Summary
Many compounds produced by fungi have relevant pharmaceutical applications. The purpose of this study was to collect and isolate endophytic fungi from different regions of Panama and then to test their potential therapeutic activities against Leishmania donovani, Plasmodium falciparum, and Trypanosoma cruzi as well as their anticancer activities in MCF-7 cells. Of the 25 fungal isolates obtained, ten of them had good anti-parasitic potential, showing selective activity against L. donovani; four had significant anti-malarial activity; and three inhibited the growth of T. cruzi. Anticancer activity was demonstrated in four isolates. Of the active isolates, Edenia sp. strain F0755, Xylaria sp. strain F1220, Aspergillus sp. strain F1544, Mycoleptodiscus sp. strain F0194, Phomopsis sp. strain F1566, Pycnoporus sp. strain F0305, and Diaporthe sp. strain F1647 showed the most promise based on their selective bioactivity and lack of toxicity in the assays.
Keywords: Leishmania, endophytic fungi, malaria, Chagas's disease, anticancer activity
Introduction
Parasitic infections are major causes of human chronic diseases in most countries of the tropics. The parasites include protozoa and helminths, infect billions of people, and the resulting diseases cause debilitating injuries such as blindness and disfigurement, or death in millions of people. According to World Health Organization (WHO) estimates, 25% of the human population is infected with parasitic worms. However, attempts to develop vaccines against these pathogens have been frustrated by the difficulty of cultivating the parasites in the laboratory, the complexity of their multicellular organization and—in many species—their multistage development, in addition to their impressive antigenic variability [http://www.who.int/vaccine_research/diseases/soa_parasitic/en/index.html].
Malaria is the most dangerous parasitic disease, as evidenced by the high rates of complications and mortality caused by the most fatal species, Plasmodium falciparum [15]. Chagas disease, or American trypanosomiasis, is a potentially life-threatening two-phase illness caused by the protozoan Trypanosoma cruzi. The acute phase persists for about two months after infection; symptoms are absent or mild and can include fever, headache, enlarged lymph glands, pallor, muscle pain, difficulty in breathing, swelling, and abdominal or chest pain. In the chronic phase, the parasites reside mainly in the heart and digestive muscle, resulting in cardiac disorders in up to 30% of patients and digestive, neurological, or mixed pathologies in up to 10%. Eventually, the infection can lead to sudden death or heart failure, caused by progressive destruction of cardiac muscle [10,15]. Leishmaniasis, a worldwide disease, is caused by several species of the flagellated protozoan parasite Leishmania. In its more severe forms, the disease causes serious disfigurement and may be fatal. The WHO estimates a worldwide prevalence of leishmaniasis of approximately 12 million cases, with an annual mortality of about 60,000 and approximately 350 million people at risk. The expansion of leishmaniasis and the alarming rise in the number of cases has been attributed to environmental changes, such as deforestation, dam construction, new irrigation schemes, and the migration of non-immune individuals to endemic areas [10,15].
At the same time, the frequency of drug-resistant parasites has greatly increased and most treatments involve highly toxic drugs. In addition, the chemotherapeutic agents used in patients with these diseases have lacked effectiveness. Thus, there is an urgent need to search for novel drugs from previously unexplored sources, including natural products, to combat the global health problems posed by parasitic infections. Cancer is another major cause of mortality worldwide; in 2008, it accounted for 7.6 million deaths. According to WHO forecasts, an increase to 11 million deaths annually is expected by 2030. The prevalence is higher in low and middle-income countries.
As a part of the on-going research activities, the Panamanian International Cooperative Biodiversity Group (ICBG) [17] recently decided to explore endophytic fungi as a source of molecules with antiparasitic and anticancer bioactivities [18,21,22]. Within the ICBG program, we have assayed the antiparasitic and in vitro anticancer activities of 25 isolates, while also analyzing the effect of the culture medium on the production of secondary metabolites by Panamanian endophytic fungi. The results of these studies are reported and discussed herein.
Materials and methods
Microorganisms and maintenance
The endophytic fungi used in this study were isolated from the leaves of nineteen plants collected in Panama's protected areas, including Coiba, Barro Colorado Islands, and Altos de Campana National Park. Harvested leaves were washed in running tap water and processed as follows: 96 adjacent 1-mm × 2-mm segments were cut from the lamina, midway between the petiole and leaf tip. The segments were surface-sterilized by washing them for 2 min in 0.525% sodium hypochlorite and then for 2 min in 70% ethanol [1]. Subsequently, 24 segments were arbitrarily selected and placed on Petri dishes containing 2% malt extract agar (MEA). The plates were incubated on laboratory benches at room temperature with ambient light. Every 3 days for 21 days, fungal growth from the leaf segments was monitored. Hyphal tips from distinct colonies emerging from leaf segments were subcultured on fresh 2% MEA plates to obtain pure colonies.
