Abstract
In 20 subjects we quantified the rate at which subthalamic nucleus deep brain stimulation effects on Parkinson’s bradykinesia “washed-out” after stimulation ceased. We found that wash-out was a two-step process, consisting of an initial fast decrease in stimulation’s therapeutic effect, followed by a further, slow decline. Moreover, the relative contribution of the fast and slow component differed between patients. Finally, we found that lateral stimulation caused more of the fast-decaying component, while medial stimulation caused more of the slow-decaying component. This implies the existence of at least two separate mechanisms by which subthalamic nucleus deep brain stimulation improves bradykinesia, associated with activation of spatially separate zones in the vicinity of the subthalamic nucleus.
Keywords: Parkinson’s, Deep Brain Stimulation, Subthalamic Nucleus, Zona Incerta, Plasticity
INTRODUCTION
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is an effective treatment for symptoms of Parkinson’s disease (PD) [Deuschl et al. 2006]. It is well known that the therapeutic effects of STN DBS do not cease instantaneously when stimulation is turned off, but, rather, decay gradually [Temperli et al. 2003]. The implications of this observation for the design of clinical trials are well recognized, but little work has been done to quantify precisely the rate of decay, or to establish how it varies from one patient to another. Temperli et al. [2003] give average figures for time to 75%, or 90% of maximum and these results justify a 1–2 hour washout period. However, Keresztenyi et al. [2007] reported much faster rates of decay. These differences could be related to study design or inter-subject variability. The present study was designed to assess inter-subject variability with respect to both rapid and slow decay of DBS effects.
In addition to its practical implications, the decay of DBS effects provides important clues to the physiological mechanisms by which DBS exerts its therapeutic effect. Temperli et al. [2003] pointed out that the slow decay of STN DBS therapeutic effect implicated physiological mechanisms capable of persistent changes. We have suggested that DBS-induced synaptic plasticity is such a mechanism [Cooper et al. 2008] [Cooper et al. 2009]. Given that current theories on DBS mechanisms propose that it overrides a native, pathological pattern of activity it is possible that slow decay of DBS therapeutic effects could reveal whether a particular DBS-induced change in neuronal activity (i.e. power in the beta frequency band) has a causal relation to DBS therapeutic effects [Eusebio & Brown 2009]: if beta-suppression causes therapeutic effects, then it should persist, after DBS ceases, for about as long as the therapeutic effects do [Bronte-Stewart et al. 2009]. While this proposition is not without controversy [Foffani et al. 2006], it does provide further motivation to understand the factors affecting the decay of DBS therapeutic effects.
In the present paper we measure STN DBS therapeutic effect on bradykinesia, and report on rates of decay of that effect after stimulation is turned off. We found that inter-subject variation was high, but non-random, exhibiting both a fast- and a slow-decaying process. Moreover, these did not represent two separate patient populations but rather two separate physiological processes which could occur simultaneously in the same patient. As a result, we found that individuals differed in the relative contributions of fast and slow processes to their net DBS effect. Finally, we associated the fast and slow processes with spatially distinct sites of stimulation. These results address ambiguities in the previous literature and point toward a better understanding of physiological mechanisms underlying the therapeutic effect of DBS.
METHODS
Subjects
Subjects were patients with Parkinson’s disease and STN DBS devices at the Cleveland Clinic. All had 1) a diagnosis of PD by a movement disorders neurologist 2) 5 or more years disease duration, 3) clear levodopa response 4) no dementia and 1) were at least 3 months post-implantation on the tested side 2) had completed the initial postoperative period of stimulator adjustments, and reached stable stimulator settings in the judgement of the treating clinician 3) were obtaining satisfactory and expected clinical benefit from the stimulation. Mean (median) time from last clinical change of stimulator settings to time of experiment was 20 (14) months.
Details of subjects’ pre- and postoperative medication regimens are given in Supplementary Material.
Surgical procedure
The initial target was MR image-based, and the angulation adjusted to avoid cortical sulci, blood vessels, and, when possible, ventricles. The target was further refined using intraoperative microelectrode recording and microstimulation. Intraoperative stimulation through the DBS electrode was used to confirm a satisfactory therapeutic window between therapeutic effects and side effects.
