Abstract
Expression of the activation antigen CD38 on T cells is a strong predictor of the risk of HIV disease progression, but it is not known whether CD38 is a marker or mediator of dysfunction. We examined the relationship between CD38 expression and responses to T-cell receptor stimulation in central memory and effector memory CD4+ T cells in HIV-infected persons and in healthy controls. Basal CD38 expression was preserved by blocking golgi transport with brefeldin A. Intracellular expression of interleukin 2, interferon γ, and CD154 was measured after stimulating peripheral blood mononuclear cells with the superantigen staphylococcal enterotoxin B with or without anti-CD28 costimulation. Interferon-γ responses were comparable or increased in stimulated CD38+ memory cells, and the interleukin 2 responses of costimulated CD38+ central memory cells were decreased in HIV infection. In CD38+ cells and especially in CD38+ cells of HIV-infected persons, stimulated memory cells more often failed to express CD154 (CD40 ligand) when induced to express cytokine. A dissociated cytokine and CD154 expression by memory CD4 T cells may impair interactions between T cells and antigen-presenting cells, contribute to impaired immunity and help explain the relationship between CD38 expression and disease progression in chronic HIV infection.
Keywords: AIDS, CD4+, T cell, immune activation
INTRODUCTION
There is growing evidence that immune activation is central to the pathogenesis of immune deficiency in chronic HIV infection.1–4 Increased levels of several soluble activation markers in blood or increased percentages of T cells expressing activation markers predict the occurrence of AIDS-defining clinical events.5–7 Particularly, the expression of the activation marker CD38 on CD8+ T cells is a strong predictor of the risk of progression to AIDS, sometimes independently of levels of HIV viremia.8–15
These findings have been taken to suggest that there is a causal link between immune activation and progressive immune deficiency that is characterized not only by progressive depletion of CD4+ T cells but also by profound impairments in the functions of these cells. For instance, even among HIV-infected persons with relatively preserved CD4+ T-cell counts, there are diminished proliferative responses to polyclonal activation,16,17 and CD4+ T cells from HIV-infected persons have impaired interferon (IFN)-γ and interleukin (IL)-2 responses after polyclonal stimulation.18,19 CD4+ T-cell proliferation and cytokine production in response to microbial antigens are also characteristically impaired in persons with HIV infection.17,18,20–22 Though immune activation and disease progression are linked, there are limited data that link immune activation and CD4+ T-cell dysfunction in HIV infection.23,24 Although a relationship between immune activation indices and functional impairments was demonstrable,17,25,26 it remains uncertain as to whether these indices are simply correlated or whether an activated cell (specifically, a cell that expresses the activation antigen CD38) is functionally impaired and if so, whether the multifunctional ectoenzyme CD38 actually renders the cell dysfunctional. In this study, we asked if expression of the activation antigen CD38 on effector memory (EM) and central memory (CM) CD4+ T cells conferred any degree of functional impairment in healthy controls or in HIV-infected persons. We hypothesized that after T-cell receptor stimulation, CD38+ CD4+ T cells would demonstrate a profound impairment in expression of the T-helper cytokines, IL-2 and IFN-γ, thereby demonstrating a deleterious consequence of immune activation on functional immunity. We found normal or increased IFN-γ expression by CD38+ CM and EM cells in HIV infection. In contrast, costimulated CD38+ CM cells had an impaired IL-2 response to T-cell receptor (TCR) stimulation, and in both memory populations, ex vivo stimulation failed to induce coordinate expression of cytokine and CD154 (CD40 ligand) in CD38+ cell populations. Thus, CD38+ memory CD4 T cells—presumably activated in vivo—tend to dysregulated expression of cytokine and the costimulatory molecule CD154. These defects may link CD38 expression to a functional impairment of immune responses and disease progression in HIV infection.
METHODS
Samples
This protocol was performed in consistence with the policies of the Institutional Review Board of University Hospitals/Case Medical Center. After informed consent was obtained, heparinized blood samples were prepared from 11 healthy controls and 11 HIV+ patients with median CD4+ T-cell counts of 391 cells per microliter [interquartile range: 277–576] and a median plasma HIV RNA level of 14, 200 copies per milliliter (interquartile range: 1845–60,250). No patient had an active opportunistic infection or malignancy, and none was receiving antiretroviral therapy or immunomodulatory drugs.
