Abstract
Tree rings have been used in various applications to reconstruct past climates as well as to assess the effects of recent climatic and environmental change on tree growth. In this paper we briefly review two ways that tree rings provide information about climate change and CO2: (i) in determining whether recent warming during the period of instrumental observations is unusual relative to prior centuries to millennia, and thus might be related to increasing greenhouse gases; and (ii) in evaluating whether enhanced radial growth has taken place in recent decades that appears to be unexplained by climate and might instead be due to increasing atmospheric CO2 or other nutrient fertilization. It is found that a number of tree-ring studies from temperature-sensitive settings indicate unusual recent warming, although there are also exceptions at certain sites. The present tree-ring evidence for a possible CO2 fertilization effect under natural environmental conditions appears to be very limited.
Longer time series than those presently available from instrumental records are needed to evaluate whether recent climatic shifts are unusual and might be evidence of anthropogenic change due to increasing CO2 and other greenhouse gases. Longer records of natural climate variability and forest growth information can also help validate climate and carbon budget models used for prediction of future climate (e.g., see ref. 1).
Large-scale changes in sources and sinks of carbon in the terrestrial biosphere (due to climatic change, direct CO2 fertilization, forest regrowth, increased decay rates, or other factors) can act as either negative or positive feedbacks to the earth’s climate system (e.g., see refs. 2 and 3). Recent studies based on isotopic measurements of atmospheric CO2 suggest that there may in fact be a large CO2 sink in the land biosphere of northern temperate latitudes (30–60°N) (4, 5).
Below we outline some of the tree-ring evidence for recent climate and forest growth changes and their relevance for studies of the global carbon cycle. We focus on two issues: (i) whether recent climatic changes during the period of instrumental observations appear to be unusual relative to the past, and (ii) whether enhanced radial growth has taken place that appears to be unexplained by climate and might be due to increasing atmospheric CO2 or other nutrient fertilization.
Do Temperature-Sensitive Tree-Ring Records Indicate that Recent Warming is Unusual?
Tree-ring measurements can help to distinguish anthropogenic from natural environmental change. These data can be used to determine whether recent climatic changes are unusual and possibly due to anthropogenic effects (specifically, increasing CO2 and other trace gases) (e.g., see ref. 6) or are still within the range of natural climate variability. Several recent studies, outlined briefly below, have evaluated tree-ring and other proxy data with this goal in mind.
Cook (7) reviewed high-resolution temperature histories from tree ring and coral proxies to evaluate to what degree the 20th century warming has been anomalous relative to prior centuries to millennia. At northern latitudes, these histories include temperature-sensitive tree-ring series for northern Alaska (8), the north Polar Urals (9), and the Arctic as a whole (10). All three of these series indicate unusual 20th century warming. Recent tree-ring data from Mongolia indicate that there is unusual warming in that region (11), in agreement with the Arctic reconstruction (10).
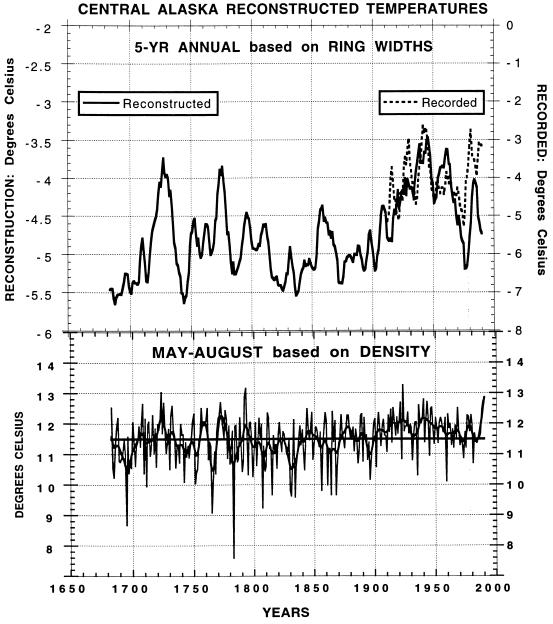
Jacoby and D’Arrigo (8) describe recent warming in Alaska relative to past tree growth variations (see Fig. 1). This study describes a summer temperature reconstruction based on maximum latewood density which shows evidence of recent warming of 0.5° to 1°C over the past century. By contrast, the ring-width data, which appear to integrate temperature conditions throughout the year (8), indicate more pronounced recent warming of annual temperatures of 2° to 3°C.
Figure 1.
