SUMMARY
Purpose
To correlate kindling-associated alterations of the neurotransmitter secretory machinery, glutamate release in the trisynaptic hippocampal excitatory pathway, and the behavioral evolution of kindling-induced epileptogenesis.
Method
Neurotransmitter release requires the fusion of vesicle and plasma membranes; it is initiated by formation of a stable, ternary complex (7SC) of SNARE [soluble N-ethylmaleimide sensitive factor (NSF) attachment protein receptor] proteins. Quantitative Western blotting was used to monitor levels of 7SC and SNARE regulators [NSF, SV2 (synaptic vesicle protein 2)] in hippocampal synaptosomes from amygdala-kindled animals. Hippocampal synaptic glutamate release was measured in vivo with a unique microelectrode array (MEA) that uses glutamate oxidase to catalyze the breakdown of glutamate into a reporter molecule.
Key Findings
Ipsilateral hippocampal accumulation of 7SC developed with onset of amygdalar kindling, but became permanent only in animals stimulated to at least Racine stage 3; the ratio peaked and did not increase with more than two consecutive stage 5 seizures. Chronic 7SC asymmetry was seen in entorhinal cortex and the hippocampal formation, particularly in dentate gyrus (DG) and CA1, but not in the other brain areas examined. There was a strong correlation between asymmetric 7SC accumulation and increased total hippocampal SV2. Following a 30-day latent period, amplitudes of spontaneous synaptic glutamate release were enhanced in ipsilateral DG and reduced in ipsilateral CA3 of kindled animals; increased volleys of synaptic glutamate activity were seen in ipsilateral CA1.
Significance
Amygdalar kindling is associated with chronic changes in the flow of glutamate signaling in the excitatory trisynaptic pathway and with early but permanent changes in the mechanics of vesicular release in ipsilateral hippocampal formation.
Keywords: Epileptogenesis, Exocytosis, Microelectrode array
It has been more than 40 years since kindling, a model of complex partial epilepsy and epileptogenesis, was first demonstrated. In 1969, Goddard et al. described this process of progressive and permanent intensification of epileptiform afterdischarges culminating in generalized seizures in response to repeated subconvulsive electrical stimulation (Goddard et al., 1969). The development of kindling in the rat is characterized by electrographic and behavioral stages: Stages 1–2 mimic human complex partial seizures and behaviors in stages 3–5 are consistent with evolution to secondarily generalized motor seizures (Racine, 1972). Once the fully kindled state is achieved, animals remain sensitized to stimulus-evoked seizures. If they experience a sufficient number of seizures, spontaneous generalized convulsions may continue throughout the lifespan of the animal. This permanently enhanced excitability is thought to result from changes both at the cellular level, through altered synaptic neurotransmission, and at a network level (Mody, 1993; McNamara, 1995). However, the precise underlying mechanism(s) remain elusive. In recent years we have evaluated altered presynaptic vesicular fusion machinery as a potential driver of the process (Matveeva et al., 2003, 2007, 2008).
Stimulus-induced release of neurotransmitter from synaptic vesicles is facilitated by docking to and fusion of the vesicle membrane with the presynaptic plasma membrane at a specialized region of the presynaptic bouton called the neuronal active zone. This membrane fusion is mediated by integral membrane proteins called SNAREs [soluble N-ethylmaleimide sensitive factor (NSF) attachment protein receptors], as well as a host of regulatory proteins that control how and when the SNARE proteins interact. Cognate SNAREs, from the synaptic vesicle (v-SNARE) and the plasma membrane (t-SNARE), form a stable, trans bilayer complex that promotes membrane fusion and neurotransmitter release (Sudhof, 2004; Brunger, 2005). The 7S SNARE complex (7SC) in neurons, a four-helical protein bundle composed of synaptobrevin/vesicle-associated membrane protein 2 (VAMP-2) from the synaptic vesicle and syntaxin 1 and synaptosomal-associated protein 25 (SNAP-25) from the neuronal active zone, is the minimal requirement for vesicle-plasma membrane fusion (Weber et al., 1998; Jahn & Scheller, 2006). Formation of stable 7SC is believed to represent one of the last steps before membrane fusion and is thus a hallmark of vesicles in a “ready-release” state. Our work has shown that the 7SC accumulate in hippocampal synapses of kindled rats and are maintained for as long as a year following cessation of kindling stimuli (Matveeva et al., 2003, 2007, 2008). This accumulation occurs ipsilateral to the stimulus and develops during kindling evoked by entorhinal cortical, amygdalar, and septal stimulation (Matveeva et al., 2007). The focus of the present study is to (1) further define the specificity of the asymmetric accumulation of 7SC, (2) better correlate these observations with behavioral measures of the kindling process, and (3) compare 7SC asymmetry to tonic and phasic glutamate release in subregions of the rat hippocampus using a new microelectrode array (MEA) technology.