Endophytic fungi identification
The fungi, most of which did not fruit in culture, were tentatively identified by molecular phylogenetic analyses of the fast-evolving nuclear ribosomal internal transcribed spacer (ITS), a ca. 600-bp locus frequently used in fungal systematics at the species level [2]. Total fungal genomic DNA was extracted directly from fresh, axenic mycelia using an SDS extraction protocol. The DNA was diluted 1:10 and then used to PCR-amplify the ITS region. The PCR products were purified, visualized on a 1% agarose gel, and sequenced in two directions on an ABI 3700 automated sequencer using the PCR primers ITS5 and ITS4. The resulting sequences were assembled into contigs and basecalls edited manually. The consensus sequences were subjected to BLAST searches of the NCBI GenBank database [http://blast.ncbi.nlm.nih.gov/Blast.cgi] for preliminary identification. The sequences for each species were deposited at the Smithsonian Tropical Research Institute. The organisms were maintained on MEA slants.
Fungal culture conditions
The fungi were grown on a small scale using four types of liquid culture media: (i) modified malt extract (MME), consisting of 10 g malt extract (Scharlau Chemie), 0.5 g mycological peptone (Fluka, Sigma-Aldich Chemie), and 10 g dextrose (Mallinckrodt Baker); (ii) potato dextrose broth (PDB, Sigma-Aldrich Chemie); (iii) Czapex Dox (CD, Sigma-Aldrich Chemie); and (iv) V8, containing 90 ml V8 vegetable juice (Campbells) and 1 g calcium carbonate (Sigma-Aldrich Chemie). Five hundred ml of each of the sterile media were placed in1-liter Erlenmeyer flasks and inoculated with small pieces of actively growing mycelium (0.5 cm2). The cultures were incubated at 30°C under orbital agitation (150 rpm) for 15 days.
Production of extracts
After incubation, the contents of the flasks were membrane-filtered (0.45-mm-pore-size, Millipore) and the filtrate then extracted exhaustively with ethyl acetate. The organic phase was vacuum-concentrated to obtain the extracts. Mycelia were lyophilized and extracted with the same solvent.
In vitro antiparasitic assays: Leishmania
Axenically grown (cell-free) amastigotes of L. donovani (LD-1S/MHOM/SD/00-strain 1S), the species responsible for the visceral and potentially lethal form of the disease, were used in the bioassay. Parasite growth and survival were measured using the fluorochrome PicoGreen, an ultrasensitive fluorescent nucleic acid strain for measuring double-stranded DNA. Samples to be screened were first tested in duplicate at a single concentration of 10 μg/ml [6]. The results were expressed as percentage of parasite inhibitory growth (% IG) compared to the control. Samples with an IG value ≥65% were considered active. The fungal compounds were assayed at six concentrations (0.00032, 0.0016, 0.08, 0.4, 2, and 10 μg/ml) to determine the IC50 values. Amphotericin B was used as a positive control; the typical IC50 response of L. donovani to this drug is 70–120 ng/μl.
In vitro antiparasitic assays: Plasmodium
Antiplasmodial activity was evaluated using a fluorometric method in which parasite DNA is detected with the fluorochrome PicoGreen in a chloroquine-resistant strain (Indocrina W2) of Plasmodium falciparum, as described in [2]. The parasites were maintained in vitro by a modification of the method of Trager and Jensen. Chloroquine served as a positive control (IC50 = 80–100 nM) [5]. The results were expressed as the %IG (as defined above) compared to the chloroquine control. Samples with IG values ≥65% were considered active.
In vitro antiparasitic assays: Trypanosoma cruzi
Antitrypanosomal bioassays were performed using a colorimetric method. The inhibition of parasite growth was assessed based on the expression of the reporter gene for β-galactosidase (β-Gal) in the recombinant Tulahuen clone C4 of T. cruzi. The assays were performed in triplicate on amastigotes—the intracellular form of the parasite infecting African green monkey kidney (Vero) cells—exposed for 120 h to different concentrations (10, 2, and 0.4 μg/ml) of the test compounds at 37°C under an atmosphere of 5% CO2/95% air [6]. The intensity of the color, indicating the amount of cleavage of chlorophenol red-β-d-galactoside (CPRG) by β-Gal expressed by the parasite, was measured at 570 nm using a Benchmark Bio-Rad microplate reader. The results were expressed as the %IG (as defined above) compared to the nifurtimox control. Samples with IG values ≥65% were considered active [4].
In vitro cytotoxicity assays
The cytotoxic activity of the test compounds against the human breast cancer cell line MCF-7 was determined following the standard protocol of the U.S. National Cancer Institute [23]. Vero cells adhering to 96-well plates were used to evaluate the toxicity of the compounds purified from Aspergillus sp. strain F1544 on the basis of the reduction of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, Sigma). The cells were treated with the test compound for 4 h at 37°C, after which cell viability was evaluated in an ELISA reader at 570 nm [23]. The results were expressed as the %IG compared to the amphotericin B control. Samples with IG values ≥65% were considered active.
Large-scale cultures for chemistry
For each culture (Edenia sp. strain F0755 and Mycosphaerella sp. nov. strain F2140), ten 1-liter Erlenmeyer flasks, each containing 0.5 l of liquid medium (CD or PD), were individually inoculated with a 1-cm2 agar plug taken from a stock culture of the respective fungus. The flasks were placed on an orbital shaker at 150 rpm and incubated at 30°C for 15 days.
