Abstract
Objective To analyse clinical outcomes with new oral anticoagulants for prophylaxis against venous thromboembolism after total hip or knee replacement.
Design Systematic review, meta-analysis, and indirect treatment comparisons.
Data sources Medline and CENTRAL (up to April 2011), clinical trials registers, conference proceedings, and websites of regulatory agencies.
Study selection Randomised controlled trials of rivaroxaban, dabigatran, or apixaban compared with enoxaparin for prophylaxis against venous thromboembolism after total hip or knee replacement. Two investigators independently extracted data. Relative risks of symptomatic venous thromboembolism, clinically relevant bleeding, deaths, and a net clinical endpoint (composite of symptomatic venous thromboembolism, major bleeding, and death) were estimated using a random effect meta-analysis. RevMan and ITC software were used for direct and indirect comparisons, respectively.
Results 16 trials in 38 747 patients were included. Compared with enoxaparin, the risk of symptomatic venous thromboembolism was lower with rivaroxaban (relative risk 0.48, 95% confidence interval 0.31 to 0.75) and similar with dabigatran (0.71, 0.23 to 2.12) and apixaban (0.82, 0.41 to 1.64). Compared with enoxaparin, the relative risk of clinically relevant bleeding was higher with rivaroxaban (1.25, 1.05 to 1.49), similar with dabigatran (1.12, 0.94 to 1.35), and lower with apixaban (0.82, 0.69 to 0.98). The treatments did not differ on the net clinical endpoint in direct or indirect comparisons.
Conclusions A higher efficacy of new anticoagulants was generally associated with a higher bleeding tendency. The new anticoagulants did not differ significantly for efficacy and safety.
Introduction
Venous thromboembolism, which encompasses deep vein thrombosis and pulmonary embolism, is responsible for the death of more than half a million people in Europe each year1 and is the third leading cause of death from cardiovascular causes only ahead of myocardial infarction and stroke.2 Additionally, 1.66 million cases of non-fatal symptomatic venous thromboembolism are diagnosed in Europe each year, with two thirds being acquired in hospital.1 Venous thromboembolism represents an important problem in patients admitted to hospital, including those undergoing major orthopaedic surgery.3 4
The therapeutic arsenal of anticoagulants available for prophylaxis against venous thromboembolism is mainly composed of parenteral agents, such as low molecular weight heparins or fondaparinux.3 These agents are effective and safe but require daily subcutaneous injections, which may be problematic in some patients. Dabigatran etexilate (Pradaxa; Boehringer Ingelheim International, Germany),5 rivaroxaban (Xarelto; Bayer Pharma, Germany),6 and apixaban (Eliquis; Bristol-Myers Squibb/Pfizer EEIG, United Kingdom),7 are new oral anticoagulants available for prophylaxis against venous thromboembolism in patients undergoing total hip or knee replacement surgery. The pivotal studies on these indications are mainly based on findings from mandatory venography of the legs, which is not routinely carried out in standard practice. Definitions for bleeding may differ between studies, however, leading to an underestimation of bleeding risk in some cases.8 9 10 Therefore the effect of the new oral anticoagulants on clinical outcomes is uncertain. In addition, no up to date head to head comparisons have been done between these new oral anticoagulants.
We systematically reviewed and meta-analysed data from randomised controlled trials of the new oral anticoagulants for prophylaxis against venous thromboembolism in patients undergoing total hip or knee replacement. We made direct comparisons with enoxaparin and indirect comparisons between the new oral anticoagulants on the clinical outcomes of symptomatic venous thromboembolism, bleeding, and death.
Methods
We considered randomised controlled trials comparing any of the approved new oral anticoagulants (rivaroxaban, dabigatran, and apixaban) with enoxaparin in patients undergoing total hip or knee replacement. At least one of the daily doses tested in the experimental arms had to correspond to the total daily dose approved for the new oral anticoagulant (dabigatran 220 mg or 150 mg, apixaban 5 mg, or rivaroxaban 10 mg). At least one of the daily doses tested in the control groups had to correspond to the approved regimens for enoxaparin: 40 mg once daily started 12 hours before surgery (Europe) or 30 mg twice daily started 12-24 hours after surgery (North America).
Trial identification and data collection
We searched Medline and CENTRAL (up to April 2011), clinical trial registries, relevant conference proceedings, and websites of regulatory agencies (see supplementary file for search strategy). No language restrictions were applied. Two investigators (AG-O and AIT-F) independently and separately assessed trials for eligibility and extracted data. If a trial was covered in more than one report we used a hierarchy of data sources: public reports from regulatory authorities (US Food and Drug Administration, European Medicines Agency), peer reviewed articles, reports from the web based repository for results of clinical studies, and other sources. Finally, we contacted sponsors or the main investigators for missing outcome data.
Study characteristics and quality
To assess whether the trials were sufficiently homogeneous to be meta-analysed we collected data on patients’ characteristics (age and sex), percentage of patients evaluable for efficacy and safety, dosage used in the experimental and control groups, duration of treatment and follow-up, inclusion and exclusion criteria, definitions of outcomes, adjudication committees of venographies and clinical events, type of surgery (total hip or knee replacement), and rate of events in the enoxaparin control group. Additionally, we assessed study quality using the Jadad scale.11
Outcome measures
The prespecified primary outcome was symptomatic venous thromboembolism—that is, symptomatic deep vein thrombosis or symptomatic pulmonary embolism. The prespecified primary safety outcome was clinically relevant bleeding—that is, major bleeding or clinically relevant non-major bleeding. The main secondary outcomes were each of the components of the primary efficacy and safety outcomes, as well as all cause death and a net clinical outcome of hard endpoints, defined as the composite of symptomatic venous thromboembolism, major bleeding, and all cause death.
Other secondary outcomes included total venous thromboembolism (venographic proximal or distal deep vein thrombosis or non-fatal pulmonary embolism) or all cause death (composite main outcome in individual studies) and major venous thromboembolism (venographic proximal deep vein thrombosis or non-fatal pulmonary embolism) or venous thromboembolism related death (composite key secondary outcome in individual studies).