Testing procedure
Testing was in the off-medication state: mean (median) delay between medication withdrawal and testing was 12.8 (12.0) hours (range: 10.5–16.5). The dominant hand and contralateral stimulator were tested.
Subjects performed three tasks, in rotation: A) a 20 second block of continuous finger-tapping (UPDRS item 23), B) a 20 second block of muscle-tone testing using the device developed by Patrick et al. [2001], and C) a 30 second block of a visual choice reaction time task (only the finger-tapping results are reported here), maintaining an interval of about 2 minutes between consecutive bradykinesia measurements. The time of each bradykinesia measurement was known to an accuracy of one second. This continued for 20 minutes constituting the initial stimulation-on period, designated Epoch 0.
At the conclusion of Epoch 0, the stimulator was turned off using a Medtronic model 8840 or 7451 programmer. Subjects then resumed performing the three tasks in rotation for a further 50 minutes with the stimulator now off: this constituted the stimulation-off period, designated Epoch 1.
At the conclusion of Epoch 1, the stimulator was turned back on again and tasks resumed in rotation with the stimulator back on again, for a further 20 minutes designated Epoch 2.
The procedure for turning on/off stimulators is detailed in Supplementary Material.
Bradykinesia measurements
To measure bradykinesia, we used an instrumented version of UPDRS item 23 (“finger tapping”), in which subjects tapped the tip of the thumb and index finger together “as fast as possible” and “as wide as possible” for 20 seconds. Angular velocity sensors(model G-1, NeuroKinetics, Edmonton, Alberta, Canada) were taped to first phalange of thumb & index finger to detect metacarpophalangeal flexion/extension. Validation of the quantitative tapping measurement procedure against UPDRS_III is presented in Supplementary Material.
Data analysis
All data analysis was done using the pylab, numpy, and scipy libraries (www.enthought.com or www.scipy.org) The angular velocity signals were sampled (PCI-6025E, National Instruments, Austin, TX) at 16 bits x 10 KHz resolution and the Euclidean sum √(x2+y2) taken. A power spectrum was then computed (Welch’s method, with window 215 = 32768 samples) and the total power computed in a band of 1.0 to 10.0 Hz.
Ideally, the subject is a stationary system, and all changes over time reflect only the dynamics of the subject’s response to stimulation. However, factors, such as fatigue or boredom may also cause changes over time. Therefore, we excluded from analysis four experiments in which bradykinesia did not improve when the stimulator was turned back on again at the end of the experiment (the Epoch-1 to Epoch-2 transition), since, in such experiments, changes during Epoch-1 could not reliably be attributed to turning off the stimulation.
Curve fitting
Curves were fit to the graph of tapping-power vs. time (see Fig 1) using Nelder-Mead iterative minimization of summed, squared error (scipy.optimize.fmin function).
Figure 1.
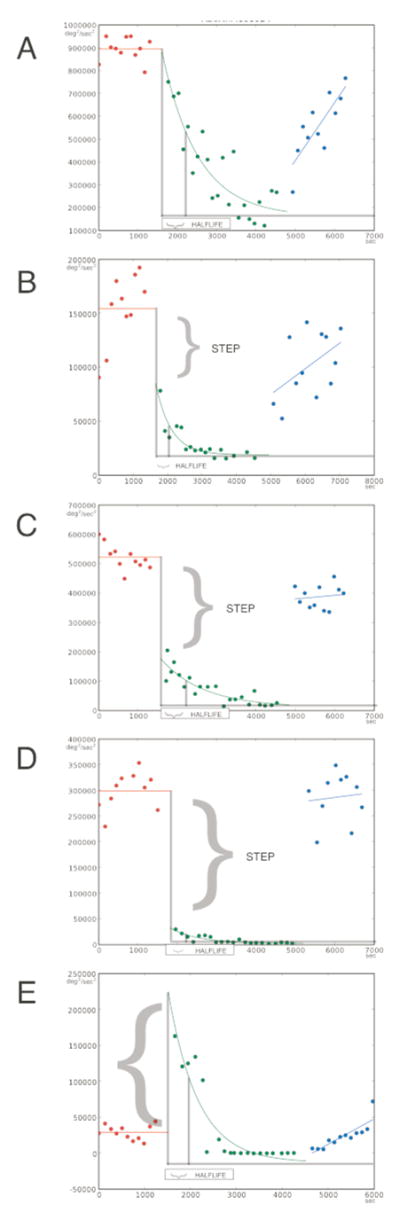
Decrease in tapping power after STN DBS is turned off, showing “Fast” and “Slow” changes for 5 individual subjects, illustrating the spectrum of results we observed. Tapping power vs time: 20 minutes baseline, followed by 50 minutes with stimulation off, during which tapping power changes, followed by a further 20 minutes with stimulation back on, during which tapping power recovers.
