Abstract
The unfolded protein response (UPR) is activated upon the accumulation of misfolded proteins in the endoplasmic reticulum (ER), that are sensed by the binding immunoglobulin protein (BiP)/glucose-regulated protein 78 (GRP78). The accumulation of unfolded proteins sequesters BiP so it dissociates from three ER-transmembrane transducers leading to their activation. These transducers are inositol requiring (IRE) 1α, PKR-like ER kinase (PERK) and activating transcription factor (ATF) 6α. PERK phosphorylates eukaryotic initiation factor 2 alpha (eIF2α) resulting in global mRNA translation attenuation, and concurrently selectively increases the translation of several mRNAs, including the transcription factor ATF4, and its downstream target CHOP. IRE1α has kinase and endoribonuclease (RNase) activities. IRE1α autophosphorylation activates the RNase activity to cleave XBP1 mRNA, to produce the active transcription factor sXBP1. IRE1α activation also recruits and activates the stress kinase JNK. ATF6α transits to the Golgi compartment where it is cleaved by intramembrane proteolysis to generate a soluble active transcription factor. These UPR pathways act in concert to increase ER content, expand the ER protein folding capacity, degrade misfolded proteins, and reduce the load of new proteins entering the ER. All of these are geared toward adaptation to resolve the protein folding defect. Faced with persistent ER stress, adaptation starts to fail and apoptosis occurs, possibly mediated through calcium perturbations, reactive oxygen species, and the proapoptotic transcription factor CHOP. The UPR is activated in several liver diseases; including obesity associated fatty liver disease, viral hepatitis and alcohol-induced liver injury, all of which are associated with steatosis, raising the possibility that ER stress-dependent alteration in lipid homeostasis is the mechanism that underlies the steatosis. Hepatocyte apoptosis is a pathogenic event in several liver diseases, and may be linked to unresolved ER stress. If this is true, restoration of ER homeostasis prior to ER stress-induced cell death may provide a therapeutic rationale in these diseases. Here we discuss each branch of the UPR and how they may impact hepatocyte function in different pathologic states.
Introduction
Described as a response to the accumulation of unfolded proteins in the endoplasmic reticulum (ER), the eponymously named unfolded protein response (UPR) is characterized by the activation of three distinct signal transduction pathways mediated by inositol requiring (IRE) 1α, PKR-like ER kinase (PERK) and activating transcription factor (ATF) 6α (Figure 1). Alterations in ER function can be induced by myriad stimuli, pharmacologically by exogenously applied chemicals or physiologically such as by increased secretory protein demand [29]. Altogether these perturbations are referred to as ER stress and are recognized by the activation of the UPR transducers (Key Message Box 1). The ER lumenal domains of all three stress sensors are bound by the ER chaperone BiP in the unstressed state. Upon ER stress, BiP binding to unfolded proteins causes dissociation from the lumenal domains of the sensors leading to the activation of IRE1α and PERK by transautophosphorylation, and ATF6α by proteolytic processing [10, 100]. Following activation, UPR signaling pathways act to induce expression of genes that encode functions to ameliorate the stressed state of the ER. These adaptive mechanisms include global attenuation of mRNA translation via phosphorylation of eIF2α. eIF2α phosphorylation dramatically decreases the functional load on the ER by reducing synthesis of new proteins that would require folding. Additionally, transcriptional activation of ER chaperones and ER expansion occur to facilitate folding of the accumulated misfolded proteins. ER-associated degradation (ERAD) components are upregulated transcriptionally as well, facilitating degradation of terminally misfolded proteins. Sustained ER stress leads to apoptosis. Though the exact mechanisms that mediate ER-stress induced apoptosis have not yet been elucidated, the transcription factor C/EBP homologous protein (CHOP), the mitogen activated protein kinase c-jun N-terminal kinase (JNK), Bcl-2 family proteins, calcium and redox homeostasis, and caspase activation have all been implicated.
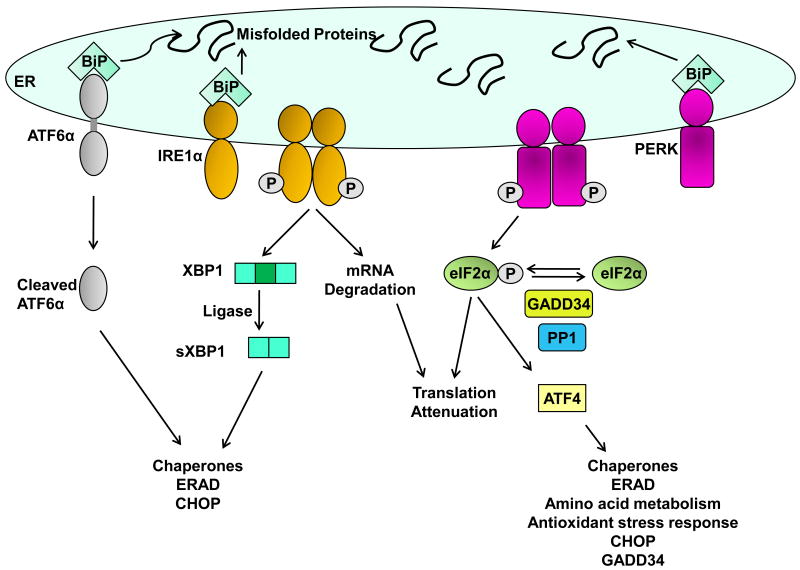
Figure 1. The unfolded protein response sensors.
Three ER membrane sensors activating transcription factor 6 α (ATF6α), inositol requiring (IRE) 1α, and PKR-like ER-localized kinase (PERK) mediate signals from the endoplasmic reticulum (ER) upon activation of the unfolded protein response (UPR). The accumulation of misfolded proteins in the ER lumen sequesters the chaperone BiP away from the lumenal domain of all three ER sensors which leads to their activation. ATF6α is activated by regulated intramembrane proteolysis in the Golgi to release the transcriptionally active 50 kDa cytosolic N-terminal domain. Cleaved ATF6α heterodimerizes with spliced XBP1 (sXBP1) to transcriptionally induce several genes encoding ER chaperones and ER-associated degradation (ERAD) proteins. IRE1α undergoes dimerization and transautophosphorylation which activates its endoribonuclease (RNase) activity. It cleaves X-box binding protein 1 (Xbp1) mRNA, which is then ligated by an uncharacterized ligase to form sXBP1 encoding a potent transcription factor, that also induces expression of ERAD proteins and chaperones. Dimerization and transautophosphorylation of PERK activates its kinase activity, leading to phosphorylation of the alpha subunit of eukaryotic initiation factor 2 (eIF2α). This leads to global translation attenuation. Selective translation of activating transcription factor 4 (ATF4) occurs following phosphorylation of eIF2α. ATF4 induces the expression of several genes including amino acid transporters, chaperones, and C/EBP homologous protein (CHOP). CHOP also induces the expression of GADD34, which associates with protein phosphatase 1 (PP1) to dephosphorylate eIF2α in a negative feedback loop, thus resuming translation.
Key Message Box 1.
In addition to the accumulation of misfolded proteins in the ER, many stimuli that perturb ER homeostasis can cause protein misfolding. The mechanisms by which some of these agents can activate the UPR are known. Tunicamycin inhibits N-linked protein glycosylation and castanospermine inhibits N-linked oligosaccharide trimming, thus both drugs alter protein folding and trafficking. Nutrient depletion presumably lowers ATP levels to disrupt chaperone-dependent protein folding reactions. Thapsigargin disrupts calcium storage which is required for protein chaperone function and protein folding. Dithiothreitol disrupts oxidative protein folding to cause protein misfolding. Brefeldin A activates the UPR by inhibiting ER to Golgi transport. In type 2 diabetes increased proinsulin biosynthesis leads to UPR due to excess demands on the folding machinery. In the neurodegenerative disorders, Alzheimer's disease and Parkinson's disease, abnormal protein aggregates are observed and are implicated in the pathogenesis of disease. Free fatty acids and reactive oxygen species can activate the UPR, though the exact mechanisms are not defined. Pharmacologic inhibition of proteasomal degradation causes misfolded proteins to accumulate.
Inositol requiring (IRE)-1α is a dual function type I transmembrane protein with Ser/Thr protein kinase and endoribonuclease activities (Key Message Box 2). It is the most archaic and conserved branch of the UPR. Initially the Ire1 gene was isolated on screening of Saccharomyces cerevisiae genetic mutants for inositol autotrophy. It was characterized as essential for expression of ER chaperones and adaptation to ER stress in yeast [23, 94]. Subsequently two mammalian genes, Ire1α and Ire1β were identified, with significant homology with yeast at the C-terminus, and divergence at the N-terminus [131, 140]. Ire1α is widely expressed, and Ire1β expression is limited to intestinal epithelium, though it may be expressed in other cell types [9]. IRE1α protein has an amino-terminal ER lumenal domain, a transmembrane region, and a carboxy-terminal domain that contains both kinase and endoribonuclease catalytic activities. Upon dissociation from BiP binding, IRE1α dimerizes, transautophosphorylates and activates the endoribonuclease (RNase). The RNase activity of IRE1α is essential for the execution of the UPR. IRE1 endoribonuclease activity splices the transcription factor Hac1 mRNA in yeast and Xbp1 mRNA in mammals. Activated IRE1α also recruits tumor necrosis factor receptor (TNFR)-associated factor-2)(TRAF2) and apoptosis signaling kinase 1 (ASK1) to mediate the activation of c-jun N-terminal kinase (JNK) and activation of nuclear factor kappa B ((NFκB) [53, 135]. The IRE1α RNase activity is also implicated in the degradation of several cellular mRNAs, a process named regulated IRE1-dependenet decay (RIDD) [51-52], including proinsulin mRNA and IRE1α mRNA itself [42, 131]. Microsomal triglyceride transfer protein (MTTP) mRNA is degraded by IRE1β [55]. X-box-binding protein 1 (Xbp1) mRNA is the only characterized target of IRE1α RNase activity that is subject to ligation; in yeast the ligation is performed by the tRNA ligase Rlg1p and the mammalian ligase is yet to be identified [126]. Xbp1 mRNA undergoes unconventional (cytoplasmic, spliceosome-independent) splicing to generate a potent basic leucine zipper domain (bZIP)-containing transcription factor [134]. Removal of 26 nucleotides from the mammalian Xbp1 mRNA results in a translational frame switch, encoding a protein containing 376 amino acids, as compared with 261 amino acids encoded by the unspliced mRNA. Both forms of XBP1 can bind the ER stress element (ERSE); however, spliced XBP1 (sXBP1) activates the UPR far more potently than its unspliced form [156]. Furthermore, upon ER stress, Xbp1 mRNA expression is enhanced by ATF6α, providing additional substrate for IRE1α to splice into the more transcriptionally active form [73, 156]. The unspliced XBP1 protein is unstable in the cell and can heterodimerize with ATF6 and sXBP1 to promote their proteasomal degradation [157]. Spliced XBP1 can bind to three cis-acting elements, ERSE, unfolded protein response element (UPRE) and ERSE-II [151]. Though ATF6α can bind some of these elements as well, sXBP1 homodimers and sXBP1-ATF6 heterodimers bind to ERSE activating the transcription of several XBP1 target genes, including Herp [151], EDEM (ER degradation-enhancing alpha-mannosidase-like protein) [155], and MDG1/ERdj4 [62]. Transcriptional targets of sXBP1 include genes that encode functions in ER protein folding and quality control, ER-associated degradation (ERAD) and ER biogenesis [128, 155].