Isolation of compounds from Edenia sp. strain F0755 and Mycosphaerella sp. nov. strain F2140
Each crude extract was subjected to open column chromatography (silica gel 60, 70–230 mesh, Merck), which yielded several fractions. These were subsequently tested for activity in antiparasitic and anticancer bioassays. Active fractions that gave rise to large peaks were further fractionated using normal-phase HPLC in the case of Edenia sp. strain F0755 and reversed-phase HPLC in the case of Mycosphaerella sp. nov. strain F2140, to yield compounds 1–7. HPLC was carried out on a Waters LC system, including a 600 pump, a 996 photodiode array detector, and a YMC-Pack SIL (150–10 mm) NP-HPLC column. All solvents were HPLC grade and were used without further purification.
Structural elucidation
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 300-MHz spectrometer at 300 MHz (1H) or 75 MHz (13C) using TMS as an internal chemical shift reference. The spectra were recorded in CDCl3, with the solvent signals at d 7.26 (for protons) and d 77.0 (for carbon) used as references. Mass spectra were obtained in a mass spectrometer APCI-HR-MS (JEOL LC-mate mass spectrometer).
Results and Discussion
Twenty five strains of endophytic fungi were isolated from the leaves of plants collected in Panama's protected areas with the aim of screening the isolates for antiparasitic and anticancer bioactivities (Table 1).
Table 1.
Sample ID, isolate code, and results of BLAST searches of NCBI-GenBank using bidirectionally sequenced, manually edited sequence data from the nuclear ribosomal internal transcribed spacer and 5.8S gene, including accession number, description, and, for those fungi whose top BLAST hits were to unnamed isolates, the highest-matching named isolate (top named hit). Taxonomy was estimated only on the basis of BLAST analyses, such that these species and genus names should be treated as estimations, rather than as the definitive identities of these fungi
| Sample | Isolate | Accession No. | Description | Top named hit | Host plant |
|---|---|---|---|---|---|
| F0194 | 3993 B1-2[3](10) | AJ542528.1 | Uncultured fungus voucher CSIRO (M) E7391 | Fungal endophyte isolate 85 | Desmotes incomparabilis (Riley) Kallunki (Rutaceae) |
| F0268 | 314B2-2 | EU040233.1 | Ochrocladosporium elatum strain CBS 146.33 | Tetrapterys sp. | |
| F0275 | 34A1(60) | EU686869.1 | Fungal endophyte isolate 1455 | Stenocarpella maydis culture-collection NRRL: 13615 | Platypodium elegans V. (Fabaceae) |
| F0305 | 3993B1(10)(72) | AB158315.1 | Trametes maxima* | Desmotes incomparabilis (Riley) Kallunki (Rutaceae) | |
| F0307 | 371B4[11](21) | FJ612751.1 | Fungal sp. ARIZ L262 | Xylariaceae sp. E6923c | Desmotes incomparabilis (Riley) Kallunki (Rutaceae) |
| F0324 | 215C1 | HM537066.1 | Fungal endophyte sp. g93 | Nigrospora sp. SGLMf43 | Ladenbergia brenesii |
| F0392 | 371B2[5](16) | AY908991.1 | Camarops ustulinoides strain PR113 | Fungal endophyte isolate 1947 | Desmotes incomparabilis (Riley) Kallunki (Rutaceae) |
| F0404 | 3994 B2-3[7](21) | EU687033.1 | Fungal endophyte isolate 2157 | Cercophora sparsa voucher JF00229 | Desmotes incomparabilis (Riley) Kallunki (Rutaceae) |
| F0407 | 3993 B1-2[1](8) | EU687033.1 | Fungal endophyte isolate 2157 | Cercophora sparsa voucher JF00229 | Desmotes incomparabilis |
| F0755 | 349B1 | EF565744.1 | Edenia gomezpompae strain C1c 18S | Petrea volubilis L (Verbenaceae) | |
| F0819 | EU687098.1 | Fungal endophyte isolate 343 | Xylariales cf. JP14-3 | Poulsenia armata | |
| F1220 | 420B4-14 | FJ799950.1 | Xylaria sp. SAB-2009a strain Q1343 | unidentified (Poaceae) | |
| F1491 | 157B2(368) | AJ558114.1 | Nectria mauritiicola | Macuna mutisiana (Kunth) DC. (Fabaceae) | |
| F1534 | 65B6(30) | DQ480346.1 | Penicillium paxilli isolate A71 | Philodendron tripartitum (Jacq.) Schott (Araceae) | |
| F1544 | 20C4(68) | HQ631021.1 | Aspergillus sp. TMS-2011 voucher BGd1p 19-4 | Unidentified | |
| F1559 | EU563525.1 | Fungal sp. 144C2i | Diaporthe eres isolate MB0839_1 | Unidentified | |
| F1566 | 20B3(4) | FJ799940.1 | Diaporthe sp. SAB-2009a strain Q1 160 | Unidentified (Cyperaceae) | |
| F1642 | 68B1(24) | FJ612855.1 | Fungal sp. ARIZ L525CLA | Xylaria sp. F-069,049 | Siparuna pauciflora (Beurl.) A. DC. (Siparunaceae) |
| F1644 | 15C1(267) | EF565744.1 | Edenia gomezpompae strain C1c | Drymonia serrulata | |