Cell Preparation
Peripheral blood mononuclear cells were prepared by density sedimentation (Ficoll-Paque, GE Healthcare Biosciences AB, Piscataway, NJ), then washed twice in RPMI 1640 medium (Lonza, Walkersville, MD), and resuspended at 106 cells per milliliter in complete RPMI medium, containing 100 U/mL Penicillin, 0.1 mg/mL Streptomycin (Penicillin/Streptomycin, Lonza), 2 mM L-glutamine (Lonza), 5 mM HEPES buffer (Lonza), and 10% heat inactivated fetal bovine serum (HyClone, Logan, UT).
Cell Stimulation
One million peripheral blood mononuclear cells were incubated in 1 mL complete medium plus 5 μg S. aureus enterotoxin B (SEB) as a polyclonal TCR-activating superantigen27 (Sigma, St. Louis, MO), or in medium + SEB and 1μg anti-CD28 agonistic monoclonal antibody (azide free, low endotoxin, BD Biosciences, San Jose, CA). Cells were incubated for 2 hours at 37°C in a 5% CO2 enriched humidified atmosphere. One μL Golgi Plug (BD Biosciences) containing brefeldin was then added, and cells were incubated for an additional 12 hours before staining. Surface levels of CD38, CD45RA, and CCR7 did not change under these conditions (see Figures, Supplemental Digital Content 1, http://links.lww.com/QAI/A85), allowing us to track cytokine expression patterns of defined CD4 T-cell subsets after TCR stimulation according to their basal (ex vivo) phenotype (ie, CD38− or CD38+).
Staining With Fluorochrome Labeled Antibodies and Flow Cytometry
After incubation, cells were washed with staining buffer (phosphate-buffered saline, 1% bovine serum albumin, 0.01% sodium azide). They were then surface stained for flow cytometric analysis by use of fluorochrome-labeled monoclonal antibodies against CD4 (Pacific Blue), CD45RA (APC), CCR7 (PE-Cy7), and CD38 (PerCP-Cy5.5) (BD Biosciences). Cells were then washed with staining buffer, fixed and permeabilized with BD FacsPERM solution (BD Biosciences), and then were stained with labeled antibodies against CD154 (PE) and antibodies against either IL-2 or IFN-γ (FITC) (BD Biosciences). Immediately after staining, cells were analyzed using an LSR-II flow cytometer (Becton Dickinson) and DIVA 4.1.2 software (Becton Dickinson, San Jose, CA).
Gates for CM and EM memory, and activated subpopulations were established using isotype controls (BD Biosciences). Gates for intracellular IL-2, IFN-γ, and CD154 measurement were established with fully stained unstimulated cells. These gates were further adjusted by clustering of unstimulated cells with the autogate algorithm of DIVA 4.1.2 software. These gates defined distinct CD154high and CD154dim populations, the latter consisting mostly of CD154− cells, as defined by staining of unstimulated cells.
Statistical Analysis
Each relevant variable (% positive cells) was compared between subject groups (HIV− and HIV+) using the Mann–Whitney rank sum test. Treatment effects (SEB or SEB plus CD28 ligation) and the effects of CD38 expression were compared for each variable using the Wilcoxon signed rank test.
