Abstract
Purpose
To understand the relationship between choroidal thickness and various disease factors in patients with age-related macular degeneration (AMD) using spectral-domain optical coherence tomography.
Design
Cross-sectional, retrospective analysis.
Methods
Fifty-seven eyes of 47 patients with wet and dry AMD seen between November 2009 and January 2010 at the New England Eye Center, Boston, Massachusetts, were analyzed. Choroidal thickness was measured by 2 independent observers at 11 sites with high-definition horizontal 1-line raster scans through the foveal center. A retrospective chart review was performed to obtain data concerning duration of disease, number of intravitreal anti–vascular endothelial growth factor injections, visual acuity, lens status, and concomitant retinal pathologic features. The Pearson correlation and Student t test were used for statistical analysis for assessment of choroidal thickness changes in wet and dry AMD.
Results
The choroid in eyes with wet and dry AMD demonstrated a wide range of thicknesses above and below the normal mean (range, 77.5 to 399.5 μm; standard deviation [SD], 90.2). Nearly one third (33.3%) of the eyes with AMD measured less than 1 SD below the mean. Eyes with wet AMD demonstrated a mean subfoveal choroidal thickness of 194.6 μm (SD, 88.4; n = 40) compared with 213.4 μm (SD, 92.2; n = 17) in the dry AMD group. The choroidal thickness in eyes with dry AMD was correlated inversely with age (r = −0.703; P = .002); however, analysis of the number of intravitreal anti–vascular endothelial growth factor injections, number of years of disease, and visual acuity failed to demonstrate any significant correlations with choroidal thickness.
Conclusions
This study demonstrated that choroidal thickness can be measured by spectral-domain optical coherence tomography and that variable choroidal thickness exists among patients with the clinical diagnosis of wet and dry AMD. However, it is unclear at this time why in some eyes, choroidal thickness either increases or decreases with the disease. Further studies need to be carried out to understand the significance of choroidal thickness with respect to visual function and disease progression over time.
Over the last decade, Optical Coherence Tomography (OCT) has become an integral tool in the field of ophthalmology.1 OCT provides a so-called optical biopsy, a high-resolution, cross-sectional, and 3-dimensional reconstructed view of the retina in vivo in a noninvasive, reproducible manner. In comparison with the original time-domain OCT devices, newer Fourier spectral-domain OCT permits faster scanning speeds, up to 52 000 A-scans/second.2,3 Dense scan patterns achievable at such high speeds, along with better tissue resolution, allow for more accurate qualitative and quantitative analysis in various macular diseases. Improvements to existing spectral-domain OCT devices, such as frame averaging, despeckling, and improved image contrast, provide even better definition of intraocular structures such as choroidal tissue.4,5
Age-related macular degeneration (AMD) is the leading cause of blindness in both developed and developing nations in patients older than 60 years.6–8 OCT generated macular thickness maps have been proven to be useful in monitoring the progression and response to treatment in wet AMD after anti–vascular endothelial growth factor (VEGF) treatment; however, little currently is known about the choroids of patients with AMD.9
The choroid in normal and disease states has been investigated by using eye tracking software, frame averaging, and image inversion to visualize the full choroidal thickness10–16; alternatively, the choroid has been measured using a device that uses increased wavelength for better choroidal signal penetration.17 It is only with the availability of the latest software upgrades of both the Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) and Cirrus-HD OCT (Carl Zeiss Meditec, Dublin, California, USA) spectral-domain devices that choroidal depth now can been visualized routinely by clinicians. Studies have demonstrated changes in choroidal structure with increasing age and conditions such as high myopia and central serous chorioretinopathy (CSCR)11–14,16,17; however, the choroidal structure in patients with AMD has not been examined. The goal of this study was to characterize choroidal thickness in patients with AMD using spectral-domain OCT and to compare the eyes to age-matched normal volunteers.