Statistical analysis
We carried out direct comparisons between dabigatran, rivaroxaban, and apixaban versus enoxaparin as well as indirect comparisons between the three drugs on an intention to treat basis, according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) recommendations.12
For the meta-analysis we calculated relative risks and their respective 95% confidence intervals for each study and for the pooled studies for each of the anticoagulants. Heterogeneity was assessed using the Cochran Q test13 and the Higgins I2 test.14 A Cochran’s Q P<0.10 and I2 >50% were considered to show significant heterogeneity.14 We used the random effects model described by Der-Simonian and Laird for the main analysis.15 We carried out subgroup analyses of trials with the different anticoagulants as well as in hip and knee replacement. P<0.05 for interaction indicates that the effect of treatment differs between the tested subgroups. As a sensitivity analysis, we calculated the results using the fixed effects method described by Mantel and Haenszel.16 Additional sensitivity analyses were done taking into account certain methodological problems that could influence the results of the meta-analysis: study phase, study quality, and duration of thromboprophylaxis. We created funnel plots showing the standard error and the effect size to evaluate publication bias. Direct comparisons were done using the RevMan statistical software, version 5.1 (Nordic Cochrane Center).17 For indirect comparisons (Bucher’s method), we used the ITC (Indirect Treatment Comparison) computer program, version 1.0.18
Results
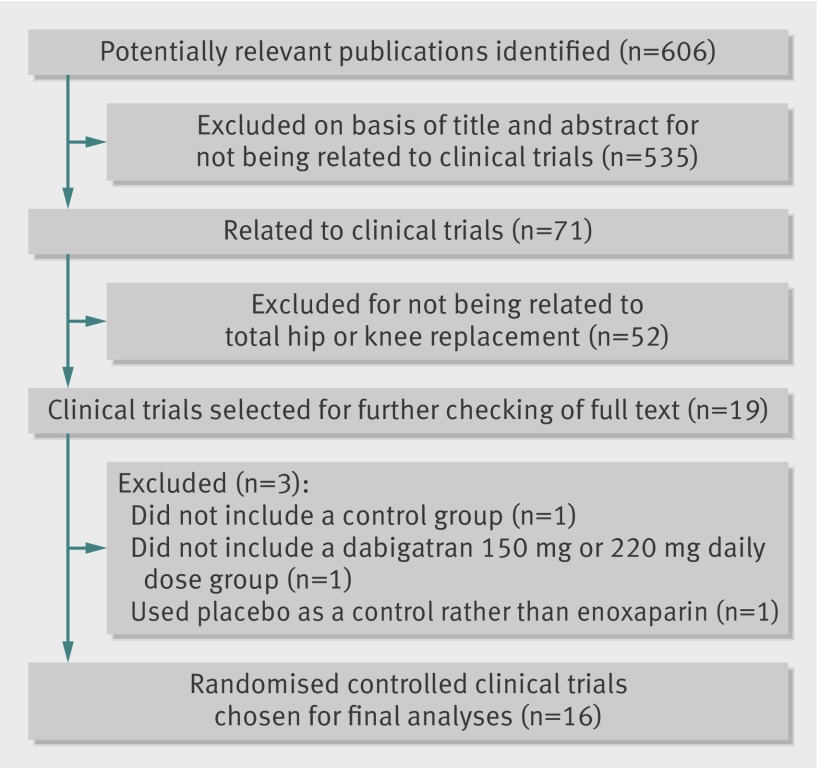
The literature search identified 606 articles, 71 of which related to clinical trials or protocols with rivaroxaban, dabigatran, or apixaban (fig 1). Of these, 19 were clinical trials in total hip or knee replacement19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 and were selected for checking as full text. Sixteen of the studies were eligible for inclusion19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 and the remaining three,35 36 37 all with dabigatran, were excluded because they did not include a control group,35 did not include a dabigatran 150 mg or 220 mg daily dose group,36 or used placebo as control rather than enoxaparin.37
Fig 1 Study identification, selection, and exclusions
Table 1 shows the characteristics of the trials and treatments. The 16 studies comprised 38 747 patients and compared dabigatran (four studies),19 20 21 22 rivaroxaban (eight studies),23 24 25 26 27 28 29 30 or apixaban (four studies)31 32 33 34 with enoxaparin in total hip replacement (eight studies)20 22 23 24 27 29 30 33 or total knee replacement (eight studies).19 21 25 26 28 31 32 34 Of these, 36 149 patients were randomised to dosages of the new anticoagulant (n=19 481) or control treatment (n=16 668) required for inclusion in the meta-analysis and therefore comprised the intention to treat population. Most of the studies (n=11) used the European enoxaparin regimen as comparator.19 20 22 23 24 25 27 29 30 32 33 Three of the eight publications of rivaroxaban trials did not include the specific method of sequence generation,27 29 30 and this information was obtained from the sponsor after request. Fifteen of the 16 studies were double blind clinical trials,19 20 21 22 23 24 25 26 28 29 30 31 32 33 34 scoring 5 points (maximum score) on the Jadad scale, and were judged to be at low risk of bias (adequate sequence generation or allocation concealment, double blinding, and clear reporting of loss to follow-up). The remaining (dose finding) study with rivaroxaban scored 3 (because it was an open label study).27 In all cases adjudication of events was blinded.
Table 1.