RESULTS
Induction of IFN-γ and IL-2 In CD38+ and CD38− Memory T Cells
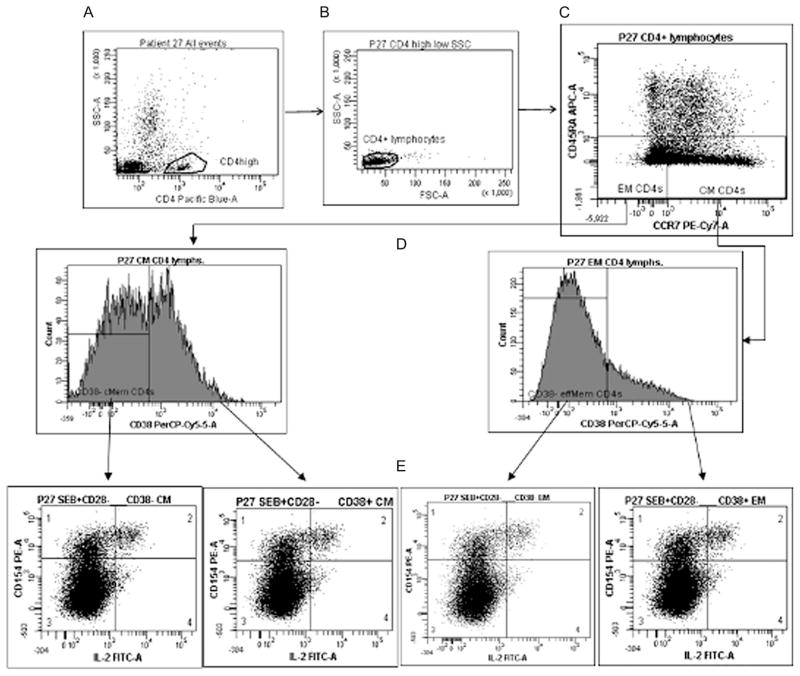
To ascertain if there are intrinsic differences in cytokine production between CD38+ and CD38− CM or EM T-cell subsets, we examined cytokine expression in these cells after stimulation with SEB or SEB plus anti-CD28 costimulatory antibodies. We utilized experimental conditions that preserved basal surface expression of CD38, CD45RA, and CCR7 after TCR stimulation (see Figures, Supplemental Digital Content 1, http://links.lww.com/QAI/A85). This enabled us to identify the ex vivo expression patterns of predefined CD4+ T-cell maturation subsets that were CD38+ or CD38−. Surface markers were utilized to delineate CM (CD45RA− CCR7+), EM (CD45RA− CCR7−) and activated (CD38+) T cells (Fig. 1A–D) and to evaluate intracellular expression of IL-2 or IFN-γ as illustrated in Fig. 1E. As intracellular expression of CD154 has been utilized to identify cells that have been activated through engagement of the T-cell receptor,28–30 phenotypically defined CD4 memory cell subsets were also examined for concurrent induction of CD154 and cytokine as shown in Figure 1E.
FIGURE 1.
Gating strategy: CD4+ lymphocytes (A, B) were subgated into maturation subpopulations (C) according to expression of CCR7 and CD45RA. Each memory subpopulation was divided into CD38− and CD38+ subsets as gated by isotype control staining (D). CD38− and CD38+ subsets of each maturation subpopulation were finally analyzed for intracellular expression of CD154 and either IFN-γ or IL-2 expression (E), according to gates established with fully stained unstimulated cells, and further adjusting for clustering of distinct populations.
EM CD4+ T Cells
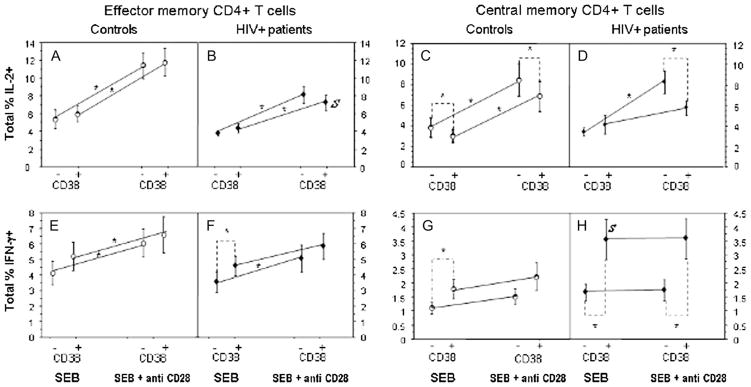
Summary data generated from the 22 subjects (11 patients, 11 controls) are shown in Figure 2. Under stimulation conditions, cytokine expression was comparable among CD38+ and CD38− EM cells (Fig. 2A, B, E), excepting the SEB-induced IFN-γ response in which CD38+ cells of patients were more frequently capable of expressing cytokine (P = 0.017, Fig. 2F) and failed to significantly increase their response to SEB when costimulated with anti-CD28 antibody. The only major difference between the HIV-infected and HIV-uninfected subjects was the presence of fewer IL-2–expressing CD38+ EM CD4 T cells in response to costimulation in the HIV-infected subjects than in uninfected controls (P = 0.0328, Fig. 2A, B).
