Synopsis
LIM-domain-binding 1 (LDB1) is a cofactor that participates in formation of transcriptional regulatory complexes involving transcription factors containing LIM domains as well as other factors. The amount of LDB1 protein in cells has previously been shown to be modulated by RNF12. RNF12 is an E3 ubiquitin ligase that can target LDB1 for poly-ubiquitination and degradation via the proteasome. We find that in HEK293 cells expression of RNF12 leads to mono-ubiquitination of LDB1 and increased levels of LDB1 protein. Mutagenesis studies identified Lys-134 of LDB1 as the residue that is mono-ubiquitinated by RNF12. Mutation of Lys-134 of LDB1 to Arg blocks the formation of mono-ubiquitinated LDB1 and surprisingly also increases LDB1 protein expression in HEK293 cells. This leads to a model in which Lys-134 of LDB1 can be either mono-ubiquitinated leading to stabilization or poly-ubiquitinated, leading to degradation by the proteasome pathway. We also find that ubiquitin-LDB1 fusion proteins are stabilized in HEK293 cells offering further evidence that mono-ubiquitination stabilizes LDB1 in these cells. Expression in Xenopus laevis embryos of an LDB1 protein in which Lys-134 is replaced with Arg, leads to enhanced expression of the mutant protein as compared to the wild type protein. These findings provide evidence that modification of Lys-134 can play a major role in regulating LDB1 expression.
Keywords: LIM-domain binding 1 (LDB1), ring finger protein 12 (RNF12), ubiquitin ligase, proteasome, protein degradation, post-translational modification
LDB1 is a highly conserved transcription cofactor that was initially identified by the ability to bind to LIM domains from LIM only and LIM homeodomain proteins [1]. A large number of studies in Drosophila by several laboratories have revealed several important aspects of LDB1 function [2–7]. These studies have led to a model in which an LDB1 dimer interacts with two LIM-homeodomain transcription factors. An important aspect of this model is the importance of the proper stoichiometry of LDB1 and LIM-homeodomain factors for forming the functional tetramer. The use of gene deletions, over-expression of proteins or protein domains and chimeric proteins have provided evidence supporting the importance of proper stoichiometry of LDB1 and LIM homeodomain factors for forming functional tetramers. This stoichiometry can also be disrupted by the LIM only protein dLMO. For instance, dLMO competes with the LIM-homeodomain factor, Apterous for binding to LDB1, disrupting the functional tetramer [3].
Interestingly, experiments in Drosophila demonstrated that LDB1 is necessary for segmentation although no LIM domain proteins are known to be required for this process [8]. This finding raised the possibility that LDB1 might have a LIM factor-independent function in modulating of transcription. This possibility was supported by subsequent experiments demonstrating the physical and functional interaction of LDB1 with a variety of non-LIM containing proteins [9–14]. The role of LDB1 as a component of transcriptional complexes including non-LIM domain proteins has been demonstrated in developing mouse neurons [15–16]. Overall there is a substantial amount of evidence suggesting that LDB1 is a component of a number of transcriptional complexes.
One of the several proteins that interacts with LDB1 is RNF12 [9]. RNF12 was found to act as a negative co-regulator for LDB1 by recruiting the Sin3A-containing histone deacetylase corepressor complex. Subsequently RNF12 was found to be an E3 ubiquitin ligase for LMOs and LDB1 [17]. This is an interesting observation in view of the importance of LDB1 protein levels for the formation of transcriptional complexes. It was also shown that RNF12 ubiquitinates LDB1 bound to LIM-homeodomain transcription factors, leading to a cofactor exchange. Studies in Xenopus have also shown that the LIM-homeodomain factor, Xlim-1, through binding to LDB1, can suppress RNF12 mediated binding and degradation of LDB1[18]. Single-stranded DNA binding protein 2 has also been shown to interact with LDB1, protecting it from RNF12-mediated degradation [19].
In the present study we report the identification of a specific lysine, Lys-134 of LDB1, as the site of RNF12-mediated ubiquitination and as a critical regulator of LDB1 stability. Somewhat surprisingly we see stabilization of LDB1 which appears to be mediated by mono-ubiquitination in the presence of RNF12 in some cells. We observe this in several tissue culture cell lines while in other cell lines we see that RNF12 expression leads to the previously observed degradation of LDB1. The mono-ubiquitination, stabilization, and degradation activities we observe depend on the RING finger domain of RNF12. Simulating mono-ubiquitination by fusion of ubiquitin to LDB1 greatly stabilizes LDB1 in HEK293 cells and this effect is also cell specific.
EXPERIMENTAL
Cell culture, DNA constructs, and transfections
Human embryonic kidney 293 cells (HEK293) and human breast adenocarcinoma cells (MCF7) were maintained in DMEM containing 10% fetal bovine serum. A reporter gene containing three LIM homeodomain protein binding sites linked to a minimal TATA box and the luciferase coding sequence has been described previously [20]. Mammalian expression vectors for GAL4 fusions have been described previously [21]. Expression constructs for 6X-His-tagged ubiquitin and Myc-His double tagged ubiquitin K48R were obtained from Dr. Hua Lu, Indiana School of Medicine. The coding sequences for mouse and human RNF12, human UBCH5 and UBCH8, and LDB1 were amplified by the polymerase chain reaction using standard protocols. Mouse Rnf12 and LDB1 were both subcloned into the expression vectors, pcDNA3 and pCS2+. The products were all confirmed by automated DNA sequencing. Cells were typically transfected with a total of 2 µg DNA and 5 µl of Lipofectamine 2000 (Invitrogen, Inc.) in 35 mm well plates, or 0.8 µg DNA and 2 µl Lipofectamine in 22 mm well plates using a protocol provided by the supplier.
Real time polymerase chain reaction
RNA and then first strand cDNA was made from transfected HEK293 cells using TRIZOL and then SuperScriptII reverse transcriptase according to the manufacturer’s instructions (Invitrogen). Real time PCR was performed using SYBR green reagent mix from Applied Biosystems with the ABI Taqman 7900HT real time PCR machine. Primers used for mouse GAPDH were: 5’-CTCTGCCACCCAGAAGACTGT and 5”-GGAAGGCCATGCCAGTGA. Primers used for FLAG-tagged mouse LDB1 were: 5’-AGCCAAGAGAGCAGATCGGAGAAT and 5’-TGCCTTGTCATCGTCGTCCTTGTA.
Preparation of cell extracts
For immunoblotting or immunoprecipitations, cells were scraped from the culture dishes in phosphate buffered saline. The cells were pelleted in a microfuge and resuspended in 100 mM sodium phosphate with 0.1% NP-40. The cells were disrupted by 4 cycles of freeze thaw using dry ice/ethanol and 37° C water baths. After centrifugation at 10,000 × g for 5 min at 4° C, the supernatant was saved as a whole cell extract. For preparation of cell extracts for luciferase assays cell monolayers were rocked for 15 minutes in 100 mM sodium phosphate, pH 7.8, 1 % Triton X-100. Cell debris was removed by transferring the extract to microfuge tubes and centrifuging for 2 min.
In vitro transcription and translation
A commercially available kit (TNT SP6 High-Yield Protein Expression System, Promega Corp.) was used to express ubiquitin fused LDB1 in vitro. A linear PCR product containing an SP6 promoter ahead of the coding sequence of epitope tagged ubiquitin fused LDB1 was used as a template as suggested by the manufacturer. Ubiquitin-aldehyde was purchased from Calbiochem.
In Vitro RNA Synthesis and Xenopus Injections
Ovulation was induced by injecting female frogs with human chorionic gonadotropin (Sigma). Capped synthetic mRNA was synthesized by in vitro transcription from linearized templates using a commercially available kit (MegaScript, Ambion, Inc.) and injected into embryos as described previously [22].
In Vivo Ubiquitination Assay
The in vivo ubiquitination assay was performed as previously described [23]. Transfected cells in 35 mm wells were collected by scraping in PBS. 10% of the cells were reserved for an immunoblotting extract as described above. The rest of the cells were disrupted by suspension in 1 ml of 6 M guanidinium-HCL, 0.1 M Na2HPO4/NaH2PO4, pH 8.0, 10 mM Tris-HCl, pH 8.0, 10 mM β-mercaptoethanol (Buffer A). 20 µl of a 50% slurry of Ni-NTA beads from Qiagen was then added and the mixture rotated at room temperature for 2 hours. The beads were then washed with 1 ml of Buffer A, then 1 ml of 8 M urea, 0.1 M Na2HPO4/NaH2PO4, pH8.0, 10 mM Tris-HCl, pH 8.0, 10 mM β-mercaptoethanol, then 1 ml of 8 M urea, 0.1 M Na2HPO4/NaH2PO4, pH8.0, 10 mM Tris-HCl, pH 6.3, 10 mM β-mercaptoethanol. Bound proteins were eluted from the Ni-NTA beads by incubation for 5 min. at room temperature with two serial 20 µl aliquots of 200 mM imidazole, 0.15 M Tris-HCl, pH 6.7, 30 % glycerol, 0.72 M β-mercaptoethanol, and 5 % SDS. Proteins bound to Ni-NTA beads were then analyzed by electrophoresis on a denaturing, polyacrylamide gel and immunoblotting as described below.
Antiserum, immunoprecipitations, and immunoblotting
Monoclonal antibody to the FLAG epitope both free and covalently attached to agarose was obtained as from Sigma, Inc. Rabbit polyclonal antiserum to LDB1 was obtained from Biovintage. Rabbit anti-AU1 antiserum and the P4D1 anti-ubiquitin monoclonal antibody were obtained from Covance. For immunoprecipitations, cell extracts were adjusted to contain 0.1% Tween-20. Aliquots containing equal amounts of total protein were combined with 15 µl of a 50% slurry of anti-FLAG agarose or rabbit anti-AU1 serum and protein A/G agarose. The immunoprecipitation mixtures were rotated for 2 h at 4° C and the agarose bound antibodies were collected by centrifugation. The agarose beads were then washed 3 times with 1 ml each of 10 mM Tris, pH 7.4, 150 mM NaCl, 0.1 % Tween-20, 0.1% Triton X-100. Proteins bound to the agarose beads were then analyzed by electrophoresis on a denaturing, polyacrylamide gel. For immunoblotting, proteins were transferred to polyvinylidene difluoride membranes (Millipore). Blocking reactions, incubation with a 1:5,000 dilution of antiserum to FLAG, ubiquitin, or LDB1, incubation with a 1:10,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antibody (Santa Cruz) and incubation with chemiluminescent reagent (Amersham Renaissance) were all performed as suggested by the suppliers.
