During the last years, transition-metal-catalyzed carbon-hydrogen bond functionalization has witnessed an explosive growth.[1] The use of C-H bond as a functional group is appealing because of shortening of reaction pathways and simplification of retrosynthetic analyses. However, most of the reports that deal with carbon-hydrogen bond conversion to carbon-carbon bonds involve either methodology development or mechanistic investigations. The applications in synthesis of natural products or their analogues are rare.[2] The limited use may be explained by the following issues. First, methods that result in functionalization of alkane C-H bonds are relatively rare.[3] Second, harsh reaction conditions are typically used that may be incompatible with sensitive functionalities. Third, methods often lack generality and require non-removable directing groups.
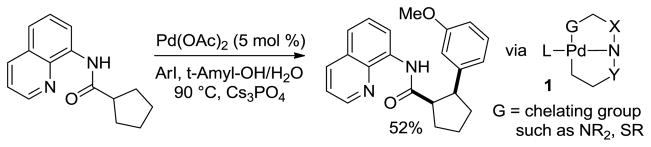
We have reported the β-arylation of carboxylic acid and γ-arylation of amine derivatives by employing an 8-aminoquinoline or picolinic acid auxiliary, catalytic Pd(OAc)2, and an aryl iodide coupling partner (Scheme 1).[4a] Subsequently, several other auxiliaries were investigated for carboxylic acid β-arylation.[4b] Use of 2-thiomethylaniline auxiliary affords selective monoarylation of methyl groups. In contrast, use of 8-aminoquinoline auxiliary allows either diarylation of methyl or monoarylation of methylene groups. The arylation regioselectivity is determined by formation of double five-membered chelate 1.
Scheme 1.
Auxiliaries for C-H Bond Arylation
Several other groups have recently used these directing groups in synthesis of natural products.[5] Corey has used the 8-aminoquinoline auxiliary to arylate sp3 C-H bonds in amino acid derivatives.[5a] However, monoarylation of alanine derivatives was not demonstrated and stereochemical integrity of arylation products as well as directing group removal was not reported. Developing new methodology for unnatural amino acid synthesis is important since they are used in drug discovery, protein engineering, peptidomimetics, glycopeptide synthesis, and click chemistry in biologically relevant systems.[6–7] Methods for preparation of chiral nonracemic unnatural α-amino acids involve synthesis of racemates followed by resolution, use of chiral auxiliaries, asymmetric hydrogenation, and biological approaches.[8] A general method for unnatural amino acid synthesis from chiral pool would expand the toolbox that is available for their preparation. We report here a method of palladium-catalyzed synthesis of protected unnatural amino acids by C-H bond functionalization that employs readily available starting materials derived from chiral pool.
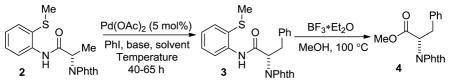
The functionalizaton of amino acid C-H bonds requires installation of a directing group and protection of the amino group. Phthaloyl group was chosen for protection of the amino functionality.[9] Directing group was installed by reacting phthaloylamino acid chlorides[10] with 8-aminoquinoline or 2-thiomethylaniline. N-Phthaloylalanine derivative 2 was arylated by PhI in the presence of a palladium catalyst and base. Subsequently, directing group was removed by treatment with BF3*Et2O in methanol at 100 °C (Table 1).[11] Nearly identical enantiomeric excess of 4 was observed by employing AgOAc, AgOCOCF3, or CsOAc bases at 60–70 °C (entries 3–8). Higher reaction temperatures resulted in erosion of product enantiomeric excess (entries 1, 4, 9), as did addition of pivalic acid (entry 2). The optimal combination of yield and enantiomeric excess was obtained by employing palladium acetate catalyst in combination with AgOAc at 60 °C (entry 5).
Table 1.