FIGURE 2.
Proportions of CD38+ and CD8−CM and EM CD4+ T cells expressing cytokine after stimulation with SEB or SEB plus anti-CD28. Data shown represent means ± SEM in experiments performed using cells of 11 HIV+ patients and 11 healthy controls. *P < 0.05 (text). S, significantly different frequency of cytokine-producing cells between HIV+ subjects and controls (P < 0.05).
CM CD4+ T Cells
High level expression of IFN-γ is not expected in phenotypically defined CM CD4+ T cells.31,32 Nonetheless, both IL-2 and IFN-γ expression were demonstrable in stimulated CM CD4+ T cells of both patients and controls. (Fig. 2C, D, G, H). SEB-stimulated CD38+ CM cells from patients more frequently expressed IFN-γ+ cells than did CD38+ CM cells from controls (P = 0.04, Fig. 2G, H), and in both patients and controls, SEB-stimulated CD38+ CM cells more frequently expressed IFN-γ than did CD38− CM cells (P = 0.0262 and 0.0164, respectively, Fig. 2G, H). This suggests that CD38+ CM cells (and especially CD38+ CM cells from HIV+ donors) are more likely to be conditioned in vivo to produce IFN-γ than are CD38− cells. Costimulation with anti-CD28 did not enhance IFN-γ production in any cell population in either patients or controls. Among patients, CD38+ costimulated CM cells more frequently expressed IFN-γ than did CD38− cells (P = 0.009), Fig. 2H).
After SEB stimulation, CM cells expressing IL-2 were significantly less frequent among controls (but not patients) CD38+ cells than among CD38− cells (P = 0.041); there were, however, no differences between patients and controls in the expression of IL-2 in either CD38+ or CD38− cells (Fig. 2C, D). Patients’ CD38+ CM cells failed to further increase their response to SEB after CD28 costimulation (Fig. 2D). Among patients and controls, CD38− costimulated cells more frequently expressed IL-2 than did CD38+ cells (P = 0.041, P = 0.01, respectively, Fig. 2C, D).
Thus, CM cells from patients produce IL-2 in response to TCR stimulation at normal frequencies, yet in both patients and controls, CD38+ CM cells less frequently express IL-2 after costimulation than do CD38− cells. In contrast, CD38+ CM cells in both patients and controls are more capable of IFN-γ production than are CD38− CM cells and among patients, these cells are also more capable of IFN-γ expression than are CD38+ CM cells of healthy controls.
Dissociation of CD154 and Cytokine Expression is More Frequent in CD38+ Memory T Cells, and Especially in HIV Infection
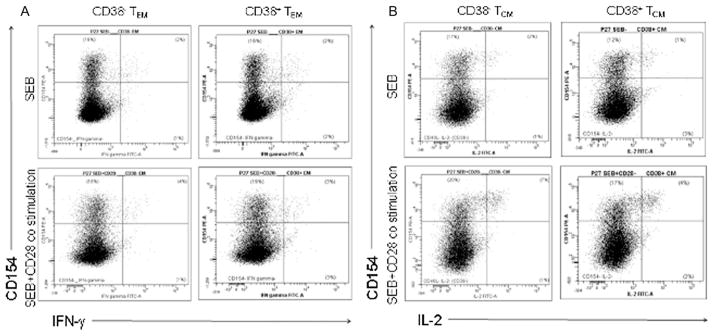
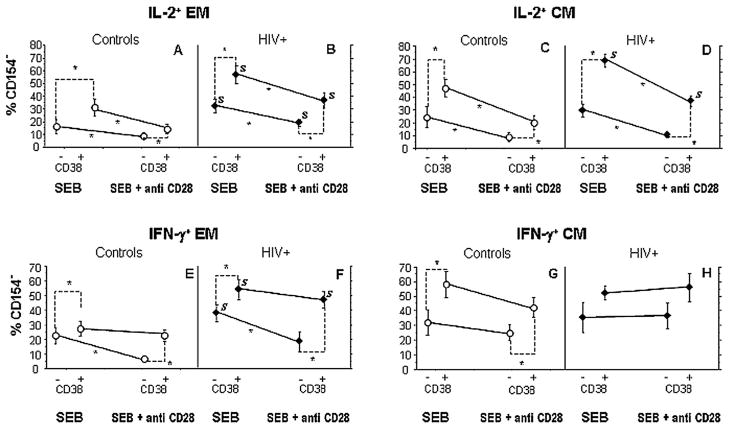
Because the induction of the important costimulator CD154 (CD40 ligand) is a sensitive and reasonably specific marker of CD4+ T cells that have been engaged through TCR stimulation,28–30 we wanted to examine the relationship between CD154 induction and cytokine expression to understand how T-cell activation after TCR engagement was coordinated in CD38+ and CD38− memory subpopulations. We, therefore, gated separately the IFN-γ and IL-2 producing cells into those that did or did not