To the three epochs of the experiment, we fit the piecewise equation
where t=time and Y = tapping power, and where toff and ton are the time stimulation was turned off, and on, respectively. f(t), g(t), and h(t) correspond to epochs 0, 1, and 2, respectively. Note that we made no a priori assumption that the equation was continuous across the boundaries between epochs, allowing for the possibility of abrupt changes when stimulation was turned on/off.
The derivation of f(t), g(t), and h(t) is discussed in detail in Supplementary Material. Briefly, for g(t) we used a simple decaying exponential (see Fig 1); the form of f(t) and h(t) did not affect our results. In this paper, we report HALFLIFE (time to decrease by a factor of 2), and STEP, defined as the fraction of total (from initial to asymptotic value) change which occurred abruptly when DBS was turned off (see Fig 1).
Statistics
Regression and tests of significance were done with the R statistical programming language [R development core team 2009]
Electrode localizations
In subjects with sufficient perioperative clinical data (see Table 1) we created a patient-specific DBS computer model using Cicerone v1.2, a freely available academic DBS research tool [Miocinovic et al. 2007], following our previously described methodology [Butson et al. 2007] (see Supplementary Material). Four subjects were excluded from the electrode localization analysis because of incomplete data due to: 1) operated at another institution (surgical records not available) 2) “frameless” stereotaxic system used (incompatible with Cicerone software 3) incomplete surgical records and 4) incomplete radiological records.
Table 1.
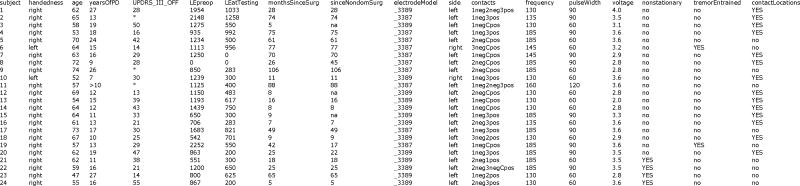 |
age in years, at time of testing. yearsOfPD years since onset of Parkinson’s. UPDRS_III_OFF UPDRS motor section total score in the off-medication, DBS-naive state. LEpreop Total daily levodopa equivalent preoperatively. LEatTesting Total daily levodopa equivalent at time of testing. monthsSinceSurg time, in months, at the time of testing, since electrode implantation, on the side tested. sinceNondomSurg time, in months, at the time of testing, since electrode implantation, on the other, nondominant side (na=subject was implanted only on the dominant side). electrodeModel type of electrode implanted (Medtronic model 3387 or 3389). side side of brain tested (finger-tapping performed contralateral to this). contacts electrode contacts stimulated (0–3, and “Case”). voltage stimulation amplitude, in volts. frequency stimulation frequency, in Hz. pulseWidth stimulation pulse width setting, microsec. Contacts, voltage, frequency, & pulse width were all the same as subject’s usual contacts, as clinically optimized prior to, and independently of the experiment. nonstationary subject failed stationarity criterion (see Methods). tremorEntrained subjects had a prominent tremor to which their finger-tapping became entrained (see Results). contactLocations location of stimulating contacts was computed in stereotactic space (see Methods section on electrode localization, below. Missing data indicated by “*" Details of how levodopa equivalent was computed are given in Supplementary Material.