RESULTS
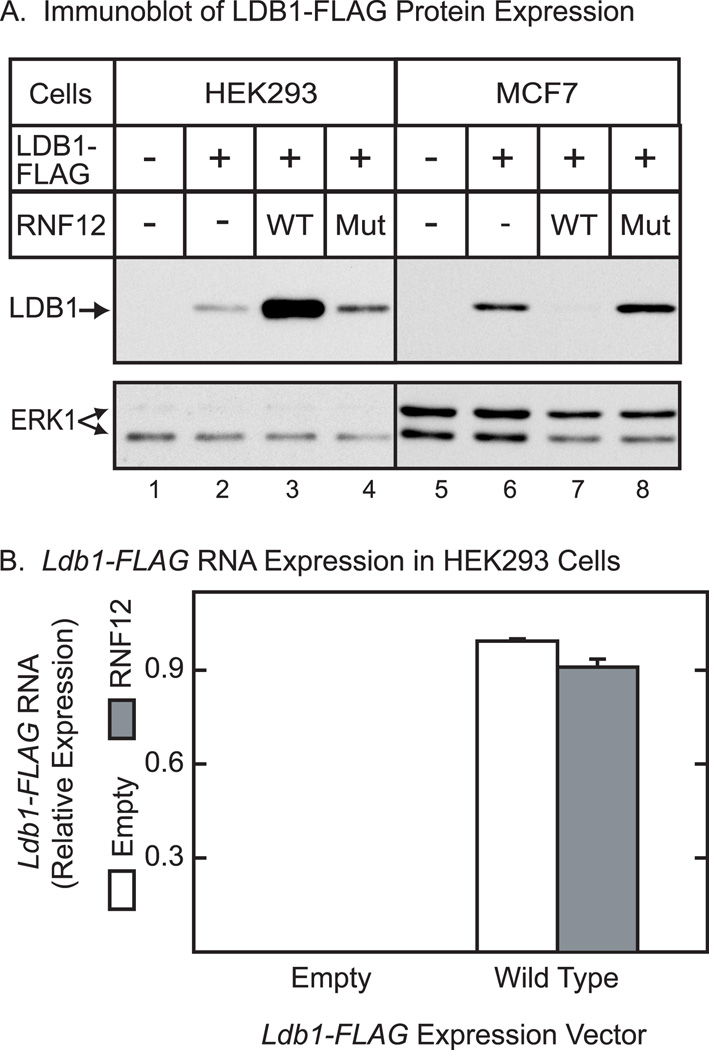
Previous studies have shown that RNF12 acts as a ubiquitin ligase for LDB1 leading to degradation of LDB1 through the 26S proteasome pathway [17–18]. Therefore, it was somewhat surprising to find that expression of RNF12 in HEK293 cells leads to an increase in the level of LDB1 protein (Fig. 1A). This increase is dependent on the RING domain of RNF12 as a mutant RNF12 protein that abolishes zinc binding and ubiquitin ligase activity [18] has little or no effect to increase LDB1 protein levels. In contrast and consistent with previous reports, RNF12 expression was found to reduce LDB1 protein levels in the MCF7 cell line. The RNF12 expression vectors had little effect on ERK1 in either HEK293 cells or MCF7 cells. The effects of RNF12 to enhance LDB1 levels in HEK293 cells appear to be at the post-transcriptional level as RNF12 did not increase the amount of Ldb1 RNA in HEK293 cells (Fig. 1B). This finding and the known activity of RNF12 as a ubiquitin ligase suggests that RNF12 probably influences LDB1 protein levels by altering proteasome-mediated degradation of LDB1. The differing effects of RNF12 on LDB1 expression in HEK293 and MCF7 might reflect differences in expression in the two cell lines. To examine this possibility, we explored a broad range of RNF12 expression levels in HEK293 cells. At all tested expression levels, RNF12 enhanced LDB1 expression in HEK293 cells (data not shown).
Figure 1. RNF12 has cell-specific effects on expression of LDB1 at a post-transcriptional level.
(A) HEK293 or MCF7 cells were transfected with an expression vector for FLAG-tagged LDB1 and either an empty expression vector (−), or vectors coding for wild type RNF12 (WT) or RNF12 with a mutation that inactivates ubiquitin ligase activity (Mut). Whole cell extracts were prepared 20 h after transfection and the extracts resolved by denaturing polyacrylamide gel electrophoresis, transferred to a membrane and then incubated with FLAG antiserum to detect epitope-tagged LDB1. A horseradish peroxidase labeled anti-rabbit secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. The immunoblots were stripped and then probed with antibody to ERK1 as a loading control. (B) HEK293 cells were transfected with expression vectors for LDB1-FLAG, RNF12 and empty expression vectors as indicated. At 20 h after transfection, RNA was prepared from cells. Ldb1 and GAPDH RNA were quantitated by real-time PCR. Ldb1 RNA expression in each sample was normalized to GAPDH in each sample and then relative RNA levels were normalized so that the wild type average value for the Ldb1 group was set to 1.0. Values are means +/− SEM for three separate transfections.
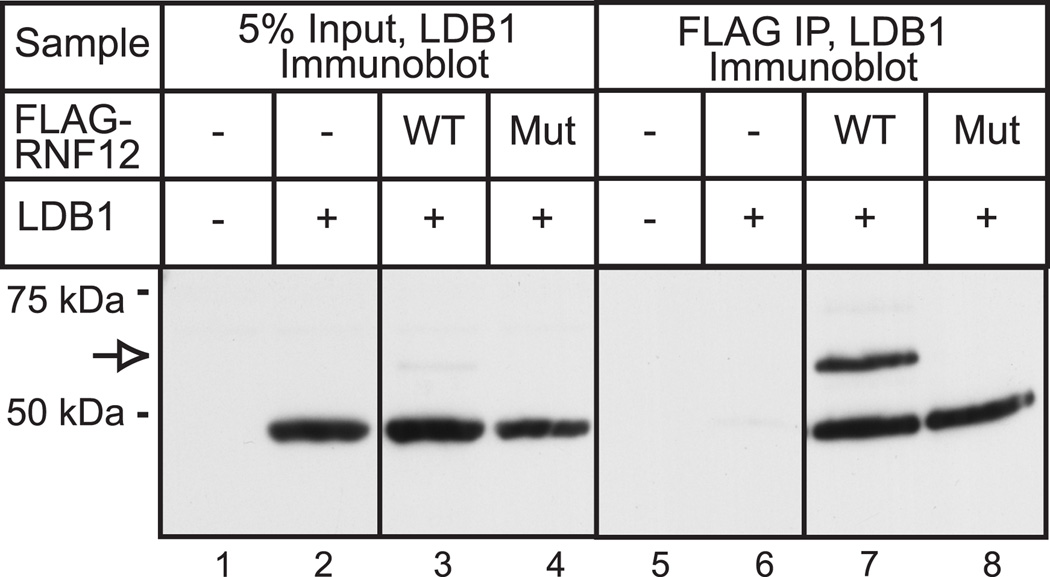
A co-immunoprecipitation study examining the interaction of the full length LDB1 with RNF12 in HEK293 cells provided a useful insight. HEK293 cells were transiently transfected with expression vectors for FLAG epitope-tagged RNF12 or RNF12 mutant and un-tagged LDB1. The tagged RNF12 was recovered using a FLAG monoclonal antibody and co-immunoprecipitated LDB1 was visualized by blotting using a polyclonal antiserum (Fig. 2). In this experiment relatively large amounts of DNA were transfected and long exposure times were used to enhance detection of relatively minor components. Using these conditions, the effects of RNF12 to stabilize LDB1 are not as evident (Fig. 2, lanes 1–4). However, these conditions did permit identification of two LDB1-immunoreactive bands that were co-immunoprecipitated with wild type RNF12 (Fig. 2, lane 7). One of the bands migrated as expected for full length LDB1 and a more slowly migrating band was also detected, corresponding to addition of an approximately 9 kiloDalton mass to LDB1. Although LDB1 was found to co-immunoprecipitate with the RNF12 mutant, the more slowly migrating band, presumably representing modified LDB1 is absent (Fig. 2, lane 8). As RNF12 has been previously demonstrated to ubiquitinate LDB1, these findings are consistent with a role of RNF12 in ubiquitination of LDB1 in HEK293 cells. Subsequent experiments provide evidence that this modification is ubiquitin (see below). Under these conditions in HEK293 cells, it appears that RNF12 leads to mono-ubiquitination which is associated with LDB1 stabilization rather than poly-ubiquitination and degradation as occurs in other cell types [17–18]. Interestingly, in the RNF12 co-immunoprecipitate, the more slowly migrating form of LDB1 is similar in intensity to the full-length, non-modified LDB1. This contrasts to the input material, where the more slowly migrating form of LDB1 is difficult to detect and therefore must be much less abundant than the non-modified LDB1. This finding suggests that RNF12 either binds preferentially to the more slowly migrating mono-ubiquitinated form of LDB1, or is able to mono-ubiquitinate bound LDB1 in the immunoprecipitation.
Figure 2. A slowly migrating form of LDB can be identified following expression of RNF12 and LDB1 in HEK293 cells.
HEK293 cells were transfected with vectors for LDB1, FLAG-tagged wild type (WT) or mutant (Mut) RNF12 or empty expression vector (−) as indicated. At 20 h after transfection, whole cell extracts were prepared. A portion of the whole cell extract was analyzed by denaturing gel electrophoresis and immunoblotting with polyclonal antisera to LDB1 to detect the input expression of the LDB1 (5% Input, lanes 1–4). FLAG-tagged-RNF12 was isolated from the cell extract by immunoprecipitation with FLAG monoclonal antibody and the immunoprecipitate was analyzed by denaturing gel electrophoresis and immunoblotting with LDB1 rabbit polyclonal antibody to detect co-immunoprecipitated LDB1 (Immunoprecipitate, lanes 5–8). Immunoblotting detected proteins with the appropriate migration for full-length LDB1 and a more slowly migrating form of the protein (open arrow).