Characteristics of included randomised controlled trials and study treatments
| Drug, trial | No in sample | Type of surgery | Trial phase | Dose, treatment duration (timing of first dose in relation to surgery) | Design of randomised controlled trial; adjudicating committee | Jadad score | Day of venography | Follow-up (days) | |
|---|---|---|---|---|---|---|---|---|---|
| Experimental drug | Control drug | ||||||||
| Dabigatran: | |||||||||
| RE-MODEL19 | 2101 | Total knee replacement | III | Dabigatran 220 mg or 150 mg once daily, 6-10 days (1-4 hours) | Enoxaparin 40 mg once daily, 6-10 days (about 12 hours*) | Double blind; Gothenburg | 5 | 6-10 | 90 |
| RE-NOVATE20 | 3493 | Total hip replacement | III | Dabigatran 220 mg or 150 mg once daily, 28-35 days (1-4 hours) | Enoxaparin 40 mg once daily, 28-35 days (aboout 12 hours*) | Multicentre, double blind; Holland | 5 | 33 | 94 |
| RE-MOBILIZE21 | 2615 | Total knee replacement | III | Dabigatran 220 mg or 150 mg once daily, 12-15 days (8-12 hours) | Enoxaparin 30 mg twice daily, 12-15 days (12-24 hours) | Double blind; Gothenburg | 5 | 14 | 90 |
| RE-NOVATE II22 | 2055 | Total hip replacement | III | Dabigatran 220 mg once daily, 28-35 days (1-4 hours) | Enoxaparin 40 mg once daily, 28-35 days (about 12 hours*) | Double blind; Holland | 5 | 32 | 90 |
| Rivaroxaban: | |||||||||
| RECORD123 | 4541 | Total hip replacement | III | Rivaroxaban 10 mg once daily, 35d (6 hours) | Enoxaparin 40 mg once daily, 35 days (about 12 hours*) | Double blind; Gothenburg | 5 | 36 | 66-71 |
| RECORD224 | 2509 | Total hip replacement | III | Rivaroxaban 10 mg once daily, 31-39 days (6 hours) | Enoxaparin 40 mg once daily, 14 days (about 12 hours*)+placebo 30 days | Double blind; Gothenburg | 5 | 32-40 | 62-75 |
| RECORD325 | 2531 | Total knee replacement | III | Rivaroxaban 10 mg once daily, 10-14 days (6 hours) | Enoxaparin 40 mg once daily, 10-14 days (about 12 hours*) | Double blind; Gothenburg | 5 | 11-15 | 41-50 |
| RECORD426 | 3148 | Total knee replacement | III | Rivaroxaban 10 mg once daily, 10-14 days (6 hours) | Enoxaparin 30 mg twice daily, 10-14 days (12-24 hours) | Double blind; Gothenburg | 5 | 11-15 | 40-49 |
| PROOF OF CONCEPT27 | 641 | Total hip replacement | IIa | Rivaroxaban 2.5, 5, 10, 20, or 30 mg twice daily, rivaroxaban 30 mg once daily, 5-9 days (6-8 hours)† | Enoxaparin 40 mg once daily, 5-9 days (about 12 hours*) | Open label; Gothenburg | 3 | 5-9 | 38-68 |
| ODIXA KNEE28 | 621 | Total knee replacement | IIb | Rivaroxaban 2.5, 5, 10, 20, or 30 mg twice daily, 5-9 days (6-8 hours)† | Enoxaparin 30 mg twice daily, 5-9days (12-24 hours) | Double blind; Gothemburg | 5 | 5-9 | 37-67 |
| ODIXA HIP (twice daily)29 | 722 | Total hip replacement | IIb | Rivaroxaban 2.5, 5, 10, 20, or 30 mg twice daily, 5-9 days (6-8 hours)† | Enoxaparin 40 mg once daily, 5-9 days (about 12 hours*) | Double blind; Gothenburg | 5 | 5-9 | 38-68 |
| ODIXA HIP (once daily)30 | 873 | Total hip replacement | IIb | Rivaroxaban 10, 20, or 30 mg once daily, 5-9 days (6-8 hours)† | Enoxaparin 40 mg once daily, 5-9 days (about 12 hours*) | Double blind; Gothenburg | 5 | 6-10 | 35-69 |
| Apixaban: | |||||||||
| ADVANCE-131 | 3195 | Total knee replacement | III | Apixaban 2.5 mg twice daily, 10-14 days (12-24 hours) | Enoxaparin 30 mg twice daily, 12 days (12-24 hours) | Double blind; Hamilton | 5 | 10-14 | 70-84 |
| ADVANCE-232 | 3057 | Total knee replacement | III | Apixaban 2.5 mg twice daily, 10-14 days (12-24 hours) | Enoxaparin 40 mg once daily, 10-14 day (about 12 hours*) | Double blind; Hamilton | 5 | 10-14 | 70-84 |
| ADVANCE-333 | 5407 | Total hip replacement | III | Apixaban 2.5 mg twice daily, 35 days (12-24 hours) | Enoxaparin 40 mg once daily, 35 days (about 12 hours*) | Double blind; Hamilton | 5 | 32-38 | 90-100 |
| APROPOS34 | 1238 | Total knee replacement | 2b | Apixaban 5, 10, or 20 mg once daily, 2.5, 5, or 10 mg twice daily, 10-14 days (12-24 hours)‡ | Enoxaparin 30 mg twice daily, 10-14 days (about 12 hours*) or warfarin (international normalised ratio 1.8-3.0§) | Double blind; Hamilton | 5 | 10-14 | 42 |
*Administered preoperatively; other first doses were administered postoperatively.
†Only data pertaining to 10 mg total daily dose (5 mg twice daily or 10 mg once daily) were included in the meta-analysis.
‡Only data pertaining to 5 mg total daily dose (2.5 mg twice daily or 5 mg once daily) were included in the meta-analysis.
§Only data pertaining to 40 mg once daily dose control group were included in the meta-analysis.
Patients’ characteristics were homogeneous across the trials, with age ranging between 61 and 68 years, a predominance of women, and body weight between 75 and 84 kg (table 2).
Table 2.
Characteristics of patients, surgery, and concomitant treatments*
| Drug, trial | Participants mean age (years), % women, mean weight (kg) | History of venous thromboembolism (%) | Use of neuraxial anaesthesia (%) | Surgery duration (minutes) | Use of elastic compression stockings | Use of intermittent pneumatic compression | Use of acetylsalicylic acid/NSAID |
|---|---|---|---|---|---|---|---|
| Dabigatran: | |||||||
| RE-MODEL19 | 68, 69, 82 | NA | 78 | 90 | Allowed | Prohibited | Allowed: acetylsalicylic acid <160 mg and NSAID of no long half life |
| RE-NOVATE20 | 64, 56, 78 | 3 | 76 | 87 | Allowed | Prohibited | Allowed: acetylsalicylic acid <160 mg and NSAID of no long half life |
| RE-MOBILIZE21 | 66, 58, 88 | NA | 48 | 90 | Allowed | Prohibited | Allowed: acetylsalicylic acid <160 mg and NSAID if half life <17 hours |
| RE-NOVATE II22 | 62, 50, 80 | 2 | 77 | 79 | NA | Prohibited | NA |
| Rivaroxaban: | |||||||
| RECORD123 | 63, 56, 78 | 2 | 70 | 91 | NA | Prohibited | Allowed |
| RECORD224 | 62, 53, 75 | 1 | 71 | 93 | NA | Prohibited | Allowed |
| RECORD325 | 68, 67, 81 | 4 | 79 | 97 | NA | Prohibited | Allowed |
| RECORD426 | 65, 64, 84 | 2 | 81 | 100 | NA | Prohibited | Allowed |
| PROOF OF CONCEPT27 | 64, 54, 79 | NA | 73 | NA | Allowed | Prohibited | Allowed if half life <17 hours |
| ODIXA KNEE28 | 66, 55, 89 | NA | 53 | 91 | Allowed | Prohibited | Allowed if half life <17 hours |
| ODIXA HIP (twice daily)29 | 65, 59, 77 | NA | 70 | 82 | Allowed | Prohibited | Allowed if half life <17 hours |
| ODIXA HIP (once daily)30 | 66, 64, 75 | NA | 62 | 84 | NA | Prohibited | Allowed if half life <17 hours |
| Apixaban: | |||||||
| ADVANCE-131 | 66, 62, 87 | 4 | 87 | 95 | NA | NA | Allowed if half life <17 hours |
| ADVANCE-232 | 67, 74, 78 | 2 | 84 | 95 | NA | NA | Allowed if half life <17 hours |
| ADVANCE-333 | 61, 55, 80 | 2 | 68 | 90 | NA | NA | Allowed if half life <17 hours |
| APROPOS34 | 67, 52, 83 | NA | 54 | 78 | NA | NA | NA |
NSAID=non-steroidal anti-inflammatory drug; NA=not available.