RESULTS
Inter-subject variation
Fig 1 shows several experiments, illustrating the range of results we obtained. Note the contrast between Fig 1A, in which tapping power declines gradually after DBS is turned off (“Slow” decay) and Fig 1D, in which tapping power drops abruptly, followed by a small residual slow decay (“Fast-slow”). Fig 1B & C show intermediate cases in which the initial abrupt drop and the subsequent slow decay were of comparable magnitude, illustrating that A and D are extremes of a continuum. This analysis was done in 20 subjects (see Methods). Finally, Fig. 1E shows an example with a prominent tremor appearing promptly when stimulation was turned off, and where tapping became entrained to tremor. In such “tremor entrained” subjects, total power measured tremor, not bradykinesia; the two tremor-entrained subjects were not included in the regression analysis (next section) for that reason. Nonetheless, we note that, in both tremor-entrained subjects, tapping power declined with similar halflife to the others.
In order to deal more rigorously with these variations, we fit a curve to each experiment’s data (see Methods section) so that the time course of DBS effects was described by two parameters STEP and HALFLIFE (Fig. 1). The resulting bivariate distribution is shown in Fig. 2. The amplitude of the STEP parameter is expressed as a fraction of the total change in bradykinesia from baseline to its asymptotic value after “washout” of DBS effect. Thus, subjects with “slow decay” had a STEP close to zero, while those with “fast-slow” decay had values ranging between zero and −1.0. (The two “tremor-entrained” subjects (inset) had large positive values greater than +1.0.) STEP was uncorrelated with (independent of) HALFLIFE.
Figure 2.
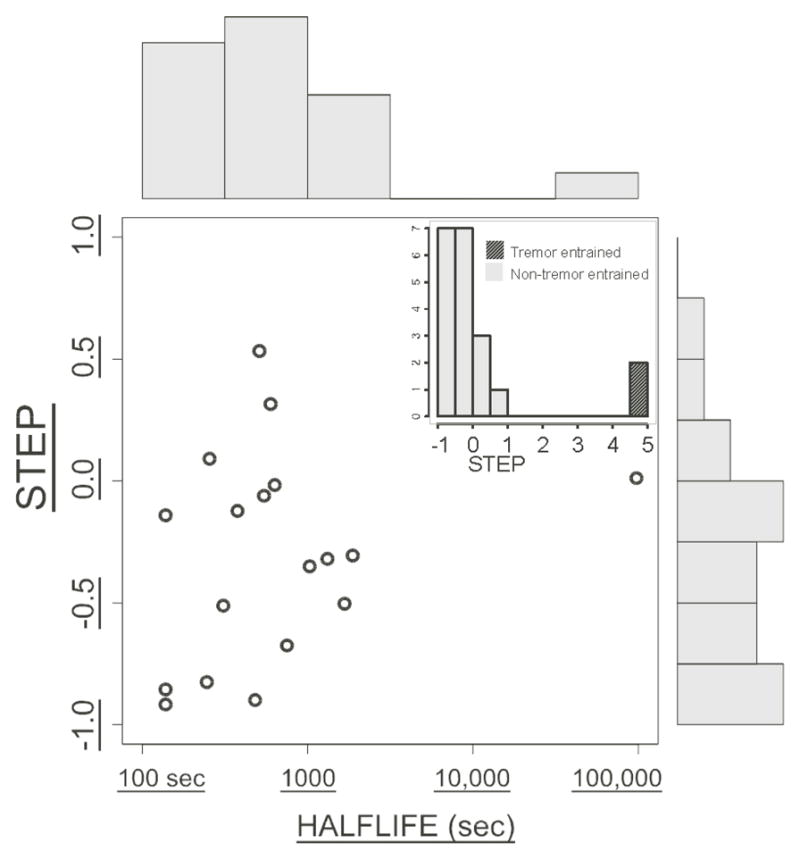
“STEP” parameter vs. HALFLIFE (semilog axis). N = 20 subjects. Histograms of STEP and HALFLIFE are shown along the vertical and horizontal axes, respectively, for non-tremor-entrained subjects (tremor entrained subjects shown in the inset).