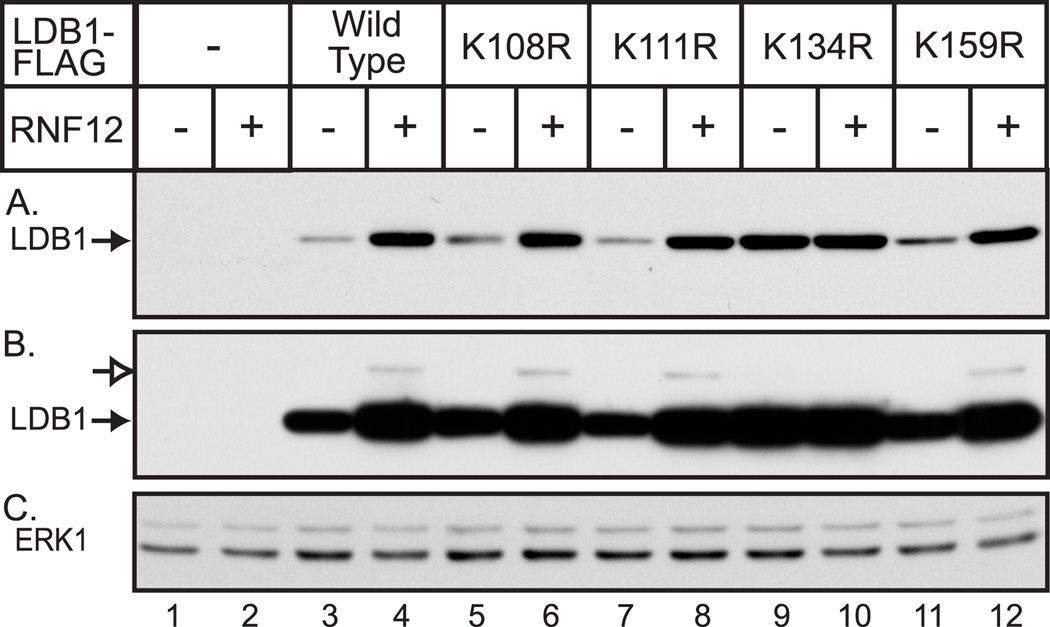
The ability of wild type RNF12 to cause the formation of a discrete, size-shifted LDB1, in HEK293 cells suggested the possibility that enhanced levels of LDB1 is due to mono-ubiquitination induced protein stabilization. The ability of RNF12 to induce the appearance of a shifted LDB1 band provided an assay to identify the relevant single lysine or lysines. Mutation of a unique lysine residue that is mono-ubiquitinated should abolish the shifted band. We initially tested a number of lysine mutations in the carboxy terminal half of LDB1 (residues 196 to 375). In the context of full-length LDB1, these mutations tended to have a relatively small effect to increase LDB1 protein levels and none of the mutations substantially reduced or eliminated the more slowly migrating form of LDB1 (data not shown). Consequently, attention was changed to focus on lysine residues in the amino-terminal region of LDB1. A preliminary amino-terminal deletion indicated that the lysine at position 40 of LDB1 was not required for the RNF12-mediated shifted LDB1 band (data not shown). Therefore, all of the other lysines within residues 1–198 of LDB1 were mutated to arginine. HEK293 cells were transiently transfected with vectors for FLAG epitope-tagged wild type or mutant LDB1 in the absence or presence of an RNF12 expression vector and cell extracts were immunoblotted with FLAG monoclonal antibody to detect LDB1 (Fig. 3A). With the exception of Lys-134, all of the lysine mutants appear to be somewhat stabilized relative to wild-type in the absence of RNF12 and were further stabilized by co-expression of RNF12. Similar results were obtained after mutation of lysine 79 or 192 to Arg (data not shown). Mutation of Lys-134 to Arg dramatically increased LDB1 protein levels relative to wild-type and this mutant LDB1 was essentially unaffected by co-expression of RNF12 (Fig. 3A, lanes 9,10). To detect the more slowly migrating form of LDB1, a long exposure of the immunoblot was used (Fig. 3B). Under these conditions the shifted LDB1 species, indicated by the open arrow, is visible for the wild-type LDB1 and for the mutants, except for the Lys-134 mutant. These findings provide evidence that expression of the E3 ubiquitin ligase, RNF12, leads to mono-ubiquitination ofLys-134 of LDB1. Lys-134 of LDB1 is apparently the major target of RNF12-mediated ubiquitination as mutation of this residue both eliminates the more slowly migrating, modified form of LDB1 and dramatically stabilizes LDB1 in HEK293 cells. In view of the finding that RNF12 expression appears to stabilize LDB1 and RNF12 also leads to mono-ubiquitination of Lys-134 of LDB1, it is surprising the LDB1-K134R mutant is stabilized rather than degraded. One model which would be consistent with this surprising finding would involve a role for mono-ubiquitination of Lys-134 leading to stabilization and competing with poly-ubiquitination of the same residue which would lead to degradation by the proteasome. In this model, RNF12-induced mono-ubiquitination of Lys-134 would reduce poly-ubiquitination and degradation. Similarly, mutation of Lys-134 would also block poly-ubiquitination.
Figure 3. Mutation of Lys-134 of LDB1 enhances LDB1 expression and also eliminates RNF12-induced formation of a slower migrating form of LDB1.
(A) HEK293 cells were transfected with vectors for wild type or mutant FLAG-tagged LDB1 as indicated. At 20 h after transfection, whole cell extracts were prepared and the expression of FLAG-tagged LDB1 was assessed by denaturing gel electrophoresis and immunoblotting with FLAG monoclonal antibody. A horseradish peroxidase labeled anti-mouse secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. (B) Longer exposure of the immunoblot in (A). Proteins with the appropriate migration for full-length, FLAG-tagged LDB1 (closed arrow) and a more slowly migrating form of the protein (open arrow) were detected. (C) The immunoblot from (A) and (B) was stripped and incubated with antibody to ERK1 as a loading control.
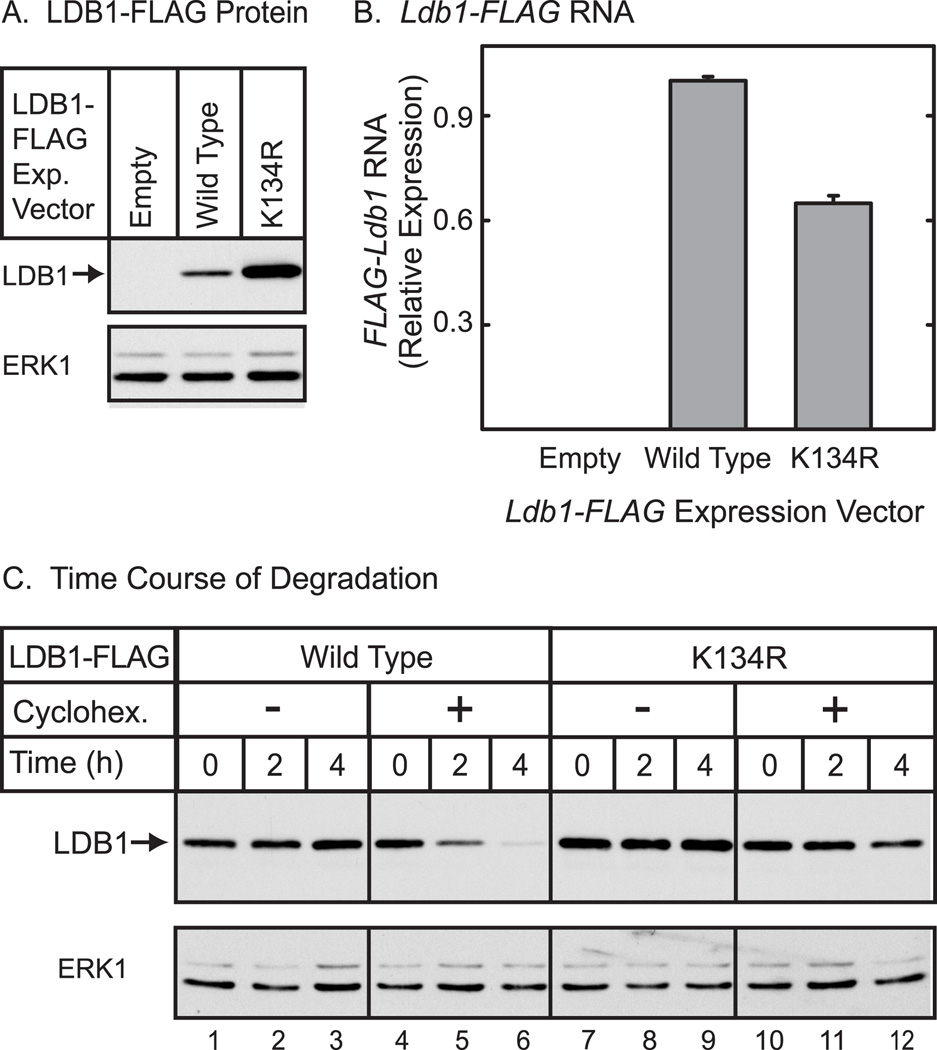
To determine if the K134R mutation affects LDB1 at a post-transcriptional level, LDB1 protein and RNA expression were assessed in the same experiment (Fig. 4A). As observed in the preceding experiment, the level of K134R mutant protein was enhanced substantially as compared to wild type LDB1. Quantitation of Ldb1 RNA (Fig. 4B) provided evidence that the RNA encoding the K134R mutant was slightly less abundant than RNA encoding wild type LDB1. Thus, changes at a post-transcriptional level must account for the increase in LDB1 protein. To explore the possibility that the K134R mutant protein is stabilized, an experiment using cycloheximide to inhibit protein synthesis was performed (Fig. 4C). In this experiment, less expression vector for LDB1-K134R than wild type LDB1 was transfected in an effort to equalize levels of the two proteins and therefore facilitate comparison of the time course of cycloheximide-induced decreases in protein levels. The results provide evidence that following inhibition of protein synthesis, wild type LDB1 decreases more rapidly than the LDB1-K134R. The immunoblot was subjected to quantitative analysis using an appropriately labeled secondary antibody and an infrared imaging system. After 4 hours of cycloheximide treatment, wild type LDB1 declined to 38% of control, while the K134R mutant decreased to 87% of control during the same time interval. Overall the findings suggest that mutation of Lys-134 to Arg increases LDB1 expression at a post-transcriptional level probably involving stabilization of the protein.
