Introduction
Growing evidence reveals the majority of critically ill patients are at risk for developing two common, dangerous, and potentially iatrogenic conditions-intensive care unit (ICU) delirium and weakness. ICU-acquired delirium and weakness not only influence a patient’s ability to survive critical illness,1,2 but are also associated with poor long-term physical, functional, and cognitive outcomes.3–6 The societal burden of these conditions is daunting. For example, the cost estimates of caring for delirious, mechanically ventilated patients in the United States alone ranges from $6.5–$20.4 billion dollars annually.7,8 Strategies to prevent and/or treat ICU-acquired delirium and weakness are urgently needed to improve both ICU patient outcomes and the resulting societal burdens.
Recently, a novel, inter-professional, bundled approach to managing ICU–acquired delirium and weakness has been proposed. A “bundle”, according to the Institute for Healthcare Improvement,9 is a set of evidence-based practices — generally three to five — that, when performed collectively and reliably, improve patient outcomes. The Awakening and Breathing Coordination, Delirium Monitoring and Management, and Early Mobility (ABCDE) bundle incorporates the best available evidence related to delirium, immobility, sedation/analgesia, and ventilator management in the ICU and tailors the pharmacologic and nonpharmacologic interventions used in prior clinical trials into a bundle that can be adopted into everyday clinical practice.10–13 The foundation of the ABCDE bundle primarily depends upon three principles: 1) improving communication among members of the ICU team, 2) standardizing care processes, and 3) breaking the cycle of over-sedation and prolonged mechanical ventilation that can subsequently lead to delirium and weakness.10
The purpose of this paper is to summarize the evidence behind the ABCDE bundle. Additionally, we aim to explain the individual components of the ABCDE bundle and provide readers an example of an ABCDE policy. Finally, we discuss the registered nurses (RNs) unique role in implementing the ABCDE bundle into clinical practice.
Evidence Supporting Nursing-Implemented Sedation Protocols and Daily Awakening
The majority of critically ill patients require some form of analgesic or sedative therapy during their ICU stay; most often, various combinations of opioids, benzodiazepines, hypnotics, and antipychotics.14 Nurses administer these medications to facilitate mechanical ventilation, improve tolerance of invasive procedures, protect the patient and staff from harm caused by aggressive or agitated patient behavior, and relieve pain and anxiety.15,16 As with any procedure, there are adverse events associated with sedation and analgesia including respiratory depression, hypotension, renal failure, and deconditioning.14 Moreover, several studies highlight the relationship between ICU-acquired delirium and the utilization of potent sedative and analgesic agents, with a notable increased risk of delirium with benzodiazepines.17–19 These safety concerns have generated a surge of interest in broadly implementing strategies to decrease patients’ exposure to sedative medications.
Nursing implemented, protocol-directed sedation is one strategy of reducing patients’ exposure to potentially harmful medications. In a randomized, controlled trial, Brook and colleagues20 found that protocol-directed sedation during mechanical ventilation reduced the duration of mechanical ventilation, decreased ICU and hospital length of stay (LOS), shortened the duration of continuous infusion of sedation, and lowered tracheostomy rates of patients’ compared to those treated with non-protocol directed sedation. Since that time, many sedation protocols and algorithms have incorporated the evaluation and management of pain and agitation within a single algorithm,16 with management intended to be under the direction of the bedside nurse. Beneficial outcomes linked to the use of nurse-managed sedation/analgesia algorithm(s) or protocol(s) in controlled studies include: more “on-target” sedation,21 less pain and agitation,22 reduced direct drug costs or medication use,23 less patient ventilator asynchrony,21 and decreased incidence of ventilator associated pneumonia.24
Another innovative way to reduce sedation in adult ICU patients is the practice of daily interruption of sedation. In 2001, Kress and colleagues25 conducted a single-center, randomized controlled trial of 128 mechanically ventilated patients comparing usual care to a sedation strategy that involved daily interruption of sedative (midazolam or propofol) and analgesic (morphine) infusions, until the patient was awake, able to follow 3 or 4 simple commands, or was agitated. They found that daily interruption of sedation, now often referred to as spontaneous awakening trials (SATs), led to a significant decrease in the duration of mechanical ventilation, a shorter ICU LOS, and use of fewer diagnostic tests for unexplained mental status changes. A retrospective analysis of this study found patients treated with SATs also experienced significantly fewer overall complications (e.g., ventilator associated pneumonia, upper gastrointestinal hemorrhage, bacteremia, barotrauma) than those treated with usual care.26 To evaluate the impact of SATs on long-term psychological outcomes, Kress and colleagues also compared the development of post-traumatic stress disorder (PTSD) symptoms in each group.27 Patients whose daily sedation was interrupted developed significantly fewer symptoms of PTSD following critical illness, suggesting that not only are SATs safe, but may have other beneficial effects on long-term outcomes of mechanically ventilated patients.28
Evidence Supporting Respiratory Therapist Driven Protocolized Spontaneous Breathing Trials
Just as SATs are used to determine a patient’s need for sedation, spontaneous breathing trials (SBTs) are used to determine if a mechanically ventilated patient is ready to breathe on her/his own.28 Support for the use of SBTs was garnered over 10 years ago when a study by Esteban et al. found this strategy was associated with reduced time to successful weaning.29 Subsequently, Ely and colleagues30 reported that a respiratory care-driven weaning protocol including SBTs significantly shortened time to extubation compared to physician-driven weaning. The data generated by the aforementioned studies established the use of SBTs as an effective way of achieving early liberation from mechanical ventilation.28
Evidence Supporting the Pairing of SATs and SBTs: Awakening and Breathing Trial Coordination