Relation to stimulating contact location
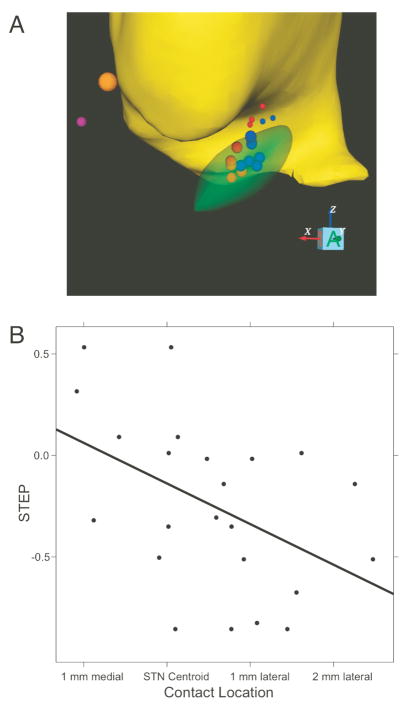
We reconstructed the location of the active electrode contacts in each hemisphere (see Methods section), and regressed the STEP parameter on the stereotaxic X (mediolateral), Y (anteroposterior), and Z (dorsoventral) coordinates, relative to the centroid of the subthalamic nucleus (Fig 3). Contacts inducing more fast effect were located lateral, and slightly anterodorsal to those causing more slow effect (Fig 3A). Regression of the STEP parameter on X, Y, and Z was statistically significant at p = 0.02, confirming that the location of stimulation determines the proportion of fast- vs. slow-decaying STN DBS effect. X,Y, and Z coefficients were 3.4, 1.1, and 1.5 standard errors respectively, suggesting that statistical significance was driven mainly by X; in keeping with this, simple regression of STEP on X was significant at (Bonferroni-corrected) p = 0.02.
Figure 3.
A: Geometrical interpretation of the regression: The subjects with the 5 highest and 5 lowest values of STEP are shown (out of a total of 14 with sufficient perioperative data to reconstruct contact locations). Smaller STEP: red; larger STEP:blue. Large spheres: negative contacts; small spheres: positive contacts. Thalamus: yellow; STN: green; anterior commissure: orange; posterior commissure: purple. The relation of STEP to X,Y,Z location of active contacts (including all 14 subjects) is statistically significant (multiple linear regression p = 0.02). In part A, only the highest, and lowest one-third of the data are shown, in order to show more clearly the physical separation between high and low values of STEP. For a more rigorous, but less visual statistical analysis including all the data, see Part B of this figure (regression on X) and the text (regression on X,Y, & Z). In part A, all contact locations are mapped into a common atlas space so that the borders of the STN can be shown. The regression of STEP on contact location is statistically significant whether this remapping is done or not. B: STEP parameter vs. location of each active contact on the X (mediolateral) axis (single linear regression p = 0.005) N = 14 subjects).
Additional details of the statistical anaylysis are given in Supplemental Material.
Relation to other clinical variables
Regression of STEP on the following variables was not statistically significant: Stimulation voltage, duration of Parkinson’s disease, total daily levodopa equivalent preoperatively, and total daily levodopa equivalent at time of testing.
DISCUSSION
In this paper, we quantified the rate at which STN DBS effects on PD bradykinesia “wash-out” after stimulation ceases. We found that wash-out is a two-step process, consisting of an initial fast decrease in DBS therapeutic effect, followed by a further, slow decline. We also found that the relative contribution of the fast and slow processes differ between patients. Finally, we found that the difference is attributable to the site of stimulation, with lateral stimulation causing more of the fast-decaying process, while medial stimulation caused more of the slow-decaying process. This has two important implications. First, it provides a way to reconcile some apparent conflicts in the literature. Second, it implies the existence of at least two distinct physiological mechanisms of STN DBS, associated with stimulation of different anatomical entities.
Potential resolution to conflicts in the literature
Do lingering effects decay in tens of minutes, or tens of seconds?