Reconstructions of central Alaska temperatures. (Upper) Five-year averaged annual (October–September) temperature reconstructed by using ring widths. (Lower) Summer (May–August) temperatures reconstructed by using maximum latewood density. Note the increase in reconstructed summer temperature over the past 100 years is only about 0.5° to 1.0°C, whereas the reconstructed annual temperature has increased about 1.5° to 2°C. The cooler period in annual temperatures prior to 1900 was broken by several warm intervals. Dashed line in Upper is 5-year recorded temperatures for central Alaska. Note that the reconstruction underestimates temperatures since about 1970. This is attributed to the effects of moisture stress (8).
Briffa et al. (12) used a 1,000-year long tree-ring temperature record from Siberia to infer that the twentieth century (1901–1990) summer warmth has been unusual relative to the past millennium. Bradley and Jones (13) reconstructed Northern Hemisphere summer temperatures back to A.D. 1400 by using a combination of historical, tree-ring, and ice-core data and found recent conditions to be very warm relative to the past. In contrast, a millennium-long record from Fennoscandia indicates that it was warmer in Fennoscandia during the so-called Medieval Warm Period (14) than it is today, possibly due to cooling of the North Atlantic (12).
In the Southern Hemisphere, a multimillennial summer temperature reconstruction from southern South America shows no evidence of unusual recent warming, in agreement with instrumental records (15, 16). However, tree-ring and other records from Tasmania and New Zealand do indicate anomalous warming in recent decades relative to the past (7, 17, 18). The Tasmanian huon pine record, which is multimillennial in length, indicates that the warming of recent decades is highly unusual, with only one marginally warmer interval over the past several thousand years. This warm-season temperature reconstruction suggests that the recent warming in Tasmania is anomalous although not entirely unprecedented (7).
In summary, a number of temperature-sensitive middle to higher latitude tree-ring records from both hemispheres show evidence that recent warming in these regions may be anomalous. A few of these series are millennial in length. These few very long records allow evaluation of temperature variations prior to the Little Ice Age cold interval, which could bias interpretation of recent warm conditions (13). Other sources of proxy data in several areas also support these indications of unusual recent warming relative to the past [e.g., ice cores (19)].
The tree-ring data used in these studies are from sites selected to amplify the climatic signal due to temperature, and are not necessarily representative of large components of the land biosphere nor indicators of large-scale enhanced carbon sequestration. Changes in radial growth in these trees do not provide information about possible shifts in respiration or allocation of carbon below-ground. Warming may also be causing negative feedbacks to forest productivity, which can counteract enhanced growth in other areas. For example, some temperature-limited sites may now be showing the negative effects of moisture stress (partially due to increased evapotranspiration caused by warmer temperatures) or insect infestation related to recent warmer conditions (ref. 8 and see Fig. 1).
Is There a CO2 Fertilization Effect in Tree Rings?
Another means by which tree rings are being used to test for anthropogenic effects is by evaluating whether direct CO2 fertilization due to increasing atmospheric CO2 (ordinarily limiting to plant growth) is presently enhancing the growth of natural vegetation. The response of plant growth to a direct CO2 fertilization effect has been demonstrated in numerous laboratory experiments, usually using seedlings (e.g., ref. 20). Modeling suggests that this enhanced growth should result in greater carbon sequestering of land ecosystems, provided that this “beta factor” is sufficiently large (e.g., refs. 21 and 22).
Little is known, however, about whether such an effect is occurring on a large scale in natural vegetation, where environmental conditions are exceedingly complex. Here we review several tree-ring studies which evaluate the possible effects of direct CO2 fertilization on radial growth of trees growing in natural environmental settings.
LaMarche et al. (23) presented one of the first studies which purported to find evidence for a possible CO2 fertilization effect in tree rings. Their study was based on ring-width chronologies of high-elevation bristlecone and limber pines growing in the southwestern United States, which show unusual enhanced growth over the past century. One reason for their conclusion that this enhanced growth is due to CO2 fertilization is that high-elevation plants may be more CO2-limited than those at lower elevations (24). Yet no quantitative modeling was presented by LaMarche et al. (23) to rule out the possible contribution of favorable climatic change to account for the growth increases.
Graumlich (25) found no such evidence for CO2 fertilization in high-elevation foxtail pine and other species in the Sierra Nevada. She based her conclusions on the observations that (i) recent trends were not unusual relative to those in the pre-anthropogenic period, and (ii) recent growth variations were largely explainable by climate–growth relationships.