Key Message Box 2.
Inositol requiring 1α (IRE1α) is conserved from yeast to higher organisms. It is activated upon dissociation from BiP by dimerization and transautophosphorylation to elicit its endoribonuclease activity. IRE1α initiates cleavage of 26 nucleotides from X-box binding protein-1 (XBP1) mRNA in the cytosol. Spliced XBP 1 mRNA is ligated by an unidentified ligase and encodes for a potent transcription factor (sXBP1) that activates a subset of UPR genes involved with ER biogenesis and ER-associated degradation (ERAD). IRE1α also recruits the adaptor protein TRAF2 (tumor necrosis factor receptor (TNFR)-associated factor-2), leading to activation of c-jun N-terminal kinase (JNK). IRE1α-dependent JNK activation has been linked to insulin resistance and apoptosis. Caspase 12 may be recruited by the IRE1α-TRAF2 complex in ER stress induced apoptosis in mice.
The genetic absence of either IRE1α or XBP1 in the mouse results in embryonic lethality after gestational day ∼11.5 that is associated with fetal liver hypoplasia in these embryos [113, 162]. In addition, hepatocyte-specific expression of a transgene encoding spliced XBP1 was able to rescue embryonic lethality associated with Xbp1 deletion, suggesting the embryonic lethality is due to a requirement for spliced XBP1 in hepatocytes [70]. However, it was recently reported that Ire1α deletion also causes placental defects that were proposed to be responsible for the embryonic lethality of conditional Ire1α-null mice [56]. Ire1β deficiency is non-lethal, though the mice are more susceptible to dextran sulfate-induced colitis and demonstrate enhanced enterocyte chylomicron secretion secondary to increased expression of MTTP [9, 55].
ATF6α is a type II ER transmembrane protein, with a cytoplasmic N-terminus that contains a basic leucine zipper motif that functions as a transcription factor following regulated intramembrane proteolysis (RIP) in ER-stressed cells [46, 154] (Key Message Box 3). The ER resident form is 90 kDa and has two Golgi localization sequences (GLS) that are masked by BiP binding [123]. Upon dissociation from BiP, ATF6α translocates to the Golgi and its C-terminal half is cleaved by site-1 protease [152]. The membrane anchored N-terminus is cleaved by site-2 protease and a 50 kDa protein is released into the cytosol. The 50 kDa N-terminus protein translocates to the nucleus to activate transcription by binding to the ATF (activating transcription factor)/cAMP response element (CRE) and ER stress response element (ERSE). Transcriptional induction of ER chaperone genes such as BiP and GRP94 (glucose regulated protein 94) is mostly mediated by ATF6α binding to the cis-acting ERSE consensus sequence CCAAT-N9-CCACG. ATF6α also transcriptionally activates ERAD components by heterodimerization with sXBP1. Two Atf6 genes, alpha(α) and beta(β) are expressed ubiquitously, with no obvious phenotype in mice lacking either Atf6α or Atf6β individual isoform [150]. However, challenge of Atf6α-null mice, but not Atf6β-null mice, with ER stress in the liver leads to hepatosteatosis and death [119]. Although ATF6α mediates UPR gene induction in response to ER stress, the gene targets for ATF6β have not been identified. Interestingly, the combined deletion of Atf6α and Atf6β causes a very early embryonic lethality, suggesting these genes provide an essential complementary function(s) in early mammalian development.
Key Message Box 3.
Activating transcription factor 6 α (ATF6α) is a basic leucine zipper domain (bZIP) family transcription factor that upon release from BiP transits to the Golgi compartment where it is processed by regulated intramembrane proteolysis. Cleaved ATF6α activates a subset of UPR genes, including XBP1, ER protein chaperones and CHOP.
PERK is a type I ER resident protein kinase that, upon ER stress is activated to phosphorylate the alpha subunit of eukaryotic translation-initiation factor 2 (eIF2α) on serine residue 51 [45] (Key Message Box 4). This leads to a rapid reduction in the initiation of mRNA translation thus reducing the load of new client proteins that require folding in the ER. Attenuation of translation promotes adaptation as PERK-null cells, as well as cells with Ser51Ala mutation at the PERK phosphorylation site in eIF2α, are unable to decrease protein synthesis upon ER stress and exhibit enhanced cell death [121]. PERK dependent eIF2α phosphorylation decreases synthesis of cyclin D1 to mediate cell cycle arrest in stressed cells [13]. PERK is expressed ubiquitously with highest levels in the pancreas [124]. Loss of function mutations in PERK are the cause of Wolcot-Rallison syndrome in humans which stems from a loss of insulin production and beta cell failure [25]. PERK deletion in mice also causes pancreatic insufficiency most prominent in the beta cell at 4 weeks of age and in acinar cells at 6-8 weeks of age [44]. Further analysis of the role of eIF2α phosphorylation, by characterization of mice with Ser51Ala homozygous mutation in eIF2α demonstrated that eIF2α phosphorylation prevents neonatal lethality due to hypoglycemia and preserves pancreatic beta cell mass [121]. In the absence of eIF2α phosphorylation, beta cells exhibit a higher rate of protein synthesis thus increasing the demand for protein folding. This includes increased proinsulin folding and misfolding; the later leads to accumulation of misfolded protein in the ER, and thereby enhanced oxidative stress [3, 122]. These observations lead to the notion that inhibition of translation by PERK-mediated eIF2α phosphorylation in response to ER stress is required for cell survival by limiting the protein-folding load thus preventing accumulation of misfolded proteins, and thereby the subsequent additional stress of oxidative protein folding. Phosphorylation of eIF2α can also be effected by three other kinases that respond to cellular and environmental stress; these kinases are protein kinase RNA-activated (PKR), heme-regulated inhibitor (HRI), and GCN2 kinase.
Key Message Box 4.
Protein kinase RNA (PKR)-like ER kinase (PERK) is activated by dimerization following dissociation from BiP in the stressed ER. It is activated by transautophosphorylation, leading to subsequent phosphorylation of its only known physiologically important substrate eukaryotic translation initiation factor α (eIF2α) on serine residue 51. This results in translational halt and reduces the client load of new proteins in the ER. Selective translation of activating transcription factor 4 (ATF4), and its downstream target C/EBP homologous protein (CHOP) occurs. ATF4 induces the expression of amino acid biosynthesis transporters and antioxidant stress response genes. CHOP activates the expression of GADD34 (growth arrest and DNA damage 34), which along with protein phosphatase 1 dephosphorylates eIF2α leading to resumption of mRNA translation.
Transcriptional profiling of homozygous Ser51Ala eIF2α mutant and wildtype cells demonstrated that the expression of several genes is dependent on PERK-mediated eIF2α phosphorylation [121]. Key among these is activating transcription factor 4(ATF4) that is up regulated at the mRNA translational level upon eIF2α phosphorylation [43]. Atf4 translation is repressed by the presence of two upstream open reading frames (uORFs). Upon eIF2α phosphorylation ribosomes scan through the upstream uORFs to initiate Atf4 translation [136]. ATF4 transcriptionally activates numerous ER-stress response genes that promote adaptation, as inhibition of transcription in ER-stressed cells impairs viability. ATF4 induces genes responsible for the antioxidant response, amino acid metabolism, and apoptosis, including the C/EBP homologous protein (CHOP). CHOP, also known as growth arrest and DNA damage-inducible gene (GADD)153, was identified as a stress-induced negative regulator of other C/EBP-family proteins [6, 12, 118]. Subsequently, CHOP expression was found to be potently induced by ER-stress inducing agents. Mice deleted in Chop develop normally; however cells isolated from these mice are resistant to ER-stress induced cell death, implicating a requirement for CHOP in the apoptotic response to ER stress [163]. CHOP upregulates the expression of GADD34, which complexes with protein phosphatase 1 (PP1c) to target dephosphorylation of eIF2α, thus forming a negative feedback loop [96]. Phosphorylation of eIF2α with subsequent translation attenuation reduces synthesis of IκB with consequent activation of the transcription factor NFκB as part of the response to stress [60, 147].
Er Stress and Apoptosis
Sustained or massive ER stress leads to apoptosis. Several apoptosis mediators are implicated in ER stress associated cell death (Figure 2). Some of these mediators are activated by the UPR sensors, whereas others are related to calcium and redox homeostasis. Although studies implicate IRE1α in modulating ER stress induced apoptosis, it is unclear in which context and by what mechanism IRE1α mediates protection versus death. IRE1α activation recruits the adaptor protein TRAF2, with subsequent activation of apoptosis signal-regulating kinase 1 (ASK1) and JNK. JNK has several proapoptotic effects, including phosphorylation-induced activation of the proapoptotic Bim, and inactivation of the antiapoptotic Bcl2 proteins [75, 149]. ASK1 interacting protein 1 (AIP1) is also a key mediator of ER stress-induced ASK1 activation downstream of IRE1α [86]. Mice deficient in AIP1 are resistant to ER stress-induced JNK activation and apoptosis, while retaining oxidative stress induced JNK activation. Bak and Bax (proapoptotic Bcl2 family members), on the other hand, associate with IRE1α on the ER membrane and potentiate its RNase activity [47]. Mice deficient in both Bak and Bax are more sensitive to tunicamycin induced liver injury in spite of diminished apoptosis, presumably due to deficient IRE1α activity [47]. Mouse embryonic fibroblasts deficient in Bak and Bax are also resistant to tunicamycin or thapsigargin-induced cell death [143]. Bax inhibitor 1 (BI-1), an ER membrane protein, that inhibits apoptosis, is a negative regulator of IRE1α activation [82]. Mice deficient in BI-1 are more sensitive to ER-stress induced apoptosis, suggesting a proapoptotic role for IRE1α [18]. However, in ER-stressed cells, XBP1 splicing and protein expression decline with time [81]. This decline correlates with cell death, and reconstitution of IRE1α activity improves cell survival. In mice, caspase 12 activation can occur via IRE1α-TRAF2 induced recruitment and activation of procaspase 12 [153]. However, in humans the caspase 12 gene has acquired loss-of-function mutations, and functional protein is not synthesized [35]. eIF2α phosphorylation causes translation attenuation that is required to protect against apoptosis in response to ER stress. Though heightened sensitivity to ER stress-induced apoptosis is observed in Perk-/- and eIF2α Ser51Ala mutant cells, the further inhibition of new protein synthesis by cycloheximide treatment in these stressed cells improves survival, thus pointing toward the role of newly synthesized proteins in further stressing the ER and promoting cell death.