Methods
Kindling
Fourteen-week-old male Sprague-Dawley rats (Harlan Laboratories, Inc., Indianapolis, IN, U.S.A.) had stimulating electrodes surgically implanted in the right amygdala [from bregma: AP −2.8; ML +4.8; DV −8.5; nose bar −3.3 (flat skull)] as previously described (Matveeva et al., 2007). Animals were tested for their afterdischarge (AD) threshold prior to kindling. An S88 dual channel stimulator (Grass Technologies, West Warwick, RI, U.S.A.) delivered a 1 s train of biphasic square wave pulses (1 ms pulse duration, 60 Hz) starting at an initial 100 μA until an AD was recorded on electroencephalography (EEG); this AD threshold was used for subsequent stimulation. About one third of animals exhibited no behavioral activity, and two-thirds experienced facial clonus (stage 1) or head nodding (stage 2) at their established AD threshold. Animals received either electrical stimulation (Slevin & Ferrara, 1985) or no electrical stimulation following sham operations. All animal procedures were approved by the Lexington Veterans Administration Medical Center Institutional Animal Care and Use Committee. Animals were housed individually in an enriched environment and maintained on a 12 h light/dark cycle with food and water available ad libitum.
Unless specifically stated, all animals were stimulated once daily, 5 days per week, until they experienced two consecutive Racine stage 5 seizures (tonic–clonic activity with loss of postural control/falling; (Racine, 1972)). For the time-course studies, 66 animals were euthanatized following a latent period of 1 or 30 days after reaching a specific behavioral stage. To determine if repeated stimulus-evoked seizures affect 7SC and SNARE regulators, 26 animals were kindled to two consecutive stage 5 seizures (AD = 227 ± 15 μA, 7.2 ± 0.4 stimuli); 12 were given an additional eight consecutive daily stimulus-evoked seizures and all animals plus 10 surgical controls then experienced a 6-month (195 ± 5 days) latent period followed by biochemical analyses. For the multisite biochemical studies, 36 animals (limbic sites = 10, hippocampal subfields = 26) were euthanitized following a 30-day latent period after experiencing two consecutive stage 5 seizures and compared to 18 (limbic sites = 4, hippocampal subfields = 14) surgical controls. Of 25 animals anesthetized for the MEA studies, 12 experienced two consecutive stage 5 seizures followed by a 30-day latent period and 13 were surgical controls; all were subsequently euthanatized for MEA placement verification. No animals experienced a spontaneous generalized seizure within at least 1 week of MEA or biochemical studies (except those in the time study euthanatized after a 1-day latency period) as verified by daily inspection of cages for evidence of excessive defecation, blood, or other sign of bodily injury and by direct visual and auditory scrutiny for up to 1 h daily. Because animals did not undergo continuous video and electroencephalographic surveillance during this time, it is possible a seizure may have gone undetected. However, we have previously demonstrated that a generalized seizure per se does not affect the 7SC ratio (Matveeva et al., 2003).