Methods
A retrospective review was performed on 57 eyes of 47 patients with the clinical diagnosis of either wet or dry AMD seen between November 2009 and January 2010 at the New England Eye Center, Tufts Medical Center, Boston, Massachusetts. All patients included in the study underwent a comprehensive ophthalmologic examination with fundus biomicroscopy, color fundus photography, best-corrected Snellen visual acuity, fluorescein angiography, and OCT. OCT imaging was performed using Cirrus-HD OCT software version 4.5. The software version allows for the acquisition of high-definition 1-line raster scans that are constructed from 20 B-scans obtained at the same location and processed using a unique Selective Pixel Profiling system (Cirrus HD-OCT; Carl Zeiss Meditec). The 1-line raster is a 6-mm line consisting of 4096 A-scans, with an axial resolution of approximately 5 to 6 μm and a transverse resolution of approximately 15 to 20 μm. These high-definition images provide increased definition of retinal layers, as well as more posterior structures, such as the choroid–sclera junction. The images were not inverted to bring the choroid in closer proximity to the 0 delay line, as has been described previously as a tool to visualize better the choroid.10 Nevertheless, in most cases, good-quality images were obtainable, allowing choroidal thickness measurements to be performed. All images included in this study were at least 6 of 10 in intensity, taken as close to the fovea as possible, and able to demonstrate clear visualization of the choroid–sclera boundary.
Choroidal thickness measurements were obtained manually using the Cirrus linear measurement tool at 500-μm intervals, 2500 μm temporal and nasal to the fovea, perpendicular from the base of the hyperreflective retinal pigment epithelium to the choroid–sclera junction. In 9 eyes with choroidal neovascularization, measurements were performed from the edge of the Bruch membrane to the top of the sclera (Figure 1). The 11 measurements were obtained from all 57 eyes by 2 independent observers (V.M., J.G.) to correlate the thickness measurements. Chart review was performed to collect information regarding duration of disease, treatments, number of intravitreal anti-VEGF injections, visual acuity (VA), lens status, and concomitant retinal pathologic features. Eyes with more than −6 diopters of refractive error were excluded because of known choroidal thinning associated with high myopia.13
Figure 1.

Choroidal thickness measurement in age-related macular degeneration. Cirrus HD optical coherence tomography (OCT) 1-line raster scan demonstrating measurement technique used to obtain choroidal thickness across the macula. This OCT scan also demonstrates the measurement from the edge of the Bruch membrane to the choroid–sclera junction in eyes with wet age-related macular degeneration and pigment epithelial detachments.
All statistics were calculated using SPSS software version 17.0 for Windows (SPSS, Inc, Chicago, Illinois, USA). Pearson correlation and the Student t test were used to correlate interobserver measurements and to correlate choroidal thickness values with various disease factors. A P value of < .05 was considered statistically significant in this study.
Results
The Study Group Included 23 Males (40%) and 34 Females (60%). Forty eyes (70%) had the diagnosis of wet AMD with a mean age of 78.6 years (standard deviation [SD], 7.0 years). Seventeen eyes (30%) had dry AMD with a mean age of 78.2 years (SD, 7.9 years). AMD is defined as a chronic, progressive degenerative disorder of the macula that affects older individuals and features central visual loss as a result of drusen deposition, geographic atrophy, serous detachment of the retinal pigment epithelium, and neovascularization. Patients were subclassified as having dry AMD if there was no evidence of neovascularization such as intraretinal or subretinal fluid at any visit time point. This group included patients with geographic atrophy, drusen, and pigment epithelial detachments. Patients were subclassified as having wet AMD if there was evidence of neovascularization such as intraretinal or subretinal fluid at any visit time point. Patients were treated by different attending physicians with a combination of treat-and-extend and as-needed regimens with standard anti-VEGF therapy. Of the 40 eyes with wet AMD, 7 of these patients had undergone prior photodynamic therapy at least once in the study eye. The average duration of disease was 3.8 years. Subfoveal choroidal thickness measurements were used for comparative purposes in this study. The pattern of choroidal thickness in the macula in the 57 eyes with AMD in this study demonstrated the thickest choroid located subfoveally, with nasal and temporal thinning, maintaining a similar pattern seen in normal eyes (Figure 2).11,16
Figure 2.
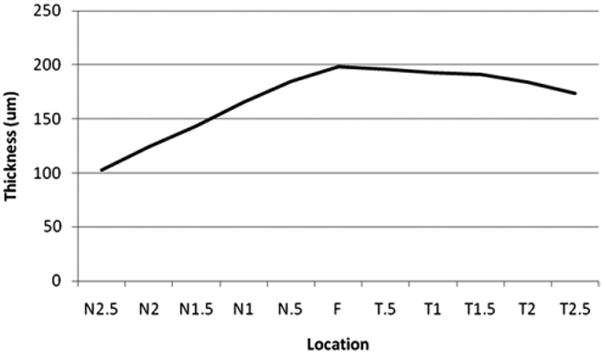
Graph showing mean macular choroidal thickness in eyes with age-related macular degeneration. Mean thickness at each of the 11 locations measured at 500-μm (0.5-mm) intervals temporal (T) and nasal (N) to the fovea (F) in all 57 eyes. The graph illustrates variation in choroidal thickness across the macula with the choroid thinnest nasally, thickening subfoveally, and thinning again temporally.