Figure 4. Mutation of Lys-134 of LDB1 alters expression of the protein at a post-transcriptional level.
(A) HEK293 cells were transfected with an empty expression vector or an expression vector for FLAG-tagged wild type or K134R mutant LDB1. Whole cell extracts were prepared 20 h after transfection from half the cells and the extracts resolved by denaturing polyacrylamide gel electrophoresis, transferred to a membrane and then incubated with FLAG antiserum to detect epitope-tagged LDB1. A horseradish peroxidase labeled anti-rabbit secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. The membrane was then incubated with antibody to ERK1 as a loading control. (B) RNA was prepared from half of the transfected cells described. Ldb1 and GAPDH RNA were quantitated by real-time PCR. Ldb1 RNA expression in each sample was normalized to GAPDH in each sample and then relative RNA levels were normalized so that the wild type average value for the Ldb1 group was set to 1.0. (C) HEK293 cells were transfected with an expression vector for FLAG-tagged wild type or K134R mutant LDB1. Twice as much expression vector for wild-type LDB1 was transfected in an attempt to equalize the amounts of wild type and mutant LDB1. The next morning cells were either untreated or treated with 25 µg/ml of cycloheximide for the times indicated. Whole cell extracts were prepared and the extracts resolved by denaturing polyacrylamide gel electrophoresis, transferred to a membrane and then incubated with FLAG antiserum to detect epitope-tagged LDB1. A horseradish peroxidase labeled anti-rabbit secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. The membrane was then incubated with antibody for ERK1 as a loading control.
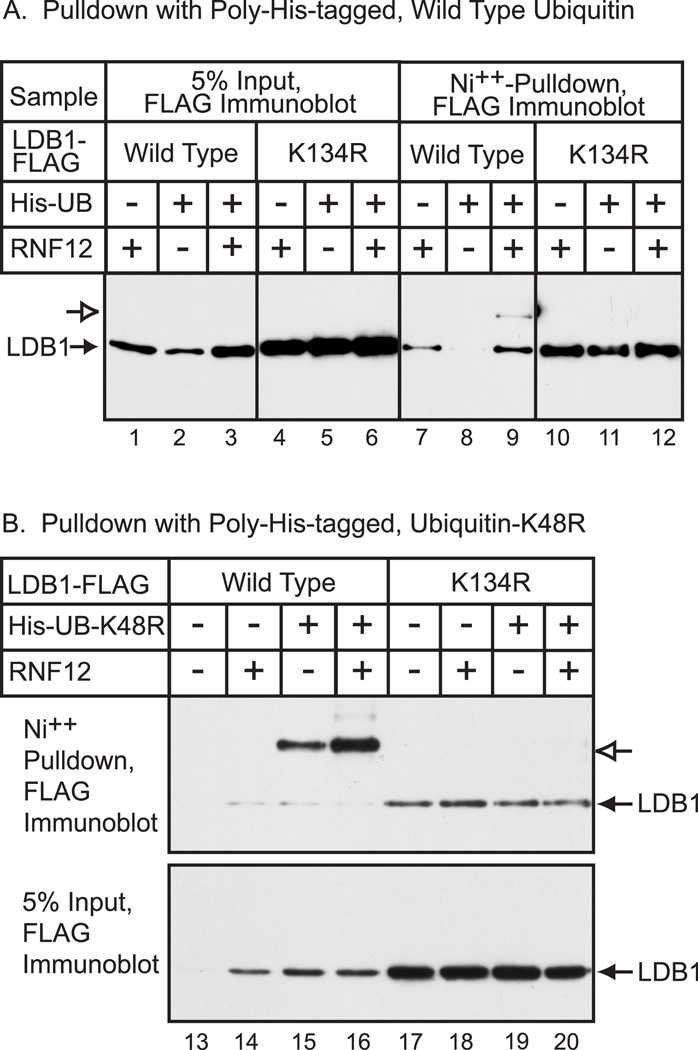
A transfection assay was used to determine if RNF12-induced modification of LDB1 Lys-134 involves ubiquitin. FLAG epitope-tagged LDB1 was expressed in HEK293 cells in the absence or presence of RNF12 and 6X histidine-tagged ubiquitin. The cells were disrupted and proteins denatured in guanidine and the extract subjected to nickel-chelate chromatography to isolate histidine-containing proteins. LDB1 in the eluate was identified by immunoblotting with FLAG monoclonal antibody (Fig. 5A). Although many proteins can bind to nickel chelate resin, 6X histidine tagged proteins bind strongly to the resin. Analysis of the input LDB1-FLAG again provided evidence for enhanced levels of LDB1-K134R as compared to wild type (Fig. 5A, lane 4–6). In the absence of 6X histidine-tagged ubiquitin, there was some background binding of LDB1-FLAG to the nickel chelate resin (Fig. 5A, lanes 7 and 10) presumably due to histidine content of LDB1. The more slowly migrating form of LDB1-FLAG was only detected with wild type LDB1 in the presence of both 6X histidine-tagged ubiquitin and RNF12 (Fig. 5A, lane 9). The LDB1-K134R mutation abolished the more slowly migrating LDB1 immunoreactive band. These findings support the view that RNF12 causes mono-ubiquitination of LDB1 at Lys-134. Interestingly, overexpression of ubiquitin appeared to decrease levels of wild type LDB1, but not the Lys-134 LDB1 mutant (Fig. 5A, lanes 1–3). This is consistent with model in which mono- or poly- ubiquitination of Lys-134 of LDB1 may cause either stabilization or degradation. To examine this further, the experiment was repeated using a 6X histidine-tagged ubiquitin-K48R. Ubiquitin Lys-48 is the major site of ubiquitin polymerization leading to proteasomal targeting [24]. Mutating this lysine of ubiquitin should block poly-ubiquitination and act to inhibit protein degradation. Transfection of a vector for ubiquitin-K48R was found to enhance the level of LDB1-FLAG similar to that obtained with RNF12 (Fig 5B, Input panel, lanes 13, 14 and 15). Following expression of the mutant ubiquitin-K48R and nickel chelate chromatography, the more slowly-migrating form of LDB1 was easily detected and indeed became the major form of LDB1 (Fig. 5B, lane 15). Expression of ubiquitin-K48R with the LDB1-K134R mutant abolished the shifted band observed with wild type LDB1. Expression of ubiquitin-K48R should essentially act to trap LDB1 that is normally poly-ubiquitinated. The finding that LDB1-K134R mutation blocks formation of the more slowly migrating form of LDB1 provides additional evidence that Lys-134 is the major site of LDB1 ubiquitination in HEK293 cells. These results are also consistent with a model involving mono-ubiquitination of Lys-134 in HEK 293 cells leading to LDB1 stabilization or poly-ubiquitination leading to proteasomal degradation.
Figure 5. Ubiquitin can modify LDB1 at Lys-134.
(A) HEK293 cells were transfected with expression vectors for LDB1-FLAG (Ldb1), poly-His-Ubiquitin (His-Ub) and AU1-RNF12 (RNF12) as indicated. At 20 h after transfection, whole cell extracts were prepared and His-containing proteins were isolated under denaturing conditions by Ni++-chelate chromatography. An aliquot of the whole-cell extract (5% input) or the proteins isolated by Ni++-chelate chromatography was resolved by denaturing gel electrophoresis. FLAG-tagged LDB1 was visualized by immunoblotting with FLAG monoclonal antibody. Proteins with the appropriate migration for full-length, FLAG-tagged LDB1 indicated with a closed arrow and a more slowly migrating form of LDB1 is indicated with an open arrow. (B) HEK293 cells were transfected with expression vectors for LDB1-FLAG (Ldb1), poly-His-Ubiquitin-K48R (His-Ub) and AU1-RNF12 (RNF12) as indicated. His-containing proteins were isolated by chromatography and analyzed by gel electrophoresis as above. Closed arrows, migration appropriate for LDB1-FLAG, open arrows, more slowly migration form of LDB1-FLAG.