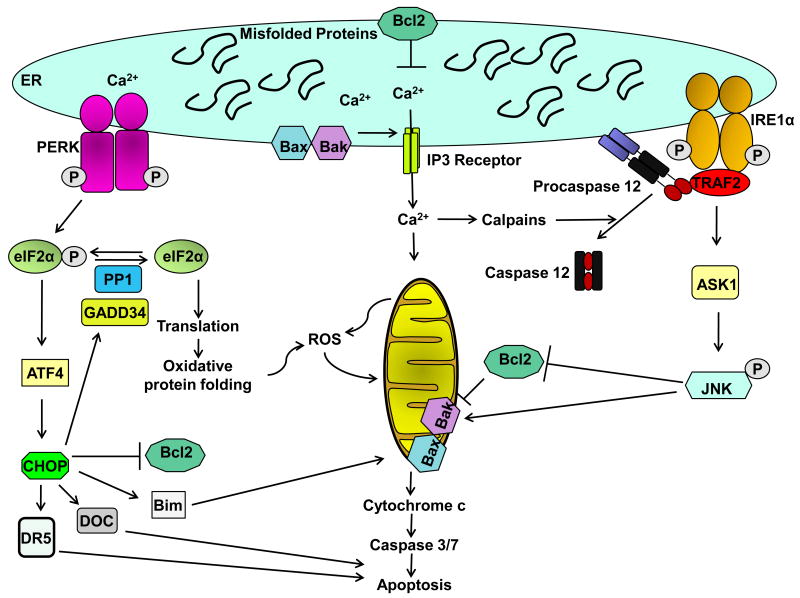
Figure 2. ER stress-induced apoptosis.
Sustained ER stress is associated with cell death. Cells that are dying upon ER stress demonstrate evidence of ongoing UPR, or a lack of resolution of the UPR. Some of the pathways that can lead to ER-stress induced apoptosis are depicted. The proapoptotic proteins Bax and Bak as well as the antiapoptotic protein Bcl-2 are localized on the ER membrane and regulate Ca2+ homeostasis. Calcium release from the ER can activate calpains, which may proteolytically activate caspase 12 to mediate apoptosis. The downstream effectors of caspase 12-induced apoptosis are not known, but presumably promote activation of terminal caspases. Calcium uptake by mitochondria leads to mitochondrial permeabilization and release of cytochrome C. CHOP can induce the expression of proapoptotic BH3-only protein Bim, the cell surface death receptor TRAIL receptor 2, other downstream of chop (DOC) mRNAs, and inhibit Bcl-2 transcription. Oxidative protein folding and mitochondrial dysfunction are associated with the accumulation of reactive oxygen species (ROS) with downstream oxidative cellular damage. JNK is activated by IRE1α via TRAF2. JNK can phosphorylate and activate proapoptotic Bcl-2 family proteins and inactivate antiapoptotic proteins.
Perturbations in ER calcium are also linked to apoptosis effectors. ER stress-inducing agents, in certain cell lines, led to sustained Ca2+ release from the ER, mitochondrial Ca2+ accumulation followed by mitochondrial permeabilization and release of apoptosis effectors from mitochondria into the cytosol [26]. The antiapoptotic protein Bcl-2 reduces resting free ER calcium and cell death when over expressed [37]. The proapoptotic proteins, Bak and Bax, also control ER calcium, as cells deficient in both have lower free ER calcium content, and reduced sensitivity to some selective death-inducing stimuli, such as hydrogen peroxide [98]. Bax Inhibitor-1 also regulates ER calcium in cell lines, promoting calcium release under acidic conditions with associated increase in cell death [66]. In other experiments, mice lacking BI-1 are sensitized to tunicamycin induced cell death [18]. Hepatocytes require mitochondrial permeabilization in order to activate terminal caspases and execute apoptosis, a process mediated by Bax and Bak. Membranes of mitochondria and ER associate via distinct junctions comprised of tethering proteins [68]. These junctions facilitate transfer of calcium and phospholipids, and may also be involved in apoptosis. However, as in other systems, the exact signaling pathways that mediate ER stress-induced apoptosis in stressed hepatocytes are not well defined.
The transcription factor CHOP/GADD153 is the most well characterized proapoptotic pathway that emanates from the stressed ER. CHOP transcription is primarily activated by ATF4 although ATF6α may contribute [33, 87]. CHOP deficient mice were protected from ER stress induced cell death in renal tubular epithelium upon challenge with tunicamycin [163]. Interestingly, renal epithelial regeneration was also impaired in the CHOP null mice, which may have been secondary to lower cell death or may be due to a direct effect of CHOP on renal epithelial regeneration. In murine models of diabetes CHOP deletion in pancreatic β cells protected against apoptosis, improved β cell survival and mitigated the severity of diabetes [102, 127]. CHOP is linked to the apoptosis machinery in several ways. CHOP transcriptionally enhances the expression of GADD34, with subsequent dephosphorylation of eIF2α [90]. Thus translation is resumed and nascent proteins can enter the ER to undergo oxidative protein folding with consequent generation of ROS. The entry of new client proteins prematurely into the ER, under conditions where ER stress has not been completely resolved, can generate reactive oxygen species (ROS) with deleterious consequences. Intriguingly, protein misfolding in the ER can lead to oxidative stress; and antioxidant treatment, Chop deletion, or translation attenuation can reduce oxidative stress and preserve ER function [3, 89, 127]. These findings suggest an intimate relationship between ER protein misfolding and ROS production. Another mechanism for ROS production is based on intracellular calcium fluxes. Protein folding in the ER can lead to release of intracellular Ca2+ from the ER via inositol 1,4,5-triphosphate receptor (IP3R) channels leading to mitochondrial Ca2+ uptake, which in turn promotes ROS production and apoptosis via multiple effects on mitochondria [26, 107].
A number of CHOP downstream target genes have been proposed to lead to apoptosis, their overall contribution in different conditions and diverse cell types remains only partially studied. CHOP can transcriptionally up regulate the expression of the death receptor TRAIL receptor 2 (also known as death receptor 5, DR5) in human cancer cell lines [148]. How enhanced expression of a cell surface death receptor sensitizes cells to ER-stress induced apoptosis is not known. Another Bcl-2 family target is the proapoptotic BH3-only protein Bim. CHOP can transcriptionally induce the expression of Bim [111]. Importantly, over expression of CHOP does not result in apoptosis, though it does sensitize cells to apoptosis [91]. Cellular glutathione depletion, generation of reactive oxygen species and decreased expression of the antiapoptotic protein Bcl-2 were associated with enhanced sensitivity to cell death in CHOP over expressing cells. Another proapoptotic protein TRB3 is transcriptionally induced in tunicamycin treated cells and is CHOP-dependent for its expression [99]. Several other ER stress-induced genes named “downstream of CHOP (DOC)” are induced by CHOP-C/EBP β heterodimers, one of these has been identified as carbonic anhydrase VI that may acidify the cytoplasm, a feature associated with apoptosis [141]. In macrophages, CHOP mediates apoptosis in an ER oxidase 1 alpha (ERO1α)-dependent manner leading to Ca2+ release from the ER [77].
Er Stress and Inflammation
The UPR has been linked to several inflammatory response pathways in many cellular models and diseases, among these are the activation of NFκB, JNK, ROS, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). The sustained activation of NFκB can occur due to inhibition of synthesis of new inhibitor of NFκB (IκB), as it has a short half life [147]. In addition NFκB and JNK can be also activated by the IRE1α branch of the UPR [53, 135]. Both of these pathways are implicated in free cholesterol-induced macrophage activation and production of TNF-α and IL-6 [79]. In endothelial cells oxidized lipids activate the UPR and inflammatory gene expression is dependent on ATF4 and XBP1 [40]. Furthermore, the immune response is dependent on XBP1 signaling. In Caenorhabditis elegans, activation of the innate immune response leads to the UPR and requires XBP1 for resolution and survival [115]. In mice XBP1 is required for differentiation of B lymphocytes to antibody secreting plasma cell [114], and XBP1 deletion in intestinal epithelia leads to spontaneous enteritis [63]. XBP1 signaling maintains Paneth cell function and mucosal responses to bacterial and chemical challenge. Furthermore, single nucleotide polymorphisms in the XBP1 gene are associated with human inflammatory bowel disease. Thus, several lines of crosstalk exist between UPR mediators and inflammatory responses in different organisms, tissues and diseases.
Er Stress in Liver Disease
Hepatocytes perform a myriad of metabolic functions, including plasma protein synthesis and secretion, lipoprotein and very low density lipoprotein (VLDL) assembly and secretion, cholesterol biosynthesis, and xenobiotic metabolism, and thus are enriched in both smooth and rough ER. These metabolic functions and their compartmentalization in the ER have been long recognized; however it is not known how ER homeostasis and signaling through the UPR sensors impacts these diverse ER functions. ATF6α is a negative regulator of gluconeogenesis under conditions of acute ER stress by its inhibitory interaction with the transcription factor CREB regulated transcription coactivator 2 (CRTC2) [142]. The ER membrane-localized hepatocyte-specific bZIP-transcription factor CREBH (cyclic-AMP-responsive-element-binding protein H) is cleaved by RIP upon ER stress, similarly to ATF6α [80, 161]. Hepatocyte nuclear factor 4α (HNF4α), a nuclear hormone receptor, essential for differentiated hepatocyte function, regulates CrebH expression in mouse liver, raising the possibility that HNF4α via CrebH regulates the acute phase response (APR) [85]. CrebH induces expression APR genes upon ER stress and is required for the expression of serum amyloid P-component (SAP), and C-reactive protein (CRP), as well as the ERAD component Herp. Furthermore, it heterodimerizes with ATF6α, forming a more potent transcription factor with regard to SAP and CRP transcription, underscoring the link between the UPR and APR upon ER stress. CREBH is also regulated by nutrient availability and regulates hepatic gluconeogenesis independent of the UPR [74]. Hepcidin, an iron regulatory hormone and an APR protein, is also induced by CREBH upon ER stress [137]. In addition to CREBH in the liver, ER dysfunction is linked to inflammatory responses in other tissues, as discussed earlier. The role of ER stress-induced inflammation in liver injury is not yet fully determined.