A contrasting view is presented by Graybill (26) and Graybill and Idso (27), who argued that CO2 fertilization is detectable in certain pine species growing at high elevations of the southwestern United States, but only if they show a strip-bark growth form. In trees with a strip-bark morphology, any added CO2 should be allocated primarily to the active cambial region, resulting in a greater response (27). Graumlich (25) speculated that the disparate conclusions for trees in the southwest might be reconciled, since the LaMarche et al. (23) trees were also of a strip-bark morphology. By contrast, the trees in her study did not show this feature. Other studies include a paper by Kienast and Luxmoore (28), who showed negative results for a CO2 fertilization effect in trees in the Rocky Mountains of Colorado.
D’Arrigo and Jacoby (29) did not find evidence for a CO2 fertilization effect at the northern treeline of North America, based on evaluation of residual trends following modeling of climate–growth relationships. One possible explanation is that a threshold level of CO2 increase is needed before an effect can be detected. Another is that other factors, including cold temperatures, a short season of cambial cell division, and nitrogen deficiency could preclude a direct CO2 response in the extreme boreal forests. The unexplained increase in growth of lodgepole pine at a high-elevation site in the San Jacinto Mountains of California (30) did not occur in limber pine near the same site and, as noted in the study, could be related to changes in winter precipitation.
One of the most thorough analyses of representative boreal forest growth involved the measurement of ring widths and density of trees in mature, closed canopy, white spruce stands at 11 locations in western Canada (31). A limited number of trees were felled, and seven disks were cut from each to obtain data on cross-sectional area and taper to enable calculations of volumetric and biomass growth rate change. Jozsa and Powell concluded that biomass productivity and annual growth layer weights are related to long-term and yearly climatic variability with possible response to spruce budworm activity (31). They do not present any indication that there is a systematic growth trend that could be related to CO2 fertilization. This is an extremely important study of mature trees in natural forest stands. Thus the results are widely relevant to real-world situations.
Discussion and Conclusions
We have briefly described some of the tree-ring evidence presently being used to assess whether recent growth changes are unusual relative to the past, and might be evidence for warming due to greenhouse gases and/or direct CO2 fertilization. A number of temperature-sensitive records, some of which date back for several millennia, do indicate unusual recent warming. Yet this is by no means taking place at all sites. Another caveat is that the trees studied here are from particular sites selected to amplify climatic signals, and are not necessarily representative of large components of the land biosphere nor enhanced sequestering of carbon (2). In addition, above-ground radial growth changes do not provide information about respiration or below-ground effects. Other changes (e.g., drought stress) could lead to negative feedback effects (e.g., refs. 2 and 8).
The evidence for CO2 fertilization is inconclusive at present for trees growing in natural settings, where there can be many other limiting and interacting factors. Controlled experiments simulating natural conditions underway at the Biosphere 2 facility will attempt to evaluate the combined effects of different environmental factors, and compare plant responses in different simulated ecosystems and between species (32). Such controlled studies may provide additional insights which can help resolve the uncertainties of the CO2 fertilization issue. Even if trees with a strip-bark growth form are most likely to show this effect, these types of trees are only a small component of the land biosphere.
The evidence described here provides only partial information regarding the behavior of the land biosphere. There are still many uncertainties, and it is unlikely that these issues will be resolved in the very near future. Additional studies and improved spatial and temporal coverage of tree-ring data are needed to decrease uncertainties about whether anthropogenic effects are presently taking place.
Acknowledgments
Research for this paper was supported by the Climate Dynamics Program of the National Science Foundation, ATM 94-06732. This is Lamont–Doherty Earth Observatory contribution no. 5594.