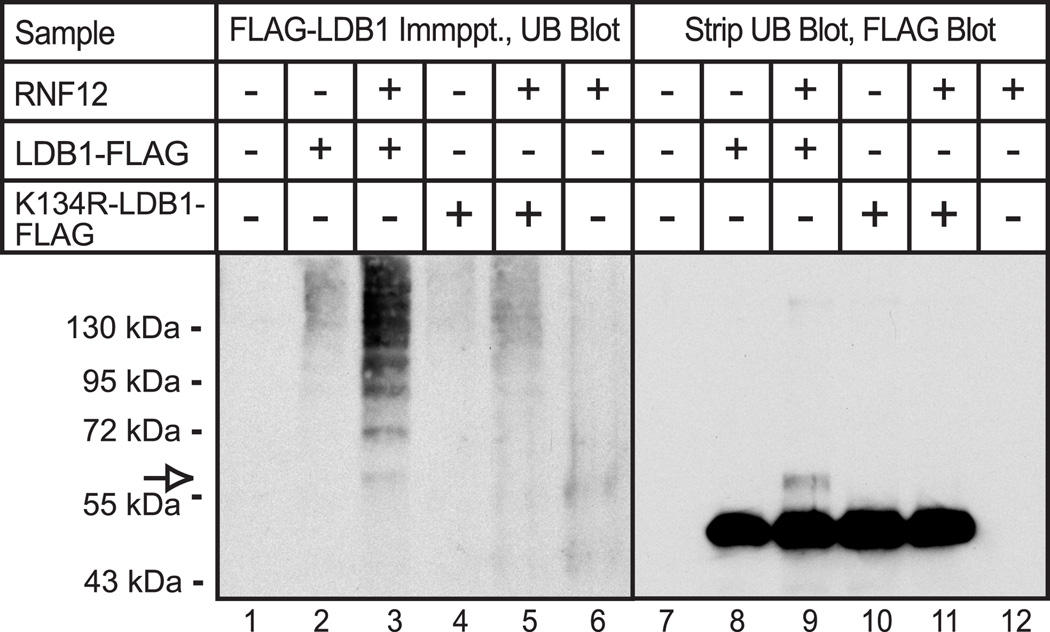
The preceding findings are consistent with a role for lysine 134 of LDB1 as the major site of both mono-ubiquitination and poly-ubiquitination. Although several of these studies have used immunoblots to detect a slowly migrating, mono-ubiquitinated form of LDB1, these blots did not detect more slowly migrating forms of LDB1 representing poly-ubiquitination. Presumably, poly-ubiquitinated LDB1 is degraded rapidly and therefore the poly-ubiquitinated form of LDB1 is difficult to detect. In an effort to address this issue we used an alternative approach. HEK293 cells were transfected with FLAG- tagged wild type or mutant LDB1, in the absence or presence of RNF12. For this experiment less LDB1-K134R expression vector than wild type LDB1 vector was used in an attempt to equalize expression of wild type and mutant LDB1. LDB1 was isolated by immunoprecipitation with monoclonal, anti-FLAG antibody. The immunoprecipitated proteins were eluted using non-denaturing conditions. This allows the bound proteins to be eluted without substantial contamination by FLAG antibody, facilitating the subsequent use of a second mouse monoclonal antibody to detect ubiquitin. The use of an antibody to ubiquitin in this experiment may enhance detection of poly-ubiquinated proteins that contain multiple copies of the antigen. The eluted proteins were analyzed by denaturing gel electrophoresis and immunoblot using a monoclonal antibody to ubiquitin. Expression of RNF12 with wild type LDB1 resulted in a “ladder” of discrete, ubiquitin-containing proteins of increasing sizes, likely representing poly-ubiquitinated proteins (Fig 6A, lane 3). The immunoprecipitated proteins in this experiment were eluted using non-denaturing conditions. Therefore, the detected poly-ubiquitinated proteins may be LDB1 and/or LDB1 associated proteins. The ubiquitin-containing protein ladder is greatly reduced for the LDB1-K134R mutant (Fig 6A, lane 5) suggesting that the poly-ubiquitinated protein may indeed be LDB1. However, we cannot rule out the possibility that mutation of Lys-134 disrupts interaction of LDB1 with another protein that is poly-ubiquitinated. The blot was stripped and probed with an antibody to LDB1 (Fig. 6B). As observed previously, a more slowly migrating form of LDB1, presumably containing mono-ubiquinated LDB1 was detected with wild type LDB1 (Fig 6B, lane 3, open arrow) and this form of LDB1 is no longer apparent upon mutation of Lys-134. Physical alignment of the films from the immunoblots indicates that the mono-ubiquitinated LDB1 detected with the antibody to LDB1 (Fig. 6B, lane 3, open arrow) migrates precisely the same as the most rapidly migrating ubiquitinated protein (Fig. 6A, lane 3, open arrow). These findings are consistent with the model that Lys-134 of LDB1 serves as a major site for ubiquitination of LDB1.
Figure 6. Lysine 134 is the major site of RNF12 mediated poly-ubiquitination of LDB1.
HEK293 cells were transfected with expression vectors for LDB1-FLAG (WT-Ldb1 or K134R-Ldb1) and AU1-RNF12 (RNF12) as indicated. At 20 h after transfection, whole cell extracts were prepared. FLAG-tagged-LDB1 was isolated from the cell extract by immunoprecipitation with FLAG monoclonal antibody and the immunoprecipitate was analyzed by denaturing gel electrophoresis and immunoblotting with a monoclonal antibody to ubiquitin (left panel). The immunoblot was stripped and probed with antibody to LDB1 (right panel). The migration of a protein with the appropriate size for mono-ubiquitinated LDB1 is indicated with an open arrow.
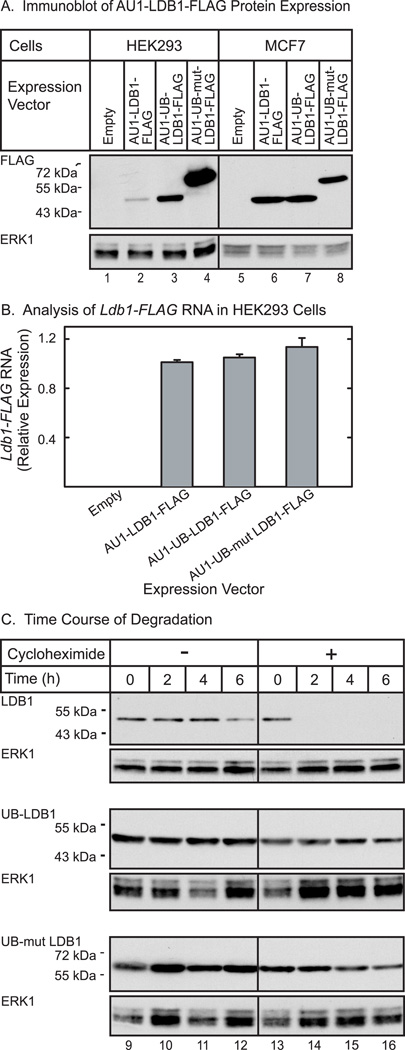
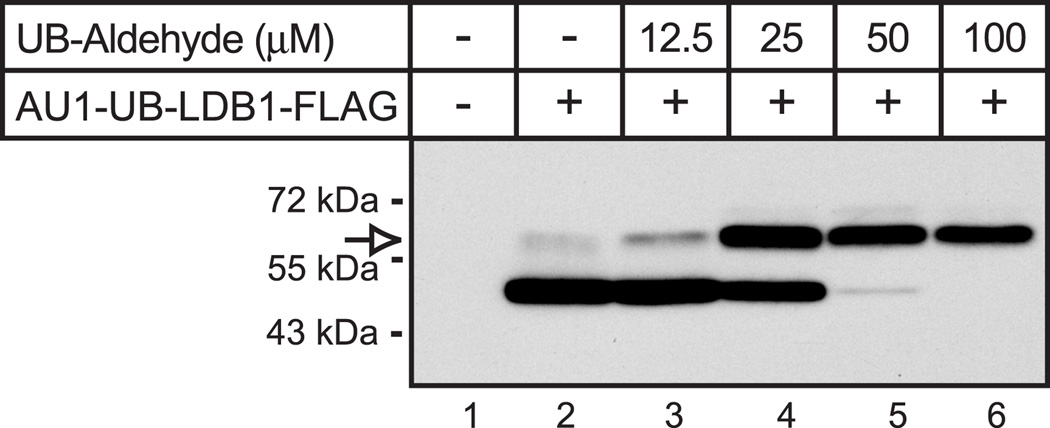
The finding that RNF12-mediated mono-ubiquitination of LDB1 in HEK293 cells is associated with increased protein levels of LDB1 suggests that this modification may play a role in stabilization of LDB1. To further test this model we prepared an expression vector for a ubiquitin-LDB1 fusion. We fused the amino-terminus of LDB1 to the carboxy terminus of either wild type ubiquitin or a ubiquitin mutant with the carboxy-terminal glycine replaced with valine (G76V). Use of the ubiquitin-G76V mutant has been shown to produce fusion proteins resistant to de-ubiquitinating enzymes [25]. Previous studies have shown that fusions to ubiquitin destabilizes proteins, presumably by enhancing poly-ubiquitination [25–27]. In contrast to the previously observed protein destabilization, transfection of the ubiquitin-LDB1 fusion vector (AU1-UB-LDB1-FLAG) in HEK293 cells led to increased protein levels as compared to non-fused LDB1( AU1-LDB1-FLAG, Fig. 7A). The product of the fusion of wild type ubiquitin to LDB1 appeared to be efficiently de-ubiquitinated as the major protein observed had the same electrophoretic mobility as LDB1 not fused to ubiquitin (Fig. 7A, lanes 2 and 3). Although the ubiquitin-LDB1 fusion appeared to be rapidly de-ubiquinated, the amount of LDB1 protein was increased as compared to LDB1 produced from an expression vector for non-fused LDB1. Ubiquitin-G76V fused LDB1 was not de-ubiquitinated and accumulated to even higher levels (Fig. 7A, lanes 2 and 4). The observation that the wild type ubiquitin-LDB1 fusion protein is readily de-ubiquitinated is consistent with published reports [25–26]. We further examined possible de-ubiquitination of the LDB1 fusion protein using ubiquitin aldehyde to inhibit de-ubiquitinating enzymes in a cell-free system [28]. Proteins were expressed using a cell-free system derived from wheat germ. In reactions programmed with RNA directing synthesis of the ubiquitin-LDB1 fusion (AU1-Ubiquitin-LDB1-FLAG) the major product had the same size as reactions programmed by AU-LDB1-FLAG RNA offering evidence that the ubiquitin-LDB1 fusion was efficiently de-ubiquitinated, (Fiq. 8, lane 2). Increasing amounts of ubiquitin aldehyde resulted in a product with the mobility of expected for the ubiquitin-LDB1 fusion protein. The findings with ubiquitin aldehyde provide evidence that the ubiquitin-LDB1 coding sequence directs the synthesis of a fusion protein as expected and that this ubiquitin-LDB1 fusion protein can be efficiently de-ubiquinated. Quantitative PCR demonstrates that the RNA levels for the ubiquitin fused LDB1 constructs transfected into HEK293 cells are all similar (Fig. 7B) suggesting changes in protein stability are responsible for the different levels of proteins observed. To further test this, we performed an experiment using cycloheximide to inhibit protein synthesis (Fig. 7C). Fusion of wild type ubiquitin ubiquitin-G76V with LDB1 greatly increased the apparent half-life of the fusion protein as compared to non-fused LDB1. We don’t see as much difference in degradation between wild type and G76V ubiquitin fused LDB1 as would be expected based on differences in steady-state levels (Fig. 7A); this may be due to increasing cell death with longer cycloheximide treatments. The half-lives of these fusion proteins appear to be longer than the time required for cycloheximide to cause significant cell death.
Figure 7. Ubiquitin fusion to LDB1 alters expression of the protein at a post-transcriptional level.