Emerging is the central role of the ER in regulation of lipid metabolism in hepatocytes. Recent studies using genetic or dietary models of insulin resistance and fatty liver have demonstrated a key interconnectedness between hepatic steatosis and ER stress (vide infra), as well as the physiologic role of the UPR sensors in lipid homeostasis. Basal IRE1α activation in mouse liver, as well as tunicamycin-challenged activation, was subject to regulation by the circadian rhythm [24]. The basal IRE1α activation rhythm was linked to the circadian regulation of lipid homeostasis. Several distinct enzymatic lipogenic pathways are compartmentalized in the ER. These include fatty acid elongation machinery, cholesterol biosynthesis, complex lipid biosynthesis, and assembly of VLDL particles. These processes are transcriptionally regulated by the transcription factors SREBP (sterol regulatory element binding protein)-1a, -1c and -2 [14]. Translation attenuation in ER stressed cells decreases levels of the protein Insig-1, thus releasing the cholesterol-sensing adaptor protein SCAP (SREBP cleavage activating protein) and SREBP (-1a and -2) from inhibitory binding. This leads to the translocation of SREBPs to the Golgi, followed by regulated intramembrane proteolysis by S1P, and S2P to generate the active transcription factors [72]. There is some evidence from in vitro studies that ER cholesterol accumulation during ER stress also contributes to the activation SREBP2 [22]. Over expression of the ER chaperone BiP in obese ob/ob mice led to amelioration of ER stress in the liver [61]. This was associated with inhibition of SREBP-1c activation, improved insulin sensitivity, and reduced steatosis. ER stress is also linked to lipid homeostasis [119]. Tunicamycin treatment leads to down regulation of transcription factors and pathways involved in lipid synthesis. In the absence of any of the individual adaptors of the UPR, steatosis develops in the tunicamycin-stressed livers, and is associated with ongoing ER stress, prolonged upregulation of CHOP expression, and inhibition of metabolic master regulators. Liver specific deletion of Xbp1 reduces serum lipids in mice and decreases de novo lipogenesis in the liver [71]. Future studies should elucidate how ER stress impacts lipid assembly and trafficking from the ER and how chronic physiological ER stress leads to fatty liver.
ER stress has been observed in a variety of liver diseases (Key Message Box 5). Some of these observations offer mechanistic insights, and present potential therapeutic targets. Whereas other associations indicate that new hypotheses are required to test the role of the UPR in liver disease. Stimuli that injure the liver can activate multiple intracellular stress responses, such as direct lysosomal damage, mitochondrial permeabilization, oxidative stress and inflammatory responses. An association of these responses with ER dysfunction is being increasingly recognized. How these intracellular stress responses and inflammatory responses interact, cooperatively or competitively, in the pathogenesis of liver injury is not well defined. In one model, it is possible that these pathways culminate on apoptotic cascades, though it is more likely that their interactions are more complex. What is known of the role of the UPR in specific liver diseases is discussed in the forthcoming sections (Figure 3).
Key Message Box 5.
ER stress is observed in many liver diseases. The activation of the UPR is linked to hepatic insulin resistance in obesity and fatty liver. Chronic viral hepatitis B and C are both associated with UPR activation. Other diseases include alcohol-induced liver injury, hyperhomocysteinemia, ischemia-reperfusion injury, acetaminophen and other acute hepatotoxins.
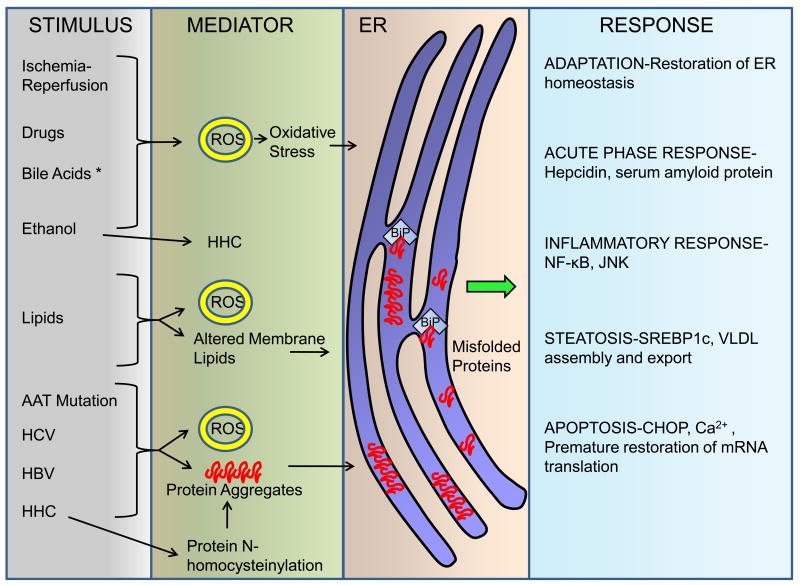
Figure 3. ER dysfunction in liver disease.
Perturbations in ER homeostasis leading to dysfunction and activation of some of the UPR sensors occur in several liver diseases. Depicted here are the stimuli that can lead to ER dysfunction. The mechanism for some of them includes generation of reactive oxygen species (ROS) leading to oxidative stress, altered membrane lipid composition, hyperhomocysteinemia (HHC) with subsequent protein N-homocysteinylation, formation of protein aggregates, or in some instances are unknown (depicted with an asterisk *). It is likely that additional mediators exist, that are yet to be characterized. In hepatocytes, ER dysfunction leads to many different responses. The UPR is activated to restore ER homeostasis. Steatosis occurs in the liver following acute ER stress, mediated by the lipid-regulatory transcription factors sterol regulatory element binding protein (SREBP)-1c and 2, as well as dysregulation of VLDL assembly and secretion. Inflammatory response cascades are activated in both acute and chronic liver diseases. In alpha-1 antitrypsin (AAT) deficiency the activation of nuclear factor-κB (NF-κB) occurs downstream of perturbations of the ER. Chronic viral hepatitis (hepatitis C virus HCV, hepatitis B virus HBV) is also associated with ER dysfunction. Sustained ER stress leads to apoptosis which may be mediated by C/EBP homologous protein (CHOP), altered Ca2+ homeostasis, and premature resumption of mRNA translation.
Nonalcoholic Fatty Liver Disease
The role of perturbations in the ER in NAFLD has become a subject of considerable interest in recent years based on studies in rodent models and humans. Activation of the UPR has been observed in the liver in several dietary and genetic models of NAFLD [103]. The activation of the stress kinase JNK was observed in liver, fat and muscle tissue of obese mice, and mice deleted in the Jnk1 gene were protected from the development of obesity and insulin resistance [50]. Following these observations JNK activation was linked to ER stress [103]. The UPR was activated in liver tissue from obese mice. Enhanced ER stress was linked to JNK activation, presumably IRE1α-dependent, and insulin resistance was shown to be a consequence of JNK-mediated inhibitory phosphorylation of serine residue 307 of insulin receptor substrate 1 (IRS-1). In liver and subcutaneous adipose tissue samples from human subjects undergoing bariatric surgery, UPR markers were elevated in the obese state and declined following weight loss; both BiP expression and eIF2α phosphorylation in the liver correlated with weight loss [41]. In another study liver samples from patients with NAFLD and NASH demonstrated increased eIF2α phosphorylation and BiP expression, though other UPR markers were not increased [110]. Several important mechanistic questions emerge from these observations. These include, though are not limited to, what is the mechanism by which ER homeostasis is disrupted?; is ER stress solely an adaptive response?; which of the UPR mediators are key players in the survival or death of hepatocytes?; which UPR pathway is linked with which specific cellular response? Using genetic rodent models with liver specific or whole body deletions in specific UPR mediators some of these questions have been addressed.
The association of ER stress signaling and hepatic steatosis has been proven through genetic modulation of the eIF2α phosphorylation pathway, the IRE1α/XBP1 pathway, and the ER protein translocation pathway. Enforced expression of GADD 34 in the liver confers a metabolic advantage to the whole mouse when challenged with a high fat diet along with systemic oxidative stress [101]. These mice demonstrated decreased steatosis. The dephosphorylation of eIF2α, in this model, was associated with a decrease in ER stress activated genes upon high fat feeding. This was associated with decreased expression of PPARγ in comparison with wildtype mice, as well as nuclear levels of C/EBPβ and C/EBPα. eIF2α phosphorylation regulates the use of alternate AUG start codons that can modify the biological activity of the translated protein. The mRNAs that encode C/EBP-α and -β harbor alternative AUG initiation codons. The product from the upstream AUG encodes a liver-specific transcriptional activator protein (LAP), whereas the product from the downstream AUG codon is a liver-specific transcriptional inhibitor protein (LIP) [27]. When eIF2α is phosphorylated, initiation at the AUG codon encoding LAP is favored [15]. Impaired nuclear translocation of sXBP1 in the leptin deficient ob/ob mouse due to loss of heterodimerization between the regulatory subunits of phosphatidyl inositol 3-kinase (PI3K) and sXBP1, which facilitates its nuclear translocation, resulted in a failure to induce chaperones and resolve ER stress in the liver [105]. Furthermore, mice deficient in the p85 regulatory subunit of P13K in a liver-specific manner displayed blunted activation of IRE1α and ATF6α [146], pointing toward a multilevel interaction between PI3K and the UPR in the liver. Mice mutated for the ER secretory pathway protein Sec61alpha1 demonstrated defects in the liver and pancreatic β-cells [83]. These mice developed hepatic steatosis, progressive liver injury with fibrosis and eventual cirrhosis upon challenge with a high fat diet. Correction of the β-cell defect by transgenic restoration of the Sec61alpha1 gene in the pancreas did not correct the hepatic defect. Sec61α1 null mice fed regular chow, thus unchallenged by a high fat diet, also demonstrated ER distension in hepatocytes and enhanced expression of BiP and CHOP mRNA in liver, indicative of ongoing ER stress. BI-1 expression in the ER was reduced in genetic and dietary mouse models of NAFLD. BI-1 over expression in hepatocytes of obese leptin deficient ob/ob or leptin receptor deficient db/db mice restored insulin sensitivity by inhibiting gluconeogenesis [4]. However, BI-1 over expression also inhibited IRE1α activity and worsened hepatic steatosis and lowered serum cholesterol and triglycerides. Thus genetic obesity is associated with an impaired UPR and persistent ER stress in the liver, and impairment in even any single branch of the UPR in the setting of a metabolic challenge with high fat feeding worsens hepatic steatosis and liver injury.
