. For example, Burgomaster et al. [26] and Talanian et al. [16] have shown that 6 to 7 sessions of HIIE had significant increases in whole body and skeletal muscle capacity for fatty acid oxidation. The excess postexercise oxygen consumption response to HIIE does not appear to have been examined, however, it is feasible that the significant levels of catecholamines generated during acute HIIE [27] could elevate postexercise fat oxidation. The significant catecholamine response to HIIE is in contrast to moderate, steady-state aerobic exercise that results in small increases in epinephrine and norepinephrine [28]. Also the high levels of catecholamines produced by HIIE may underlie its ability to reduce visceral fat, as catecholamines have been shown to drive lipolysis and are mainly responsible for fat release from visceral fat stores [29]. Also significantly, more β-adrenergic receptors have been found in visceral compared to subcutaneous fat [30] suggesting that
HIIE may have greater potential than steady-state exercise (e.g., jogging, cycling) to reduce visceral fat. Furthermore, increased fat oxidation after HIIE may occur as a result of the need to remove lactate and H+ and to resynthesize glycogen. Uncoupled respiration, protein turnover, and sympathetic nervous system activity may also contribute to increased energy expenditure and fat oxidation after exercise [9]. Finally, HIIE may also have a suppressive effect on appetite as exposing rats to hard exercise has been repeatedly reported to reduce food intake [31].
As this HIIE program required minimal time commitment, it has implications regarding subject compliance with exercise interventions. Thus, physical activity prescriptions, which require the least effort, while still producing adequate reductions in subcutaneous and visceral fat are likely to be optimal [9] and HIIE would seem to fall under this category as subject's total exercise commitment was 60 min per week. In conclusion, 20 min of HIIE, performed three times per week for 12 weeks, resulted in significant reductions in total body, abdominal, trunk, and visceral fat and a significant increase in fat-free mass of overweight young males.
Acknowledgments
The authors wish to thank Diabetes Australia for supporting this project (Grant no. RM06599) and also would like to thank Chau Tran, Joshua Lane, Roger Burrell, and Lucas Webb for help with data collection.
References
- 1.Speiser PW, Rudolf MCJ, Anhalt H, Comacho-Hubner C, Chiarelli F, Elakim A, et al. Childhood obesity. Journal of Clinical Endocrinology & Metabolism. 2005;90:1871–1887. doi: 10.1210/jc.2004-1389. [DOI] [PubMed] [Google Scholar]
- 2.Jakicic JM, Clark K, Coleman E, et al. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Medicine and Science in Sports and Exercise. 2001;33(12):2145–2156. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Ross R, Dagnone D, Jones PJH, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. Annals of Internal Medicine. 2000;133(2):92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- 4.Evans EM, Saunders MJ, Spano MA, Arngrimsson A, Lewis RD, Cureton KJ. Effects of diet and exercise on the density and composition of the fat- free mass in obese women. Medicine and Science in Sports and Exercise. 1999;31(12):1778–1787. doi: 10.1097/00005768-199912000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Wu T, Gao X, Chen M, Van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obesity Reviews. 2009;10(3):313–323. doi: 10.1111/j.1467-789X.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 6.Boutcher SH, Dunn SL. Factors that may impede the weight loss response to exercise-based interventions. Obesity Reviews. 2009;10(6):671–680. doi: 10.1111/j.1467-789X.2009.00621.x. [DOI] [PubMed] [Google Scholar]
- 7.Boutcher SH. High-intensity intermittent exercise and fat loss. Journal of Obesity. 2011;2011 doi: 10.1155/2011/868305. Article ID 868305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. International Journal of Obesity. 2008;32(4):684–691. doi: 10.1038/sj.ijo.0803781. [DOI] [PubMed] [Google Scholar]
- 9.Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Medicine. 2006;36(3):239–262. doi: 10.2165/00007256-200636030-00005. [DOI] [PubMed] [Google Scholar]