| F1647 | 164B2(1) | EU563572.1 | Fungal sp. 192c2g | Diaporthe phaseolorum strain AK25A | Castilla elastica Sessé & Cerv. (Moraceae) |
| F1664 | 86A2(86) | FJ434202.1 | Hypocrea sp. LY 30.1 | Cecropia longipes Pitt. (Cecropiaceae) | |
| F1717 | 125B1(107) | EU490050.1 | Uncultured ascomycete clone C31_C08 | Shiraia bambusicola | Sloanea zuliaensis Pittier (Elaeocarpaceae) |
| F1815 | 59A1(219) | EU686869.1 | Fungal endophyte isolate 1455 | Phomopsis sp. WF167W | Vitis tiliifolia Humb. & Bonpl. (Vitaceae) |
| F2140 | 433B1-4 | EU167598.2 | Mycosphaerella stromatosa** | Psychotria sp. (Rubiaceae) |
Some of these isolates were tentatively identified on the basis of careful evaluation of sequence similarity to known species available through GenBank. Others could not be identified due to a lack of sporulation and because there were no closely related species available in online databases for phylogenetic analysis. Alignments of the ITS regions of 12 isolates showed 100% identity with sequences deposited in GenBank, while for 13 other isolates this same high identity was lacking. Based on the molecular identification, four of the isolates obtained were Xylaria sp., three were Diaporthe sp., two were Cercophora sp., two were Edenia sp., and the remaining 14 belonged to different families. These results reflect the great diversity of fungal endophytes associated with various Panamanian plants. They also suggest that the composition and distribution of fungal endophytes are notably affected by the specific environment and by the fungi's hosts.
Most of the fungal families identified in this study have been previously reported as endophytes in other plants. Colletotrichum species are involved in plant diseases; in fact, this genus is listed among the most destructive post-harvest pathogens of cereals, legumes, fruits, and vegetables [11]. Diaporthe species, which are commonly found as endophytes, are also considered as pathogens, causing important economic losses in some countries [29]. Xylaria is often found in dead wood; note that one species of this family, isolated from Siparuna sp. (Siparunaceae), reportedly shows antimalarial activity [18].
Following isolation of the endophytes, fermentation was carried out on small scale. The resulting culture broths were separated from the mycelia by filtration under vacuum and the filtrate submitted to liquid-liquid partition with ethyl acetate (EtOAc). The organic solvent was evaporated under reduced pressure to dryness to yield an EtOAc extract. Mycelia were extracted with the same solvent. Because of their chromatographic similarities, as determined by thin-layer chromatography, the extracts were pooled to yield a single fungal extract for each of the different culture media. The activities of these extracts were evaluated against three pathogenic parasites, L. donovani, P. falciparum, and T. cruzi, and against the MCF-7 cancer cell line. The antiparasitic and anticancer activities of the fungal extracts are summarized in Table 2.
Table 2.
The growth-inhibitory effect (% IG) of total extracts from active endophytic fungi against tropical parasites and MCF-7 cancer cell line
| Isolate | CM | L. donovani | P. falciparum | T. cruzi | MCF-7 |
|---|---|---|---|---|---|
| F0275 | A | 83.0 | I | I | I |
| B | 71.1 | 76.2 | 92.7 | I | |
| D | 72.1 | I | I | I | |
| F0755 | A | 98.5 | I | I | I |
| B | 98.1 | I | I | I | |
| C | 96.8 | I | I | I | |
| D | 97.4 | I | I | I | |
| F1220 | D | 65.3 | I | I | I |
| F1534 | B | I | 98.0 | I | I |
| C | 92.9 | I | 76.5 | I | |
| F1544 | A | 70.1 | I | I | I |
| B | 72.9 | I | I | I | |
| C | 77.2 | I | I | I | |
| F1566 | C | 98.3 | I | 86.7 | I |
| D | 98.1 | I | I | I | |
| F1642 | C | I | I | I | 74.0 |
| F1647 | C | 80.0 | I | I | I |
| D | 71.0 | I | I | I | |
| F0194 | B | 93.4 | I | I | I |
| F1491 | B | 76.2 | I | 81.1 | 79.9 |
| C | 97.2 | I | I | I | |
| F1815 | C | I | 75.8 | I | I |
| F0305 | A | I | 70.2 | I | I |
| F0307 | A | I | 70.6 | I | I |
| B | 66.2 | 68.3 | I | 93.8 | |
| C | I | I | I | 90.4 | |
| F2140 | A | 94.4 | I | 91.9 | 86.5 |
| B | 93.6 | I | 94.3 | I | |
| C | 96.7 | 100.0 | 95.9 | 87.3 | |
| D | 96.8 | 88.7 | 92.8 | 80.3 | |
| F1644 | B | 95.4 | I | I | I |
| C | 96.0 | I | I | I | |
| F0268 | A | I | 77.2 | 80.4 | I |
| B | I | I | I | 78.7 |
CM, culture medium used for fungal growth. A, malt extract. B, potato dextrose broth. C, Czapek Dox. D, V8 juice. I, inactive.