Reaction Optimization.
 | ||||
|---|---|---|---|---|
| Entry | Base | Temp, °C | % conv, 3 | % ee, 4 |
| 1[a] | CsOAc | 110 | 68 | 77 |
| 2[a–c] | CsOAc | 90 | 61 | 55 |
| 3[a] | CsOAc | 60 | 51 | 92 |
| 4[d] | AgOAc | 70 | 90 | 88 |
| 5[b],[d] | AgOAc | 60 | 78 | 92 |
| 6[d] | AgOCOCF3 | 70 | 78 | 91 |
| 7[d] | AgOCOCF3 | 60 | 77 | 92 |
| 8[d–e] | AgOCOCF3 | 70 | 59 | 93 |
| 9[b],[d];f] | AgOCOCF3 | 110 | 82 | 67 |
Toluene solvent.
Isolated yield.
Pivalic acid additive.
No solvent.
Pd(OCOCF3)2 catalyst.
Reaction time 12 h. See supporting information for details.
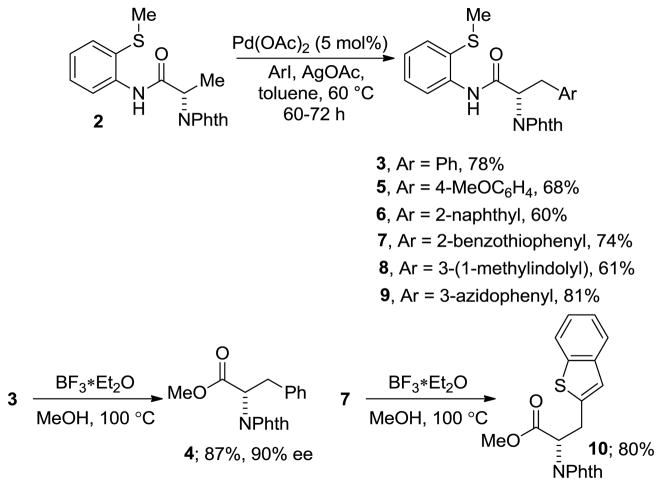
Use of 2-thiomethylaniline derivative allows for a selective monoarylation of methyl group in 2 (Scheme 2). Arylation of 2 by iodobenzene affords 3 in 78% yield. 4-Methoxyiodobenzene is reactive and the arylation product 5 was isolated in 68% yield. 2-Iodonaphthalene and 2-iodobenzothiophene afforded the products in good yields. β-(2-Naphthyl)alanine-containing peptides are highly specific Pin1 inhibitors.[12] Interestingly, 3-iodo-1-methylindole can be coupled with 2 to give an N-methylated tryptophan derivative 8 in 61% yield. An azido functionality is tolerated and 3-azidophenylalanine derivative 9 was obtained in 81% yield. Thus, a wide variety of substituted phenylalanines can be made from a readily available, single starting material 2 in a convergent fashion. Two of the arylated derivatives were subjected to cleavage of directing group. N-Phthaloylphenylalanine methyl ester 4 was obtained in 87% yield and 90% ee. The benzothiophene derivative 10 was obtained in 80% yield.
Scheme 2.
Synthesis of Modified Phenylalanine Derivatives.