The Awakening and Breathing Controlled (ABC) Trial conducted by Girard and colleagues31 advanced the science of sedation and ventilator management by integrating the roles of nurses’ sedation management with that of respiratory therapists’ ventilator management by studying the pairing of SATs with SBTs. This randomized controlled trial included 336 patients at 5 medical centers in North America. The intervention group in the ABC trial was managed with the “wake up and breathe” protocol, consisting of protocolized coordination of nurse directed SATs and respiratory therapist directed SBTs, whereas the control group received patient-targeted sedation according to “usual care” combined with SBTs. The SBTs were accomplished by allowing the patient to breathe through either a T-tube circuit or a ventilator circuit with continuous positive airway pressure of 5 cm H20 or pressure support ventilation of less than 7 cm H20. Patients in the intervention arm spent significantly more days breathing without ventilator assistance, were discharged from the ICU and hospital earlier, had shorter duration of coma, and experienced a 14% absolute reduction in the risk of death up to 1 year after study enrollment. Although more patients in the intervention group self-extubated than in the control, the number of patients who required reintubation was similar. Few other differences were noted in in-hospital adverse events between groups31 or long-term cognitive or psychological outcomes.32
Evidence Supporting Delirium Monitoring and Management
Research conducted over the last decade has consistently shown that delirium, often-referred to as acute brain dysfunction, is a significant problem in the ICU setting. The prevalence of delirium in mechanically ventilated adults is as high as 83%.1 Delirium in the ICU setting is associated with multiple unfavorable outcomes including: higher ICU and hospital costs,7 longer ICU admissions and overall LOS,33,34 greater use of continuous sedation and physical restraints,35 increased self-removal of catheters and self-extubation,36 and higher ICU mortality.2 Additionally, the impact of delirium extends to the post-discharge period. Post-discharge sequelae of delirium include: greater likelihood of discharge to a place other than home,3 greater functional decline,3 higher six month and 1-year mortality,14 and long-term neurocognitive impairment.5
It is essential that providers routinely use valid and reliable sedation and delirium screening tools. Multiple studies report that without the use of these instruments clinicians miss the vast majority of ICU delirium cases.37,38 One potential reason clinicians fail to notice delirium in critically ill patients is because the syndrome is frequently “invisible”. For example, the hypoactive form of delirium, characterized by a depressed level of consciousness and lack of psychomotor agitation, was found to be far more prevalent (64% and 60%) compared to the mixed (9% and 6%) or purely hyperactive (0% and 1%) forms in mechanically ventilated surgical and trauma intensive care unit patients.18
Fortunately, there are a number of valid and reliable tools available to screen for delirium in the ICU setting. Two of the most widely used tools39 include the Confusion Assessment Method- ICU (CAM-ICU)40 and the Intensive Care Delirium Screening Checklist (ICDSC).41 Developed for use in critically ill, nonvocal patients, the CAM-ICU defines delirium in terms of four diagnostic features. Delirium is deemed present when a patient displays an acute change or fluctuating course of mental status (Feature 1), inattention (Feature 2), and either an altered level of consciousness (Feature 3), or disorganized thinking (Feature 4). The ICDSC contains eight items that are scored as either 1 (present) or 0 (absent). A total score of 4 or greater is considered a positive screen for delirium. Extensive information on how to successfully use these quick delirium-screening tools is available at www.icudelirium.org.42
Evidence Supporting Early Mobility
A strategy for whole-body rehabilitation, accomplished by the use of SATs and early exercise and mobilization, was recently found to be safe and well tolerated by critically ill patients.6 Schweickert and colleagues randomly assigned subjects to exercise and mobilization (with physical and occupational therapy, N=49) beginning on the day of enrollment (intervention) or to standard care (N=55) with physical therapy and occupational therapy delivered as ordered by the primary care team. Both groups were managed by goal-directed sedation and underwent daily interruption of sedatives. Patients in the intervention group were found to have significantly shorter duration of delirium and coma and more ventilator free days during the 28-day follow-up period than did controls. They also found intervention subjects were more likely to return to independent functional status at hospital discharge than controls. This liberation and animation strategy led to improvements in functional and cognitive outcomes even though only 33% of intubated patients moved from bed to chair and 15% ambulated. Active movements in bed, dangling and grooming were the most frequently performed animation activities with intubated patients,-tasks the nurse can incorporate into the patient’s bath.43
Significant improvement in patient outcomes were also found in a recent quality improvement project that involved the formation of a multidisciplinary team focused on reducing heavy delivery of sedatives, conducting delirium screenings, and increasing the functional mobility of mechanically ventilated patients.44 The major changes involved in this project included: 1) modifying the standardized MICU admission orders to change the default activity level from “bed rest” to “as tolerated”, 2) encouraging a change in sedation practice from using continuous IV infusions to “as needed” bolus doses, 3) establishing and disseminating simple guidelines for PT and OT consultation, 4) developing safety-related guidelines, 5) changing staffing to include a full-time PT and OT and a part-time rehabilitation assistant, 6) consulting a physiatrist for MICU patients receiving rehabilitation therapy, and 7) increasing consultations to neurologists for MICU patients with muscle weakness deemed severe or prolonged. When compared to the pre-intervention time period, the quality improvement project demonstrated benzodiazepine use decreased markedly, patients had improved sedation and delirium status, there were a greater median number of rehabilitation treatments per patient with a higher level of functional mobility (treatments involving sitting or greater mobility), and a decrease in ICU and hospital LOS. This project further demonstrates that a multicomponent, interdisciplinary approach, which includes early mobility, is an important consideration for any ICU.