Apoptosis and inflammation are key features of progressive NASH, and are both linked to the UPR as well. Apoptosis can be induced by free fatty acids, and this is a possible mechanism in the pathogenesis of NASH [88]. The exact mechanisms by which free fatty acids induce apoptosis of steatotic hepatocytes are not fully defined. Long chain free fatty acids can activate the UPR in several cell types, including hepatocytes [144]. Palmitic acid induces expression of CHOP in hepatocyte cell lines, and cells deficient in CHOP expression are protected from palmitate-induced apoptosis [17, 108]. Expression of the proapoptotic protein PUMA requires CHOP [17]. Metformin inhibits palmitic acid-induced ER stress and apoptosis [65]. Individual fatty acids may be important in their ability to induce the UPR or mitigate it, as mice compromised in their ability to synthesize monounsaturated fatty acids due to a deletion of stearoyl-CoA desaturase-1 (Scd1) exhibit activation of the UPR in their livers due to a deficiency of mono-unsaturated fatty acids [36]. Free fatty acid composition of triacylglycerol (TAG), the predominant lipid class, and diacylglycerol (DAG) in patients with NAFLD and NASH, demonstrated a trend for higher palmitic acid and oleic acid with a concomitant decrease in polyunsaturated fatty acids [109]. Hepatic free cholesterol increased progressively in patients, from NAFLD to NASH, however cholesterol esters were unchanged. Phosphatidylcholine was depleted as well. CD154, a platelet-derived inflammatory mediator, promotes XBP1 splicing, presumably enhancing ER stress adaptation, and reduces cell death upon oleic acid challenge in vitro (Villeneuve et al. Hepatology, accepted manuscript online). Furthermore, upon exposure to a high olive oil diet, CD154 null mice demonstrated decreased VLDL secretion and enhanced steatosis. This suggests the intriguing possibility that an inflammatory mediator promotes adaptation to ER stress, thus abrogating hepatic steatosis. Thus, patients with NAFLD and NASH demonstrate a constellation of findings, including apoptosis, inflammation, UPR activation, and altered lipid composition. Several mechanistic questions are generated from these observations. What is the role of protein misfolding in ER stress-associated steatosis? Does UPR occur before the onset of steatosis, and in fact is steatosis a consequence of the UPR? What is the role of each lipid class? Is free fatty acid induced apoptosis related to unresolved UPR? What is the contribution of cholesterol?
Protein Conformational Diseases
Mutations that lead to protein misfolding can result in the aggregation of abnormal proteins leading to their accumulation within the ER lumen. Some monogenic inherited dominant disorders result from mutations in single alleles that cause protein misfolding. The misfolded and/or aggregated protein can have a dominant-negative effect due to gain of a toxic function. In contrast, autosomal recessive genetic diseases frequently result from a loss of protein function. Alpha-1 antitrypsin deficiency is a protein conformational disorder affecting the liver. In alpha-1 antitrypsin (AAT) deficiency proteins encoded by mutated alleles are unable to undergo proper folding, and accumulate in the ER of hepatocytes. The Z mutant allele PiZ occurs most frequently in patients with liver disease due to AAT deficiency. The mutant protein is aggregation prone, accumulates in the ER of hepatocytes, and leads to progressive liver injury, cirrhosis and hepatocellular carcinoma. The accumulation of mutant protein in hepatocytes does not activate the UPR, and the ER remains sensitive to other UPR activating agents, thus the failure of UPR activation may be protective in hepatocytes. However, accumulation of PiZZ mutant protein in peripheral blood monocytes from human subjects with AAT deficiency activates the UPR, and is associated with enhanced production of inflammatory cytokines. This may play a role in the pathogenesis of both lung and liver disease [16]. In a cell culture system the expression of an ER chaperone SepS1 reduced PiZZ-induced ER stress [64]. Protein aggregates in the liver are removed by autophagy in AAT deficiency, and perhaps inhibition of autophagy might activate the UPR due to even greater accumulation of aggregated proteins. Alternatively, would rapamycin treatment to activate autophagy have a beneficial effect in individuals with PiZZ AAT. This was recently demonstrated in cell culture and mouse models using carbamazepine to enhance autophagy [48]. The activation of NF-κB with associated inflammatory gene regulation occurs due to ER accumulation of mutant proteins, and leads to the characteristic chronic inflammatory liver disease and confers cancer risk. Thus in AAT deficiency the ER activates inflammatory signals via NF-κB, without overt UPR activation, and it remains to be seen if increasing functional capacity of the ER in this condition, would mitigate inflammation and liver disease. In addition, other liver protein conformational diseases that involve mutations leading to protein misfolding such as hereditary hemochromatosis and progressive familiar intrahepatic cholestasis type II (PFIC II), are characterized by the accumulation of mutant proteins in the ER with subsequent loss of protein function [69, 139]. Over expression of the mutant HFE C282Y protein in cell culture activated the UPR. However, the exact role of the UPR in disease pathogenesis is not defined. These diseases may also benefit from therapies that improve protein folding and secretion.
Cholestasis
Toxic hydrophobic bile acids are retained in the liver in cholestasis. One such bile acid sodium deoxycholate induces the expression of the UPR genes BiP and CHOP in vitro [8]. Hepatocytes from CHOP deficient mice exhibit reduced cell death when treated with a toxic bile acid glycochenodeoxycholic acid (GCDCA) [130]. Utilizing the bile-duct-ligated mouse model of cholestasis it was demonstrated that CHOP protected hepatocytes from cell death, and CHOP null mice had less severe liver injury and fibrosis [130]. In a genetic model of intrahepatic cholestasis, the accumulation of bile acids in the liver was associated with ER stress [11]. Furthermore, the transgenic expression of the mutant Z allele of alpha-1 antitrypsin in the bile duct-ligated mice increased liver injury and fibrosis [92]. UPR, as assessed by mRNA expression of CHOP and BiP, was comparable to the transgenic non-ligated control group. Importantly, this suggests that the accumulation of a misfolded protein in the liver can sensitize to other injurious stimuli, possibly indicating at genetic alterations that disrupt protein folding in the ER, and thus may contribute to liver injury.
Chronic Viral Hepatitis
Hepatitis C virus (HCV) replication in infected host cells is dependent on several viral proteins that are folded in the ER, and synthesized in ribonucleoprotein complexes in association with the ER [54]. Over expression of the nonstructural 4B (NS4B) protein in hepatocyte cell lines activated the UPR and induced ROS [78]. In a yeast two-hybrid assay NS4B interacted with both ATF6β and ATF6α [132]. In an HCV replicon model ER stress was induced by viral replication and further sensitized cells to oxidative stress [21]. HCV envelope proteins, E1 and E2, when over expressed, form disulfide aggregates, induce ER stress and the expression of ATF4 and CHOP as well as XBP1 splicing [19]. Expression of HCV core protein causes ER stress, ER calcium depletion and apoptosis [7]. In cell culture systems it has also been shown that E2 protein can act as a pseudosubstrate for PERK, thus preventing eIF2α phosphorylation and subsequent translation attenuation in infected cells [106]. In liver biopsy samples from patients with chronic hepatitis C clusters of hepatocytes with abnormally dilated ER have been observed, suggesting ER stress[1]. The presence of ground glass hepatocytes is a characteristic feature of chronic hepatitis B virus (HBV) infection. These ground glass inclusions consist of accumulated surface antigen within the ER lumen though it is not known if this accumulation plays a pathologic role [129]. Mutations can be detected in the ER accumulated surface antigen leading to the possibility that the mutated proteins are entrapped in the ER due to their inability to undergo proper folding. HBV proteins too utilize the ER protein folding machinery and the cellular secretory pathway [2]. The UPR can be activated by hepatitis B x protein (HBx) in cell culture system over expressing the HBx protein [76]. Thus, HCV viral replication is intimately linked to the ER [93]. HCV viral proteins both activate and subvert the ER stress response, how this occurs temporally during the course of a chronic infection, and the role it plays in promoting or ameliorating apoptosis or acute stress response of infected hepatocytes is not known. Steatosis, a common feature of chronic HCV infection could also be related to ER stress. Similarly, the pathogenic role of the UPR in hepatitis B infection, if any, is not well defined.
Hyperhomocysteinemia
Hyperhomocysteinemia is caused by mutations in the enzymes involved in homocyteine metabolism or nutritional deficiencies of B vitamins that function as cofactors for these enzymes [116, 133]. Elevated homocysteine levels induce ER stress in many cell types, including hepatocytes and vascular endothelial cell [145, 160]. Activation of the UPR in this disorder is associated with activation of the lipogenic transcription factor SREBP-1, and increased cholesterol and triglyceride accumulation in the liver. The later occurs due to enhanced biosynthesis, and is not due to decreased VLDL export from the liver. Cells loaded with homocysteine in vitro recapitulate the cholesterol accumulation and enhanced secretion seen in vivo [97]. Along with activation of the UPR, hyperhomocysteinemia also leads to oxidative injury in the liver with accumulation of protein carbonyls, MDA and 4-HNE [116]. Homocysteine is elevated in the ethanol-fed mouse model of liver injury. By administering betaine the metabolism of homocysteine to methionine is enhanced and reduces ER stress and liver injury. Liver and plasma N-homocysteinylated proteins are elevated in mice with hyperhomocysteinemia [57]. The accumulation of N-homocysteinylated proteins in the ER could prevent their folding, and thus activate the UPR. Thus, in hyperhomocysteinemia, the associated steatosis occurs secondary to the activation of the UPR; and this in turn can be induced by accumulation of N-homocysteinylated proteins and oxidative stress.