coexpress intracellular CD154 after stimulation (Fig. 3) and expressed the data as the percentage of phenotypically defined cells that were induced to express cytokine in the absence of concurrent CD154 expression (Fig. 4)—an indicator of a dysregulated T-cell response. Typically, only a minority of cells induced to express cytokine failed to express CD154 concordantly, but among HIV-infected patients and particularly among CD38+ cells, the proportions of dysregulated responses, that is, cytokine-producing cells that failed to express CD154 tended consistently to be higher. Specifically among EM cells, CD38+ cells were more likely to express IL-2 or IFN-γ without CD154 in both patients and controls (Fig. 4A, B), and in all conditions, but one (CD38− cells producing IFN-γ in response to SEB plus anti-CD28 stimulation), patients’ cells producing cytokine more often failed to express CD154 than did controls’ cells. In all settings excepting expression of IFN-γ by CD38+ cells, addition of CD28 costimulation decreased the proportions of dysregulated cell activation (cells that expressed cytokine in the absence of CD154 induction) underscoring the importance of CD28 costimulation in inducing coordinated immune responses.
FIGURE 3.
Representative dot plots showing IFN-γ and CD154 expression among EM CD4+ T cells (A) and IL-2 and CD154 expression among CM CD4+ T cells under the experimental conditions (SEB and SEB + CD28 costimulation). Dissociated cytokine responses correspond to the lower right quadrant of each plot. Within each quadrant, the percentages of events are shown.
FIGURE 4.
A dysregulated response—expression of cytokine in the absence of CD154 coexpression is more frequent in CD38+ cells and in HIV infection after SEB or SEB/CD28 stimulation. Values correspond to the percentage of CD154− cells among total cytokine-producing cells (means ± SEM in experiments performed using cells of 11 HIV+ patients and 11 healthy controls). *P < 0.05 (text). S, significant difference between HIV+ subjects and controls (P < 0.05).
Similar tendencies were observed among CM cells, where in all settings except IFN-γ expression by cells of HIV+ subjects, CD38+ cells making cytokine more often failed to express CD154 than did CD38− cells. Only in assays of IL-2 production though and only among CD38+ cells were cells of patients more often CD154 negative. Finally, only in assays of IL-2 production did CD28 costimulation reliably decrease the frequency of cells that expressed cytokine in the absence of CD154 induction.
DISCUSSION
Given the consistent and strong correlation between CD38 expression on T cells and immune suppression in HIV infection,2,4,15,33,34 we hypothesized that expression of the activation antigen CD38 confers lower functionality to CM and EM CD4+ T-cell subsets in persons with HIV infection. The ability to produce IFN-γ was preserved and often increased in CD38+ CD4 T cells in HIV infection, whereas the ability of CD38+ CM CD4 T cells to express IL-2 after CD28 costimulation was diminished in HIV infection.