The authors' recent paper demonstrated a mean subfoveal choroidal thickness of 272 ± 81 μm in normal eyes using the Cirrus HD-OCT 1-line raster scan in patients with an average age of 51.1 years.16 In a study utilizing the Heidelberg Spectralis, normal subfoveal choroidal thickness of 287 ± 76 μm in patients with a mean age of 50.4 years was reported.11 The mean age of the 57 eyes in this study was 78.4 years (SD, 7.3 years); therefore, an age-adjusted normal subfoveal choroidal thickness of 229 μm was used for comparison because of an approximate 1.56-μm decline in choroidal thickness per year of life.11 A strong interobserver correlation of choroidal thickness measurements in all 57 eyes was found at each of the 11 locations measured along the horizontal scans of the macula. The correlation values of interobserver measurements ranged from 0.909 to 0.981, with P values of < .01 at each of the 11 measured points. The subfoveal choroidal measurements performed on the Cirrus HD-OCT 1-line raster images by the 2 separate observers (V.M., J.G.) were averaged.
The results of the choroidal thickness measurements in the 57 eyes with wet and dry AMD demonstrated considerable variation in thickness. The measurements were plotted according to SDs from the mean age-adjusted normal subfoveal choroidal thickness (Figure 3). Twenty-seven eyes (47.3%) fell outside the range of 1 SD from the mean and 2 eyes (3.5%) had significantly thickened choroids, measuring more than 2 SDs above the mean. Of the 2 eyes more than 2 SDs above the mean, 1 eye was categorized as having dry AMD and 1 was categorized as having wet AMD (Figure 4).
Figure 3.

Bar graph showing the distribution of subfoveal choroidal thickness in eyes with wet and dry age-related macular degeneration. The distribution of mean subfoveal choroidal thickness measurements in the studied eyes with wet and dry age-related macular degeneration is compared with a normal age-adjusted mean of 229 μm, with a standard deviation of 81 μm, based on recently published data.16 Dark gray column = wet age-related macular degeneration; light gray column = dry age-related macular degeneration.
Figure 4.
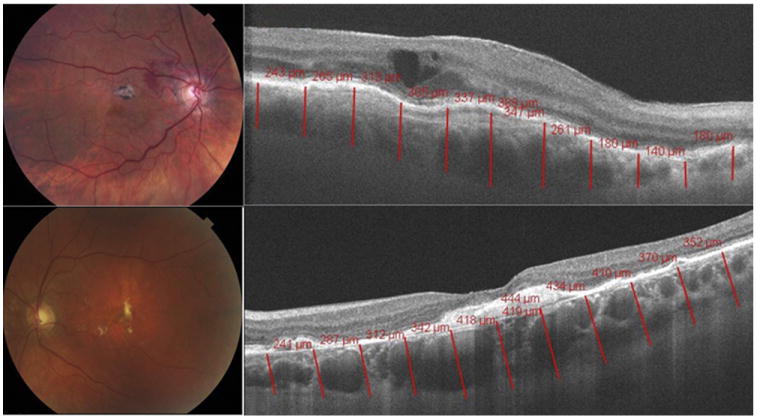
Choroidal thickness measured to be >2 standard deviation above the mean in age-related macular degeneration. (Top left) Color fundus photograph and (Top right) high-definition Cirrus HD-OCT 1-line raster scan demonstrating thickened choroid in an 84-year-old woman with a history of wet age-related macular degeneration. (Bottom left) Color fundus photograph and (Bottom right) high-definition Cirrus HD-OCT 1-line raster scan demonstrating increased choroidal thickness in a 71-year-old woman with a history of dry age-related macular degeneration.