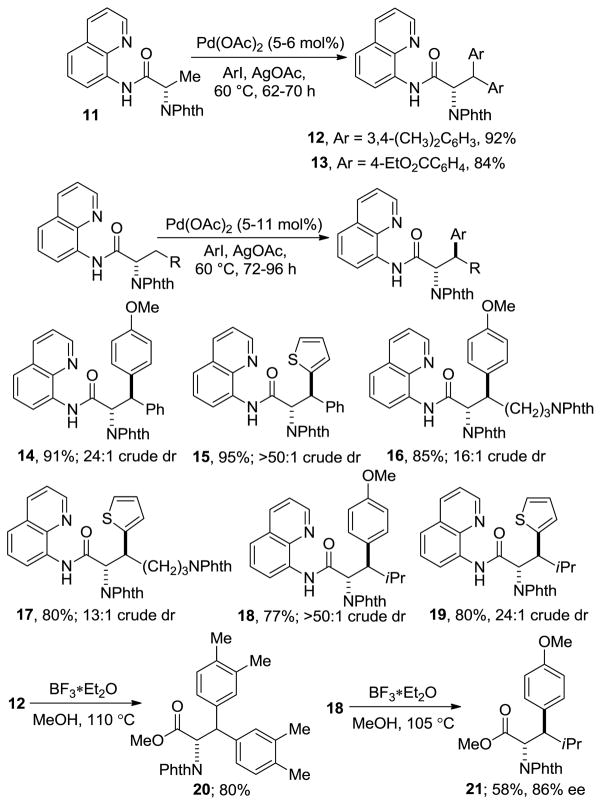
8-Aminoquinoline directing group can be used for diarylation of methyl and monoarylation of methylene functionalities (Scheme 3). Diarylation of 11 was accomplished by 3,4-dimethyl-1-iodobenzene and 4-iodobenzoic acid ethyl ester and the products 12 and 13 were isolated in excellent yields. Interestingly, arylation of methylene groups occurs with high diastereoselectivity favoring the anti diastereomers. Protected phenylalanine can be arylated by 4-iodoanisole to give 91% of the product 14 with crude diastereomer ratio 24:1. Similarly, arylation by 2-iodothiophene results in formation of a single diastereomer 15 in 95% yield. Protected lysine can be arylated by 4-iodoanisole and 2-iodothiophene in high yields and diastereoselectivities. Arylation of a leucine derivative affords products 18 and 19 in high yields. The reactions were typically run on a 0.5 mmol scale. A 5.55 mmol scale p-methoxyphenylation of the leucine derivative afforded 18 in 67% yield. Cleavage of directing group was investigated for 12 and 18. Methyl esters 20 and 21 were obtained in 80 and 58% yields, respectively. Compound 21 was produced in 86% ee that could be upgraded to 95% ee (85% recovery) by one recrystallization. Additionally, relative stereochemistry of 21, which is a derivative of highly constrained β-isopropyltyrosine,[13] was verified by X-ray crystallography.
Scheme 3.
Aminoquinoline Auxiliary.
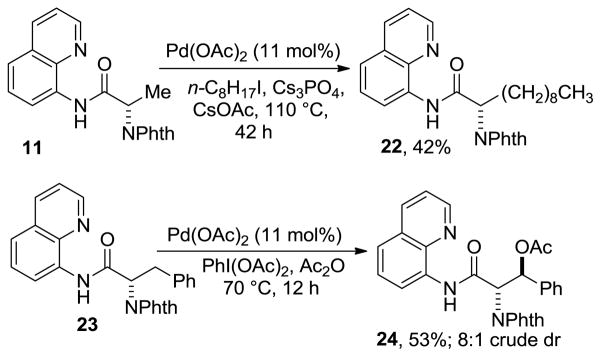
Preliminary results in alkylation and acetoxylation of amino acid C-H bonds are reported in Scheme 4. Thus, alanine derivative 11 was alkylated by 1-iodooctane affording 22 in 42% yield. Compound 22 is a derivative of a lipidic amino acid which has shown tumor cell growth inhibitor activity.[14] Acetoxylation of 23 gave 24 in 53% yield.[15–16]
Scheme 4.
Alkylation and Acetoxylation.