Analysis of 7S SNARE complexes (7SC)
Animals were euthanitized by decapitation, their brains were rapidly removed, and the hippocampi were excised on ice. Dissected hippocampi of some animals were placed directly on a chilled surface beneath a stereo-dissecting scope and then subdissected into three regions: enriched DG, CA3, and CA1 (Blalock et al., 2003; Kadish et al., 2009). Percoll gradient purified synaptosomes were prepared from individual hippocampi or hippocampal regions as described previously (Dunkley et al., 1986, 1988). Extracts were prepared by incubating equal quantities of synaptosomal protein in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer for 30 min at 37°C. Under these conditions, monomeric SNAREs are denatured but the thermally stable 7SC fail to disassemble (Hayashi et al., 1995). After separation by SDS-PAGE and transfer to polyvinylidene flouride (PVDF) membranes (Millipore Corp., Billerica, MA, U.S.A.), these complexes were probed by Western blotting using antibodies against the t-SNARE, syntaxin 1 (heme carrier protein-1; (Inoue & Akagawa, 1992)) as previously described (Matveeva et al., 2003). Western blot–based detection of 7SC was performed using alkaline phosphatase coupled secondary antibodies with Vistra ECF for visualization, and images were obtained using a Typhoon 9400 imager (GE Healthcare Bio-Sciences, Piscataway, NJ, U.S.A.). The raw data were the integrated fluorescence intensities for all pixels in a given immunodecorated protein band as determined by ImageQuant 5.2 software (GE Healthcare Bio-Sciences) in arbitrary units (AUs). We have observed that the fluorescence intensity ratio between actin and syntaxin 1 monomer is always the same. As a modification from earlier studies (Matveeva et al., 2003) and as a means of avoiding potential increased inaccuracy of introducing the additional step of actin immunostaining, to normalize for protein loading the fluorescence intensity of all 7SC bands was standardized to the syntaxin 1 monomer, rather than the actin band of the same lane.
Analysis of secretory machinery protein levels
Analysis of other secretory machinery proteins was performed by Western blotting using the methods above. NSF was detected with the 2E5 monoclonal antibody (Tagaya et al., 1993; Whiteheart et al., 1994), and α-SNARE was detected with the 4E4 monoclonal antibody (Gamma One Laboratory Inc., Lexington, KY, U.S.A.); mouse monoclonal antibody to actin was from Sigma Chemical Corp. (St. Louis, MO, U.S.A.). Mouse monoclonal antibody to SV2 (which detects both A and B isoforms) was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health & Human Development and maintained by the University of Iowa, Department of Biological Sciences, (Iowa City, IA, U.S.A.). Fluorescence intensities of the bands in each lane were normalized to the intensity of actin and cross-validated with the syntaxin 1 band in the same lane (see above). For the hippocampal subfield study, tissue from two animals was pooled to provide sufficient sample. Synaptosomes prepared from all 6 hippocampal subfields (right and left DG, CA3 and CA1) of the pooled tissue were loaded on a single gel.
Surgical implantation of the microelectrode array
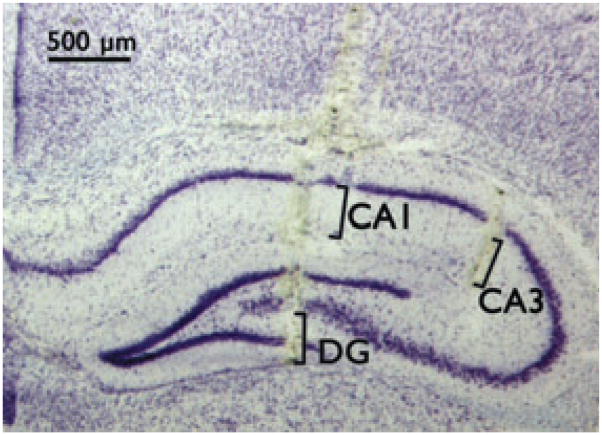
A craniotomy was performed to provide access for recordings, using the coordinates of Paxinos & Watson (2005). To confirm correct placement, an MEA with an attached pipette was used to locally apply green waterproof drawing ink, brains were collected from euthanitized animals, fresh frozen in crushed dry ice, and kept at −80°C until cryostat sectioning (Fig. 3).
Figure 3.
Cresyl violet–stained 20 μm section of hippocampus shows location of MEA tracks in DG, CA3, and CA1. Brackets indicate specific recording site locations on individual electrodes. Coordinates for MEA tip (anterior/posterior, medial/lateral, dorsal/ventral): DG = −2.3, ±1.0, −2.1; CA3 = −2.3, ±2.75, −1.4; CA1 = −2.3, ±1.5, −2.14 (Paxinos & Watson, 2005).