Pulling the Evidence Together-The ABCDE Bundle
Despite the accumulating evidence of the benefits of SATs, SBTs, delirium monitoring and management, and early mobility protocols over the last decade, this evidence has not been widely applied in the ICU setting.10 For example, a recent survey of 1,384 ICU physicians, nurses, respiratory therapists, and pharmacists found 40% of participants did not screen for delirium and almost one-third of the respondents did not use a sedation protocol.45 Very few (22%) ICU healthcare providers in this survey reported using SATs on a daily basis; with the majority reporting SATs occurring on fewer than 75% of all ICU days.45 Similarly, the use of SBTs among academic ICUs appears low, with rates ranging from 31–42%.46 The use of exercise and early mobility protocols in the ICU is also lacking. For example, one study of critically ill patients found that 20% patients received no physical activity while another 15% received only passive range-of-motion exercises during their ICU stay.47
To address these deficiencies, leading critical care researchers have promulgated a unified approach to managing ICU-acquired delirium and weakness. First proposed as a model for preventing acute and chronic brain dysfunction in young and elderly ICU patients,11 the overarching purpose of the ABCDE bundle is to reduce the frequency and magnitude of the negative outcomes associated with ICU-acquired delirium and weakness. There are several guiding principles behind the ABCDE bundle.10
In order for the ABCDE bundle to have its full impact,10 we recommend healthcare providers consider using the bundle every day, in every adult patient admitted to an ICU. In the context of a hospital’s busy ICU care environment, there will be some patients on any given day that, for legitimate medical or even psychological reasons, may need to refrain or be excluded from participating in certain components of the ABCDE care bundle. Fortunately, the ABCDE bundle was developed in such a way that these patients can be safely identified. Bundle utilization should not depend on an individual physician’s order but rather structured as a daily part of care with clearly defined safety guidelines (e.g. an “opt-out” rather than “opt-in” approach to care delivery).10 These safety guidelines should be based upon prior research while maintaining enough flexibility for institutions to adapt them to meet the needs of their special populations (e.g., neurosurgical or burn unit patients). When medically indicated, the prescriber can opt out of individual components of the ABCDE protocol. Documentation of the individual reasons will further enable the implementation team understand potential barriers to implementation, and allow for iterative modification of the protocol or training to meet the needs of the local environment. The default must be delivery of the full ABCDE bundle, which puts a premium on coordinated, interdisciplinary care.
Successful implementation of the ABCDE bundle requires effective, frequent communication among a number of different ICU team members and an evolution in critical care team roles.10 The evidence that nurse, respiratory therapist, and physical therapist-driven sedation, mechanical ventilation, and physical therapy protocols are safe and effective is compelling.10,20,26,48 The contribution of a clinical pharmacist is essential in not only developing ICU sedation guidelines, but may also assist in monitoring and improving compliance with such guidelines.49
As stated previously, the ABCDE bundle is comprised of three distinct, yet highly interconnected, components including: 1) Awakening and Breathing trial Coordination, 2) Delirium monitoring and management, and 3) Early mobility. The interventions in the ABCDE bundle are operationalized. The bundle contains several essential elements that must be carried out for it to be effective. However, the bundle is flexible enough for adaptations needed to meet the needs of patients and staff. In the following section, we outline the essential elements of the ABCDE bundle and give examples of how one institution is currently implementing policies regarding the ABCDE bundle.
Procedure-Awakening and Breathing Trial Coordination
According to the ABCDE bundle, every mechanically ventilated patient should be evaluated with the ABC protocol (Table 1, Figure 1). This requires establishing a coordinated routine that relies on a number of team members making informed decisions. For example, a RN is primarily responsible for performing the SAT. A respiratory therapist (RT) is primarily responsible for performing the SBT. A licensed prescriber makes the decision to extubate the patient. Effective, frequent communication among professionals is necessary for successful implementation of the coordinated SAT and SBT.
Table 1.