Anti-leishmanial activity
Of the 25 fungi tested, seven (Edenia sp. strain F0755, Penicillium sp. strain F1534, Diaporthe sp. strain F1566, unidentified sp. strain F0194, Nectria sp. strain F1491, Mycosphaerella sp. strain F2140 and Hypocrea sp. strain F1644) showed marked anti-leishmanial activity, with at least one extract of each of these fungi showing >90% inhibition. Other strains, such as Stenocarpella sp. strain F0275, Xylaria sp. strain F1220, Aspergillus sp. strain F1544, Diaporthe sp. strain F1647, and Xylariaceae sp. strain F0307, showed moderate antileishmanial activity (at least one extract of each fungus showed >60% inhibition). These results suggest that new antileishmanial drugs can be obtained from endophytic fungi.
Thus far, only a few natural fungal-derived compounds with antileishmanial activity have been described. IB-01212, an antitumoral cyclodepsipeptide isolated from the marine fungus Clonostachys sp., was shown to have leishmanicidal activity on promastigote and amastigote forms of the parasite at low micromolar concentrations [20]. Chaetoxanthone C, isolated from the marine fungus Chaetomium sp., was reported to exhibit moderate activity against T. cruzi, with an IC50 value of 1.5 μg/ml [26].
Antimalarial activity
Only three strains (Stenocarpella sp. strain F0275, Nectria sp. strain F1491, and Mycosphaerella sp. strain F2140) had antimalarial activity (>90% inhibition of the parasite). Another five isolates (Penicillium sp. strain F1534, Phomopsis sp. strain F1815, Trametes sp. strain F0305, Xylariaceae sp. strain F0307, and Ochrocladosporium sp. strain F0268) showed moderate inhibition (Table 1).
Antimalarial activity has been demonstrated in compounds isolated previously from endophytic fungi. Monocerin and 11-hydroxymonocerin, natural products isolated from the endophytic fungus Exserohilum rostratum, are active against P. falciparum (K1, multidrug-resistant strain) with IC50 values of 0.68 and 7.70 μM, respectively [28]. The benzoquinone metabolites 2-chloro-5-methoxy-3-methylcyclohexa-2,5-diene-1,4-dione and xylariaquinone A, obtained from Xylaria sp., are active in vitro against P. falciparum K1 strain, with IC50 values of 1.84 and 6.68 μM, respectively [30]. Phomoxanthones A and B, isolated from Phomopsis sp. BCC 1323, show significant activity against Plasmodium falciparum (K1, multi drug-resistant strain) [16].
Anti-trypanosomiasis activity
Most of the extracts in this study failed to significantly inhibit Trypanosoma cruzi. Among the 25 isolates, high activity against this parasite was determined only in Mycosphaerella sp. strain F2140. However, moderate inhibition was obtained with extracts from Stenocarpella sp. strain F0275, Penicillium sp. strain F1534, Diaporthe sp. strain F1566, Nectria sp. strain F1491, and Ochrocladosporium sp. strain F0268 (Table 1).
As with leishmaniasis, only a few fungally derived compounds able to inhibit trypanosomiasis have been described. Ascofuranone, isolated from the fungus Ascochyta viciae, has shown good therapeutic effects for African trypanosomiasis in mice [25].
Anticancer activity in vitro
In the anticancer assay, the pattern was similar to that observed in the bioassay against T. cruzi. In fact, good anticancer activity was confirmed in only two strains (Xylariaceae sp. strain F0307 and Mycosphaerella sp. strain F2140). Of the strains tested, four (Diaporthe sp. strain F1566, Xylaria sp. strain F1642, Nectria sp. strain F1491, and Ochrocladosporium sp. strain F0268) produced metabolites that moderately (60–80%) inhibited the cancer cell line MCF-7 (Table 1).
Endophytic microorganisms are major reservoirs of anticancer secondary metabolites. For example, two new norsesquiterpene peroxides (talaperoxides B and D), isolated from the mangrove endophytic fungus Talaromyces flavus are cytotoxic against five human cancer cell lines (MCF-7, MDA-MB-435, HepG2, HeLa, and PC-3), with IC50 values ranging from 0.70 to 2.78 μg/mL [19]. The cyclic depsipeptide 1962A, isolated from an unknown mangrove endophytic fungus, is weakly active against human breast cancer MCF-7 cells [13]. Moderate activity against four cancer cell lines, NCI-H460 (non-small cell lung cancer), MCF-7 (breast cancer), SF-268 (CNS glioma), and MIA Pa Ca-2 (pancreatic carcinoma), and normal human fibroblast cells (WI-38) [3], was determined for globosumones A and B, isolated from Chaetomium globosum, an endophyte of Ephedra fasciculata.