Rates of symptomatic venous thromboembolism in the enoxaparin control group were low and similar across studies. Therefore data on symptomatic venous thromboembolism were considered suitable for meta-analysis. However, major bleeding rates reported in the four pivotal RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes) studies with rivaroxaban23 24 25 26 were 7-8 times lower than those in the enoxaparin groups of the remaining studies, which was attributed to the exclusion of most wound bleedings from the definition of major bleeding, as previously reported.8 9 10 This issue prevented the pooling of data on major bleeding reported in the publications of the RECORD studies. However, the major bleeding rates in the RECORD studies without excluding major wound bleedings were reported in an FDA review,38 and were similar to the major bleeding rates of the remaining studies. Finally, we used the major bleeding data of RECORD studies from the FDA in the main analysis and major bleeding data from the publications as an additional sensitivity analysis.
Primary efficacy outcome
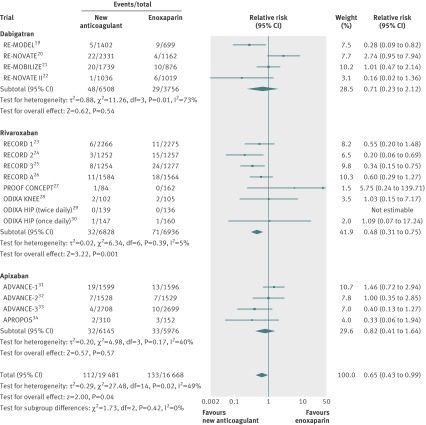
Rivaroxaban was associated with a significant reduction in risk of symptomatic venous thromboembolism compared with enoxaparin (relative risk 0.48, 95% confidence interval 0.31 to 0.75; P=0.001) (fig 2). Compared with enoxaparin, neither dabigatran (0.71, 0.23 to 2.12; P=0.54) nor apixaban (0.82, 0.41 to 1.64; P=0.57) reduced the risk of symptomatic venous thromboembolism (fig 2).
Fig 2 Symptomatic venous thromboembolism
No evidence of statistical heterogeneity for symptomatic venous thromboembolism was found among studies comparing rivaroxaban or apixaban with enoxaparin. However, there was evidence of statistical heterogeneity for symptomatic venous thromboembolism among the dabigatran trials (P=0.01; I2=73%) (fig 2). The source of heterogeneity could not be identified after investigating dabigatran daily dose, enoxaparin regimen, type of surgery, adjudicating committee, or the presence of an outlier study. The effect on symptomatic venous thromboembolism compared with enoxaparin was similar with dabigatran doses of 220 mg (0.70, 0.18 to 2.76; P=0.61) and 150 mg (0.86, 0.31 to 2.35; P=0.63).
After including symptomatic venous thromboembolism events that occurred during follow-up, the results were similar than those of the main analysis (not including post-treatment events): rivaroxaban (0.53, 0.37 to 0.77; P=0.0008), dabigatran (0.90, 0.45 to 1.80; P=0.76), and apixaban (0.69, 0.30 to 1.57; P=0.37) compared with enoxaparin.
Secondary efficacy outcomes
Rivaroxaban was associated with a significantly lower risk of symptomatic deep vein thrombosis than was enoxaparin (relative risk 0.40, 95% confidence interval 0.22 to 0.72; P=0.002), whereas this trend was not significant for symptomatic pulmonary embolism (0.89, 0.30 to 2.67; P=0.84). Rivaroxaban also decreased the risk for total venous thromboembolism or all cause death (0.56, 0.39 to 0.80; P=0.002) as well as for major venous thromboembolism or venous thromboembolism related death (0.42, 0.21 to 0.86; P=0.02).
Compared with enoxaparin, dabigatran was not associated with a different risk of symptomatic deep vein thrombosis (0.82, 0.17 to 3.99; P=0.81) or pulmonary embolism (0.69, 0.31 to 1.54; P=0.36). Dabigatran was associated with a trend towards a higher risk of total venous thromboembolism or all cause death than enoxaparin (1.08, 0.93 to 1.25; P=0.31) and a similar risk of major venous thromboembolism or venous thromboembolism related death (0.89, 0.63 to 1.25; P=0.49). The risk of total venous thromboembolism or all cause death was similar between dabigatran 220 mg and enoxaparin (1.00, 0.87 to 1.15; P=0.98) but it was higher with the dabigatran 150 mg dose than with enoxaparin (1.21, 1.05 to 1.39; P=0.009). Major venous thromboembolism or venous thromboembolism related death did not differ significantly between the dabigatran 220 mg daily dose v enoxaparin (0.80, 0.54 to 1.17; P=0.24) or between the dabigatran 150 mg daily dose v enoxaparin (1.12, 0.81 to 1.54; P=0.49).
Apixaban decreased the risk of symptomatic deep vein thrombosis compared with enoxaparin (0.41, 0.18 to 0.95; P=0.04) but was associated with a numerical increase in cases of pulmonary embolism (apixaban 24 v enoxaparin 14; relative risk 1.25, 0.38 to 4.15; P=0.72) with borderline heterogeneity (P=0.11; I2=51%). The results for pulmonary embolism were homogeneous within the two pivotal studies on total knee replacement surgery (P=0.37; I2=0%), in which the risk of symptomatic pulmonary embolism with apixaban was significantly higher than that with enoxaparin (2.56, 1.10 to 5.98; P=0.03). On the contrary, apixaban was associated with a lower risk of total venous thromboembolism or all cause death (0.63, 0.42 to 0.95; P=0.03) and a trend towards a lower risk of major venous thromboembolism or venous thromboembolism related death (0.61, 0.32 to 1.14; P=0.12) than enoxaparin. (See supplementary figures A1-7 for the full results of the secondary efficacy outcomes).