(A) HEK293 or MCF7 cells were transfected with an empty expression vector or an expression vector for epitope-tagged wild type, wild type ubiquitin fused, or G76V mutant ubiquitin fused LDB1. Whole cell extracts were prepared 20 h after transfection from half the cells and the extracts resolved by denaturing polyacrylamide gel electrophoresis, transferred to a membrane and then incubated with FLAG antiserum to detect epitope-tagged LDB1. A horseradish peroxidase labeled anti-rabbit secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. The membrane was then incubated with antibody to ERK1 as a loading control. (B) RNA was prepared from half of the transfected cells described. Ldb1 and GAPDH RNA were quantitated by real-time PCR. Ldb1 RNA expression in each sample was normalized to GAPDH in each sample and then relative RNA levels were normalized so that the wild type average value for the Ldb1 group was set to 1.0. (C) HEK293 cells were transfected with an expression vector for epitope-tagged wild type, wild type ubiquitin fused, or G76V mutant ubiquitin fused LDB1. Twice as much expression vector for wild-type LDB1 was transfected in an attempt to equalize the amounts of wild type and ubiquitin fused LDB1. The next morning cells were either untreated or treated with 25 µg/ml of cycloheximide for the times indicated. Whole cell extracts were prepared and the extracts resolved by denaturing polyacrylamide gel electrophoresis, transferred to a membrane and then incubated with FLAG antiserum to detect epitope-tagged LDB1. A horseradish peroxidase labeled anti-rabbit secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. The membrane was then incubated with antibody for ERK1 as a loading control.
The effects of RNF12 on LDB1 stability are cell specific as demonstrated in figure 1. We tested the effects of ubiquitin fusion to LDB1 in MCF7 cells in figure 7A. In contrast to what is seen in HEK293 cells both the wild type and G76V ubiquitin fused LDB1 are expressed at a similar level as un-fused LDB1. This suggests that like the ability of RNF12 to mono-ubiquitinate and stabilize LDB1, stabilization of LDB1 by ubiquitin fusion is cell specific.
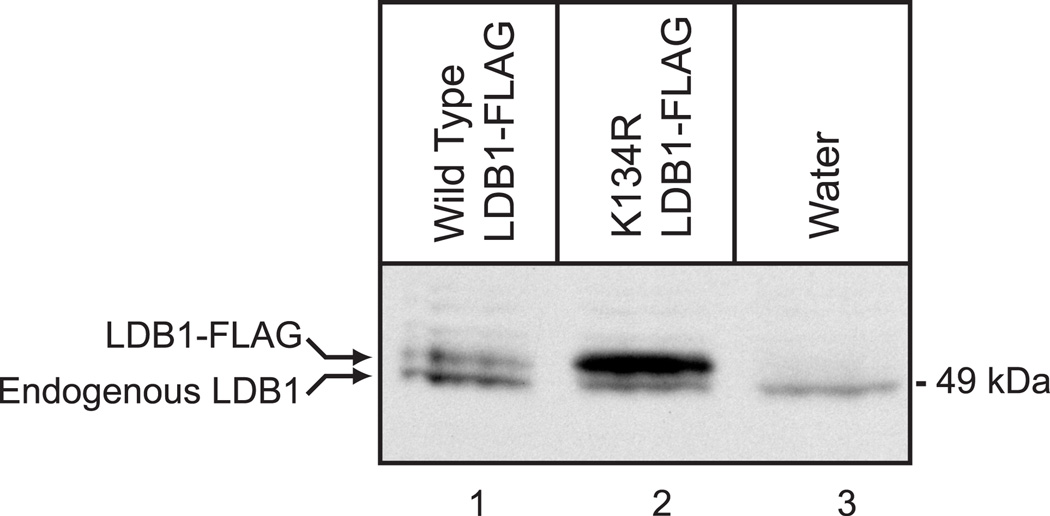
To further test the physiological significance of Lys-134 in regulating expression of LDB1, we injected RNA for epitope-tagged wild type and K134R Xenopus laevis LDB1 into developing Xenopus laevis embryos. Murine and Xenopus LDB1 differ at just five amino acids and Lys-134 is conserved. Embryos were injected dorsally at the four cell stage with 4 ng of capped RNA. Embryos were collected at stage 11 and equal amounts of protein extracts were analyzed by denaturing electrophoresis and immunoblotting with a polyclonal antiserum to LDB1 (Fig. 9). The K134R mutant Xenopus LDB1 was found to be present in a greater amount than wild type LDB1 (Fig. 9, lanes 1 and 2). A slightly more rapidly migrating band that is presumably endogenous LDB1 was also detected in this experiment and as the endogenous LDB1 is expressed at about the same level as the LDB1-FLAG offers evidence that tagged LDB1 is not substantially overexpressed. The endogenous LDB1 also serves as a loading control. These findings provide evidence that in a physiological setting, Lys-134 plays an important role in regulating expression of LDB1.
Figure 9. Xenopus LDB1 K134R is stabilized in developing Xenopus laevis embryos.
cRNA (4ng) for epitope-tagged, wild type and K134R Xenopus laevis LDB1 was injected dorsally at the four cell stage into developing Xenopus laevis embryos. Embryos were collected at stage 11 and equal amounts of protein extracts were analyzed by denaturing electrophoresis and immunoblotting with a polyclonal antiserum to LDB1.
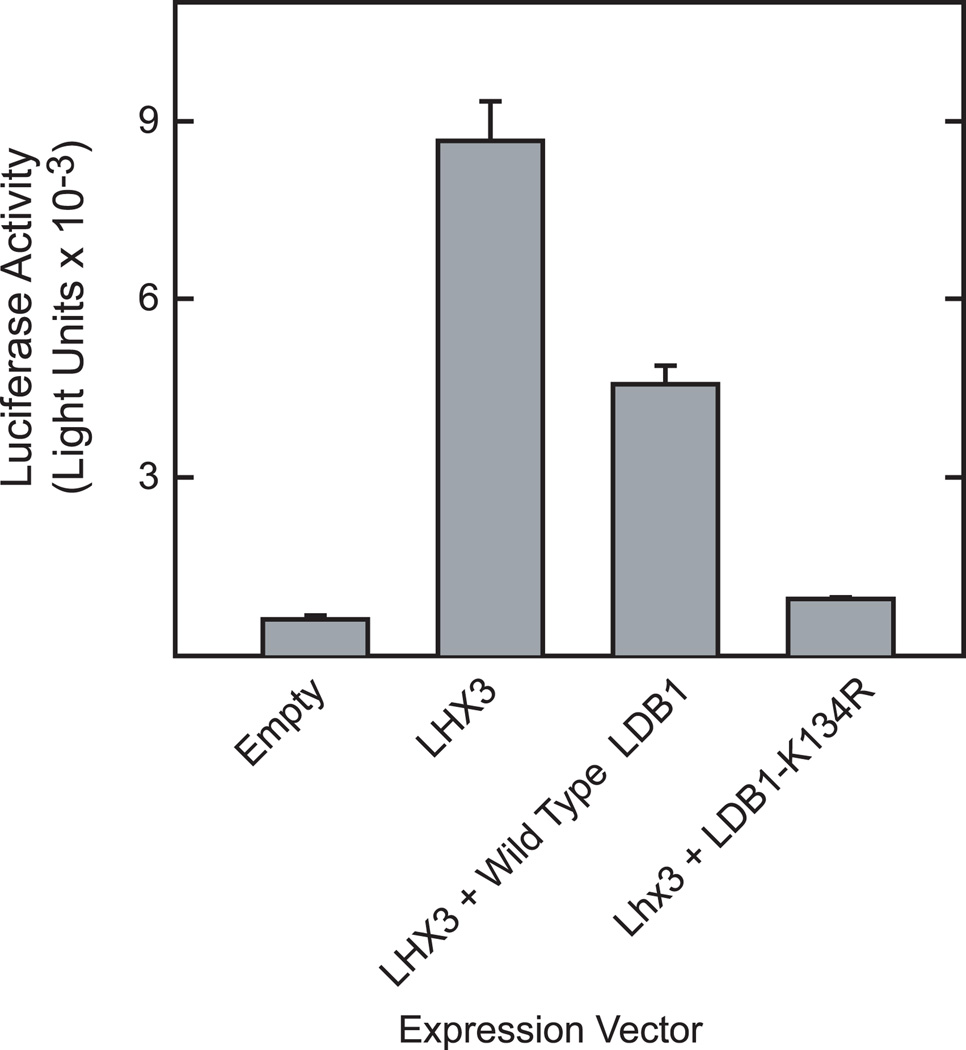
As we have found that RNF12-mediated mono-ubiquitination leads to substantial increases in LDB1 expression in HEK293 cells, it seems likely that these changes in expression would influence the ability of LDB1 to function as transcriptional cofactor. As mentioned previously, the stoichiometry of LDB1 to interacting transcription factors is important [2–7]. Over-expression of LDB1 as compared to LIM homeodomain transcription factors can inhibit transcription, presumably by disrupting the formation of tetramer complexes involving LDB1 dimers interacting with the LIM homeodomain factors. To test this we used a reporter gene containing 3 copies of the LIM homeodomain binding sequence from the glycoprotein hormone α-subunit promoter. The glycoprotein hormone α-subunit promoter has been shown to be activated by LIM homeodomain transcription factors [29–30]. HEK293 cells were transfected with luciferase reporter gene containing 3 copies of an LIM homeodomain binding site and a minimal promoter and expression vectors for Lhx3 and vectors encoding wild type LDB1 or LDB1-K134R (Fig. 10). As expected, over-expression of wild type LDB1 in HEK293 inhibited the activity of the α-subunit reporter gene. Expression of LDB1-K134R resulted in greater inhibition of the α-subunit reporter gene consistent with the substantially higher level expression of the mutant protein and the inhibitory effects of LDB1 overexpression.
Figure 10. Functional effects of replacement of Lys-134 of LDB1 with Arg.
HEK293 cells were transfected with a reporter gene containing 3 copies of an Lhx3 binding site upstream of a minimal promoter linked to the luciferase coding sequence and expression vectors for Lhx3, wild type LDB1, LDB1-K134R or empty vector as indicated as well as an expression vector for β-galactosidase as an internal standard for transfection efficiency. Cell extracts were prepared 20 h later and assayed for luciferase and β-galactosidase activity. Luciferase activity was corrected for β-galactosidase activity and the results are presented as means +/− SEM obtained from three separate transfections.