All patients who were studied in this work had demonstrable viremia with a median plasma HIV RNA level of 14,200 copies per milliliter. Likewise, most all had sufficiently advanced disease to have abnormally low numbers of circulating CD4+ T cells with a median count of 391 cells per microliter. Whether persons with more advanced disease might demonstrate more profound impairments in function of CD38+ T cells remains to be seen, but it should be noted that the relationship between CD38 expression and disease progression risk has been noted in patient populations with a similar stage of disease as the patients studied herein.14
When cytokine induction was examined according to the concordant expression of CD154, in both patients and controls, CD38+ T cells more frequently failed to express CD154 when induced to express either IL-2 or IFN-γ, and this failure was particularly evident among patients’ cells. These observations are consistent with the overall failure of CD154 induction after TCR engagement that has been observed in the setting of HIV infection,35 and here we demonstrate that this is primarily a phenomenon seen among CD38+ T cells.
It is tempting to speculate that among CD38+ CM CD4+ T cells, T-cell receptor stimulation induces substantial inflammation (here reflected in interferon gamma expression) but may fail to induce sufficient IL-2 to support expansion of this population—a phenotype that may link the predictive value of CD38 expression7,13,15,36 to the importance of CM expansion failure37,38 to clinical outcomes in lentiviral infection. We cannot discern in these studies whether this defect reflects an effect of sustained HIV replication on T-cell function or whether this is a consequence of CD4 lymphocytopenia. Additional experiments in patient populations with discordant immunologic and virologic responses to antiretroviral therapy will help to address this.
Although APC function was not examined directly in the current in vitro studies, where cytokine induction was largely unimpaired in HIV infection, we propose that a failure of CD154 induction on peptide stimulated CD4+ T cells will fail to provide costimulatory signals to professional APCs in chronic HIV infection,35,39 thereby, contributing to the maturation and functional defects that have been described in these populations.40–42 We, therefore, suspect that a poorly coordinated interplay between activated CD38+ CD4+ T memory cells and the professional APCs with which they interact may contribute to the impaired anamnestic response that we and others have described in chronic HIV infection.43–50
It should be noted that only 11 patients were studied here, and this may have limited our ability to demonstrate a broader inhibitory effect of CD38 expression on cytokine expression. We think that this is not likely, however, because the intersubject variation in cytokine induction was small, the results according to CD38 expression were generally concordant in the 11 patients and in the 11 controls, and in most settings, IFN-γ expression tended to be higher in the CD38+ population than among the CD38− populations (Figure 2).
In summary, we could not find evidence that expression of the activation antigen CD38 is a mediator or a marker of impaired cytokine expression in CD4+ T cells in HIV infection. Although overall induction of cytokine expression by SEB stimulation was largely comparable in HIV infection and among controls, a disproportionally high frequency of cytokine-producing cells that failed to upregulate CD154 was observed in HIV infection. The failure to induce CD154 after TCR engagement that was seen among memory CD4+ T-cell populations in HIV infection also may contribute to the APC dysfunction and impaired anamnestic responses that characterize chronic HIV infection.
Acknowledgments
Supported by the Center for AIDS Research at Case Western Reserve University AI-36219 and by grants AI-68636 and AI-76174 from the National Institutes of Health.
Footnotes
Portions of these data were presented at Keystone Symposium HIV Immunobiology: From Infection to Immune Control, March 22–27, 2009, Keystone, CO.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions this article on the journal’s Web site (www.jaids.com).