Primary safety outcome
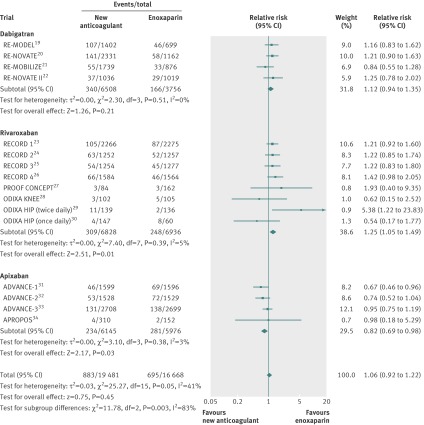
Rivaroxaban was associated with a significant increase in risk of clinically relevant bleeding (relative risk 1.25, 95% confidence interval 1.05 to 1.49; P=0.01) (fig 3). Dabigatran did not show a significant increase compared with enoxaparin (1.12, 0.94 to 1.35; P=0.21). The risk was similar in the comparison of dabigatran 220 mg with enoxaparin (1.12, 0.92 to 1.38; P=0.26) and dabigatran 150 mg with enoxaparin (1.12, 0.89 to 1.40; P=0.34). On the contrary, apixaban was associated with a significantly reduced risk of clinically relevant bleeding compared with enoxaparin (0.82, 0.69 to 0.98; P=0.03). No evidence of statistical heterogeneity was found for this outcome among studies comparing rivaroxaban, dabigatran, or apixaban with enoxaparin (fig 3).
Fig 3 Clinically relevant bleeding
Secondary safety outcomes
Rivaroxaban was associated with a non-significant trend towards a higher risk of major bleeding than was enoxaparin (relative risk 1.29, 95% confidence interval 0.98 to 1.69; P=0.07) and clinically relevant non-major bleeding (1.21, 0.98 to 1.50; P=0.07). Compared with enoxaparin, dabigatran was associated with a similar risk of major bleeding (0.94, 0.58 to 1.52; P=0.79) and a non-significant trend towards a higher risk of clinically relevant non-major bleeding (1.19, 0.96 to 1.48; P=0.11). Apixaban showed a non-significant trend towards a low risk of major bleeding than did enoxaparin (0.81, 0.45 to 1.43; P=0.46), which was in the limit of statistical significance for clinically relevant non-major bleeding (0.83, 0.68 to 1.00; P=0.05). No significant trends were found in risk of death between the new anticoagulants and enoxaparin. (See supplementary figures A8-10 for the full results of the secondary safety outcomes).
Net clinical endpoint
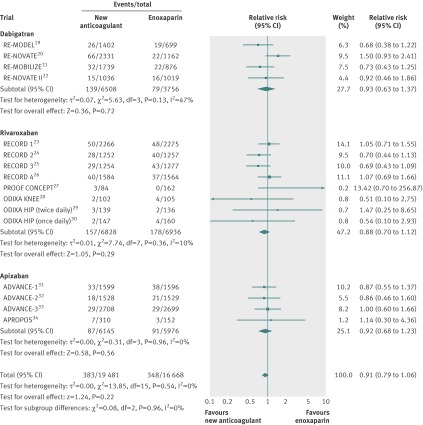
No statistically significant differences were found between the new anticoagulants and enoxaparin on the net clinical endpoint (symptomatic venous thromboembolism, major bleeding, and death) (fig 4). No evidence of statistical heterogeneity was found between studies.
Fig 4 Net clinical endpoint
Main outcomes by type of surgery
No statistically significant interaction of the type of surgery (total hip or knee replacement) was found for symptomatic venous thromboembolism, clinically relevant bleeding, and net clinical endpoint (table 3). Overall, the net clinical benefit of the new anticoagulants tended to be better in total knee replacement surgery than in total hip replacement surgery.
Table 3.
Symptomatic venous thromboembolism, clinically relevant bleeding, and net clinical endpoint by type of surgery
| Variables | No of events/No in group | Relative risk (95%CI) | Weight (%) | P value* | |||
|---|---|---|---|---|---|---|---|
| New anticoagulant | Enoxaparin | ||||||
| Symptomatic venous thromboembolism | |||||||
| Dabigatran: | |||||||
| Hip20 22 | 23/3367 | 10/2181 | 0.78 (0.05 to 12.35) | 42.5 | 0.83 | ||
| Knee19 21 | 25/3141 | 19/1575 | 0.56 (0.16 to 1.98) | 57.5 | |||
| Rivaroxaban: | |||||||
| Hip23 24 27 29 30 | 11/3888 | 27/3990 | 0.52 (0.18 to 1.45) | 35.6 | 0.93 | ||
| Knee25 26 28 | 21/2940 | 44/2946 | 0.49 (0.29 to 0.83) | 64.4 | |||
| Apixaban: | |||||||
| Hip33 | 4/2708 | 10/2699 | 0.40 (0.13 to 1.27) | 23 | 0.14 | ||
| Knee31 32 34 | 28/3437 | 23/3277 | 1.08 (0.56 to 2.06) | 77 | |||
| Clinically relevant bleeding | |||||||
| Dabigatran: | |||||||
| Hip20 22 | 178/3367 | 87/2181 | 1.22 (0.95 to 1.58) | 51.8 | 0.36 | ||
| Knee19 21 | 162/3141 | 79/1575 | 1.01 (0.74 to 1.39) | 48.2 | |||
| Rivaroxaban: | |||||||
| Hip23 24 27 29 30 | 186/3888 | 152/3990 | 1.25 (0.90 to 1.75) | 59.6 | 0.90 | ||
| Knee25 26 28 | 123/2940 | 96/2946 | 1.29 (0.99 to 1.67) | 40.4 | |||
| Apixaban: | |||||||
| Hip33 | 131/2708 | 138/2699 | 0.95 (0.75 to 1.19) | 52.2 | 0.09 | ||
| Knee31 32 34 | 103/3437 | 143/3277 | 0.71 (0.55 to 0.91) | 47.8 | |||
| Net clinical endpoint | |||||||
| Dabigatran: | |||||||
| Hip20 22 | 81/3367 | 38/2181 | 1.26 (0.80 to 1.98) | 49.3 | 0.06 | ||
| Knee19 21 | 58/3141 | 41/1575 | 0.71 (0.48 to 1.05) | 50.7 | |||
| Rivaroxaban: | |||||||
| Hip23 24 27 29 30 | 86/3888 | 94/3990 | 0.92 (0.60 to 1.41) | 53.2 | 0.76 | ||
| Knee25 26 28 | 71/2940 | 84/2946 | 0.85 (0.60 to 1.19) | 46.8 | |||
| Apixaban: | |||||||
| Hip33 | 29/2708 | 29/2699 | 1.00 (0.60 to 1.66) | 32.8 | 0.70 | ||
| Knee31 32 34 | 58/3437 | 62/3277 | 0.88 (0.62 to 1.26) | 67.2 | |||
*Random effects model, subgroup differences.