Steps Involved in Awakening and Breathing Trial Coordination*@
Step 1 –SAT Safety Screen-RN Driven- The RN will determine if it is safe to interrupt sedation by responding to a set of predefined safety screen questions. For example,
|
Step 2- Perform SAT-RN Driven- The RN will determine if the patient tolerated interruption of sedation by assessing if the patient demonstrates any predefined SAT failure criteria. For example,
|
Step 3- SBT Safety Screen- Respiratory Therapist (RT) Driven-The RT will determine if is safe to perform a SBT by responding to a set of predefined safety questions. For example,
|
Step 4-Perform SBT-RT Driven- The RT will determine if the patient tolerated the SBT by assessing if the patient demonstrates any predefined SBT failure criteria. For example,
|
Criteria used in the Awakening and Breathing Controlled Trial (Evidence-based)31
Criteria added by example institution based on interdisciplinary discussion
Figure 1.
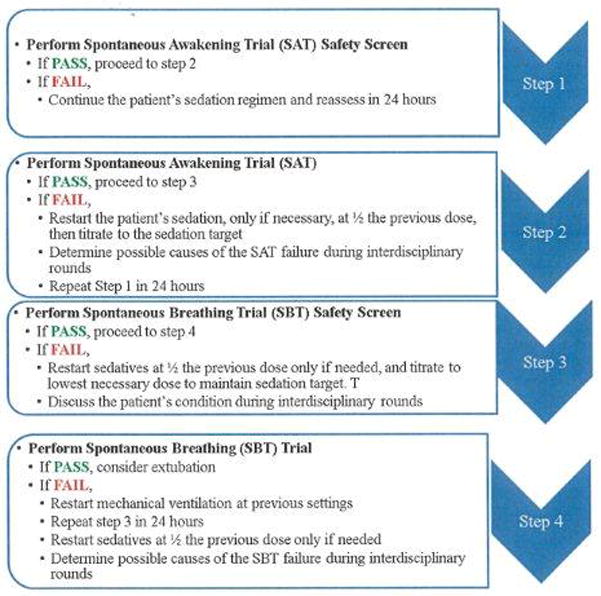
Steps in Awakening and Breathing Trial Coordination
There are four major steps in the Awakening and Breathing Trial Coordination process (Table 1). The evidence supporting the ABCs is mainly derived from the Awakening and Breathing Controlled Trial.31 Step 1 is the SAT Safety Screen. In this step, a RN determines if it is safe to interrupt sedation by responding to a set of predefined safety questions (Table 1). If any of the SAT Safety Screen questions are answered YES, the RN should conclude it is NOT SAFE to shut off the patient’s continuous sedative infusions. In the case it is determined to be unsafe, the RN should continue the patient’s sedation regimen and reassess in 24 hours. The interdisciplinary team should also discuss the patient’s condition during rounds. If all of the SAT Safety questions are answered NO, the RN will conclude it is SAFE to perform a SAT and proceed to step 2.
Step 2 involves the RN performing a SAT. A SAT involves the RN shutting off all continuous sedative infusions. Continuous analgesic infusions are maintained only if needed for active pain. During the SAT, the RN should also hold all sedative boluses. If the patient should complain or demonstrate signs/symptoms of pain while the continuous sedative infusion is shut off, the RN may administer bolus doses of analgesics as needed/ordered.
Next, the RN determines if the patient tolerated interruption of sedation by assessing if the patient demonstrates any of the SAT failure criteria described in Table 1. If the patient displays any of the SAT failure criteria, the RN should conclude the patient has failed the SAT. The RN should then restart the patient’s sedation, if necessary, at ½ the previous dose, then titrate to the sedation target. The RN will repeat Step 1 in 24 hours. The interdisciplinary team will determine possible causes of the SAT failure during rounds.
At the point that the patient is able to open his/her eyes to verbal stimulation while tolerating the sedatives turned off (i.e. without failure criteria)-regardless of trial length- the RN will conclude the patient has passed the SAT and ask the RT to immediately perform a SBT Safety Screen. A SAT is also considered “successful” in those patients who after four hours do not respond to verbal stimulation, but do not display any of the failure criteria. In this case, the RN would also ask the RT to proceed to step 3.
Step 3 is the SBT Safety Screen. In this step, the respiratory therapist will determine if it is safe to perform a SBT by responding to a set of predefined safety questions (Table 1). If any of the SBT Safety Screen questions are answered YES, the RT will conclude it is NOT SAFE to perform a SBT. The RT will continue mechanical ventilation and repeat step 3 in 24 hours. The RT will ask the RN to restart sedatives at ½ the previous dose only if needed, and titrate to lowest necessary dose to maintain sedation target. The interdisciplinary team will discuss the patient’s condition during rounds. If all of the above questions are answered NO, the RT will conclude it is SAFE to perform a SBT and proceed to step 4.
Step 4 involves performing a SBT. In this step, the RT will place the patient on a SBT (e.g., change ventilator settings to CPAP pressure support 5, PEEP 5, use T-piece). The RT will determine if the patient tolerated the SBT by assessing if the patient demonstrates any of the spontaneous breathing trial failure criteria (Table 1). If the patient displays any of the SBT failure criteria, the RT will conclude the patient has failed the SBT and restart mechanical ventilation at previous settings. The RT will inform the nurse of the SBT failure and remind him/her to restart sedatives at ½ the previous dose only if needed. The RN and RT will evaluate the patient again in 24 hours starting with Step 1. The interdisciplinary team will determine possible causes of the spontaneous breathing trial failure during rounds. If the patient tolerates spontaneous breathing for 30–120 minutes without failure criteria, the RT will inform the RN and physician that the patient has passed their SBT. The ABC trial used the 2 hour time frame for establishing extubation readiness while Esteban and colleagues11 found patients who were extubated after successfully completing a 30-min SBT had similar reintubation rates to those who were not extubated until they completed a 120-min trial. At this time, the physician should consider extubation.