Alcohol-Induced Liver Injury
Ethanol-fed murine models have been utilized to understand the molecular mediators and cellular responses that mediate alcohol-induced liver injury. With respect to the ER, apart from ethanol metabolism via cytochrome P450 2E1 (Cyp2E1), ethanol itself induces this enzyme, favoring the formation of reactive oxygen species and leading to a state of oxidative stress. Gene expression profiling of ethanol fed mice demonstrated enhanced mRNA abundance of ER chaperones, BiP and Grp 94, CHOP as well as caspase 12, as early as 2 weeks and persisted up to 6 weeks of ethanol feeding [58]. On a protein level, BiP was minimally induced, CHOP induction was significant, and procaspase 12 was processed as well. Homocysteine levels were elevated in ethanol-fed mice, and treating with betaine lowered homocysteine levels and ameliorated the UPR. Utilizing CHOP null mice, ethanol feeding demonstrated that hepatocyte apoptosis in this model was CHOP-dependent [59]. Both CHOP null and wildtype mice developed comparable levels of steatosis and ER stress. This suggests a role for CHOP-mediated hepatocyte apoptosis in ethanol-induced liver injury. In addition in micropigs fed alcohol, liver steatosis and apoptosis were associated with increased mRNA levels of CYP2E1, GRP79, SREBP-1c, increased protein levels of CYP2E1, GRP78, nuclear SREBP-1c, and activated caspase 12 [32]. While many different pathways have been implicated in ethanol-induced steatosis [159], the occurrence of ER stress in ethanol-fed mice suggests that activation of the UPR may also mediate this process.
Ischemia Reperfusion
Ischemia-reperfusion (IR) injury in the liver activates many cellular cascades, including inflammatory response signaling, oxidative stress, increased intracellular Ca2+, apoptotic cascades, calpains and ER stress [120]. Using hemorrhagic shock followed by reperfusion in rats, XBP1 splicing was detected early (at 40 minutes) following reperfusion and persisted through 18 hours following reperfusion, indicative of ongoing ER stress [30]. In human liver samples from ischemic and reperfused livers biphasic activation of UPR pathways was observed [31]. IRE1α was activated during the ischemic phase, and upon reperfusion this was further increased. PERK and eIF2α phosphorylation decreased with ischemia within hepatocytes and was enhanced with reperfusion primarily in sinusoidal endothelial cells. Though the exact role of BI-1 in ER stress-induced apoptosis is unclear, BI-1 is induced by ischemia-reperfusion. Mice lacking BI-1 developed more severe liver injury, XBP1 splicing, ATF6α cleavage and CHOP expression, though BiP induction was similar [5]. Thus, the IRE1α branch of the UPR is activated by IR-associated ER dysfunction based on these studies, though its role in ER dysfunction in ischemia-reperfusion injury is not fully defined, IRE1α could potentially play a role in the inflammatory response as well as cell death.
Acute Toxins
There is evidence that the UPR is activated by acute insults to the liver. Toxins such as such as N,N-dimethylformamide and carbon tetrachloride induce ER stress and activate the UPR [67]. Acetaminophen depletes ER glutathione content inducing redox stress, eIF2α phosphorylation and JNK phosphorylation in mice [95]. In acute or chronic iron loading in rats, the UPR is activated in the liver and heart [84]. Using a reporter mouse, acute heavy metal toxicity was shown to reversibly activate the UPR in liver and kidney, the target organs for heavy metal toxicity [49]. Clinically used drugs such as Bortezomib, also activate the UPR [28]. In the murine liver this drug induces ER stress and also leads to hepatic steatosis [119]. Thus, ER stress maybe the unrecognized mechanism underlying steatosis that is common to the toxicity of several drugs.
Heptocellular Carcinoma
The UPR is activated in cancers arising from diverse tissues, and is implicated in cancer biology at multiple steps. Cancers are characterized by high proliferative rates that are dependent on increased protein synthesis. Activation of p38 mitogen activated protein kinase is associated with PERK-eIF2α mediated translational arrest, leading to growth arrest and dormancy promoting resistance to conventional chemotherapy [112]. The UPR also promotes adaptation to hypoxia, thus promoting survival of cancer cells under hypoxic conditions [34]. XBP1 transcription and splicing are induced by hypoxia and are essential for tumor formation [117]. BiP/GRP 78 plays a role in carcinogenesis [39]. With regard to hepatocellular carcinoma, BiP was found to be constitutively over expressed in tumor samples from patients in comparison with surrounding non-tumorous tissue [125]. BiP expression correlated with XBP1 splicing, and both BiP and nuclear ATF6α levels correlated with the histologic grade of the tumors. Manipulation of the UPR has been studied as primary or adjuvant chemotherapy as well. Indeed, several chemotherapeutic agents, e.g., Bortezomib, lead to ER stress-induced apoptosis [38]. Liver cancer-derived cell lines are sensitive to tunicamycin-induced apoptosis, and this may be of clinical relevance as drugs that selectively induce ER stress become available [20].
Therapeutic Interventions
Several ER modulating therapeutic interventions are possible, such as, improving protein folding by the use of chemical chaperones, or the use of antioxidants to ameliorate ROS production from oxidative protein folding. This too, is an area of intense research. Some preliminary studies support the use of ER stress modulating agents. In mice undergoing ischemia-reperfusion injury the chemical chaperone sodium 4-phenylbutyrate (4PBA) ameliorated ER stress and associated caspase 12 activation and liver injury [138]. It also improved survival. The use of 4PBA and taurine-conjugated ursodeoxycholic acid in the ob/ob mouse improved insulin sensitivity, and ameliorated the UPR [104]. This was associated with reversal of fatty liver and reduction in transaminases. These chemical chaperones activate multiple cellular pathways and there is no direct evidence that improved protein folding is the mechanism for the observed improvement in fatty liver. Utilizing ER stress-activated indicator (ERAI) transgenic mice it was demonstrated that pioglitazone treatment reduced ER stress followed by improvements in insulin sensitivity and hepatic steatosis [158]. The liver is being explored as a therapeutic site for gene therapy, utilizing hepatocytes for the synthesis and secretion of proteins, most of which undergo oxidative folding in the ER with the potential for generation of excess ROS. Over expression of an aggregation prone mutant of coagulation factor VIII led to activation of the UPR and steatosis [119]. On the other hand, even over expressed wild type VIII, led to oxidative stress in the liver with associated protein misfolding, UPR activation and apoptosis [89]. Furthermore, the use of antioxidants ameliorated oxidative stress and the UPR along with improvement in protein secretion. As CHOP deletion is protective in models of diabetes and protein over expression, partially by reduction of ROS production and preventing apoptosis of stressed cells, CHOP antagonism is a rational target for drug therapy as well [127]. Other interventions that prevent apoptosis emanating from the stressed ER may be of therapeutic potential, by perpetuating the adaptive arm of the UPR. Improved protein folding would be of benefit in disorders of malfolded proteins such as alpha-1 antitrypsin deficiency. Thus agents that ameliorate ER stress by promoting adaptive UPR signaling or inhibiting ER stress-induced apoptosis offer a therapeutic opportunity.
Conclusions
The UPR is a conserved signaling response limited to IRE1α in yeast, and expanded to include the ATF6α and PERK pathways in higher organisms. The UPR is activated in many acute and chronic liver diseases. Steatosis, a common feature of many liver disorders, may be due to the regulation of lipogenic transcription factors downstream of the UPR. The ER is also a key mediator of the acute stress response, via the transcription factor CREBH. The use of liver-specific genetic rodent models with deletions or over expression of specific components of the UPR has shown that in the absence of the function of any one UPR sensor, ER stress is prolonged, and sensitizes to cell death. Furthermore, improvements in metabolic homeostasis and fatty liver observed with the use of chemical chaperones which reduced ER stress confirms the pathogenic role of the UPR in nonalcoholic fatty liver disease.
Concomitant with the expanding role of the ER in homeostasis and disease, one must look at definitions and paradigms as well. Ideally, the UPR would be assessed by a direct measure of accumulated unfolded proteins. However, in the absence of such a technique the UPR should be assessed by activation of its proximal sensors; IRE1α activation by XBP1 splicing, PERK activation by its own phosphorylation along with eIF2α phosphorylation, and finally ATF6α by its nuclear translocation. When all three are activated, the canonical UPR has occurred. However, what defines ER stress, and what the signatures of ER stress are is not well defined at the moment. What is known is that there are perturbations in ER structure and homeostasis in several disorders. These are associated with the accumulation of chaperones alone, or the isolated activation of one branch of the UPR, or two branches of the UPR, none of which should equal the UPR, and should simply be signaling pathways activated by a perturbed ER. Lastly, a suggestion that in the absence of a consensus definition of the term ER stress and its signature, the specific signaling pathways and processes that are activated by the perturbed ER be themselves used in place of the term ER stress.
Acknowledgments
We would like to thank Ms. Janet Mitchell for her excellent secretarial support and help in preparing this manuscript. We are also thankful to members of the Kaufman laboratory who reviewed this manuscript and provided valuable input.
Portions of this work were supported by NIH grants DK042394, HL052173 and HL057346 (R.J.K.). Portions of this work were supported by NIH grants P30 DK084567 and T32 DK07198 (H.M.).