Indirect comparisons
Rivaroxaban tended to be associated with the lowest risk for symptomatic venous thromboembolism, whereas apixaban seemed to achieve the lowest risk for clinically relevant bleeding (table 4). No differences were found between treatments on the net clinical outcome.
Table 4.
Indirect comparisons between rivaroxaban, dabigatran, and apixaban*
| Outcomes | Relative risk (95% CI) | ||
|---|---|---|---|
| Rivaroxaban v dabigatran | Rivaroxaban v apixaban | Apixaban v dabigatran | |
| Symptomatic venous thromboembolism | 0.68 (0.21 to 2.23) | 0.59 (0.26 to 1.33) | 1.16 (0.31 to 4.28) |
| Clinically relevant bleeding | 1.12 (0.87 to 1.44) | 1.52 (1.19 to 1.95) | 0.73 (0.57 to 0.94) |
| Major bleeding | 1.37 (0.79 to 2.39) | 1.59 (0.84 to 3.02) | 0.86 (0.41 to 1.83) |
| Net clinical endpoint | 0.95 (0.61 to 1.48) | 0.96 (0.66 to 1.40) | 0.99 (0.61 to 1.61) |
*Random effects model, events while receiving treatment.
Absolute difference in events per 1000 patients treated
The numbers of symptomatic venous thromboembolic events avoided per 1000 patients treated with rivaroxaban versus enoxaparin, dabigatran, or apixaban were generally similar to those of the additional resultant major bleeds (table 5). No significant absolute differences were apparent between treatments on the net clinical outcome.
Table 5.
Direct and indirect comparisons: absolute difference in events per 1000 patients treated*
| Comparison | Risk difference (95% CI) | |||
|---|---|---|---|---|
| Symptomatic venous thromboembolism | Clinically relevant bleeding | Major bleeding | Net clinical endpoint | |
| Direct comparisons: | ||||
| Rivaroxaban v enoxaparin | −5 (−9 to −1) | 9 (2 to 17) | 4 (−0.4 to 8) | −3 (−9 to 3) |
| Dabigatran v enoxaparin | −2 (−9 to 5) | 5 (−4 to 13) | −1 (−6 to 5) | −1 (−9 to 7) |
| Apixaban v enoxaparin | −1 (−4 to 2) | −8 (−15 to −1) | −1 (−7 to 5) | −1 (−6 to 3) |
| Indirect comparisons: | ||||
| Rivaroxaban v dabigatran | −3 (−11 to 4) | 5 (−7 to 16) | 4 (−2 to 11) | −2 (−12 to 9) |
| Rivaroxaban v apixaban | −4 (−9 to 1) | 18 (7 to 28) | 5 (−2 to 12) | −2 (−9 to 6) |
| Apixaban v dabigatran | 1 (−7 to 8) | −13 (−24 to −2) | 0 (−8 to 7) | 0 (−9 to 9) |
*Random effects model, events while receiving treatment.
Sensitivity analyses
Sensitivity analyses were consistent with those of the main analysis for the direct comparisons between the new anticoagulants and enoxaparin on symptomatic venous thromboembolism, clinically relevant bleeding, and the net clinical endpoint, regardless of the assumption of the statistical model and study quality, phase, or duration (see supplementary tables A1-3). Acceptance of the definition for major bleeding as reported in the publications (accepting the exclusion of major wound bleedings in the RECORD studies), had a significant impact on the apparent efficacy and safety of rivaroxaban, as it would have been declared superior to enoxaparin in the net clinical endpoint (0.68, 0.50 to 0.91; P=0.01) (table A4 of the supplementary appendix). In sensitivity analyses of indirect comparisons (tables A5 to A7 of the supplementary appendix), the use of the fixed effects model led to closer confidence intervals than those obtained using random effects, suggesting a lower risk of symptomatic venous thromboembolism with rivaroxaban than with dabigatran (0.53, 0.29 to 0.99) or apixaban (0.51, 0.27 to 0.96).
Publication bias
The visual inspection of funnel plots showed no evidence of publication bias (see supplementary figure A11).
Role of funding
All studies were sponsored by pharmaceutical companies. The sponsor was responsible for the collection and statistical analysis of the data. In all cases the sponsor was involved in the design and oversight of the study with or without the collaboration of a scientific committee, and at least one of the authors of the publications were employees of the sponsor.
Discussion
This systematic review and meta-analysis indicates that a higher efficacy of the new type of anticoagulant is generally associated with a higher bleeding tendency in patients undergoing total hip or knee replacement surgery. At the time of balancing efficacy (symptomatic venous thromboembolism) and safety (major bleed and deaths), the different anticoagulants did not differ significantly.
Rivaroxaban seems more effective than enoxaparin in preventing symptomatic venous thromboembolism but at the cost of an increase in clinically relevant bleeds. These results were consistent across different studies, without evidence of heterogeneity.
Dabigatran seems at least as effective as enoxaparin in the risk of symptomatic venous thromboembolism, but the results are noticeable by heterogeneity and wide confidence intervals. Surrogate venographic data on major and total venous thromboembolism indicates that the high dose (220 mg) is consistently non-inferior to enoxaparin. The low dabigatran dose (150 mg) may provide an alternative in patients with anticipated increased exposure to dabigatran,39 such as those aged more than 75 years and those with moderate renal impairment.5 In our meta-analysis, the risk of clinically relevant bleeding was not significantly different between dabigatran and enoxaparin. The upper limit of the 95% confidence interval, however, indicates that a relative risk of clinically relevant bleeding with dabigatran versus enoxaparin by 35% cannot be excluded.