DISCUSSION
These studies have used RNF12 as a tool to identify Lys-134 of LDB1 as a residue that can be modified by ubiquitination leading to changes in LDB1 protein levels. We find that mutation of Lys-134 of LDB1 to Arg has substantial effects on LDB1 levels in both cultured cells and Xenopus embryos. Combined with the finding that Lys-134 is required for both mono-ubiquitination and poly-ubiquitination of LDB1, the studies are consistent with a model in which mono-ubiquitination of Lys-134 can enhance stability of LDB1 while poly-ubiquitination leads to increased degradation of LDB1. The view that LDB1 mono-ubiquitination can stabilize LDB1 is supported by the observation that a fusion of ubiquitin to LDB1 causes substantial stabilization of LDB1. As LDB1 protein levels and LDB1 stoichiometry relative to binding partners is very important [2–4, 6–7], the ability of ubiquitination of Lys-134 to alter LDB1 levels would be expected to have important consequences for LDB1 function. In a study of the developmental expression pattern of RNF12 and LDB1 it was found that protein expression levels varied more than the nearly ubiquitous RNAs for these factors suggesting substantial post transcriptional regulation [31]. Cell-specific differences in regulation of LDB1 degradation are likely one of the post-transcriptional mechanisms contributing to tissue-specific differences in LDB1 protein expression. The present studies identify Lys-134 as an important residue regulating LDB1 expression and this finding provides insights into regulation of transcriptional complexes that include LDB1.
There are a number of interesting questions about RNF12-mediated ubiquitination of LDB1 that await future studies. An interesting mechanistic question concerns the ability of RNF12 to decrease LDB1 expression in MCF7 cells and increase LDB1 expression in HEK293 cells. Presumably there is some altered or unique component of the ubiquitination machinery that leads to this change in outcome. There is evidence that ubiquitin chain initiation and elongation are kinetically separable processes [32] which may be mediated by different E2 ubiquitin conjugating enzymes acting sequentially [33–34]. Therefore, it is possible that differences in expression of specific E2 ubiquitin conjugating enzymes in HEK293 cells versus MCF7 cells leads to differences in mono-ubiquitination versus poly-ubiquintation. It has been reported that UBCH5 or UBCH8 can act as an E2 ubiquitin conjugating enzyme for RNF12 [17, 35]. In HEK293 cells, expression vectors for UBCH5 or UBCH8 had relatively small effects on LDB1 expression (Howard, unpublished). A number of other E2 enzymes have been shown to interact with RNF12 [36] and these enzymes remain candidates as mediating cell-specific effects of RNF12. In addition, some E2 enzymes function with specific E3 ligase proteins even though a physical interaction between them is not observed in typical experimental screens [37]. Thus, further studies of a possible role for specific E2 enzymes in mono-ubiquitination of LDB1will likely require a comprehensive analysis of E2 expression in different cells and tissues as well as a test of functional role through over-expression and knockdown approaches. Another possibility for tissue-specific effects of RNF12 could involve differential expression of another E3 ubiquitin ligase. While RNF12 has been shown to be a ubiquitin ligase, it is possible that RNF12 works indirectly to recruit another protein that plays a role in direct ubiquitination of LDB1. It has been shown that RING domain proteins can interact with a second RING domain protein to enhance the ubiquitin ligase activity of the second protein [38–40]. Thus, cell-specific differences in the expression of E2 or E3 enzymes might account for cell-specific differences in mono-ubiquitination.
Similarly, the mechanisms permitting mono-ubiquitination to stabilize LDB1 are unknown. One possibility could involve interaction of ubiquitin-binding proteins with mono-ubiquinated LDB1. This binding could act to block poly-ubiquitination [41]. It is conceivable that the physical interaction of RNF12 with LDB1 under some circumstances blocks poly-ubiquitination. Interestingly, although RNF12 expression increases the level of LDB1 protein, only a relatively small fraction of LDB1 appears to be mono-ubiquitinated. How can this small amount of modified LDB account for substantial changes in LDB1 expression? It should be noted that this observation is not without precedent. There are other examples of regulation by ubiquitin or ubiquitin-like proteins that appear to involve modification of a small fraction of the relevant proteins [42–43]. It is quite interesting that we find that transfection of HEK293 cells with an expression vector for a ubiquitin-LDB1 fusion lead to increased levels of the LDB1 portion of the fusion protein despite the fact that the amino-terminal ubiquitin moiety appeared to be rapidly removed. The ubiquitin-LDB1 fusion construct introduces no additional amino acid between the carboxy-terminal glycine of ubiquitin and the initiation methionine of LDB1. As the second amino acid of LDB1 is leucine, a relatively large amino acid, the amino-terminal methionine is not predicted to be removed [44]. Thus after de-ubiquitination of the ubiquitin-LDB1 fusion the resulting LDB1 protein should have the same amino acid sequence as protein produced by the expression vector encoding the unfused LDB1. These findings are consistent with the possibility that transient mono-ubiquitination induces a long lasting change in LDB1 that blocks poly-ubiquitination and degradation. For instance, interaction with ubiquitin binding protein might lead to changes in subcellular location so that LDB1 was not available for poly-ubiquitination. There are some similarities in the present studies of LDB1 with other recent studies of the ubiquitination of transcription factors. It was found that RNF12 binds and ubiquitinates the estrogen receptor α , but does not stimulate degradation of the receptor [45]. The E3-ubiquitin ligase, RNF31, can mono-ubiquitinate and stabilize the transcriptional regulatory factor, DAX-1 [46]. Mono-ubiquitination is known to control processes such as membrane transport, protein localization, and protein-protein interactions [41, 43, 47–48]. In addition there is evidence that mono-ubiquitination can enhance transcription factor activity [49–50].
Perhaps the most interesting question about RNF12 and LDB1 concerns a possible physiological role of RNF12 to increase LDB1 expression. Previous studies have shown that RNF12 can decrease LDB1 protein levels due to the action of RNF12 working as a ubiquitin ligase leading to poly-ubiquitination and degradation LDB1 [17–18]. The present studies have confirmed that in some cells, RNF12 can lead to LDB1 degradation in an RNF12 RING finger-dependent manner. Our studies examining expression of LDB1-K134R in Xenopus embryos demonstrate that mutation of this residue enhances the level of LDB1. This finding is consistent with a net effect in the whole embryo of poly-ubiquitination at Lys-134 leading to degradation of LDB1. However, the present studies also demonstrate that in HEK293 cells, expression of RNF12 has the opposite effect, enhancing LDB1 levels. The present findings raise the possibility that in some cells during development or under particular conditions, RNF12 does not cause LDB1 degradation, but rather stabilizes LDB1. This would provide an additional level of LDB1 regulation. We have been unable to determine if endogenous RNF12 is required to enhance LDB1 expression in HEK293 cells, as knockdown experiments were not informative. Perhaps future studies of mice with genetic disruption of RNF12 will be informative. If RNF12 does play a physiological role in some cells or tissues to enhance LDB1 levels, it would likely have substantial biological significance.
Figure 8. Ubiquitin-aldehyde protects ubiquitin fused LDB1 from de-ubiquitination in vitro.
A wheat germ based in vitro coupled transcription/translation system was used to express epitope tagged ubiquitin fused LDB1. All reactions contained the same reagents and template with the exception of ubiquitin-aldehyde which was present at the indicated concentrations. Aliquots of the reactions were resolved by denaturing polyacrylamide gel electrophoresis, transferred to a membrane and then incubated with FLAG antiserum to detect epitope-tagged LDB1. A horseradish peroxidase labeled anti-rabbit secondary antibody was used with a chemiluminescent detection reagent to visualize the immunoreactive proteins. The migration of a protein the appropriate size for the tagged-ubiquitin-LDB1 fusion is indicated with an open arrow.
ACKNOWLEDGEMENTS
We thank Bobbi Maurer and Tiffani Howard for assistance in preparing this manuscript.
FUNDING
This work was supported by American Cancer Society Research Scholar Award RSG-04-038-01-DDC (to DGR) and National Institutes of Health Grant DK062779 (to RAM).
Abbreviations used
- LDB1
LIM-domain binding 1
- RNF12
ring finger protein 12
- GST
glutathione S-transferase
- DMEM
Dulbecco’s Modified Eagle’s Medium
Footnotes
AUTHOR CONTRIBUTION
The experimental plan was designed by Paul Howard, David Ransom and Richard Maurer. The research was performed by Paul Howard and Shall Jue. The data were analyzed and the paper prepared by Paul Howard, David Ransom and Richard Maurer.