Abbreviations
- ATF4
Activating transcription factor-4
- ATF6α
Activating transcription factor-6α
- ATF6β
Activating transcription factor-6β
- AMP
Adenosine monophosphate
- ALT
Alanine aminotransferase
- ASK1
Apoptosis signal regulated kinase 1
- BI-1
Bax inhibitor-1
- CHOP
C/EBP homologues protein
- CREBH
Cyclic-AMP responsive element binding protein H
- ER
Endoplasmic reticulum
- ERAD
ER associated degradation
- ERSE
ER stress response element
- eIF2α
Eukaryotic initiation factor 2 alpha
- GRP78
Glucose regulated protein 78
- BiP
Binding immunoglobulin protein
- GRP94
Glucose regulated protein 94
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- 4HNE
4-Hydroxynonenal
- IP3R
Inositol 1,4,5-triphosphate receptor
- IRE1α
Inositol requiring 1α
- IL-6
Interleukin-6
- JNK
C-jun N-terminal kinase
- MDA
Malondialdehyde
- MTTP
Microsomal triglyceride transfer protein
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- PFIC II
Progressive familiar intrahepatic cholestasis type II
- PERK
Protein kinase RNA (PKR)-like ER kinase
- ROS
Reactive oxygen species
- RIP
Regulated intramembrane proteolysis
- RIDD
Regulated IRE1-dependenet decay
- S1P
Site-1 protease
- S2P
Site-2 protease
- SREBP
Sterol regulatory element binding protein
- sXBP1
Spliced X-box binding protein 1
- TNF-α
Tumor necrosis factor alpha
- TRAF2
Tumor necrosis factor receptor-associated factor-2
- XBP1
X-box binding protein 1
- UPR
Unfolded protein response
- VLDL
Very low density lipoprotein
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest with respect to the information presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asselah T, Bieche I, Mansouri A, Laurendeau I, Cazals-Hatem D, Feldmann G, et al. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J Pathol. 2010;221(3):264–274. doi: 10.1002/path.2703. [DOI] [PubMed] [Google Scholar]
- 2.Awe K, Lambert C, Prange R. Mammalian BiP controls posttranslational ER translocation of the hepatitis B virus large envelope protein. FEBS Lett. 2008;582(21-22):3179–3184. doi: 10.1016/j.febslet.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, et al. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailly-Maitre B, Belgardt BF, Jordan SD, Coornaert B, von Freyend MJ, Kleinridders A, et al. Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem. 2010;285(9):6198–6207. doi: 10.1074/jbc.M109.056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, et al. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006;103(8):2809–2814. doi: 10.1073/pnas.0506854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batchvarova N, Wang XZ, Ron D. Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153) EMBO J. 1995;14(19):4654–4661. doi: 10.1002/j.1460-2075.1995.tb00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benali-Furet NL, Chami M, Houel L, De Giorgi F, Vernejoul F, Lagorce D, et al. Hepatitis C virus core triggers apoptosis in liver cells by inducing ER stress and ER calcium depletion. Oncogene. 2005;24(31):4921–4933. doi: 10.1038/sj.onc.1208673. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein H, Payne CM, Bernstein C, Schneider J, Beard SE, Crowley CL. Activation of the promoters of genes associated with DNA damage, oxidative stress, ER stress and protein malfolding by the bile salt, deoxycholate. Toxicol Lett. 1999;108(1):37–46. doi: 10.1016/s0378-4274(99)00113-7. [DOI] [PubMed] [Google Scholar]
- 9.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, et al. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107(5):585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2(6):326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 11.Bochkis IM, Rubins NE, White P, Furth EE, Friedman JR, Kaestner KH. Hepatocyte-specific ablation of Foxa2 alters bile acid homeostasis and results in endoplasmic reticulum stress. Nat Med. 2008;14(8):828–836. doi: 10.1038/nm.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasier AR, Ron D, Tate JE, Habener JF. A family of constitutive C/EBP-like DNA binding proteins attenuate the IL-1 alpha induced, NF kappa B mediated trans-activation of the angiotensinogen gene acute-phase response element. EMBO J. 1990;9(12):3933–3944. doi: 10.1002/j.1460-2075.1990.tb07614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A. 2000;97(23):12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 15.Calkhoven CF, Muller C, Leutz A. Translational control of C/EBPalpha and C/EBPbeta isoform expression. Genes Dev. 2000;14(15):1920–1932. [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll TP, Greene CM, O'Connor CA, Nolan AM, O'Neill SJ, McElvaney NG. Evidence for unfolded protein response activation in monocytes from individuals with alpha-1 antitrypsin deficiency. J Immunol. 2010;184(8):4538–4546. doi: 10.4049/jimmunol.0802864. [DOI] [PubMed] [Google Scholar]
- 17.Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, Gores GJ. CHOP and AP-1 Cooperatively Mediate PUMA Expression During Lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2010 doi: 10.1152/ajpgi.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chae HJ, Kim HR, Xu C, Bailly-Maitre B, Krajewska M, Krajewski S, et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15(3):355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Chan SW, Egan PA. Hepatitis C virus envelope proteins regulate CHOP via induction of the unfolded protein response. FASEB J. 2005;19(11):1510–1512. doi: 10.1096/fj.04-3455fje. [DOI] [PubMed] [Google Scholar]
- 20.Chiang PC, Hsu JL, Yeh TC, Pan SL, Guh JH. Elucidation of susceptible factors to endoplasmic reticulum stress-mediated anticancer activity in human hepatocellular carcinoma. Naunyn Schmiedebergs Arch Pharmacol. 2008;377(2):167–177. doi: 10.1007/s00210-007-0249-4. [DOI] [PubMed] [Google Scholar]
- 21.Ciccaglione AR, Marcantonio C, Tritarelli E, Equestre M, Vendittelli F, Costantino A, et al. Activation of the ER stress gene gadd153 by hepatitis C virus sensitizes cells to oxidant injury. Virus Res. 2007;126(1-2):128–138. doi: 10.1016/j.virusres.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Colgan SM, Tang D, Werstuck GH, Austin RC. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2. Int J Biochem Cell Biol. 2007;39(10):1843–1851. doi: 10.1016/j.biocel.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73(6):1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 24.Cretenet G, Le Clech M, Gachon F. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1alpha pathway controls lipid metabolism in mouse liver. Cell Metab. 2010;11(1):47–57. doi: 10.1016/j.cmet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25(4):406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 26.Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27(3):285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 27.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 28.Dong H, Chen L, Chen X, Gu H, Gao G, Gao Y, et al. Dysregulation of unfolded protein response partially underlies proapoptotic activity of bortezomib in multiple myeloma cells. Leuk Lymphoma. 2009;50(6):974–984. doi: 10.1080/10428190902895780. [DOI] [PubMed] [Google Scholar]
- 29.Dorner AJ, Wasley LC, Kaufman RJ. Increased synthesis of secreted proteins induces expression of glucose-regulated proteins in butyrate-treated Chinese hamster ovary cells. J Biol Chem. 1989;264(34):20602–20607. [PubMed] [Google Scholar]
- 30.Duvigneau JC, Kozlov AV, Zifko C, Postl A, Hartl RT, Miller I, et al. Reperfusion does not induce oxidative stress but sustained endoplasmic reticulum stress in livers of rats subjected to traumatic-hemorrhagic shock. Shock. 2010;33(3):289–298. doi: 10.1097/SHK.0b013e3181aef322. [DOI] [PubMed] [Google Scholar]
- 31.Emadali A, Nguyen DT, Rochon C, Tzimas GN, Metrakos PP, Chevet E. Distinct endoplasmic reticulum stress responses are triggered during human liver transplantation. J Pathol. 2005;207(1):111–118. doi: 10.1002/path.1798. [DOI] [PubMed] [Google Scholar]
- 32.Esfandiari F, Villanueva JA, Wong DH, French SW, Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G54–63. doi: 10.1152/ajpgi.00542.2004. [DOI] [PubMed] [Google Scholar]
- 33.Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J. 1999;339(Pt 1):135–141. [PMC free article] [PubMed] [Google Scholar]
- 34.Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5(7):723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 35.Fischer H, Koenig U, Eckhart L, Tschachler E. Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun. 2002;293(2):722–726. doi: 10.1016/S0006-291X(02)00289-9. [DOI] [PubMed] [Google Scholar]
- 36.Flowers MT, Keller MP, Choi Y, Lan H, Kendziorski C, Ntambi JM, et al. Liver gene expression analysis reveals endoplasmic reticulum stress and metabolic dysfunction in SCD1-deficient mice fed a very low-fat diet. Physiol Genomics. 2008;33(3):361–372. doi: 10.1152/physiolgenomics.00139.2007. [DOI] [PubMed] [Google Scholar]
- 37.Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2000;97(11):5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fribley A, Wang CY. Proteasome inhibitor induces apoptosis through induction of endoplasmic reticulum stress. Cancer Biol Ther. 2006;5(7):745–748. doi: 10.4161/cbt.5.7.2971. [DOI] [PubMed] [Google Scholar]
- 39.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5(7):741–744. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 40.Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26(11):2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 41.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, et al. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138(3):562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 44.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7(6):1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 45.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 46.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10(11):3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312(5773):572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 48.Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329(5988):229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 49.Hiramatsu N, Kasai A, Du S, Takeda M, Hayakawa K, Okamura M, et al. Rapid, transient induction of ER stress in the liver and kidney after acute exposure to heavy metal: evidence from transgenic sensor mice. FEBS Lett. 2007;581(10):2055–2059. doi: 10.1016/j.febslet.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 50.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420(6913):333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 51.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313(5783):104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 53.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26(8):3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hugle T, Fehrmann F, Bieck E, Kohara M, Krausslich HG, Rice CM, et al. The hepatitis C virus nonstructural protein 4B is an integral endoplasmic reticulum membrane protein. Virology. 2001;284(1):70–81. doi: 10.1006/viro.2001.0873. [DOI] [PubMed] [Google Scholar]
- 55.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, et al. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7(5):445–455. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iwawaki T, Akai R, Yamanaka S, Kohno K. Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc Natl Acad Sci U S A. 2009;106(39):16657–16662. doi: 10.1073/pnas.0903775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakubowski H, Perla-Kajan J, Finnell RH, Cabrera RM, Wang H, Gupta S, et al. Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. FASEB J. 2009;23(6):1721–1727. doi: 10.1096/fj.08-127548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124(5):1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 59.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29(8):1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, et al. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23(16):5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119(5):1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanemoto S, Kondo S, Ogata M, Murakami T, Urano F, Imaizumi K. XBP1 activates the transcription of its target genes via an ACGT core sequence under ER stress. Biochem Biophys Res Commun. 2005;331(4):1146–1153. doi: 10.1016/j.bbrc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 63.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134(5):743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly E, Greene CM, Carroll TP, McElvaney NG, O'Neill SJ. Selenoprotein S/SEPS1 modifies endoplasmic reticulum stress in Z variant alpha1-antitrypsin deficiency. J Biol Chem. 2009;284(25):16891–16897. doi: 10.1074/jbc.M109.006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim DS, Jeong SK, Kim HR, Chae SW, Chae HJ. Metformin regulates palmitate-induced apoptosis and ER stress response in HepG2 liver cells. Immunopharmacol Immunotoxicol. 2010;32(2):251–257. doi: 10.3109/08923970903252220. [DOI] [PubMed] [Google Scholar]
- 66.Kim HR, Lee GH, Ha KC, Ahn T, Moon JY, Lee BJ, et al. Bax Inhibitor-1 Is a pH-dependent regulator of Ca2+ channel activity in the endoplasmic reticulum. J Biol Chem. 2008;283(23):15946–15955. doi: 10.1074/jbc.M800075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim TH, Kim YW, Shin SM, Kim CW, Yu IJ, Kim SG. Synergistic hepatotoxicity of N,N-dimethylformamide with carbon tetrachloride in association with endoplasmic reticulum stress. Chem Biol Interact. 2010;184(3):492–501. doi: 10.1016/j.cbi.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 68.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science. 2009;325(5939):477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawless MW, Mankan AK, White M, O'Dwyer MJ, Norris S. Expression of hereditary hemochromatosis C282Y HFE protein in HEK293 cells activates specific endoplasmic reticulum stress responses. BMC Cell Biol. 2007;8:30. doi: 10.1186/1471-2121-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24(24):4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320(5882):1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee JN, Ye J. Proteolytic activation of sterol regulatory element-binding protein induced by cellular stress through depletion of Insig-1. J Biol Chem. 2004;279(43):45257–45265. doi: 10.1074/jbc.M408235200. [DOI] [PubMed] [Google Scholar]