Mean subfoveal choroidal thickness was found to be 194.6 μm (SD, 88.4; n = 40) in the wet AMD group and 213.4 μm (SD, 92.2; n = 17) in the dry AMD group. A weak correlation (r = −0.367; P = .005) was found between subfoveal choroidal thickness and age when all 57 eyes were considered together. However, when analyzed separately, eyes with wet AMD showed a weak correlation with age (r = −0.203; P = .208) that was not statistically significant, whereas eyes with dry AMD demonstrated a statistically significant correlation (r = −0.703; P = .002). This relationship in dry eyes was found to be stronger than the correlation between subfoveal choroidal thickness and age found in normal eyes (r = −0.61; P = .0001).16
Analysis of the relationship between the number of intravitreal anti-VEFG injections (mean number of injections, 5.4; SD, 4.6) and subfoveal choroidal thickness in the eyes with wet AMD demonstrated no correlation (r = −0.037; P = .821). Correlation of disease duration defined as the number of years since initial diagnosis of macular degeneration (mean number of years of disease, 3.8; SD, 2.7 years) also demonstrated no relationship (r = −0.046; P = .737) with subfoveal choroidal thickness. In subgroup analysis, there was no significant correlation found between duration of disease and choroidal thickness within the wet AMD group (r = −0.066; P = .684) or the dry AMD group (r = 0.134; P = .608) with relation to disease duration and choroidal thickness.
Analysis also was performed to examine the relationship between VA and subfoveal choroidal thickness. All Snellen visual acuities were converted to logarithm of the minimal angle of resolution (logMAR) for statistical analysis. Overall mean VA was 20/65 (logMAR units, 0.52). Within the subgroups, the wet eyes had a mean VA of 20/71 (logMAR units, 0.55), whereas the dry eyes had a mean VA of 20/54 (logMAR units, 0.43). No correlation was found overall (r = −0.009; P = .948) between choroidal thickness and VA, nor within the wet AMD eyes (r = −0.126; P = .446) and dry AMD eyes (r = 0.245; P = .327) when analyzed separately.
Discussion
The Choroid is a Highly Vascular Structure that varies in thickness with intraocular and perfusion pressure and is subject to regulation by various vasoactive factors, including nitric oxide, endothelins, and autonomic innervation.18–23 The choroid provides nutrients to the photoreceptors and removes waste products from the retinal pigment epithelium. The macula is the region of highest metabolic demand in the retina, and this has been postulated to be the reason for the greatest choroidal thickness beneath the fovea.
Choroidal structure is of particular interest in AMD because abnormalities of the choroidal circulation have been hypothesized to contribute to the development of AMD.24 Some studies suggest that AMD may be a vascular disease, with inadequate choroidal perfusion leading to hypoxia and ischemia of the retinal pigment epithelium with the subsequent production of VEGF, which ultimately may lead to the formation of choroidal neovascularization.25,26 Studies using Doppler techniques also have shown that foveolar choroidal blood flow in normal eyes decreases with increasing age.27 Compared with normal eyes, eyes with dry AMD have been shown to have decreased blood volume and abnormal flow and further worsening of blood flow with increasing disease severity.24,28,29 The decreased blood flow observed in the eyes of patients with AMD has been theorized to be a combination of narrowing of the choriocapillaries lumen, loss of cellularity, and thinning of the choroid, especially the choriocapillaries layer.24 Furthermore, decreased nitric oxide has been demonstrated in patients with AMD, presumably leading to vasoconstriction and hypoxia.30 Therefore, it is not unexpected that roughly one third of the patients in this study had thinner than average choroids compared with those of age-matched normal volunteers, possibly suggesting a role for choroidal thinning in the pathogenesis or progression of AMD.
Certainly, the choroid has a dynamic role in the pathogenesis of AMD, and up to this point there have been few studies examining the thickness of the choroid in AMD. This study implements the latest in OCT imaging to measure choroidal thickness to understand better if the observed changes can be related to disease progression and to aid in the identification of those patients with AMD who are at greater risk of advanced disease.
The results of this study demonstrated variation of OCT choroidal measurements among patients with the clinical diagnosis of AMD. Half (50.8%) of the measured eyes demonstrated choroid thickness of more than 1 SD away from the mean, with 33% of the eyes having thinner choroids than age adjusted normal eyes, and a small subset of eyes (3.5%) having choroidal thickness of more than 2 SDs above the mean.
The eyes with a thicker choroid were re-examined to see if there were any unique characteristics about this group. Based on the OCT appearance, it is difficult to determine whether these patients with a thicker choroid are a variant of normal, belong to a unique subset of AMD, or have a different disease entity altogether, such as chronic CSCR, which reportedly features very thick choroid.14 Examination of fundus photographs and fluorescein angiography of the eyes with increased choroidal thickness in this study dating back to the initial visit did not demonstrate any obvious characteristic changes suggesting the diagnosis of CSCR or polypoidal choroidal vasculopathy. Based on clinical appearance, chronic CSCR in elderly patients, however, is not differentiated easily from wet AMD; assessing choroidal thickness by OCT may help to distinguish these 2 possible diagnoses.