Essential Elements of Delirium Monitoring and Management
According to the ABCDE bundle, every patient admitted to an adult ICU should undergo routine sedation/agitation and delirium assessment using standardized, validated assessment tools. We suggest the RN perform and record the results of a validated sedation scale every 2–4 hours with vital signs. We also suggest the RN perform and record the results of the delirium assessment (CAM-ICU or ICDSC) at least once per shift and whenever a patient experiences a change in mental status.
To facilitate communication among the interdisciplinary team, the ICU team should set a “target” sedation/agitation score at which the patient should be maintained for the following 24 hours. Each day during interdisciplinary rounds, the RN will inform the team of the patient’s: 1) “target” sedation score, 2) actual sedation/agitation score, 3) delirium status, and 4) sedative and analgesic medication exposure (Figure 2). There are a number of valid and reliable tools that can be used to facilitate goal-directed titration of sedative medications including the Richmond Agitation Sedation Scale,50 Sedation-Agitation Scale,51 Adaption to the Intensive Care Enviornment,52 Motor Activity Assessment Scale,53 Vancouver Interaction and Calmness Scale54 and others.
Figure 2.

Facilitating Inter-professional Communication during Intensive Care Unit Rounds-The Brain Road Map
Because the management of delirium is focused on identifying and treating the actual cause of the syndrome, each day during interdisciplinary rounds, the team will also discuss possible causes of the patient’s delirium. One useful acronym for the team is to “THINK” when a patient is delirious (Table 2), a cognitive script meant to prompt the team to “think” of the underlying cause(s) contributing to the patients newly developed or ongoing delirium. Finally, while it is beyond the scope of this paper to address the nonpharmacologic management of delirium, there are a number of excellent references that specifically address this issue.55,56 Though most nonpharmacologic delirium interventions have been studied in geriatric populations, they should still be considered in the routine care of all critically ill patients. We suggest the interdisciplinary team should always consider the use of nonpharmacologic strategies and modification of risks first when caring for a patient with delirium.
Table 2.
What to “THINK” When Your Patient is Delirious
| Toxic situations and medications: congestive heart failure, shock, dehydration, new organ failure (e.g., liver, kidney), deliriogenic medications |
| Examples of deliriogenic medications include benzodiazepines, anticholinergic medications, and steroids |
| Hypoxemia |
| Infection/sepsis (nosocomial), inflammation, immobilization |
| Non-pharmacological interventions |
| K+ or other electrolyte interventions |
Essential Elements of Early Mobility
In the ABCDE bundle, patients are candidates for mobilization when they meet certain criteria (Table 3). These criteria were developed from some of the evidence supporting early mobility protocols.44,47,57,58,58 We suggest exceptions to these criteria should only be permitted by specific written order by the prescriber (for example, skin integrity issues). The interdisciplinary care team assesses the patient’s readiness for mobility. The team includes a physical therapist who assesses the patient’s physical ability to participate; a nurse who assesses physiologic stability; and a respiratory therapist who is responsible for maintaining the patient’s airway.59 In addition, a critical care physician confirms that there are no clinical contraindications to physical activity.
Table 3.
| N – Neurologic |
| R – Respiratory |
C – Circulatory/Central lines/Contraindications
|
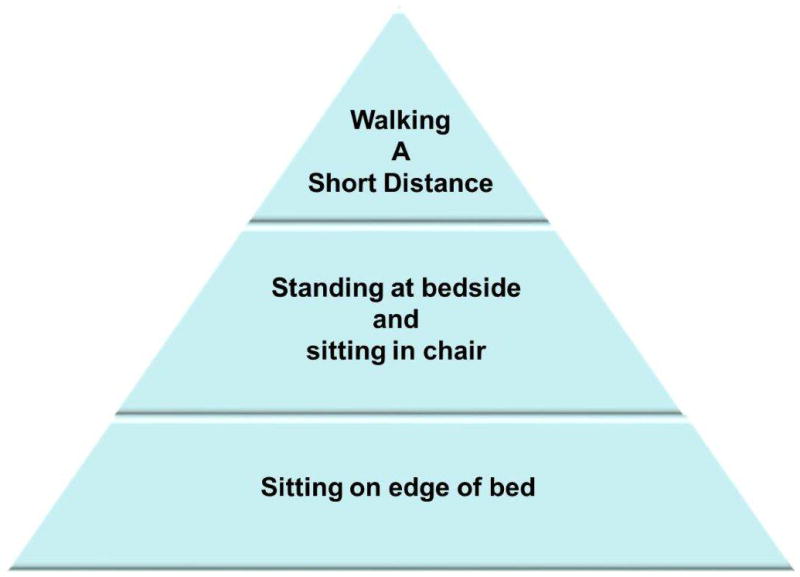
There are a number of resources describing early mobility procedures found in the ICU literature.60,58,61,62 An example protocol, that incorporates the best of this evidence, is also provided by the Agency for Healthcare Research and Quality.59 According to this protocol, each patient is assessed upon admission to the ICU, and those who qualify immediately begin the protocol. Those who are not eligible are reassessed during daily rounds. If activity has been halted due to an acute event (see examples Table 4) the patient is reevaluated each day until the protocol can be reinstated. Each eligible patient is encouraged to be mobile at least once a day, with the specific level of activity geared to his or her readiness. Patients progress through a three-step process, embarking on the highest level of physical activity they can tolerate, as outlined in Figure 3. The authors suggest that the use of the protocol ends when the patient is discharged from the ICU.