References
- 1.Mitchell J F B, Johns T C, Gregory J M, Tett S F B. Nature (London) 1995;376:501–504. [Google Scholar]
- 2.Jacoby G C, D’Arrigo R D. In: Biotic Feedbacks in the Global Climatic System: Will the Warming Feed the Warming? Woodwell G M, Mackenzie F T, editors. New York: Oxford Univ. Press; 1995. pp. 108–118. [Google Scholar]
- 3.Woodwell G M, Mackenzie F T, editors. Biotic Feedbacks in the Global Climatic System: Will the Warming Feed the Warming? New York: Oxford Univ. Press; 1995. [Google Scholar]
- 4.Ciais P, Tans P, Torlier M, White J W C, Francey R J. Science. 1995;269:1098–1102. doi: 10.1126/science.269.5227.1098. [DOI] [PubMed] [Google Scholar]
- 5.Denning A S, Fung I Y, Randall D. Nature (London) 1995;376:240–243. [Google Scholar]
- 6.Wigley T M L, Jones P D, Raper S C B. Proc Natl Acad Sci USA. 1997;94:8314–8320. doi: 10.1073/pnas.94.16.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook E R. Climate Dynamics. 1995;11:211–222. [Google Scholar]
- 8.Jacoby G C, D’Arrigo R D. Global Biogeochemical Cycles. 1995;9:227–234. [Google Scholar]
- 9.Graybill D A, Shiyatov S G. In: Climate Since A. D. 1500. Bradley R S, Jones P D, editors. London: Routledge; 1992. pp. 393–414. [Google Scholar]
- 10.D’Arrigo R D, Jacoby G C. Climatic Change. 1993;25:163–177. [Google Scholar]
- 11.Jacoby G C, D’Arrigo R D, Davaajamts T. Science. 1996;273:771–773. doi: 10.1126/science.273.5276.771. [DOI] [PubMed] [Google Scholar]
- 12.Briffa K R, Jones P D, Schweingruber F H, Shiyatov S G, Cook E R. Nature (London) 1995;375:156–159. [Google Scholar]
- 13.Bradley R S, Jones P D. The Holocene. 1993;3:367–376. [Google Scholar]
- 14.Hughes M K, Diaz H F. Climatic Change. 1994;26:109–142. [Google Scholar]
- 15.Lara A, Villalba R. Science. 1993;260:1104–1106. doi: 10.1126/science.260.5111.1104. [DOI] [PubMed] [Google Scholar]
- 16.Villalba R, Boninsegna J A, Lara A, Veblen T T, Roig F A, Aravena J C, Ripalta A. In: Climatic Variations and Forcing Mechanisms of the Last 2000 Years. Jones P D, Bradley R S, Jouzel J, editors. New York: Springer; 1996. pp. 161–189. [Google Scholar]
- 17.Cook E R, Bird T, Peterson M, Buckley B, D’Arrigo R, Francey R. The Holocene. 1992;2:205–217. [Google Scholar]
- 18.D’Arrigo R D, Buckley B, Cook E R, Wagner W S. Palaeogeography, Palaeoclimatology, Palaeoecology. 1995;119:293–300. [Google Scholar]
- 19.Thompson L G. In: Climate Since A. D. 1500. Bradley R S, Jones P D, editors. London: Routledge; 1992. pp. 517–548. [Google Scholar]
- 20.Allen L H, Amthor J S. In: Biotic Feedbacks in the Global Climatic System: Will the Warming Feed the Warming? Woodwell G M, Mackenzie F T, editors. New York: Oxford Univ. Press; 1995. pp. 51–84. [Google Scholar]
- 21.Reynolds J F, Harley P C, Thomas R B, Strain B R. Plant, Cell and Environment. 1992;15:271–282. [Google Scholar]
- 22.Wullschleger S D, Post W M, King A W. In: Biotic Feedbacks in the Global Climatic System: Will the Warming Feed the Warming? Woodwell G M, Mackenzie F T, editors. New York: Oxford Univ. Press; 1995. pp. 85–107. [Google Scholar]
- 23.LaMarche V C, Jr, Graybill D A, Fritts H C, Rose M R. Science. 1984;225:1019–1021. doi: 10.1126/science.225.4666.1019. [DOI] [PubMed] [Google Scholar]
- 24.Cooper C F. Science. 1986;231:859–860. doi: 10.1126/science.231.4740.859. [DOI] [PubMed] [Google Scholar]
- 25.Graumlich L J. Ecology. 1991;72:1–11. [Google Scholar]
- 26.Graybill D A. In: Proceedings of the International Symposium on Ecological Aspects of Tree-Ring Analysis. Jacoby G C, Hornbeck J W, editors. Springfield, VA: U.S. Dept. of Commerce; 1987. pp. 463–474. [Google Scholar]
- 27.Graybill D A, Idso S B. Global Biogeochem Cycles. 1993;7:81–95. [Google Scholar]
- 28.Kienast F, Luxmoore R J. Ecology. 1988;76:487–495. doi: 10.1007/BF00397859. [DOI] [PubMed] [Google Scholar]
- 29.D’Arrigo R D, Jacoby G C. Global Biogeochem Cycles. 1993;7:525–535. [Google Scholar]
- 30.Jacoby, G. C. (1986) in Climate-Vegetation Interactions, NASA Conference Publication 2440, eds. Rosenzweig, C. & Dickinson, R. (NASA, Greenbelt, MD), pp. 114–118.
- 31.Jozsa L A, Powell J M. Can J For Res. 1987;17:1075–1079. [Google Scholar]
- 32.Nelson M, Burgess T, Alling A, Alvarez-Romo N, Dempster W, Walford R, Allen J. Bioscience. 1993;43:225–236. [PubMed] [Google Scholar]