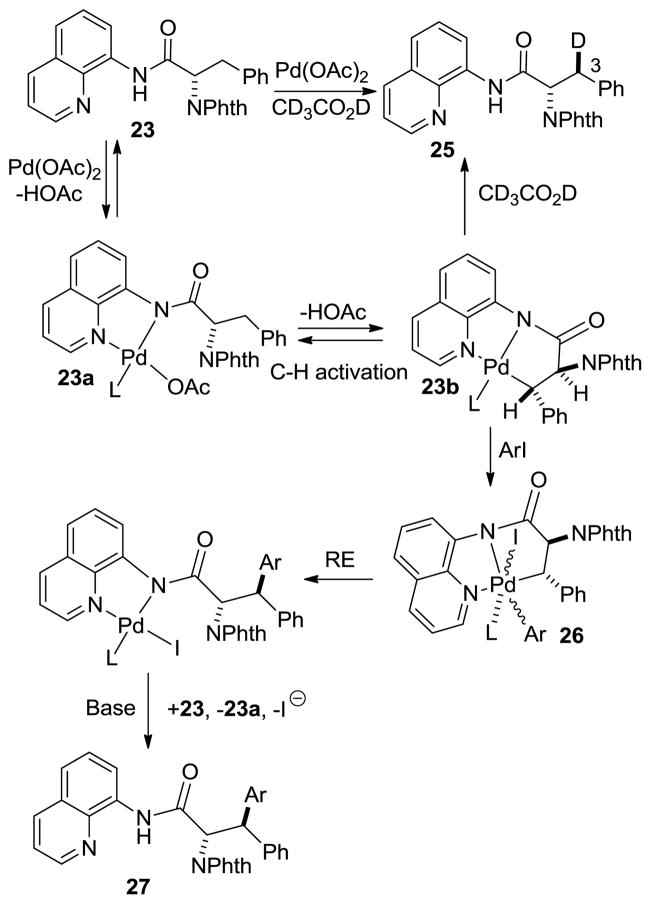
The arylation diastereoselectivity is set either at the C-H activation or, less likely, at reductive elimination step.[17] The H/D exchange in 23 was examined by heating the substrate with catalytic Pd(OAc)2 in CD3CO2D-toluene-d8 mixture. (Scheme 5). After 5 hours at 100 °C, 64% of deuterium incorporation was observed at 3S position with minimal (<10%) incorporation at 3R position. A generalized reaction mechanism can be proposed. Formation of a palladium amide 23a is followed by the C-H activation that affords 23b. The complex 23b then can be protonated or deuterated leading to 25. Since protonation likely occurs with retention of configuration,[18] it can be assumed that 23b has a trans arrangement of phthaloyl and phenyl groups and that the diastereoselectivity of the arylation is set at the stage of palladation. Oxidative addition to give a high-valent[19] Pd intermediate 26 is followed by reductive elimination that proceeds with retention of configuration. Oxidative addition of aryl iodides to palladium(II) may be facilitated by the silver salts since they are known to complex aryl iodides.[20] Ligand exchange affords 27 and regenerates 23a.
Scheme 5.
Mechanistic Considerations.
In conclusion, we have shown that synthesis of a number of substituted phenylalanine derivatives is possible by using C-H bond functionalization methodology. The syntheses are highly convergent and employ N-phthaloylalanine possessing a 2-thiomethylaniline directing group. The use of 8-aminoquinoline directing group allows for the diarylation of methyl and diastereoselective monoarylation of amino acid methylene groups. Acetoxylation and alkylation of amino acid derivative C-H bonds is also possible.
Experimental Section
(S)-N-(3-(Benzothiophene-2-yl)-2-phthalimidopropionyl)-2-methylthioaniline (7)
To a 1-dram vial was added (S)-N-(2-phthalimidopropionyl)-2-methylthioaniline (170 mg, 0.5 mmol), Pd(OAc)2 (6 mg, 0.027 mmol), 2-iodobenzothiophene (785 mg, 3.0 mmol), AgOAc (209 mg, 1.25 mmol), and toluene (0.4 mL). The mixture was stirred at 60 °C for 64 h. After cooling to room temperature, the reaction mixture was diluted with CH2Cl2 (25 mL) and extracted with brine (15 mL). The aqueous layer was extracted with CH2Cl2 (2×15 mL). Combined organic layers were dried over MgSO4. Evaporation to remove the organic solvents followed by purification by flash chromatography in hexanes/EtOAc (100% hexanes to 3:1) and preparative HPLC in hexanes/EtOAc 4:1 gave 175 mg of colorless oil (74%). Rf = 0.45 (SiO2, 1/2 EtOAc/hexanes). 1H NMR (500 MHz, CDCl3, ppm) δ 8.95 (s, 1H) 8.37-8.31 (m, 1H) 7.87-7.82 (m, 2H) 7.75-7.67 (m, 3H) 7.62-7.58 (m, 1H) 7.45-7.41 (m, 1H) 7.33-7.20 (m, 4H) 7.80-7.04 (m, 1H) 5.40 (dd, 1H, J= 5.7, 10.3 Hz) 4.03 (dd, 1H, J= 5.1, 14.9 Hz) 4.11 (dd, 1H, J= 10.9, 15.5 Hz) 2.13 (s, 3H). 13C NMR (125 MHz, CDCl3, ppm) 167.8, 165.8, 140.1, 139.9, 138.0, 134.7, 133.6, 131.5, 129.4, 125.5, 125.0, 124.3, 124.1, 123.9, 123.4, 123.3, 122.3, 120.6, 55.9, 29.7, 19.2 Signal for one carbon could not be located. FT-IR (neat, cm−1) 1715, 1513, 1436, 1380. Calcd for C26H20N2O3S2 (472.58 g/mol) C: 66.08; H: 4.27; N: 5.93; Found C: 65.82; H: 4.45; N: 5.72.