References
- 1.Hunt PW. Role of immune activation in HIV pathogenesis. Curr HIV/AIDS Rep. 2007;4:42–47. doi: 10.1007/s11904-007-0007-8. [DOI] [PubMed] [Google Scholar]
- 2.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvestri G, Paiardini M, Pandrea I, et al. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri G, Feinberg MB. Turnover of lymphocytes and conceptual paradigms in HIV infection. J Clin Invest. 2003;112:821–824. doi: 10.1172/JCI19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Cumberland WG, Hultin LE, et al. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:332–340. doi: 10.1097/00042560-199808010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz JL, Czerniewski MA, Edinger M, et al. Multisite comparison of methods for the quantitation of the surface expression of CD38 on CD8(+) T lymphocytes. The ACTG Advanced Flow Cytometry Focus Group. Cytometry. 2000;42:174–179. [PubMed] [Google Scholar]
- 9.Giorgi JV, Liu Z, Hultin LE, et al. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 10.Giorgi JV, Ho HN, Hirji K, et al. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ J Infect Dis. 1994;170:775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Cumberland WG, Hultin LE, et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Plaeger S, Bass HZ, Nishanian P, et al. The prognostic significance in HIV infection of immune activation represented by cell surface antigen and plasma activation marker changes. Clin Immunol. 1999;90:238–246. doi: 10.1006/clim.1998.4646. [DOI] [PubMed] [Google Scholar]
- 13.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 14.Giorgi JV, Lyles RH, Matud JL, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr. 2002;29:346–355. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 16.Miedema F, Petit AJ, Terpstra FG, et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Invest. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieg SF, Harding CV, Lederman MM. HIV-1 infection impairs cell cycle progression of CD4(+) T cells without affecting early activation responses. J Clin Invest. 2001;108:757–764. doi: 10.1172/JCI12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCloskey TW, Haridas V, Pontrelli L, et al. Response to superantigen stimulation in peripheral blood mononuclear cells from children perinatally infected with human immunodeficiency virus and receiving highly active antiretroviral therapy. Clin Diagn Lab Immunol. 2004;11:957–962. doi: 10.1128/CDLI.11.5.957-962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieg SF, Bazdar DA, Harding CV, et al. Differential expression of interleukin-2 and gamma interferon in human immunodeficiency virus disease. J Virol. 2001;75:9983–9985. doi: 10.1128/JVI.75.20.9983-9985.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knox KS, Day RB, Kohli LM, et al. Functional impairment of CD4 T cells despite normalization of T cell number in HIV. Cell Immunol. 2006;242:46–51. doi: 10.1016/j.cellimm.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lascar RM, Lopes AR, Gilson RJ, et al. Effect of HIV infection and antiretroviral therapy on hepatitis B virus (HBV)-specific T cell responses in patients who have resolved HBV infection. J Infect Dis. 2005;191:1169–1179. doi: 10.1086/428502. [DOI] [PubMed] [Google Scholar]
- 22.Betts MR, Ambrozak DR, Douek DC, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez S, Price P, McKinnon EJ, et al. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol. 2006;120:163–170. doi: 10.1016/j.clim.2006.04.570. [DOI] [PubMed] [Google Scholar]
- 24.van Baarle D, Tsegaye A, Miedema F, et al. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005;97:19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Echaniz P, Arrizabalaga J, Iribarren JA, et al. CD8+CD38+ and CD8+DR+ peripheral blood lymphoid subsets of HIV-infected intravenous drug abusers correlate with CD4+ cell counts and proliferation to mitogens. Cell Immunol. 1993;150:72–80. doi: 10.1006/cimm.1993.1179. [DOI] [PubMed] [Google Scholar]
- 26.Badley AD, Parato K, Cameron DW, et al. Dynamic correlation of apoptosis and immune activation during treatment of HIV infection. Cell Death Differ. 1999;6:420–432. doi: 10.1038/sj.cdd.4400509. [DOI] [PubMed] [Google Scholar]