Effect of culture media on parasitic activity
Endophytic fungi are a potential source of a variety of new metabolites, which can be produced by a single strain following systematic modification of its cultivation parameters, a phenomenon known as the OSMAC approach (one strain of many compounds) [12]. This approach reflects the fact that very small changes in the growing conditions of a microorganism can completely alter its metabolic profile. Consequently, the same strain can express various activities depending on the culture medium and/or the specific culture environment [12]. Based on these observations, the fungi examined in this study were grown on four media containing different amounts of nutrients and the effects on bioactivity were determined.
Indeed, each medium was found to have a particular effect on biological activity. Extracts with greater activity were consistently obtained when the fungi were grown on nutrient-poor Czapek Dox medium. Nutrient limitations are a typical form of stress in fungi, as in other organisms, and the transduction of these stress signals induces a protective response to allow survival in a hostile environment. Of the three nutrient-rich media used in this study, potato dextrose broth yielded much more active extracts than obtained with the other two media, most likely due to differences in the amounts of carbon and nitrogen sources [9,31].
Isolation of bioactive compounds from Edenia sp. strain F0755 and Mycosphaerella sp. nov. strain F2140
The main goal of our research group is to find alternatives for the treatment of parasitic diseases, which the WHO still consideres as neglected diseases. Parasitic diseases and cancer are widespread, affecting populations in several regions of the world [10,14]. The drugs used to treat these diseases are highly toxic, such as the arsenic-based compounds prescribed for the treatment of Leishmania [10]. In fact, in some cases patients die from the effects of the drug itself, rather than directly from the disease.
Endophytic fungi are a promising source of novel therapeutic agents and are of particular interest in the treatment of leishmaniasis and malaria. Many studies have shown that they are a promising source of new, biologically active compounds, including secondary metabolites of biological utility. In light of these observations, we chemically analyzed Edenia sp. strain F0755 and Mycosphaerella sp. nov. strain F2140; those studies are summarized below.
Edenia sp. strain F0755
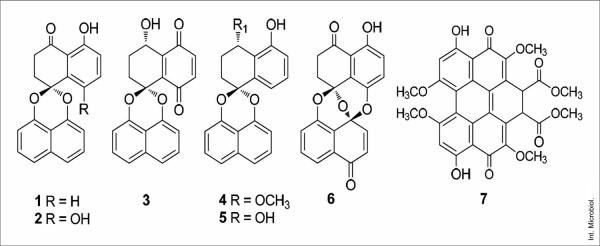
Figure 1 shows the active compounds isolated from this fungus. The six compounds were identified as non-symmetrical dimers of 1,8-dihydroxynaphthalene. Compounds 2, 3, and 4 are new natural products but all six compounds significantly inhibited the growth of Leishmania donovani in the amastigote form, with IC50 values of 3.93, 1.34, 0.62, 11.6, 8.40, and 0.12 μM, respectively. However, at concentrations of 10 μg/ml none of the compounds was active against Plasmodium falciparum and Trypanosoma cruzi, which suggested the selective activity of all of them against Leishmania. In addition, compounds 1–6 showed weak cytotoxicity to Vero cells (IC50 of 162, 174, 152, 140, 9, and 150 μM, respectively). The therapeutic window of these compounds was determined to be highly significant, with, respectively, 41, 130, 245, 18, 75 and 12 times greater anti-leishmanial activity than cytotoxicity [21,22].
Fig. 1.
Antiparasitic metabolites isolated from Edenia sp. strain F0755 (1–6) and from Mycosphaerella sp. nov. strain F2140 (7). (1) Palmarumycin CP2; (2) palmarumycin CP17; (3) palmarumycin CP18; (4) palmarumycin CP19; (5) CJ-12,371 and (6) preussomerin EG1, (7) cercosporin.
Mycosphaerella sp. nov. strain F2140
Our chemical investigations of a new endophytic fungus, Mycosphaerella sp. nov. strain F2140, associated with the foliage of the plant Psychotria horizontalis (Rubiaceae), resulted in the isolation of cercosporin (Fig. 1, compound 7), an antiparasitic compound, and of four other compounds, as major components. The isolated compounds were tested in vitro to determine their antiparasitic activity against the causal agents of malaria (Plasmodium falciparum), leishmaniasis (Leishmania donovani), and Chagas disease (Trypanosoma cruzi), but only cercosporin showed potent antiparasitic activity. To evaluate the influence of the hydroxyl groups of cercosporin on its anti-parasitic activity, the acetylated derivative of this compound was prepared. Cercosporin and its acetylated derivative were highly potent against L. donovani (IC50 0.46 and 0.64 μM, respectively), T. cruzi (IC50 1.08 and 0.78 μM, respectively), and P. falciparum (IC50 1.03 and 2.99 μM, respectively). Both compounds also showed potent cytotoxic activity against the human breast cancer cell line MCF-7 (IC50 4.68 and 3.56 μM, respectively). This result suggests that the high antiparasitic activity of cercosporin is due to its high cytotoxicity [24].