REFERENCES
- 1.Agulnick AD, Taira M, Breen JJ, Tanaka T, Dawid IB, Westphal H. Interactions of the LIM-domain-binding factor Ldb1 with LIM homeodomain proteins. Nature. 1996;384:270–272. doi: 10.1038/384270a0. [DOI] [PubMed] [Google Scholar]
- 2.Milan M, Diaz-Benjumea FJ, Cohen SM. Beadex encodes an LMO protein that regulates Apterous LIM-homeodomain activity in Drosophila wing development: a model for LMO oncogene function. Genes Dev. 1998;12:2912–2920. doi: 10.1101/gad.12.18.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milan M, Cohen SM. Regulation of LIM homeodomain activity in vivo: a tetramer of dLDB and apterous confers activity and capacity for regulation by dLMO. Molecular Cell. 1999;4:267–273. doi: 10.1016/s1097-2765(00)80374-3. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Funez P, Lu CH, Rincon-Limas DE, Garcia-Bellido A, Botas J. The relative expression amounts of apterous and its co-factor dLdb/Chip are critical for dorso-ventral compartmentalization in the Drosophila wing. EMBO J. 1998;17:6846–6853. doi: 10.1093/emboj/17.23.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Meyel DJ, O'Keefe DD, Jurata LW, Thor S, Gill GN, Thomas JB. Chip and Apterous physically interact to form a functional complex during Drosophila development. Molecular Cell. 1999;4:259–266. doi: 10.1016/s1097-2765(00)80373-1. [DOI] [PubMed] [Google Scholar]
- 6.van Meyel DJ, O'Keefe DD, Thor S, Jurata LW, Gill GN, Thomas JB. Chip is an essential cofactor for apterous in the regulation of axon guidance in Drosophila. Development. 2000;127:1823–1831. doi: 10.1242/dev.127.9.1823. [DOI] [PubMed] [Google Scholar]
- 7.Rincon-Limas DE, Lu C-H, Canal I, Botas J. The level of DLDB/CHIP controls the activity of the LIM homeodomain protein Apterous: evidence for a functional tetramer complex in vivo. EMBO J. 2000;19:2602–2614. doi: 10.1093/emboj/19.11.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morcillo P, Rosen C, Baylies MK, Dorsett D. Chip, a widely expressed chromosomal protein required for segmentation and activity of a remote wing margin enhancer in Drosophila. Genes Dev. 1997;11:2720–2740. doi: 10.1101/gad.11.20.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bach I, Rodriguez-Esteban C, Carriere C, Bhushan A, Krones A, Rose DW, Glass CK, Andersen B, Belmonte JCI, Rosenfeld MG. RLIM inhibits functional activity of LIM homeodomain transcripiton factors via recruitment of the histone deacetylase complex. Nature Genetics. 1999;22:394–399. doi: 10.1038/11970. [DOI] [PubMed] [Google Scholar]
- 10.Torigoi E, Bennani-Baiti IM, Rosen C, Gonzalez K, Morcillo P, Ptashne M, Dorsett D. Chip interacts with diverse homeodomain proteins and potentiates bicoid activity in vivo. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2686–2691. doi: 10.1073/pnas.050586397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gause M, Morcillo P, Dorsett D. Insulation of enhancer-promoter communication by a gypsy transposon insert in the Drosophila cut gene: cooperation between suppressor of hairy-wing and modifier of mdg4 proteins. Mol Cell Biol. 2001;21:4807–4817. doi: 10.1128/MCB.21.14.4807-4817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramain P, Khechumian R, Khechumian K, Arbogast N, Ackermann C, Heitzler P. Interactions between chip and the achaete/scute-daughterless heterodimers are required for pannier-driven proneural patterning. Mol Cell. 2000;6:781–790. doi: 10.1016/s1097-2765(05)00079-1. [DOI] [PubMed] [Google Scholar]
- 13.Heitzler P, Vanolst L, Biryukova I, Ramain P. Enhancer-promoter communication mediated by Chip during Pannier-driven proneural patterning is regulated by Osa. Genes Dev. 2003;17:591–596. doi: 10.1101/gad.255703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Meyel DJ, Thomas JB, Agulnick AD. Ssdp proteins bind to LIM-interacting co-factors and regulate the activity of LIM-homeodomain protein complexes in vivo. Development. 2003;130:1915–1925. doi: 10.1242/dev.00389. [DOI] [PubMed] [Google Scholar]
- 15.Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM Factor Lhx3 Contributes to the Specification of Motor Neuron and Interneuron Identity through Cell-Type-Specific Protein-Protein Interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee SK, Pfaff SL. Synchronization of neurogenesis and motor neuron specification by direct coupling of bHLH and homeodomain transcription factors. Neuron. 2003;38:731–745. doi: 10.1016/s0896-6273(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 17.Ostendorff HP, Peirano RI, Peters MA, Schluter A, Bossenz M, Scheffner M, Bach I. Ubiquitination-dependent cofactor exchange on LIM homeodomain transcription factors. Nature. 2002;416:99–103. doi: 10.1038/416099a. [DOI] [PubMed] [Google Scholar]
- 18.Hiratani I, Yamamoto N, Mochizuki T, Ohmori SY, Taira M. Selective degradation of excess Ldb1 by Rnf12/RLIM confers proper Ldb1 expression levels and Xlim-1/Ldb1 stoichiometry in Xenopus organizer functions. Development. 2003;130:4161–4175. doi: 10.1242/dev.00621. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Meng X, Cai Y, Liang H, Nagarajan L, Brandt SJ. Single-stranded DNA-binding proteins regulate the abundance of LIM domain and LIM domain-binding proteins. Genes Dev. 2007;21:942–955. doi: 10.1101/gad.1528507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glenn DJ, Maurer RA. MRG1 binds to the LIM domain of LHx2 and may function as a coactivator to stimulate glycoprotein hormone α-subunit gene expression. J. Biol. Chem. 1999;274:36159–36167. doi: 10.1074/jbc.274.51.36159. [DOI] [PubMed] [Google Scholar]
- 21.Sun P, Maurer RA. An inactivating point mutation demonstrates that interaction of the cAMP response element binding protein (CREB) with the CREB binding protein is not sufficient for transcriptional activation. J. Biol. Chem. 1995;270:7041–7044. doi: 10.1074/jbc.270.13.7041. [DOI] [PubMed] [Google Scholar]
- 22.Moon R, Christian JL. Microinjection and expression of synthetic mRNAs in Xenopus embryos. Technique. 1989;1:76–89. [Google Scholar]
- 23.Xirodimas D, Saville MK, Edling C, Lane DP, Lain S. Different effects of p14ARF on the levels of ubiquitinated p53 and Mdm2 in vivo. Oncogene. 2001;20:4972–4983. doi: 10.1038/sj.onc.1204656. [DOI] [PubMed] [Google Scholar]
- 24.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 25.Johnson ES, Bartel B, Seufert W, Varshavsky A. Ubiquitin as a degradation signal. EMBO J. 1992;11:497–505. doi: 10.1002/j.1460-2075.1992.tb05080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stack JH, Whitney M, Rodems SM, Pollok BA. A ubiquitin-based tagging system for controlled modulation of protein stability. Nat Biotechnol. 2000;18:1298–1302. doi: 10.1038/82422. [DOI] [PubMed] [Google Scholar]
- 27.Cadima-Couto I, Freitas-Vieira A, Nowarski R, Britan-Rosich E, Kotler M, Goncalves J. Ubiquitin-fusion as a strategy to modulate protein half-life: A3G antiviral activity revisited. Virology. 2009;393:286–294. doi: 10.1016/j.virol.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Hershko A, Rose IA. Ubiquitin-aldehyde: a general inhibitor of ubiquitin-recycling processes. Proc Natl Acad Sci U S A. 1987;84:1829–1833. doi: 10.1073/pnas.84.7.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberson MS, Schoderbek WE, Tremml G, Maurer RA. Activation of the glycoprotein hormone α-subunit promoter by a LIM-homeodomain transcription factor. Mol. Cell. Biol. 1994;14:2985–2993. doi: 10.1128/mcb.14.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoderbek WE, Roberson MS, Maurer RA. Two different DNA elements mediate gonadotropin releasing hormone effects on expression of the glycoprotein hormone α-subunit gene. J. Biol. Chem. 1993;268:3903–3910. [PubMed] [Google Scholar]
- 31.Ostendorff HP, Tursun B, Cornils K, Schluter A, Drung A, Gungor C, Bach I. Dynamic expression of LIM cofactors in the developing mouse neural tube. Dev Dyn. 2006;235:786–791. doi: 10.1002/dvdy.20669. [DOI] [PubMed] [Google Scholar]
- 32.Petroski MD, Deshaies RJ. Mechanism of lysine 48-linked ubiquitin-chain synthesis by the cullin-RING ubiquitin-ligase complex SCF-Cdc34. Cell. 2005;123:1107–1120. doi: 10.1016/j.cell.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, King RW, Peters JM, Kirschner MW. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr Biol. 1996;6:455–466. doi: 10.1016/s0960-9822(02)00513-4. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigo-Brenni MC, Morgan DO. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell. 2007;130:127–139. doi: 10.1016/j.cell.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 35.Kramer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, Brill B, Groner B, Bach I, Heinzel T, Gottlicher M. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. Embo J. 2003;22:3411–3420. doi: 10.1093/emboj/cdg315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markson G, Kiel C, Hyde R, Brown S, Charalabous P, Bremm A, Semple J, Woodsmith J, Duley S, Salehi-Ashtiani K, Vidal M, Komander D, Serrano L, Lehner P, Sanderson CM. Analysis of the human E2 ubiquitin conjugating enzyme protein interaction network. Genome Res. 2009;19:1905–1911. doi: 10.1101/gr.093963.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen DE, Brzovic PS, Klevit RE. E2-BRCA1 RING interactions dictate synthesis of mono- or specific polyubiquitin chain linkages. Nat Struct Mol Biol. 2007;14:941–948. doi: 10.1038/nsmb1295. [DOI] [PubMed] [Google Scholar]
- 38.Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, Yabuki Y, Ogata H, Ohta T. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276:14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 40.Linares LK, Hengstermann A, Ciechanover A, Muller S, Scheffner M. HdmX stimulates Hdm2-mediated ubiquitination and degradation of p53. Proc Natl Acad Sci U S A. 2003;100:12009–12014. doi: 10.1073/pnas.2030930100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–621. doi: 10.1038/nrm1701. [DOI] [PubMed] [Google Scholar]
- 42.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- 44.Mogk A, Schmidt R, Bukau B. The N-end rule pathway for regulated proteolysis: prokaryotic and eukaryotic strategies. Trends Cell Biol. 2007;17:165–172. doi: 10.1016/j.tcb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Johnsen SA, Gungor C, Prenzel T, Riethdorf S, Riethdorf L, Taniguchi-Ishigaki N, Rau T, Tursun B, Furlow JD, Sauter G, Scheffner M, Pantel K, Gannon F, Bach I. Regulation of estrogen-dependent transcription by the LIM cofactors CLIM and RLIM in breast cancer. Cancer Res. 2009;69:128–136. doi: 10.1158/0008-5472.CAN-08-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ehrlund A, Anthonisen EH, Gustafsson N, Venteclef N, Robertson Remen K, Damdimopoulos AE, Galeeva A, Pelto-Huikko M, Lalli E, Steffensen KR, Gustafsson JA, Treuter E. E3 ubiquitin ligase RNF31 cooperates with DAX-1 in transcriptional repression of steroidogenesis. Mol Cell Biol. 2009;29:2230–2242. doi: 10.1128/MCB.00743-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnell JD, Hicke L. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J Biol Chem. 2003;278:35857–35860. doi: 10.1074/jbc.R300018200. [DOI] [PubMed] [Google Scholar]
- 48.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greer SF, Zika E, Conti B, Zhu XS, Ting JP. Enhancement of CIITA transcriptional function by ubiquitin. Nat Immunol. 2003;4:1074–1082. doi: 10.1038/ni985. [DOI] [PubMed] [Google Scholar]
- 50.Ferdous A, Sikder D, Gillette T, Nalley K, Kodadek T, Johnston SA. The role of the proteasomal ATPases and activator monoubiquitylation in regulating Gal4 binding to promoters. Genes Dev. 2007;21:112–123. doi: 10.1101/gad.1493207. [DOI] [PMC free article] [PubMed] [Google Scholar]