Supplementary Material
Acknowledgments
We thank the Welch Foundation (Grant No. E-1571), NIGMS (Grant No. R01GM077635), and Camille and Henry Dreyfus Foundation for supporting this research. We also thank Dr. James Korp for collecting and solving the X-ray structure of 21.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Reviews: Colby AD, Bergman RG, Ellman JA. Chem Rev. 2010;110:624. doi: 10.1021/cr900005n.Ackermann L, Vicente R, Kapdi AR. Angew Chem Int Ed. 2009;48:9792. doi: 10.1002/anie.200902996.Angew Chem. 2009;121:9976.Chen X, Engle KM, Wang DH, Yu JQ. Angew Chem Int Ed. 2009;48:5094. doi: 10.1002/anie.200806273.Angew Chem. 2009;121:5196.Seregin IV, Gevorgyan V. Chem Soc Rev. 2007;36:1173. doi: 10.1039/b606984n.Lyons TW, Sanford MS. Chem Rev. 2010;110:1147. doi: 10.1021/cr900184e.Daugulis O, Do HQ, Shabashov D. Acc Chem Res. 2009;42:1074. doi: 10.1021/ar9000058.Alberico D, Scott ME, Lautens M. Chem Rev. 2007;107:174. doi: 10.1021/cr0509760.Messaoudi S, Brion JD, Alami M. Eur J Org Chem. 2010:6495.Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d.Sun CL, Li BJ, Shi ZJ. Chem Commun. 2010:677. doi: 10.1039/b908581e.Li CJ. Acc Chem Res. 2009;42:335. doi: 10.1021/ar800164n.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem-Eur J. 2010;16:2654. doi: 10.1002/chem.200902374.Catellani M, Motti M, Della Ca’ N, Ferraccioli R. Eur J Org Chem. 2007:4153.
- 2.a) O’Malley SJ, Tan KL, Watzke A, Bergman RG, Ellman JA. J Am Chem Soc. 2005;127:13496. doi: 10.1021/ja052680h. [DOI] [PubMed] [Google Scholar]; b) Wang DH, Yu JQ. J Am Chem Soc. 2011;133:5767. doi: 10.1021/ja2010225. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Mandal D, Yamaguchi AD, Yamaguchi J, Itami K. J Am Chem Soc. 2011;133:19660. doi: 10.1021/ja209945x. [DOI] [PubMed] [Google Scholar]; d) Marcé P, Díaz Y, Matheu I, Castillón S. Org Lett. 2008;10:4735. doi: 10.1021/ol801791g. [DOI] [PubMed] [Google Scholar]; e) Larivée A, Mousseau JJ, Charette AB. J Am Chem Soc. 2008;130:52. doi: 10.1021/ja710073n. [DOI] [PubMed] [Google Scholar]; f) Chaumontet M, Piccardi R, Baudoin O. Angew Chem Int Ed. 2009;48:179. doi: 10.1002/anie.200804444. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2009;121:185. [Google Scholar]; g) Dangel BD, Godula K, Sezen B, Sames D. J Am Chem Soc. 2002;124:11856. doi: 10.1021/ja027311p. [DOI] [PubMed] [Google Scholar]; h) Ahrendt KA, Bergman RG, Ellman JA. Org Lett. 2003;5:1301. doi: 10.1021/ol034228d. [DOI] [PubMed] [Google Scholar]; i) Bowie AL, Jr, Hughes CC, Trauner D. Org Lett. 2005;7:5207. doi: 10.1021/ol052033v. [DOI] [PubMed] [Google Scholar]; j) Leblanc M, Fagnou K. Org Lett. 2005;7:2849. doi: 10.1021/ol0505959. [DOI] [PubMed] [Google Scholar]