- 73.Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16(4):452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee MW, Chanda D, Yang J, Oh H, Kim SS, Yoon YS, et al. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab. 2010;11(4):331–339. doi: 10.1016/j.cmet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 75.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100(5):2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li B, Gao B, Ye L, Han X, Wang W, Kong L, et al. Hepatitis B virus X protein (HBx) activates ATF6 and IRE1-XBP1 pathways of unfolded protein response. Virus Res. 2007;124(1-2):44–49. doi: 10.1016/j.virusres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 77.Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186(6):783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S, Ye L, Yu X, Xu B, Li K, Zhu X, et al. Hepatitis C virus NS4B induces unfolded protein response and endoplasmic reticulum overload response-dependent NF-kappaB activation. Virology. 2009;391(2):257–264. doi: 10.1016/j.virol.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 79.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280(23):21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 80.Liang G, Audas TE, Li Y, Cockram GP, Dean JD, Martyn AC, et al. Luman/CREB3 induces transcription of the endoplasmic reticulum (ER) stress response protein Herp through an ER stress response element. Mol Cell Biol. 2006;26(21):7999–8010. doi: 10.1128/MCB.01046-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318(5852):944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33(6):679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lloyd DJ, Wheeler MC, Gekakis N. A point mutation in Sec61alpha1 leads to diabetes and hepatosteatosis in mice. Diabetes. 2010;59(2):460–470. doi: 10.2337/db08-1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lou LX, Geng B, Chen Y, Yu F, Zhao J, Tang CS. Endoplasmic reticulum stress involved in heart and liver injury in iron-loaded rats. Clin Exp Pharmacol Physiol. 2009;36(7):612–618. doi: 10.1111/j.1440-1681.2008.05114.x. [DOI] [PubMed] [Google Scholar]
- 85.Luebke-Wheeler J, Zhang K, Battle M, Si-Tayeb K, Garrison W, Chhinder S, et al. Hepatocyte nuclear factor 4alpha is implicated in endoplasmic reticulum stress-induced acute phase response by regulating expression of cyclic adenosine monophosphate responsive element binding protein H. Hepatology. 2008;48(4):1242–1250. doi: 10.1002/hep.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo D, He Y, Zhang H, Yu L, Chen H, Xu Z, et al. AIP1 is critical in transducing IRE1-mediated endoplasmic reticulum stress response. J Biol Chem. 2008;283(18):11905–11912. doi: 10.1074/jbc.M710557200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318(5):1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 88.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 89.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105(47):18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21(4):1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mencin A, Seki E, Osawa Y, Kodama Y, De Minicis S, Knowles M, et al. Alpha-1 antitrypsin Z protein (PiZ) increases hepatic fibrosis in a murine model of cholestasis. Hepatology. 2007;46(5):1443–1452. doi: 10.1002/hep.21832. [DOI] [PubMed] [Google Scholar]
- 93.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5(6):453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 94.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74(4):743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 95.Nagy G, Szarka A, Lotz G, Doczi J, Wunderlich L, Kiss A, et al. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2010;243(1):96–103. doi: 10.1016/j.taap.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 96.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O K, Lynn EG, Chung YH, Siow YL, Man RY, Choy PC. Homocysteine stimulates the production and secretion of cholesterol in hepatic cells. Biochim Biophys Acta. 1998;1393(2-3):317–324. doi: 10.1016/s0005-2760(98)00086-1. [DOI] [PubMed] [Google Scholar]
- 98.Oakes SA, Opferman JT, Pozzan T, Korsmeyer SJ, Scorrano L. Regulation of endoplasmic reticulum Ca2+ dynamics by proapoptotic BCL-2 family members. Biochem Pharmacol. 2003;66(8):1335–1340. doi: 10.1016/s0006-2952(03)00482-9. [DOI] [PubMed] [Google Scholar]
- 99.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24(6):1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oikawa D, Kimata Y, Kohno K, Iwawaki T. Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp Cell Res. 2009;315(15):2496–2504. doi: 10.1016/j.yexcr.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 101.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7(6):520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109(4):525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 104.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, et al. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010;16(4):429–437. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pavio N, Romano PR, Graczyk TM, Feinstone SM, Taylor DR. Protein synthesis and endoplasmic reticulum stress can be modulated by the hepatitis C virus envelope protein E2 through the eukaryotic initiation factor 2alpha kinase PERK. J Virol. 2003;77(6):3578–3585. doi: 10.1128/JVI.77.6.3578-3585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010;1201:183–188. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- 108.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298(5):E1027–1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 110.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 111.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 112.Ranganathan AC, Adam AP, Zhang L, Aguirre-Ghiso JA. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5(7):729–735. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000;14(2):152–157. [PMC free article] [PubMed] [Google Scholar]
- 114.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412(6844):300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 115.Richardson CE, Kooistra T, Kim DH. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature. 2010;463(7284):1092–1095. doi: 10.1038/nature08762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Robert K, Nehme J, Bourdon E, Pivert G, Friguet B, Delcayre C, et al. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology. 2005;128(5):1405–1415. doi: 10.1053/j.gastro.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 117.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64(17):5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 118.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6(3):439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 119.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15(6):829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sakon M, Ariyoshi H, Umeshita K, Monden M. Ischemia-reperfusion injury of the liver with special reference to calcium-dependent mechanisms. Surg Today. 2002;32(1):1–12. doi: 10.1007/s595-002-8105-8. [DOI] [PubMed] [Google Scholar]
- 121.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7(6):1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 122.Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med. 2005;11(7):757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 123.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3(1):99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 124.Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18(12):7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, et al. Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol. 2003;38(5):605–614. doi: 10.1016/s0168-8278(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 126.Sidrauski C, Cox JS, Walter P. tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell. 1996;87(3):405–413. doi: 10.1016/s0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 127.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118(10):3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167(1):35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Su IJ, Wang HC, Wu HC, Huang WY. Ground glass hepatocytes contain pre-S mutants and represent preneoplastic lesions in chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2008;23(8 Pt 1):1169–1174. doi: 10.1111/j.1440-1746.2008.05348.x. [DOI] [PubMed] [Google Scholar]
- 130.Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, et al. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol. 2008;294(2):G498–505. doi: 10.1152/ajpgi.00482.2007. [DOI] [PubMed] [Google Scholar]
- 131.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12(12):1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tong WY, Nagano-Fujii M, Hidajat R, Deng L, Takigawa Y, Hotta H. Physical interaction between hepatitis C virus NS4B protein and CREB-RP/ATF6beta. Biochem Biophys Res Commun. 2002;299(3):366–372. doi: 10.1016/s0006-291x(02)02638-4. [DOI] [PubMed] [Google Scholar]
- 133.Ubbink JB, van der Merwe A, Delport R, Allen RH, Stabler SP, Riezler R, et al. The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J Clin Invest. 1996;98(1):177–184. doi: 10.1172/JCI118763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uemura A, Oku M, Mori K, Yoshida H. Unconventional splicing of XBP1 mRNA occurs in the cytoplasm during the mammalian unfolded protein response. J Cell Sci. 2009;122(Pt 16):2877–2886. doi: 10.1242/jcs.040584. [DOI] [PubMed] [Google Scholar]
- 135.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 136.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101(31):11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vecchi C, Montosi G, Zhang K, Lamberti I, Duncan SA, Kaufman RJ, et al. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325(5942):877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vilatoba M, Eckstein C, Bilbao G, Smyth CA, Jenkins S, Thompson JA, et al. Sodium 4-phenylbutyrate protects against liver ischemia reperfusion injury by inhibition of endoplasmic reticulum-stress mediated apoptosis. Surgery. 2005;138(2):342–351. doi: 10.1016/j.surg.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 139.Wang L, Dong H, Soroka CJ, Wei N, Boyer JL, Hochstrasser M. Degradation of the bile salt export pump at endoplasmic reticulum in progressive familial intrahepatic cholestasis type II. Hepatology. 2008;48(5):1558–1569. doi: 10.1002/hep.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17(19):5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, et al. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17(13):3619–3630. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460(7254):534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wei Y, Wang D, Gentile CL, Pagliassotti MJ. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem. 2009;331(1-2):31–40. doi: 10.1007/s11010-009-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107(10):1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med. 2010;16(4):438–445. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu S, Tan M, Hu Y, Wang JL, Scheuner D, Kaufman RJ. Ultraviolet light activates NFkappaB through translational inhibition of IkappaBalpha synthesis. J Biol Chem. 2004;279(33):34898–34902. doi: 10.1074/jbc.M405616200. [DOI] [PubMed] [Google Scholar]
- 148.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279(44):45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 149.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19(12):8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13(3):365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 151.Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K. Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem. 2004;136(3):343–350. doi: 10.1093/jb/mvh122. [DOI] [PubMed] [Google Scholar]
- 152.Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6(6):1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 153.Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276(17):13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 154.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273(50):33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- 155.Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 2003;4(2):265–271. doi: 10.1016/s1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 156.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 157.Yoshida H, Uemura A, Mori K. pXBP1(U), a negative regulator of the unfolded protein response activator pXBP1(S), targets ATF6 but not ATF4 in proteasome-mediated degradation. Cell Struct Funct. 2009;34(1):1–10. doi: 10.1247/csf.06028. [DOI] [PubMed] [Google Scholar]
- 158.Yoshiuchi K, Kaneto H, Matsuoka TA, Kasami R, Kohno K, Iwawaki T, et al. Pioglitazone reduces ER stress in the liver: direct monitoring of in vivo ER stress using ER stress-activated indicator transgenic mice. Endocr J. 2009;56(9):1103–1111. doi: 10.1507/endocrj.k09e-140. [DOI] [PubMed] [Google Scholar]
- 159.Zeng T, Xie KQ. Ethanol and liver: recent advances in the mechanisms of ethanol-induced hepatosteatosis. Arch Toxicol. 2009 doi: 10.1007/s00204-009-0457-4. [DOI] [PubMed] [Google Scholar]
- 160.Zhang C, Cai Y, Adachi MT, Oshiro S, Aso T, Kaufman RJ, et al. Homocysteine induces programmed cell death in human vascular endothelial cells through activation of the unfolded protein response. J Biol Chem. 2001;276(38):35867–35874. doi: 10.1074/jbc.M100747200. [DOI] [PubMed] [Google Scholar]
- 161.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124(3):587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 162.Zhang K, Wong HN, Song B, Miller CN, Scheuner D, Kaufman RJ. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115(2):268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12(7):982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]