Apixaban was associated with a lower rate of clinically relevant bleeding than enoxaparin, mainly in knee pivotal studies, but associated with an increase in cases of pulmonary embolism, also in knee pivotal studies. Symptomatic pulmonary embolism occurs earlier in knee replacement surgery than in hip replacement surgery,40 41 which might theoretically result in an increase in risk of early pulmonary embolism if the first dose of the anticoagulant is delayed. Whether the benefit in bleeding and the numerical increase in pulmonary embolism in knee studies are a chance finding or due to the delay of the first apixaban dose about 18 hours after surgery (mean in pivotal trials) deserves further scrutiny. Doctors may consider the potential benefits of earlier anticoagulation for venous thromboembolism prophylaxis as well as the risks of post-surgical bleeding in deciding on when to administer within the approved time window (12 to 24 hours after surgery for apixaban).7
Our meta-analysis also shows that the definition of major bleeding may have a significant impact on the apparent safety of the anticoagulants and that even difficult to perceive changes in the definitions may lead to different conclusions in the benefit-risk balance.
Strengths of the review
Our study represents the most comprehensive meta-analysis of new oral anticoagulants carried out in total hip or knee replacement surgery up to date. It is based on data from more than 30 000 patients enrolled in 16 randomised clinical trials, 15 of them using a double blind design and all including an independent and blinded assessment of outcomes. The studies were published between 2005 and 2011 and evidence of publication bias was lacking. Sensitivity analyses suggest that the results are robust. It is unlikely that a clinical trial comparing two new oral anticoagulants in total hip or knee replacement surgery would be carried out in the near future. Therefore our results provide a useful estimate of expected relative differences on clinically relevant events between rivaroxaban, dabigatran, and apixaban in total hip or knee replacement surgery.
Comparison with other reports
Few previous studies have indirectly compared dabigatran with rivaroxaban.42 43 44 Only one of them indirectly compared rates of symptomatic venous thromboembolism,42 but it did not include the RE-NOVATE II trial,22 which was published afterwards. One of these reports included studies with dabigatran, rivaroxaban, and apixaban,44 but the comparison was limited to the endpoint of total venous thromboembolism plus all cause death (mainly driven by asymptomatic venographic deep vein thrombosis), and only pivotal trials were included. The study showed better venographic outcomes with rivaroxaban and apixaban than with dabigatran.44
Limitations of the review
Our systematic review has limitations. The main efficacy outcome in our study (symptomatic venous thromboembolism) was a secondary outcome in all studies. Therefore the results on symptomatic venous thromboembolism are exploratory. Nevertheless, all events were adjudicated blindly and independently, which adds robustness to the results obtained. However, symptomatic venous thromboembolism events are more representative of what would be expected in standard clinical practice than are venographic (mainly asymptomatic) events.8 Direct comparisons between rivaroxaban or apixaban versus enoxaparin for major or total venous thromboembolism are based on studies in which venograms were adjudicated by the same committee (Gothenburg committee in the rivaroxaban studies and Hamilton committee in the apixaban studies), whereas two committees (Gothenburg and Holland) were used in the dabigatran studies. Given the double blind adjudication, it can be reasonably expected that the calculated relative risk of direct comparisons would have provided an unbiased estimate. However, we decided not to report indirect comparisons on major and total venous thromboembolism because the differences in venographic assessment reported between different adjudicating committees42 45 was considered a factor that might bias the indirect comparison.46
At the time of translating the results from these clinical trials into practice, some considerations are necessary. In absolute terms it is expected that patients in standard clinical practice would have a higher risk for symptomatic venous thromboembolism and bleeding than those included in clinical trials, because of the exclusion criteria applied in clinical trials (that is, severe renal or hepatic insufficiency, chronic use of vitamin K antagonists, concomitant treatment with non-steroidal anti-inflammatory drugs of long half life, strong CYP3A4 inhibitors, history of bleeding, and so on), as well as by other differences in personal characteristics.47 48 It is worth mentioning that the risk of bleeding increases with age and in other special situations to a greater extent than does the risk of symptomatic venous thromboembolism.48 Therefore one of the main uncertainties about the use of the new anticoagulants is related to their real bleeding risk in standard clinical practice,49 50 51 which emphasises the need for appropriate use according to product labelling to minimise such risk.5 6 7
Conclusions
Our meta-analysis indicates that a higher efficacy of the new type of anticoagulants was generally associated with a higher bleeding tendency, but the anticoagulants did not differ significantly for efficacy and safety.
What is already known on this topic
Pivotal trials in venous thromboprophylaxis are usually based on a surrogate venographic (usually asymptomatic) primary endpoint of efficacy
Rivaroxaban, dabigatran, and apixaban are new oral anticoagulants licensed for prophylaxis against venous thromboembolism after total hip or knee replacement surgery
The effect of these anticoagulants on symptomatic outcomes and their relative efficacy and safety are uncertain
What this study adds
Rivaroxaban was associated with a lower risk of symptomatic venous thromboembolism than enoxaparin but at the cost of an increase in clinically relevant bleeding
The risk of symptomatic venous thromboembolism was similar in patients receiving dabigatran or apixaban than enoxaparin, whereas apixaban was associated with a lower risk of clinically relevant bleeding than enoxaparin
A higher efficacy of the new type of anticoagulant was generally associated with a higher bleeding tendency and the new anticoagulants did not differ significantly for efficacy and safety
Contributors: AG-O, AIT-F, EV-C, and MLS-G conceived and designed the study. AG-O and AIT-F collected the data. AG-O carried out the statistical analysis and drafted the manuscript. EV-C supervised the study. All authors analysed and interpreted the data and critically revised the manuscript for important intellectual content. The contents of this study are solely the responsibility of the authors and do not necessarily represent the official view of their institutions or any other party. AG-O and EV-C are the guarantors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data available.
Cite this as: BMJ 2012;344:e3675
Web Extra. Extra material supplied by the author
Search strategy
Results of secondary efficacy outcomes
Results of secondary safety outcomes
Sensitivity analyses of direct comparisons
Sensitivity analyses of indirect comparisons
Funnel plots
References
- 1.Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 2007;98:756-64. [DOI] [PubMed] [Google Scholar]
- 2.Mackman N. Triggers, targets and treatments for thrombosis. Nature 2008;451:914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th ed). Chest 2008;133(6 suppl):S381-453. [DOI] [PubMed] [Google Scholar]
- 4.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446-55. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Agency. Pradaxa ®—summary of product characteristics. 2011. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000829/WC500041059.pdf.
- 6.European Medicines Agency. Xarelto ®—summary of product characteristics. 2011. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf.
- 7.European Medicines Agency. Eliquis ®—summary of product characteristics. 2011. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002148/WC500107728.pdf.