Table 4.
Example Criteria for Halting Early Mobility*
| Symptomatic drop in mean arterial pressure |
| Heart rate <50 or >130 beats per minute × 5 minutes |
| Respiratory rate <5 or >40 breaths per minute × 5 minutes |
| Systolic blood pressure >180 mmgHg × 5 minutes |
| Pulse oximetry reading of <88% × 5 minutes |
| Marked ventilator dysynchrony |
| Patient distress |
| New arrhythmia |
| Concern for myocardial ischemia |
| Concern for airway device integrity |
| Fall to knees |
| Endotracheal tube removal |
Figure 3.
Early Mobility Hierarchy
Conclusion-Nurses unique contribution
The successful implementation of a complex bundle requires: 1) high quality, timely, and reliable completion of independent tasks by trained individuals, 2) effective communication between individuals to ensure the proper order and sequence of the individual components, and 3) effective leadership that can mold and adapt implementation to meet the needs of the local culture/environment and provide ongoing support, resources, and training.
The ABCDE bundle is indeed complex, although successful implementation holds potential for tremendous benefit to our sickest patients. Nurses play a unique role in the implementation of ABCDE as they are critical to all requirements for successful implementation. Registered nurses lead protocol-guided sedation efforts that include daily spontaneous awakening trials and measurement of delirium and sedation/agitation using validated instruments. The nurse is also the communication link between each of the individual specialties. Decisions to advance to subsequent steps of the ABCDE bundle with SBT, early mobility, and extubation are dependent upon the RN’s assessments of level of consciousness, pain, and other clinical parameters communicated to RTs, PTs, and MDs respectively. Finally, and equally important, RNs are well suited to the leadership roles required to individualize the ABCDE bundle to the institution. Meaning, RNs understand the local context for implementation, and can provide critical insights into the resources and training required for implementation efforts.
In conclusion, the health of our patients depends upon the successful integration of many moving parts. The development or prevention of ICU-acquired delirium and weakness exemplifies the failure or success of a coordinated approach to care. Similarly, successful implementation of the ABCDE bundle will reflect effective coordination and leadership, a role that RNs are uniquely positioned to fill.
Acknowledgments
Funding and financial Disclosure: Drs. Balas and Burke are Co-Principle Investigators of a Robert Wood Johnson Foundation Interdisciplinary Nursing Quality Research Initiative grant entitled “Implementation and Dissemination of an Interdisciplinary Nurse-Led Plan to Manage Delirium in Critically Ill Adults”. Funding for Drs. Ely and Vasilevskis has been provided by the National Institutes of Health (K23AG040157), the Veterans Affairs Clinical Research Center of Excellence, and the Tennessee Valley GRECC. Dr. Ely consulted for or received honoraria/grants from Hospira, Eli Lilly, Cumberland, and Masimo. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institutes of Health, or the U.S. Department of Veterans Affairs. Ms. Boehm and Ms. Pun have received honorarium from Hospira. Dr. Olsen receives grant funding from Carmel-Pharma, Astellas Inc, Sanofi-Pasteur, and Otuska Inc.
The authors would like to acknowledge the support of the Robert Wood Johnson Foundation Interdisciplinary Nursing Quality Research Initiative and the Nebraska Medical Center for their assistance in implementing the ABCDE bundle into ICU practice.
Biography
Dr. Balas is an assistant professor at the UNMC, College of Nursing and a former John A. Hartford Foundation Building Academic Geriatric Nursing Capacity Predoctoral Scholar and Postdoctoral Fellow. Dr. Vasilevskis is an assistant professor at Vanderbilt University Medical Center and the GRECC at the VA Tennessee Valley Healthcare System. Dr. Burke is a Professor of Psychiatry and Vice-Chair for Research at the UNMC. Leanne Boehm is a Research Nurse with the ICU Delirium and Cognitive Impairment Study Group. Brenda Pun is a Program Clinical Manager at Vanderbilt University Medical Center. Dr. Olsen is a Professor and Chair of the Department of Pharmacy Practice at the UNMC. Dr. Peitz is a Pharmacy Coordinator and Clinical Assistant Professor for Pharmacy Practice at the UNMC. Dr. Ely is a Professor at Vanderbilt University School of Medicine and the Associate Director of Aging Research for the Tennessee Valley Veterans Administration GRECC.
Contributor Information
Michele C. Balas, University of Nebraska Medical Center, College of Nursing, Work address: 985330 Nebraska Medical Center, Omaha, NE, 68198-5330, Home address: 4903 North 142nd Street, Omaha, NE 68164.