- 27.Choi YW, Kotzin B, Herron L, et al. Interaction of Staphylococcus aureus toxin “superantigens” with human T cells. Proc Natl Acad Sci U S A. 1989;86:8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–1117. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 29.Frentsch M, Arbach O, Kirchhoff D, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 30.Stubbe M, Vanderheyde N, Pircher H, et al. Characterization of a subset of antigen-specific human central memory CD4+ T lymphocytes producing effector cytokines. Eur J Immunol. 2008;38:273–282. doi: 10.1002/eji.200737611. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Appay V, Van Lier RA, Sallusto F, et al. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 33.Giorgi JV, Liu Z, Hultin LE, et al. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1993;6:904–912. [PubMed] [Google Scholar]
- 34.Liu Z, Cumberland WG, Hultin LE, et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Subauste CS, Subauste A, Wessendarp M. Role of CD40-dependent down-regulation of CD154 in impaired induction of CD154 in CD4(+) T cells from HIV-1-infected patients. J Immunol. 2007;178:1645–1653. doi: 10.4049/jimmunol.178.3.1645. [DOI] [PubMed] [Google Scholar]
- 36.Giorgi JV, Lyles RH, Matud JL, et al. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J Acquir Immune Defic Syndr. 2002;29:346–355. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 37.Sieg SF, Rodriguez B, Asaad R, et al. Peripheral S-phase T cells in HIV disease have a central memory phenotype and rarely have evidence of recent T cell receptor engagement. J Infect Dis. 2005;192:62–70. doi: 10.1086/430620. [DOI] [PubMed] [Google Scholar]
- 38.Okoye A, Meier-Schellersheim M, Brenchley JM, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–2185. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subauste CS, Wessendarp M, Smulian AG, et al. Role of CD40 ligand signaling in defective type 1 cytokine response in human immunodeficiency virus infection. J Infect Dis. 2001;183:1722–1731. doi: 10.1086/320734. [DOI] [PubMed] [Google Scholar]
- 40.Chougnet C. Role of CD40 ligand dysregulation in HIV-associated dysfunction of antigen-presenting cells. J Leukoc Biol. 2003;74:702–709. doi: 10.1189/jlb.0403171. [DOI] [PubMed] [Google Scholar]
- 41.Moir S, Ogwaro KM, Malaspina A, et al. Perturbations in B cell responsiveness to CD4+ T cell help in HIV-infected individuals. Proc Natl Acad Sci U S A. 2003;100:6057–6062. doi: 10.1073/pnas.0730819100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolthers KC, Otto SA, Lens SM, et al. Functional B cell abnormalities in HIV type 1 infection: role of CD40L and CD70. AIDS Res Hum Retroviruses. 1997;13:1023–1029. doi: 10.1089/aid.1997.13.1023. [DOI] [PubMed] [Google Scholar]
- 43.Elrefaei M, McElroy MD, Preas CP, et al. Central memory CD4+ T cell responses in chronic HIV infection are not restored by antiretroviral therapy. J Immunol. 2004;173:2184–2189. doi: 10.4049/jimmunol.173.3.2184. [DOI] [PubMed] [Google Scholar]
- 44.Lange CG, Lederman MM, Medvik K, et al. Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV-1 infection. AIDS. 2003;17:2015–2023. doi: 10.1097/00002030-200309260-00002. [DOI] [PubMed] [Google Scholar]
- 45.Lange CG, Xu Z, Patterson BK, et al. Proliferation responses to HIVp24 during antiretroviral therapy do not reflect improved immune phenotype or function. AIDS. 2004;18:605–613. doi: 10.1097/00002030-200403050-00004. [DOI] [PubMed] [Google Scholar]
- 46.Pahwa R, McCloskey TW, Aroniadis OC, et al. CD8+ T cells in HIV disease exhibit cytokine receptor perturbation and poor T cell receptor activation but are responsive to gamma-chain cytokine-driven proliferation. J Infect Dis. 2006;193:879–887. doi: 10.1086/500471. [DOI] [PubMed] [Google Scholar]
- 47.Sandberg JK, Fast NM, Jordan KA, et al. HIV-specific CD8+ T cell function in children with vertically acquired HIV-1 infection is critically influenced by age and the state of the CD4+ T cell compartment. J Immunol. 2003;170:4403–4410. doi: 10.4049/jimmunol.170.8.4403. [DOI] [PubMed] [Google Scholar]
- 48.Valdez H, Smith KY, Landay A, et al. Response to immunization with recall and neoantigens after prolonged administration of an HIV-1 protease inhibitor-containing regimen. ACTG 375 team. AIDS Clinical Trials Group. AIDS. 2000;14:11–21. doi: 10.1097/00002030-200001070-00002. [DOI] [PubMed] [Google Scholar]
- 49.Valdez H, Mitsuyasu R, Landay A, et al. Interleukin-2 Increases CD4+ lymphocyte numbers but does not enhance responses to immunization: results of A5046s. J Infect Dis. 2003;187:320–325. doi: 10.1086/346056. [DOI] [PubMed] [Google Scholar]
- 50.Kim HN, Harrington RD, Van Rompaey SE, et al. Independent clinical predictors of impaired response to hepatitis B vaccination in HIV-infected persons. Int J STD AIDS. 2008;19:600–604. doi: 10.1258/ijsa.2007.007197. [DOI] [PMC free article] [PubMed] [Google Scholar]