Temperli et al. [2003] found that 60–90 minutes were required for STN DBS effect on bradykinesia to decay by 90% after STN DBS ceased (equivalent to a half-life of about 15–30 minutes, for single-exponential decay). That is, DBS effects “lingered” for a while after stimulation ceased. Lopiano et al. [2003] and Waldau et al. [2011] obtained similar results but Keresztenyi et al. [2007] measured time constants between 15 & 30 seconds (half lives about 10–20 sec). We propose that their subject populations, like ours, exhibited both fast and slow processes, but that differences in experimental design led to their differing results: Keresztenyi et al. [2007] (measurement over 5 minute after DBS turned off ) could not exclude an additional slower decay over tens of minutes. Conversely, Temperli et al. [2003], (measurements over hours, but at intervals of tens of minutes) could not exclude additional changes with a half life in the 10–20 second range.
Lingering effects and beta oscillations
Lingering STN DBS effects may test hypotheses about the relation of therapeutic effects to patterns of neuronal activity. For example, Kuhn et al [2008] and Bronte-Stewart et al [2009] measured power in the beta frequency band of LFPs recorded from STN after STN DBS ceased, and reported that suppression of beta power persisted after stimulation ceased, just as therapeutic effects on bradykinesia do. From this, they argued that a causal relation existed between the two. However, Foffani et al [2006] observed no such lingering beta-suppression. As we report here, when STN DBS is turned off, some patients experience a very rapid return of bradykinesia, while in others it returns more gradually. Thus, the differences among the above cited papers may be due to differences among the patients studied (or their electrode locations).
Candidate physiological mechanisms for lingering effects
The “slow” effect may reflect DBS-induced, long term potentiation at glutamatergic synapses [Cooper et al 2008], [Cooper et al 2009]. “Early-phase,” non-protein-synthesis-dependent LTP [Raymond 2007] has a decay time constant approaching that of lingering effects [Abraham & Otani 1991], making it a candidate mechanism for “slow” STN DBS effects. Alternatively, extracellular glutamate accumulation might mediate the slow effects. Lee et al [2007] found that STN DBS increased local extracellular glutamate concentration, and estimated a time constant of 19 minutes (1140 sec) for decay of this effect, which is in good agreement with our value for the “slow” half life.
Anatomical targets associated with fast and slow effects
Origin of the slow-decaying effect
Slow-decaying bradykinesia effects evoked from medial contacts might result from activation of medial STN, although this region is more limbic than motor [Benarroch 2008]. Alternatively, the volume of tissue activated might extend beyond the boundaries of the nucleus to regions medial to it. It is noteworthy that this region contains both pallidothalamic fibers and the zona incerta (ZI). It is known that stimulation of pallidothalamic fibers, at their source in globus pallidus pars interna, is as effective as STN DBS for symptoms of Parkinson’s Disease in human patients [Follett et al 2010]. It has also been reported that stimulation in or near ZI is clinically equally or more effective than stimulation of the STN proper for treatment of Parkinson’s symptoms [Guehl et al 2008], [Henderson et al 2002], [Yelnik et al 2003], [Godinho et al 2006], [Plaha et al 2006]
Origin of the fast-decaying effect
Fast-decaying bradykinesia effects evoked from lateral contacts likely result from stimulation of lateral, somatomotor STN, though the volume of tissue activated might extend to laterally adjacent internal capsule. It is noteworthy that recent studies have implicated antidromic activation of corticosubthalamic axons in DBS therapeutic effects [Gradinaru et al 2009].
In summary, our results do not enable us to say exactly what structures were responsible for the fast- and slow-decaying effects. However, our finding that two distinguishable therapeutic effects were obtained from spatially separate zones indicates that STN DBS exerts its therapeutic effects at mulitple sites not all necessarily within STN proper.
Limitations of the present study
Effect of turning off DBS
In this study, turning off stimulation resulted in worsening of bradykinesia, consistent with previous literature; however, this is not a conclusion of our study, since subjects were aware that their stimulator settings were changed. Rather we conclude that the dynamics (fast vs. slow) of worsening bradykinesia is related to electrode position, regarding which subjects were blinded.