Overall, the wet AMD group demonstrated an average subfoveal choroidal thickness of 194.6 μm (n = 40), whereas the dry AMD patients had an average thickness of 213.4 μm (n = 17). One could postulate that continued thinning of the choroid secondary to disease progression may cause the conversion from dry to wet form of AMD. The eyes with dry AMD demonstrated a correlation between choroidal thickness and age (r = −0.703; P = .002), with the older patients having thinner choroids, similar to normal patients.11 However, the eyes with wet AMD demonstrated a weaker correlation between choroidal thickness and age, which may indicate a change in the pathophysiologic process occurring as eyes convert from dry to wet AMD or alternatively may be a result of the small sample size and variability in the choroid of these patients. Spraul and associates reported a difference in the density and diameter of choroidal blood vessels in eyes with and without AMD with an increased choriocapillaries density in the submacular area of eyes with advanced AMD.31
The present study found no correlations between choroidal thickness and the number of intravitreal anti-VEGF injections, duration of time since first diagnosis, and visual acuity. It is difficult to draw conclusions from these results because of the cross-sectional design of this study, because it is unknown if there have been changes in choroidal thickness over time in our study eyes. Moreover, it may also be that these factors do not play a role in choroidal thickness in AMD and that further studies are needed to investigate alternative features of the disease to explain these differences.
There are important limitations to this study. This study is a retrospective review of nonconsecutive patients who already carried the diagnosis of AMD. Patients were excluded from this study if full choroidal thickness was unable to be visualized adequately to perform the 11 measurements as described above. One hundred fifty eyes of patients with AMD were assessed to identify 57 eyes (38%) that could be measured reliably and could be included in this study. The fact that so many eyes were excluded because of poor acquisition may make the data less reliable. Some eyes with an extremely thickened choroid were excluded because of poor visualization of the choroid–sclera junction as a result of signal roll off distal to the 0-delay line, the point of highest signal detection. Therefore, it is likely that insufficient signal penetration artificially lowered the total number of eyes in the thickest choroid group. In addition, with no automated computer software available to calculate choroidal thickness, 2 independent observers performed the measurements manually, which may limit accuracy; however, the strong interobserver correlations between measurements suggests that this is unlikely. In addition, patients with more than 6 diopters of myopia were excluded from the study because of the known association of high myopia and choroidal thinning.13 Even with the exclusion of high myopes, the lack of axial length measurements and average refractive error does weaken the data and its interpretation.
Using the newest OCT software, which enables measurement of choroidal thickness, this study demonstrates variable choroidal thickness among groups of patients carrying the same diagnosis. Most AMD patients in this study demonstrated thinner choroids than age-adjusted normal volunteers; one third of these patients have choroidal thinning of 1 SD or more below the mean. Eyes with wet AMD demonstrated thinner average choroidal thickness than eyes with dry AMD. No direct correlation was observed between choroidal thickness and duration of disease, number of intravitreal anti-VEGF injections, or visual acuity.
Acknowledgments
Publication of this Article was Supported in Part by a Research to Prevent Blindness Challenge Grant to the New England Eye Center/Department of Ophthalmology, Tufts University School of Medicine, Boston, Massachusetts; Grants R01-EY11289-23, R01-EY13178-10, and R01-EY013516-07 from the National Institutes of Health, Bethesda, Maryland; Grants FA9550-07-1-0101 and FA9550-07-1-0014 from the Air Force Office of Scientific Research, Arlington, Virginia; and the Massachusetts Lions Eye Research Fund, Inc, New Bedford, Massachusetts. Dr Duker receives research support from Carl Zeiss Meditec, Inc, Optovue Corporation, and Topcon Medical Systems, Inc. Dr Fujimoto receives royalties from intellectual property owned by MIT and licensed to Carl Zeiss Meditec, Inc, and LightLabs Imaging. Dr Fujimoto also is a scientific advisor and has stock options in Optovue. The authors did not receive any financial support for their work on this study. Involved in Design and conduct of study (V.M., J.G., J.S.D.); Collection, management, analysis, and interpretation of data (V.M., J.G., J.S.D.); and Preparation and review of manuscript (V.M., J.G., J.G.F., J.S.D.). The study was approved by the Institutional Review Board of the Tufts Medical Center and complied with the Health Insurance Portability and Accountability Act.
Contributor Information
Varsha Manjunath, New England Eye Center, Tufts Medical Center, Boston, Massachusetts.
Jordana Goren, New England Eye Center, Tufts Medical Center, Boston, Massachusetts.