In conclusion, endophytic fungi may be a promising source of bioactive natural products, since 64% of the strains analyzed in this study exhibited antiparasitic activity. Nine of these strains (Edenia sp. strain F0755, Xylaria sp. strain F1220, Aspergillus sp. strain F1544, Diaporthe sp. strain F1647, unidentified sp. strain F0194, Edenia sp. strain F1644, Phomopsis sp. strain F1815, Trametes sp. strain F0305 and Xylaria sp. strain F1642) showed promising selective activity.
In previous studies of chemicals extracted from selected endophytic fungi, we obtained several compounds with potent anti-parasitic activity [16,20,25,26,28,30], which justifies the screening of endophytic fungi as a source of leading compounds for the development of antiparasitic drugs. Based on the excellent antiparasitic activity of the endophytic fungi Edenia sp. strains F0755 and Mycosphaerella sp. nov. strain F2140 in our preliminary bioassay, these isolates were subjected to further chemical study and their bioactive compounds further characterized.
The ongoing development of new antiparasitic agents is important to overcome the limitations related to the high toxicity of the drugs currently available for the treatment of diseases caused by tropical parasites [7]. The use of these toxic chemotherapeutic compounds, such as sodium stibogluconate and meglumine antimonate, is associated with numerous, potentially fatal side effects [7]. While endophytic fungi are an abundant and reliable source of metabolites with medicinal and agrochemical applications, they have been only scarcely explored as sources for antiparasitic agents. As evidenced by our findings, Panamanian plants might provide a wealth of new antiparasitic and anticancer compounds.
Acknowledgements
We thank G. Keller, I. Martinez and L. Segundo for their support in fungal isolation, and P.D. Coley and T.A. Kursar for scientific input. Funding was provided from the Fogarty Center's International Cooperative Biodiversity Groups Program (grant number 1U01 TW 006634-01), from the NIH, the National Science Foundation, and the U.S. Department of Agriculture.
Footnotes
Competing interests. None declared.
References
- 1.Arnold AE, Maynard Z, Gilbert G, Coley PD, Kursar TA. Are tropical fungal endophytes hyperdiverse? Ecol Lett. 2000;3:267–274. [Google Scholar]
- 2.Arnold AE, Miadlikowska J, Higgins KL, Sarvate SD, Gugger P, Way A, Hofstetter V, Kauff F, Lutzoni F. A phylogenetic estimation of trophic transition networks for ascomycetous fungi: Are lichens cradles of symbiotrophic fungal diversification? System Biol. 2009;58:283–297. doi: 10.1093/sysbio/syp001. [DOI] [PubMed] [Google Scholar]
- 3.Bashyal BP, Wijeratne EM, Faeth SH, Gunatilaka AA. Globosumones A–C, cytotoxic orsellinic acid esters from the Sonoran Desert endophytic fungus Chaetomium globosum. J Nat Prod. 2005;68:724–728. doi: 10.1021/np058014b. [DOI] [PubMed] [Google Scholar]
- 4.Buckner FS, Verlinde CLMJ, La Flamme AC, Van Voorhis WC. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob Agents Chemother. 1996;40:2592–2597. doi: 10.1128/aac.40.11.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett Y, Herrera L, Gonzalez J, Cubilla L, Capson TL, Coley PD, Kursar TA, Romero LI, Ortega-Barria E. A novel DNA-based microfluorimetric method to evaluate antimalarial drug activity. Am J Trop Med Hyg. 2004;70:119–124. [PubMed] [Google Scholar]
- 6.Calderón A, Romero LI, Ortega-Barría E, Brun R, Correa MD, Gupta MP. Evaluation of larvicidal and in vitro antiparasitic activities of plants in a biodiversity plot in the Altos de Campana National Park, Panama. Pharm Biol. 2006;44:487–498. [Google Scholar]
- 7.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crous PW. Taxonomy and phylogeny of the genus Mycosphaerella and its anamorphs. Fungal Divers. 2009;38:1–24. [Google Scholar]
- 9.Demain AL. Regulation of secondary metabolism in fungi. Pure & Appl Chem. 1986;58:219–226. [Google Scholar]
- 10.Fernandes NF, Kovarik CL. Cutaneous manifestations of systemic tropical parasitic diseases. Dermatol Nurs. 2009;21:243–254. [PubMed] [Google Scholar]
- 11.Garcia-Pajon CM, Collado IG. Secondary metabolites isolated from Colletotrichum species. Nat Prod Rep. 2003;20:426–431. doi: 10.1039/b302183c. [DOI] [PubMed] [Google Scholar]
- 12.Grond S, Papastavrou I, Zeeck A. Novel α-l-rhamnopyranosides from a single strain of Streptomyces by supplement-induced biosynthetic steps. Eur J Org Chem. 2002;2002:3237–3242. [Google Scholar]