Bradykinesia measurements
To measure bradykinesia, we used a version of the UPDRS finger tapping item 23 which replaced the semiquantitative scale with a continuous, fully quantitative one. We have previously used this approach [Butson et al. 2007], and in the present paper, present additional data validating it against the UPDRS item 23 rating. It is clear from our results that performance of finger tapping worsened, with the dynamics we describe, after turning off STN DBS, and we feel justified in describing this as worsening of bradykinesia.
Bradykinesia vs other symptoms
Temperli et al [2003] found that, when STN DBS was turned off, bradykinesia, rigidity, tremor, and axial symptoms differ in the rate at which they return. Therefore, it remains to be established to what extent our findings extend to other Parkinson’s symptoms.
Interpretation of STEP
We have interpreted STEP as the proportion of DBS therapeutic effect due to the fast process, and (1 - STEP) as the proportion due to the slow process. Since STEP is correlated with electrode position, (1 - STEP) necessarily is correlated as well. It is possible that, during stimulation, the fast process contributes 100% of therapeutic effect, and that the slow process is only “unmasked” after stimulation ceases. This would occur, for example, if the fast process were due to direct driving of action potentials in STN efferents, while the slow process were due to synaptic changes “upstream” of STN efferent axons. Under this interpretation, it remains true that a more prominent slow-decaying effect is associated with more medial electrode placement.
Sample size
We analyzed 20 subjects, which is comparable to the 35 of Temperli et al [2003] and more than other studies in the literature [Lopiano et al 2003], [Keresztenyi et al 2007]. Insufficient sample size could result in a statistical Type II error but this is inapplicable, since our results were statistically significant. It is, of course possible that this was a statistical Type I error (wrongly rejecting the null hypothesis), but this possibility occurs with any test of statistical significance. It is also possible, that additional effects not observed in the present study, might have been apparent with a larger sample size.
Time resolution
To measure fast processes requires measurements repeated at short intervals. In our experiments, the interval between turning off DBS and making the first “stimulation-off” measurements was about 200 seconds. Thus, for the time constant of the “fast” process, we can only say it was less than 200 seconds. It seems likely, however, that it corresponds to the value of 15–30 sec measured by Keresztenyi et al. [2007].
Conversely, to measure slow processes requires measurements continued for a long time. Our measurements continued for 50 minutes with DBS off. Therefore, for processes with longer time scales we can only set a lower limit. Our half lives were about 1000 sec, but other, even slower processes may also play a role in STN DBS effects. For example, our clinical experience suggests that STN DBS effects may evolve over hours to weeks after a change in stimulator settings. Indeed, our distribution of half lives included one subject with a half life of nearly 100,000 sec. Studies of such hyper-slow processes pose technical challenges, but the present study shows they may be informative.
Supplementary Material
Research Highlight.
STN DBS wash-out is a fast decrease, followed by further slow decline.
The relative contributions of the fast and slow processes differ between patients.
The fast process is associated with lateral, and the slow with medial stimulation.
This implies there are at least two distinct physiological mechanisms of STN DBS.