James G. Fujimoto, Department of Electrical Engineering and Computer Science, Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, Massachusetts
Jay S. Duker, New England Eye Center, Tufts Medical Center, Boston, Massachusetts
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27(1):45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Potsaid B, Gorczynska I, Srinivasan V, et al. Ultrahigh speed spectral/Fourier domain OCT ophthalmic imaging at 70,000 to 312,500 axial scans per second. Opt Express B. 2008;16(19):15149–15169. doi: 10.1364/oe.16.015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sander B, Larsen M, Thrane L, Hougaard JL, Jorgensen TM. Enhanced optical coherence tomography imaging by multiple scan averaging. Br J Ophthalmol. 2005;89(2):207–212. doi: 10.1136/bjo.2004.045989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson RD, Hammer DX, Paunescu LA, Beaton S, Schuman JS. Tracking optical coherence tomography. Opt Lett. 2004;29(18):2139–2141. doi: 10.1364/ol.29.002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada M, Hiratsuka Y, Roberts CB, et al. Prevalence of visual impairment in the adult Japanese population by cause and severity and future projections. Ophthalmic Epidemiol. 2010;17(1):50–57. doi: 10.3109/09286580903450346. [DOI] [PubMed] [Google Scholar]
- 7.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Xu L, Jonas JB, Yang H, Ma Y, Li J. Prevalence of age-related maculopathy in the adult population in China: the Beijing eye study. Am J Ophthalmol. 2006;142(5):788–793. doi: 10.1016/j.ajo.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser PK, Blodi BA, Shapiro H, Acharya NR. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114(10):1868–1875. doi: 10.1016/j.ophtha.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147(5):801–810. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148(3):445–450. doi: 10.1016/j.ajo.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009;147(4):644–652. doi: 10.1016/j.ajo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150(3):325–329. doi: 10.1016/j.ajo.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikuno Y, Kawaguchi K, Yasuno Y, Nouchi T. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010;51(4):2173–2176. doi: 10.1167/iovs.09-4383. [DOI] [PubMed] [Google Scholar]
- 18.Kiel JW, van Heuven WA. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1995;36(3):579–585. [PubMed] [Google Scholar]
- 19.Reiner A, Zagvazdin Y, Fitzgerald ME. Choroidal blood flow in pigeons compensates for decreases in arterial blood pressure. Exp Eye Res. 2003;76(3):273–282. doi: 10.1016/s0014-4835(02)00316-0. [DOI] [PubMed] [Google Scholar]
- 20.Kiel JW. Modulation of choroidal autoregulation in the rabbit. Exp Eye Res. 1999;69(4):413–429. doi: 10.1006/exer.1999.0717. [DOI] [PubMed] [Google Scholar]
- 21.Kiel JW. Endothelin modulation of choroidal blood flow in the rabbit. Exp Eye Res. 2000;71(6):543–550. doi: 10.1006/exer.2000.0911. [DOI] [PubMed] [Google Scholar]
- 22.Lutjen-Drecoll E. Choroidal innervation in primate eyes. Exp Eye Res. 2006;82(3):357–361. doi: 10.1016/j.exer.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Flugel-Koch C, Kaufman P, Lutjen-Drecoll E. Association of a choroidal ganglion cell plexus with the fovea centralis. Invest Ophthalmol Vis Sci. 1994;35(13):4268–4272. [PubMed] [Google Scholar]
- 24.Grunwald JE, Hariprasad SM, DuPont J. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1998;39(2):385–390. [PubMed] [Google Scholar]
- 25.Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137(3):496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Friedman E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 1997;124(5):677–682. doi: 10.1016/s0002-9394(14)70906-7. [DOI] [PubMed] [Google Scholar]
- 27.Grunwald JE, Hariprasad SM, DuPont J. Effect of aging on foveolar choroidal circulation. Arch Ophthalmol. 1998;116(2):150–154. doi: 10.1001/archopht.116.2.150. [DOI] [PubMed] [Google Scholar]
- 28.Pournaras CJ, Logean E, Riva CE, et al. Regulation of subfoveal choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(4):1581–1586. doi: 10.1167/iovs.05-0434. [DOI] [PubMed] [Google Scholar]
- 29.Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46(3):1033–1038. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- 30.Bhutto IA, Baba T, Merges C, McLeod DS, Lutty GA. Low nitric oxide synthases (NOSs) in eyes with age-related macular degeneration (AMD) Exp Eye Res. 2010;90(1):155–167. doi: 10.1016/j.exer.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(13):2724–2735. [PubMed] [Google Scholar]