- 3.a) Dyker G. Angew Chem Int Ed Engl. 1994;33:103. [Google Scholar]; Angew Chem. 1994;106:117. [Google Scholar]; b) Barder TE, Walker SD, Martinelli JR, Buchwald SL. J Am Chem Soc. 2005;127:4685. doi: 10.1021/ja042491j. [DOI] [PubMed] [Google Scholar]; c) Chaumontet M, Piccardi R, Audic N, Hitce J, Peglion JL, Clot E, Baudoin O. J Am Chem Soc. 2008;130:15157. doi: 10.1021/ja805598s. [DOI] [PubMed] [Google Scholar]; d) Lafrance M, Gorelsky SI, Fagnou K. J Am Chem Soc. 2007;129:14570. doi: 10.1021/ja076588s. [DOI] [PubMed] [Google Scholar]; e) Liégault B, Fagnou K. Organometallics. 2008;27:484. [Google Scholar]; f) Wang DH, Wasa M, Giri R, Yu JQ. J Am Chem Soc. 2008;130:7190. doi: 10.1021/ja801355s. [DOI] [PubMed] [Google Scholar]; g) Chen X, Goodhue CE, Yu JQ. J Am Chem Soc. 2006;128:12634. doi: 10.1021/ja0646747. [DOI] [PubMed] [Google Scholar]; h) Wasa M, Engle K, Yu JQ. J Am Chem Soc. 2009;131:9886. doi: 10.1021/ja903573p. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Yoo EJ, Wasa M, Yu JQ. J Am Chem Soc. 2010;132:17378. doi: 10.1021/ja108754f. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Wasa M, Yu JQ. Tetrahedron. 2010;66:4811. doi: 10.1016/j.tet.2010.03.111. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Wasa M, Engle KM, Yu JQ. J Am Chem Soc. 2010;132:3680. doi: 10.1021/ja1010866. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Watanabe T, Oishi S, Fujii N, Ohno H. Org Lett. 2008;10:1759. doi: 10.1021/ol800425z. [DOI] [PubMed] [Google Scholar]; m) Stowers KJ, Fortner KC, Sanford MS. J Am Chem Soc. 2011;133:6541. doi: 10.1021/ja2015586. [DOI] [PMC free article] [PubMed] [Google Scholar]; n) Deng G, Zhao L, Li CJ. Angew Chem Int Ed. 2008;47:6278. doi: 10.1002/anie.200801544. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2008;120:6374. [Google Scholar]; o) Hasegawa N, Charra V, Inoue S, Fukumoto Y, Chatani N. J Am Chem Soc. 2011;133:8070. doi: 10.1021/ja2001709. [DOI] [PubMed] [Google Scholar]
- 4.a) Zaitsev VG, Shabashov D, Daugulis O. J Am Chem Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; b) Shabashov D, Daugulis O. J Am Chem Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Reddy BVS, Reddy LR, Corey EJ. Org Lett. 2006;8:3391. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]; b) Feng Y, Chen G. Angew Chem Int Ed. 2010;49:958. doi: 10.1002/anie.200905134. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2010;122:970. [Google Scholar]; c) He G, Chen G. Angew Chem Int Ed. 2011;50:5192. doi: 10.1002/anie.201100984. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2011;123:5298. [Google Scholar]; d) Gutekunst WR, Baran PS. J Am Chem Soc. 2011;133:19076. doi: 10.1021/ja209205x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Kast P. ChemBioChem. 2011;12:2395. doi: 10.1002/cbic.201100533. [DOI] [PubMed] [Google Scholar]; b) Young TS, Schultz PG. J Biol Chem. 2010;285:11039. doi: 10.1074/jbc.R109.091306. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Nestor JJ., Jr Curr Med Chem. 2009;16:4399. doi: 10.2174/092986709789712907. [DOI] [PubMed] [Google Scholar]
- 7.Sommer S, Weikart ND, Brockmeyer A, Janning P, Mootz HD. Angew Chem Int Ed. 2011;50:9888. doi: 10.1002/anie.201102531. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2011;123:10062. [Google Scholar]