Change in neuronal oscillation with DBS might relate to slow but not fast mechanism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Otani S. Macromolecules and the maintenance of long-term potentiation. In: Morel F, editor. Kindling and synaptic plasticity; the legacy of Graham Goddard. Boston, MA: Birkhauser; 1991. pp. 92–109. [Google Scholar]
- Benarroch EE. Subthalamic nucleus and its connections: Anatomic substrate for the network effects of deep brain stimulation. Neurology. 2008;70(21):1991–1995. doi: 10.1212/01.wnl.0000313022.39329.65. [DOI] [PubMed] [Google Scholar]
- Bronte-Stewart H, Barberini C, Koop MM, et al. The STN beta-band pro le in Parkinson’s disease is stationary and shows prolonged attenuation after deep brain stimulation. Exp Neurol. 2009;215(1):20–28. doi: 10.1016/j.expneurol.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, et al. Patient specific analysis of the volume of tissue activated during deep brain stimulation. Neuroimage. 2007;34(2):661–670. doi: 10.1016/j.neuroimage.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SE, Hahn PJ, McIntyre CC. Synaptic plasticity in a subthalamopallidal network model of deep brain stimulation. Society for Neurosicence Annual Meeting; Washington, DC. 2008. Abstract 139.10. [Google Scholar]
- Cooper SE, Hahn PJ, McIntyre CC. Model STN DBS induced synaptic strength changes have “lingering” effects on neuronal activity. Society for Neurosicence Annual Meeting; Chicago, IL. 2009. Abstract 326.10. [Google Scholar]
- Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- Foffani G, Ardolino G, Egidi M, et al. Subthalamic oscillatory activities at beta or higher frequency do not change after high-frequency DBS in Parkinson’s disease. BrainRes Bull. 2006;69(2):123–130. doi: 10.1016/j.brainresbull.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- Godinho F, Thobois S, Magnin M, et al. Subthalamic nucleus stimulation in Parkinson’s disease : anatomical and electrophysiological localization of active contacts. J Neurol. 2006;253(10):1347–1355. doi: 10.1007/s00415-006-0222-z. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, et al. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324(5925):354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guehl D, Vital A, Cuny E, et al. Postmortem proof of effectiveness of zona incerta stimulation in Parkinson disease. Neurology. 2008;70(16 Pt 2):1489–1490. doi: 10.1212/01.wnl.0000310426.18409.11. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Pell M, O’Sullivan DJ, et al. Postmortem analysis of bilateral subthalamic electrode implants in Parkinson’s disease. Mov Disord. 2002;17(1):133–137. doi: 10.1002/mds.1261. [DOI] [PubMed] [Google Scholar]
- Keresztenyi Z, Valkovic P, Eggert T, et al. The time course of the return of upper limb bradykinesia after cessation of subthalamic stimulation in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(7):438–442. doi: 10.1016/j.parkreldis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Kuhn AA, Kempf F, Brucke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28(24):6165– 6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Kristic K, van Hoff R, et al. High-frequency stimulation of the subthalamic nucleus increases glutamate in the subthalamic nucleus of rats as demonstrated by in vivo enzyme-linked glutamate sensor. Brain Res. 2007;1162:121–129. doi: 10.1016/j.brainres.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Lopiano L, Torre E, Benedetti F, et al. Temporal changes in movement time during the switch of the stimulators in Parkinson’s disease patients treated by subthalamic nucleus stimulation. Eur Neurol. 2003;50(2):94–99. doi: 10.1159/000072506. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Noecker AM, Maks CB, et al. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir Suppl. 2007;97(Pt 2):561–567. doi: 10.1007/978-3-211-33081-4_65. [DOI] [PubMed] [Google Scholar]
- Patrick SK, Denington AA, Gauthier MJ, et al. Quantification of the UPDRS RigidityScale. IEEE Trans Neural Syst Rehabil Eng. 2001;9(1):31–41. doi: 10.1109/7333.918274. [DOI] [PubMed] [Google Scholar]
- Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129(Pt 7):1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2009. http://www.R-project.org. [Google Scholar]
- Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30(4):167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Temperli P, Ghika J, Villemure J-G, et al. How do parkinsonian signs return after discontinuation of subthalamic DBS. Neurology. 2003;60:78–81. doi: 10.1212/wnl.60.1.78. [DOI] [PubMed] [Google Scholar]
- Waldau B, Clayton DA, Gasperson LB, Turner DA. Analysis of the Time Course of the Effect of Subthalamic Nucleus Stimulation upon Hand Function in Parkinson’s Patients. Stereotact Funct Neurosurg. 2011;89(1):48–55. doi: 10.1159/000323340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullner U, Kassubek J, Odin P, Schwarz M, Naumann M, Hack HJ, et al. Transdermal rotigotine for the perioperative management of Parkinson’s disease. J Neural Transm. 2010;117(7):855–859. doi: 10.1007/s00702-010-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelnik J, Damier P, Demeret S, et al. Localization of stimulating electrodes in patients with Parkinson disease by using a three-dimensional atlas-magnetic resonance imaging coregistration method. J Neurosurg. 2003;99(1):89–99. doi: 10.3171/jns.2003.99.1.0089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.