- 13.Huang H, She Z, Lin Y, Vrijmoed LL, Lin W. Cyclic peptides from an endophytic fungus obtained from a mangrove leaf (Kandelia candel) J Nat Prod. 2007;70:1696–1699. doi: 10.1021/np0605891. [DOI] [PubMed] [Google Scholar]
- 14.Hudson SV, Hahn KA, Ohman-Strickland P, Cunningham RS, Miller SM, Crabtree BF. Breast, colorectal and prostate cancer screening for cancer survivors and non-cancer patients in community practices. J Gen Intern Med. 2009;24:487–490. doi: 10.1007/s11606-009-1036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioset J-R. Natural products for neglected diseases: A review. Curr Org Chem. 2008;12:643–666. [Google Scholar]
- 16.Isaka M, Jaturapat A, Rukseree K, Danwisetkanjana K, Tanticharoen M, Thebtaranonth Y. Phomoxanthones A and B, novel xanthone dimers from the endophytic fungus Phomopsis species. J Nat Prod. 2001;64:1015–1018. doi: 10.1021/np010006h. [DOI] [PubMed] [Google Scholar]
- 17.Kursar TA, Capson TL, Coley PD, Corley DG, Gupta MB, Harrison LA, Ortega-Barría E, Windsor DM. Ecologically guided bioprospecting in Panama. Pharm Biol. 1999;37:114–126. [Google Scholar]
- 18.Jimenez-Romero J, Ortega-Barria E, Arnold AE, Cubilla L. Activity against Plasmodium falciparum of lactones isolated from the endophytic fungus Xylaria sp. Pharm Biol. 2008;46:1–4. [Google Scholar]
- 19.Li H, Huang H, Shao C, Huang H, Jiang J, Zhu X, Liu Y, Liu L, Lu Y, Li M, Lin Y, She Z. Cytotoxic norsesquiterpene peroxides from the endophytic fungus Talaromyces flavus isolated from the mangrove plant Sonneratia apetala. J Nat Prod. 2011;74:1230–1235. doi: 10.1021/np200164k. [DOI] [PubMed] [Google Scholar]
- 20.Luque-Ortega JR, Cruz LJ, Albericio F, Rivas L. The antitumoral depsipeptide IB-01212 kills Leishmania through an apoptosis-like process involving intracellular targets. Mol Pharmaceutics. 2010;7:1608–1617. doi: 10.1021/mp100035f. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Luis S, Della-Togna G, Coley PD, Kursar TA, Gerwick WH, Cubilla-Rios L. Antileishmanial constituents of the Panamanian endophytic fungus Edenia sp. J Nat Prod. 2008;71:2011–2014. doi: 10.1021/np800472q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Luis S, Cherigo L, Spadafora C, Gerwick WH, Cubilla-Rios L. Additional anti-leishmanial constituents of the panamanian endophytic fungus Edenia sp. Rev Latinoamer Quim. 2009;37:104–114. [Google Scholar]
- 23.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 24.Moreno E, Varughese T, Spadafora C, Arnold AE, Coley PD, Kursar TA, Gerwick WH, Cubilla-Rios L. Chemical constituents of the new endophytic fungus Mycosphaerella sp. nov. and their anti-parasitic activity. Nat Prod Commun. 2011;6:835–840. [PMC free article] [PubMed] [Google Scholar]
- 25.Osterhage C, Kaminsky R, König GM, Wright AD. Ascosalipyrrolidinone A, an antimicrobial alkaloid, from the obligate marine fungus Ascochyta salicorniae. J Org Chem. 2000;65:6412–6417. doi: 10.1021/jo000307g. [DOI] [PubMed] [Google Scholar]
- 26.Pontius A, Krick A, Kehraus S, Brun R, König GM. Antiprotozoal activities of heterocyclic-substituted xanthones from the marine-derived fungus Chaetomium sp. J Nat Prod. 2008;71:1579–1584. doi: 10.1021/np800294q. [DOI] [PubMed] [Google Scholar]
- 27.Rungjindamai N, Pinruan U, Choeyklin R, Hattori T, Jones EBG. Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Divers. 2008;33:139–16. [Google Scholar]
- 28.Sappapan R, Sommit D, Ngamrojanavanich N, Pengpreecha S, Wiyakrutta S, Sriubolmas N, Pudhom K. 11-Hydroxymonocerin from the plant endophytic fungus Exserohilum rostratum. J Nat Prod. 2008;71:1657–1659. doi: 10.1021/np8004024. [DOI] [PubMed] [Google Scholar]
- 29.Smit WA, Wingfield BD, Wingfield MJ. Integrated approach to controlling Diaporthe canker of deciduous fruit in South Africa. Recent Res Dev Plant Pathol. 1998;2:43–62. [Google Scholar]
- 30.Tansuwan S, Pornpakakul S, Roengsumran S, Petsom A, Muangsin N, Sihanonta P, Chaichit N. Antimalarial benzoquinones from an endophytic fungus, Xylaria sp. J Nat Prod. 2007;70:1620–1623. doi: 10.1021/np0701069. [DOI] [PubMed] [Google Scholar]
- 31.Tormo JR, García JB, DeAntonio M, Feliz J, Mira A, Díez MT, Hernández P, Peláez F. A method for the selection of production media for actinomycete strains based on their metabolite HPLC profiles. J Ind Microbiol Biotechnol. 2003;30:582–588. doi: 10.1007/s10295-003-0084-7. [DOI] [PubMed] [Google Scholar]