- 8.a) Ager DJ. Unnatural Amino Acids. In: Gadamasetti K, Braish T, editors. Process Chemistry in the Pharmaceutical Industry. Vol. 2. CRC Press; 2008. pp. 157–179. [Google Scholar]; b) Au-Yeung TT-L, Chan S-S, Chan ASC. In: Transition Metals for Organic Synthesis. Beller M, Bolm C, editors. Vol. 2. Wiley-VCH; 2004. pp. 14–28. [Google Scholar]; c) Perdih A, Dolenc MS. Curr Org Chem. 2011;15:3750. [Google Scholar]
- 9.Wen S-J, Yao Z-J. Org Lett. 2004;6:2721. doi: 10.1021/ol049065n. [DOI] [PubMed] [Google Scholar]
- 10.Ma T, Gao Q, Chen Z, Wang L, Liu G. Bioorg Med Chem Lett. 2008;18:1079. doi: 10.1016/j.bmcl.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Miltsov S, Rivera L, Encinas C, Alonso J. Tetrahedron Lett. 2003;44:2301. [Google Scholar]
- 12.Wildemann D, Erdmann F, Alvarez BH, Stoller G, Zhou XZ, Fanghänel J, Schutkowski M, Lu KP, Fischer G. J Med Chem. 2006;49:2147. doi: 10.1021/jm060036n. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Liao S, Han Y, Qiu W, Hruby VJ. Tetrahedron: Asymmetry. 1997;8:3213. [Google Scholar]
- 14.Wilke DV, Jimenez PC, Araujo RM, Barbosa da Silva WM, Pessoa ODL, Silveira ER, Pessoa C, Odorico de Moraes M, Skwarczynski M, Simerska P, Toth I, Costa-Lotufo LV. Bioorg Med Chem. 2010;18:7997. doi: 10.1016/j.bmc.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Gou F-R, Wang X-C, Huo P-F, Bi H-P, Guan Z-H, Liang Y-M. Org Lett. 2009;11:5726. doi: 10.1021/ol902497k. [DOI] [PubMed] [Google Scholar]
- 16.a) Desai LV, Hull KL, Sanford MS. J Am Chem Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]; b) Giri R, Liang J, Lei JG, Li JJ, Wang DH, Chen X, Naggar IC, Guo C, Foxman BM, Yu JQ. Angew Chem Int Ed. 2005;44:7420. doi: 10.1002/anie.200502767. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2005;117:7586. [Google Scholar]
- 17.a) Stille JK. In: The Chemistry of the Metal-Carbon Bond. Hartley FR, Patai S, editors. Vol. 2. Wiley; New York: 1985. chap. 9. [Google Scholar]; b) Netherton MR, Fu GC. Angew Chem Int Ed. 2002;41:3910. doi: 10.1002/1521-3773(20021018)41:20<3910::AID-ANIE3910>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.a) Fryzuk MD, Bosnich B. J Am Chem Soc. 1979;101:3043. doi: 10.1021/ja00461a014. [DOI] [PubMed] [Google Scholar]; b) Labinger JA, Hart DW, Seibert WE, III, Schwartz J. J Am Chem Soc. 1975;97:3851. [Google Scholar]
- 19.Intermediate 25 is drawn as a PdIV complex. However, intermediacy of PdIII complexes cannot be excluded. Powers DC, Geibel MAL, Klein JEMN, Ritter T. J Am Chem Soc. 2009;131:17050. doi: 10.1021/ja906935c.Deprez NR, Sanford MS. J Am Chem Soc. 2009;131:11234. doi: 10.1021/ja904116k.Canty AJ. Acc Chem Res. 1992;25:83.Amatore C, Catellani M, Deledda S, Jutand A, Motti E. Organometallics. 2008;27:4549.
- 20.Powell J, Horvath M, Lough A. J Chem Soc, Chem Commun. 1993:733. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.