Eduard E. Vasilevskis, Vanderbilt University Medical Center, Division of General Internal Medicine and Public Health – Section of Hospital Medicine and the Geriatric Research, Education and Clinical Center – VA Tennessee Valley Healthcare System, Work address: 1215 21st Ave. S., 6006 MCE, NT 37232-8300, Home address: 9151 Demery Ct. Brentwood, TN 37027.
William J. Burke, Vice-Chair for Research, Department of Psychiatry University of Nebraska Medical Center, Work address: 986150 Nebraska Medical Center, Omaha, NE 68198-6150, Home address.
Leanne Boehm, Vanderbilt University Medical Center, Division of Allergy, Pulmonary, and Critical Care Medicine, Work address: 1215 21st Avenue South, Suite 6100 MCE Nashville, TN 37232, Home: 1880 Portway Road Spring Hill, TN 37174.
Brenda T. Pun, Vanderbilt University Medical Center, Work address: 1215 21st Avenue South, Suite 6100 MCE Nashville, TN 37232, Home address: 5515 Tahoe Drive Durham, NC 27713.
Keith M. Olsen, Department of Pharmacy Practice, University of Nebraska Medical Center, Work address: 986045 Nebraska Medical Center, Omaha, NE 68198-6045, Home address: 11538 Todd Drive, Blair, NE 68008.
Gregory J. Peitz, Department of Pharmacy Practice, University of Nebraska Medical Center, Work address: 981090 Nebraska Medical Center, Omaha, NE, 68198-1090, Home address: 6235 William St. Omaha, NE 68106.
E. Wesley Ely, Department of Medicine, Division of Pulmonary and Critical Care at Vanderbilt University School of Medicine and the Associate Director of Aging Research for the Tennessee Valley Veterans Administration Geriatric Research Education Clinical Center, Work address: 1215 21st Avenue South, Medical Center East Suite 6100, Nashville, TN 37232, Home address: 3619 Hampton Avenue, Nashville, TN 37215.
References
- 1.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Lin S, Liu C, Wang C, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 3.Balas MC, Happ MB, Yang W, Chelluri L, Richmond T. Outcomes associated with delirium in older patients in surgical ICUs. Chest. 2009;135(1):18–25. doi: 10.1378/chest.08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness P. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 8.Halpern NA, Pastores SM. Critical care medicine in the united states 2000–2005: An analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38(1):65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Healthcare Improvement. 5 million lives campaign. getting started kit: Prevent ventilator associated pneumonia. Updated 2008.
- 10.Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness--crossing the quality chasm. Chest. 2010;138(5):1224–1233. doi: 10.1378/chest.10-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasilevskis EE, Pandharipande PP, Girard TD, Ely EW. A screening, prevention, and restoration model for saving the injured brain in intensive care unit survivors. Crit Care Med. 2010;38(10):S683–S691. doi: 10.1097/CCM.0b013e3181f245d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: The ‘ABCDE’ approach. Curr Opin Crit Care. 2011;17(1):43–49. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 13.Pandharipande P, Banerjee A, McGrane S, Ely EW. Liberation and animation for ventilated ICU patients: The ABCDE bundle for the back-end of critical care. Crit Care. 2010;14(3):157–157. doi: 10.1186/cc8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30(1):119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Kress JP, Pohlman AS, Hall JB. Sedation and analgesia in the intensive care unit. Am J Respir Crit Care Med. 2002;166(8):1024–1028. doi: 10.1164/rccm.200204-270CC. [DOI] [PubMed] [Google Scholar]
- 16.Sessler CN, Pedram S. Protocolized and target-based sedation and analgesia in the ICU. Crit Care Clin. 2009;25(3):489. doi: 10.1016/j.ccc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: The MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 18.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness P, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med. 2009;37(1):177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27(12):2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Richman PS, Baram D, Varela M, Glass PS. Sedation during mechanical ventilation: A trial of benzodiazepine and opiate in combination. Crit Care Med. 2006;34(5):1395–1401. doi: 10.1097/01.CCM.0000215454.50964.F8. [DOI] [PubMed] [Google Scholar]
- 22.Chanques G, Jaber S, Barbotte E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34(6):1691–1699. doi: 10.1097/01.CCM.0000218416.62457.56. [DOI] [PubMed] [Google Scholar]
- 23.Mascia MF. Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Med. 2000;28(7):2300. doi: 10.1097/00003246-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Quenot J, Ladoire S, Devoucoux F, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med. 2007;35(9):2031–2036. doi: 10.1097/01.ccm.0000282733.83089.4d. [DOI] [PubMed] [Google Scholar]
- 25.Kress JP, Pohlman AS, Hall JB. Effects of sedative interruption in critically ill, mechanically ventilated patients receiving midazolam or propofol. JCOM. 2001;8(2):33–39. [Google Scholar]
- 26.Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32(6):1272–1276. doi: 10.1097/01.ccm.0000127263.54807.79. [DOI] [PubMed] [Google Scholar]