- 8.Gómez-Outes A, Lecumberri R, Pozo C, Rocha E. New anticoagulants: focus on venous thromboembolism. Curr Vasc Pharmacol 2009;7:309-29. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Outes A, Suárez-Gea ML, Blázquez-Pérez A, Pozo-Hernández C, Vargas-Castrillón E. Rivaroxaban versus enoxaparin after total knee arthroplasty. Lancet 2009;374:682. [DOI] [PubMed] [Google Scholar]
- 10.Dahl OE, Quinlan DJ, Bergqvist D, Eikelboom JW. A critical appraisal of bleeding events reported in venous thromboembolism prevention trials of patients undergoing hip and knee arthroplasty. J Thromb Haemost 2010;8:1966-75. [DOI] [PubMed] [Google Scholar]
- 11.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29. [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed] [Google Scholar]
- 17.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.1; Copenhagen, 2011.
- 18.Wells GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect treatment comparison [computer program]. Version 1.0. Canadian Agency for Drugs and Technologies in Health; Ottawa, 2009.
- 19.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178-85. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007;370:949-56. [DOI] [PubMed] [Google Scholar]
- 21.The RE-MOBILIZE Writing Committee. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1-9. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II). A randomised, double-blind, non-inferiority trial. Thromb Haemost 2011;105:721-9. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75. [DOI] [PubMed] [Google Scholar]
- 24.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31-9. [DOI] [PubMed] [Google Scholar]
- 25.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776-86. [DOI] [PubMed] [Google Scholar]
- 26.Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673-80. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Dose-escalation study of rivaroxaban (BAY 59-7939)—an oral, direct Factor Xa inhibitor—for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Res 2007;120:685-93. [DOI] [PubMed] [Google Scholar]
- 28.Turpie AG, Fisher WD, Bauer KA, Kwong LM, Irwin MW, Kälebo P, et al. BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost 2005;3:2479-86. [DOI] [PubMed] [Google Scholar]
- 29.Eriksson BI, Borris L, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Oral, direct Factor Xa inhibition with BAY 59-7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost 2006;4:121-8. [DOI] [PubMed] [Google Scholar]
- 30.Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation 2006;114:2374-81. [DOI] [PubMed] [Google Scholar]
- 31.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009;361:594-604. [DOI] [PubMed] [Google Scholar]
- 32.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010;375:807-15. [DOI] [PubMed] [Google Scholar]
- 33.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010;363:2487-98. [DOI] [PubMed] [Google Scholar]
- 34.Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost 2007;5:2368-75. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson BI, Dahl OE, Ahnfelt L, Kälebo P, Stangier J, Nehmiz G, et al. Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO I. J Thromb Haemost 2004;2:1573-80. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson BI, Dahl OE, Büller HR, Hettiarachchi R, Rosencher N, Bravo ML, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 2005;3:103-11. [DOI] [PubMed] [Google Scholar]
- 37.Fuji T, Fuijita S, Ujihira, Sato R. Dabigatran etexilate prevents venous thromboembolism after total knee arthroplasty in Japanese patients with a safety profile comparable to placebo. J Arthroplasty 2010;25:1267-74. [DOI] [PubMed] [Google Scholar]
- 38.FDA Cardiovascular & Renal Drugs Advisory Committee. Meeting ID: 2009-4418. www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm181524.pdf.
- 39.Dahl OE, Kurth AA, Rosencher N, Noack H, Clemens A, Eriksson BI. Thromboprophylaxis with dabigatran etexilate in patients over seventy-five years of age with moderate renal impairment undergoing or knee replacement. Int Orthop 2012;36:741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kearon C. Natural history of venous thromboembolism. Circulation 2003;107(23 suppl 1):I22-30. [DOI] [PubMed] [Google Scholar]
- 41.Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br 2006;88:386-91. [DOI] [PubMed] [Google Scholar]
- 42.Trkulja V, Kolundzic R. Rivaroxaban vs dabigatran for thromboprophylaxis after joint-replacement surgery: exploratory indirect comparison based on meta-analysis of pivotal clinical trials. Croat Med J 2010;51:113-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loke YK, Kwok CS. Dabigatran and rivaroxaban for prevention of venous thromboembolism—systematic review and adjusted indirect comparison. J Clin Pharm Ther 2011;36:111-24. [DOI] [PubMed] [Google Scholar]
- 44.Maratea D, Fadda V, Trippoli S, Messori A. Prevention of venous thromboembolism after major orthopedic surgery: indirect comparison of three new oral anticoagulants. J Thromb Haemost 2011;9:1868-70. [DOI] [PubMed] [Google Scholar]
- 45.Quinlan DJ, Eikelboom JW, Dahl OE, Eriksson BI, Sidhu PS, Hirsh J. Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery. J Thromb Haemost 2007;5:1438-43. [DOI] [PubMed] [Google Scholar]
- 46.Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al. Indirect comparisons of competing interventions. Health Technol Assess 2005;9:1-134. [DOI] [PubMed] [Google Scholar]
- 47.Waddell J, Johnson K, Hein W, Raabe J, FitzGerald G, Turibio F. Orthopaedic practice in total hip arthroplasty and total knee arthroplasty: results from the Global Orthopaedic Registry (GLORY). Am J Orthop (Belle Mead NJ) 2010;39(9 suppl):5-13. [PubMed] [Google Scholar]
- 48.Deitelzweig SB, Lin J, Lin G. Preventing venous thromboembolism following orthopedic surgery in the United States: impact of special populations on clinical outcomes. Clin Appl Thromb Hemost 2011;17:640-50. [DOI] [PubMed] [Google Scholar]
- 49.Jensen CD, Steval A, Partington PF, Reed MR, Muller SD. Return to theatre following total hip and knee replacement, before and after the introduction of rivaroxaban: a retrospective cohort study. J Bone Joint Surg Br 2011;93:91-5. [DOI] [PubMed] [Google Scholar]
- 50.Legrand M, Mateo J, Aribaud A, Ginisty S, Eftekhari P, Huy PT, et al. The use of dabigatran in elderly patients. Arch Intern Med 2011;171:1285-6. [DOI] [PubMed] [Google Scholar]
- 51.European Medicines Agency updates on safety of Pradaxa (EMA/CHMP/903767/2011). Press release, 18 Nov 2011. www.ema.europa.eu/docs/en_GB/document_library/Press_release/2011/11/WC500117818.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy
Results of secondary efficacy outcomes
Results of secondary safety outcomes
Sensitivity analyses of direct comparisons
Sensitivity analyses of indirect comparisons
Funnel plots