- 10.Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P, Gautier J-F. Absence of exercise-induced variations in adiponectin levels despite decreased abdominal adiposity and improved insulin sensitivity in type 2 diabetic men. European Journal of Endocrinology. 2003;149(5):421–424. doi: 10.1530/eje.0.1490421. [DOI] [PubMed] [Google Scholar]
- 11.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Canadian Journal of Sport Sciences. 1992;17(4):338–345. [PubMed] [Google Scholar]
- 12.Lohman T, Slaughter M, Boileau R, Lussier L. Bone mineral measurements and their relation to body density in children, youth and adults. Human Biology. 1984;56(4):667–679. [PubMed] [Google Scholar]
- 13.Borg GA. Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 14.Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54(2):333–339. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- 15.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) The Journal of the American Medical Association. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Talanian JL, Galloway SDR, Heigenhauser GJF, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. Journal of Applied Physiology. 2007;102(4):1439–1447. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- 17.Weston M, Helsen W, MacMahon C, Kirkendall D. The impact of specific high-intensity training sessions on football referees’ fitness levels. The American Journal of Sports Medicine. 2004;32(1):54S–61S. doi: 10.1177/0363546503261421. [DOI] [PubMed] [Google Scholar]
- 18.Gorostiaga E, Walter C, Foster C, Hickson R. Uniqueness of interval and continuous training at the same maintained exercise intensity. European Journal of Applied Physiology and Occupational Physiology. 1991;63(2):101–107. doi: 10.1007/BF00235177. [DOI] [PubMed] [Google Scholar]
- 19.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. British Journal of Sports Medicine. 2009;43(1):1–2. [PubMed] [Google Scholar]
- 20.Mourier A, Gautier J-F, Kerviler ED, et al. Mobilization of visceral adipose tissue related to the improvement in insulin sensitivity in response to physical training in NIDDM: effects of branched-chain amino acid supplements. Diabetes Care. 1997;20(3):385–391. doi: 10.2337/diacare.20.3.385. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. International Journal of Obesity. 2007;31(12):1786–1797. doi: 10.1038/sj.ijo.0803683. [DOI] [PubMed] [Google Scholar]
- 22.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14(2):336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 23.Okauchi Y, Nishizawa H, Funahashi T, et al. Reduction of visceral fat is associated with decrease in the number of metabolic risk factors in Japanese men. Diabetes Care. 2007;30(9):2392–2394. doi: 10.2337/dc07-0218. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly JE, Hill JO, Jacobsen DJ, et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the midwest exercise trial. Archives of Internal Medicine. 2003;163(11):1343–1350. doi: 10.1001/archinte.163.11.1343. [DOI] [PubMed] [Google Scholar]
- 25.Saris W. The role of exercise in the dietary treatment of obesity. International Journal of Obesity. 1993;17(1):S17–S21. [PubMed] [Google Scholar]
- 26.Burgomaster KA, Hughes SC, Heigenhauser GJF, Bradwell SN, Gibala MJ. Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. Journal of Applied Physiology. 2005;98(6):1985–1990. doi: 10.1152/japplphysiol.01095.2004. [DOI] [PubMed] [Google Scholar]
- 27.Trapp EG, Chisholm DJ, Boutcher SH. Metabolic response of trained and untrained women during high-intensity intermittent cycle exercise. American Journal of Physiology. 2007;293(6):R2370–R2375. doi: 10.1152/ajpregu.00780.2006. [DOI] [PubMed] [Google Scholar]
- 28.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Medicine. 2008;38(5):401–423. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]
- 29.Issekutz B. Role of beta-adrenergic receptors in mobilization of energy sources in exercising dogs. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1978;44(6):869–876. doi: 10.1152/jappl.1978.44.6.869. [DOI] [PubMed] [Google Scholar]
- 30.Rebuffé-Scrive M, Andersson B, Olbe L, Björntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism. 1989;38(5):453–458. doi: 10.1016/0026-0495(89)90198-4. [DOI] [PubMed] [Google Scholar]
- 31.Bilski J, Teległów A, Zahradnik-Bilska J, Dembiński A, Warzecha Z. Effects of exercise on appetite and food intake regulation. Medicina Sportiva. 2009;13(2):82–94. [Google Scholar]