- 27.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168(12):1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 28.Hooper MH. Sedation and weaning from mechanical ventilation: Linking spontaneous awakening trials and spontaneous breathing trials to improve patient outcomes. Crit Care Clin. 2009;25(3):515. doi: 10.1016/j.ccc.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Esteban A, Alía I, Gordo F, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. the spanish lung failure collaborative group. Am J Respir Crit Care Med. 1997;156(2):459–465. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- 30.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335(25):1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 31.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): A randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 32.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. Am J Respir Crit Care Med. 2010;182(2):183–191. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomason JWW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: A prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9(4):R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Micek ST, Anand NJ, Laible BR, Shannon WD, Kollef MH. Delirium as detected by the CAM-ICU predicts restraint use among mechanically ventilated medical patients. Crit Care Med. 2005;33(6):1260–1265. doi: 10.1097/01.ccm.0000164540.58515.bf. [DOI] [PubMed] [Google Scholar]
- 36.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: A study of risk factors. Intensive Care Med. 2001;27(8):1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 37.Pun BT, Gordon SM, Peterson JF, et al. Large-scale implementation of sedation and delirium monitoring in the intensive care unit: A report from two medical centers. Crit Care Med. 2005;33(6):1199–1205. doi: 10.1097/01.ccm.0000166867.78320.ac. [DOI] [PubMed] [Google Scholar]
- 38.Devlin JW, Fong JJ, Schumaker G, O’Connor H, Ruthazer R, Garpestad E. Use of a validated delirium assessment tool improves the ability of physicians to identify delirium in medical intensive care unit patients. Crit Care Med. 2007;35(12):2721–2724. doi: 10.1097/01.ccm.0000292011.93074.82. [DOI] [PubMed] [Google Scholar]
- 39.Devlin JW, Fong JJ, Howard EP, et al. Assessment of delirium in the intensive care unit: Nursing practices and perceptions. Am J Crit Care. 2008;17(6):555–565. [PubMed] [Google Scholar]
- 40.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 41.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: Evaluation of a new screening tool. Intensive Care Med. 2001;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 42.Available at www.icudelirium.org
- 43.Pohlman MC, Schweickert WD, Pohlman AS, et al. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38(11):2089–2094. doi: 10.1097/CCM.0b013e3181f270c3. [DOI] [PubMed] [Google Scholar]
- 44.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: A quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: Survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3):825–832. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahn JM. Intensivist physician staffing and the process of care in academic medical centres. Qual Saf Health Care. 2007;16(5):329. doi: 10.1136/qshc.2007.022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomsen GE. Patients with respiratory failure increase ambulation after transfer to an intensive care unit where early activity is a priority. Crit Care Med. 2008;36(4):1119. doi: 10.1097/CCM.0b013e318168f986. [DOI] [PubMed] [Google Scholar]
- 48.Needham DM. Mobilizing patients in the intensive care unit: Improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–1690. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 49.Devlin JW, Holbrook AM, Fuller HD. The effect of ICU sedation guidelines and pharmacist interventions on clinical outcomes and drug cost. Ann Pharmacother. 1997;31(6):689–695. doi: 10.1177/106002809703100604. [DOI] [PubMed] [Google Scholar]
- 50.Sessler CN, Gosnell MS, Grap MJ, et al. The richmond agitation-sedation scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 51.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the sedation-agitation scale for adult critically ill patients. Crit Care Med. 1999;27(7):1325–1329. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 52.De Jonghe B, Cook D, Griffith L, et al. Adaptation to the intensive care environment (ATICE): Development and validation of a new sedation assessment instrument. Crit Care Med. 2003;31(9):2344–2354. doi: 10.1097/01.CCM.0000084850.16444.94. [DOI] [PubMed] [Google Scholar]
- 53.Devlin JW, Boleski G, Mlynarek M, et al. Motor activity assessment scale: A valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med. 1999;27(7):1271–1275. doi: 10.1097/00003246-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 54.de Lemos J, Tweeddale M, Chittock D. Measuring quality of sedation in adult mechanically ventilated critically ill patients. the vancouver interaction and calmness scale. sedation focus group. J Clin Epidemiol. 2000;53(9):908–919. doi: 10.1016/s0895-4356(00)00208-0. [DOI] [PubMed] [Google Scholar]
- 55.Pun BT, Boehm L. Delirium in the intensive care unit: Assessment and management. AACN Adv Crit Care. 2011;22(3):225–237. doi: 10.1097/NCI.0b013e318220c173. [DOI] [PubMed] [Google Scholar]
- 56.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 57.Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 58.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 59.Available at http://www.innovations.ahrq.gov/content.aspx?id=2442)
- 60.Ross AG, Morris PE. Safety and barriers to care. Crit Care Nurse. 2010;30(2):S11–S13. doi: 10.4037/ccn2010118. [DOI] [PubMed] [Google Scholar]
- 61.Hopkins RO. Strategies for promoting early activity in critically ill mechanically ventilated patients. AACN Adv Crit Care. 2009;20(3):277. doi: 10.1097/NCI.0b013e3181acaef0. [DOI] [PubMed] [Google Scholar]
- 62.Bailey PP, Miller, Russell R, 3rd, Clemmer TP. Culture of early mobility in mechanically ventilated patients. Crit Care Med. 2009;37(10):S429–S435. doi: 10.1097/CCM.0b013e3181b6e227. [DOI] [PubMed] [Google Scholar]