Epilepsia © ILAE
MEA preparation
To achieve glutamate measures, glutamate-oxidase (Glu-OX) was utilized to catalyze the breakdown of glutamate into a reporter molecule, hydrogen peroxide, which can be oxidized by the MEA. The MEA consists of four platinum recording sites, 15 × 333 μm, arranged in dual pairs. This allows one to employ the self-referencing technique for more selective glutamate measures as described previously (Burmeister & Gerhardt, 2001; Day et al., 2006). One pair of recording sites was configured for glutamate measures, whereas the other pair was configured to measure any background current that can be subtracted out. A 10 μl solution of 1% bovine serum albumin (BSA), 0.125% glutaraldehyde, and 1% GluOX was prepared. Using a dissecting microscope, a microsyringe was used to manually apply a small drop (~0.1 μl) of the enzyme solution onto the lower pair of platinum recording sites. The same procedure was used to coat the top pair of platinum recording sites with a solution containing 1% BSA and 0.125% glutaraldehyde. After coating, the MEAs were cured for at least 48 h in a low-humidity environment. A size exclusion layer of 1,3-phenylenediamine (mPD) was electroplated to the recording sites to increase the selectivity for glutamate. The size exclusion layer allows passage of small molecules such as hydrogen peroxide, but prevents the passage of large molecules such as ascorbic acid and dopamine from reaching the platinum recording sites. A 5 mM mPD solution was prepared in 100 ml of nitrogen bubbled 50 mM phosphate-buffered saline (PBS). The MEA was connected to the FAST-16 MKII system (Fast Analytical Sensor Technology Mark II, Quanteon; LLC, Lexington, KY, U.S.A.), and the tip of the MEA was placed in the mPD solution. The electroplating tool applied a potential as a triangular wave with an offset of −0.5 V, peak to peak amplitude of ±0.25 V at a frequency of 0.05 Hz for 20 min to electroplate the mPD onto the platinum recording sites.
A pre-pulled single-barrel micropipette [1 mm external diameter, 0.58 mm internal diameter (i.d.) glass; A-M Systems Inc., Everett, WA, U.S.A.) was attached to each ceramic microelectrode with Sticky Wax (Kerr Lab Corporation, Orange, CA, U.S.A.). This allows for intracranial application of an isotonic KCl solution (70 mM KCl, 79 mM NaCl, 2.5 mM CaCl2, pH 7.4) to study stimulus-evoked glutamate release. The tips of the micropipettes were typically pulled to an i.d. of 10 μm, and positioned 70 ± 20 μm away from the microelectrode surface, centered in the 100 μm space between the dorsal and ventral platinum recording pairs.
In vivo anesthetized recording
Rats were anesthetized with 2% isoflurane and placed in a stereotaxic frame, and body temperature was maintained at 37°C with a water pad connected to a recirculating water bath (Gaymar Ind., Orchard Park, NY, U.S.A.). A craniotomy was performed and the overlying dura was removed to provide access for recordings in the DG and hippocampal areas CA3 and CA1 (see Fig. 3). A miniature Ag/AgCl reference was implanted into a site that was remote from the recording areas. For local application of isotonic high-KCl, a 1 ml tuberculin syringe with a pulled needle was used to fill the attached micropipette; solutions were filtered using a sterile filter (0.20 μm). The micropipette was attached with tubing to a Picospritzer III (Parker Hannifin Corp., Lexington, KY, U.S.A.) to control ejection volume, with the use of a stereomicroscope fitted with a reticule to monitor volume displacement (Friedemann & Gerhardt, 1992).
Data analyses
The ipsilateral/contralateral 7SC band intensity ratios and quantified secretory machinery proteins determined from hippocampi of kindled versus surgical controls were analyzed by analysis of variance (ANOVA) and post hoc t-tests using Fisher’s protected least significant differences procedure, as more fully described previously (Matveeva et al., 2008). For the hippocampal subregion analysis of synaptosomal proteins, levels were compared among the three regions using an ANOVA for a randomized block design with gel serving as the block, since protein bands from all three regions appeared on each gel and there was considerable variability among the eight gels. Post hoc comparison of means depended on t-tests derived from this design. Statistical significance was set at 0.05.
Amperometric data and event markers were analyzed using custom exported Excel-based software (Quanteon, LLC, Nicholasville, KY, U.S.A.), to determine the parameters: resting glutamate levels, maximum amplitudes of phasic (KCl-evoked or spontaneous transients) glutamate, peak area (KCl-evoked release), exponential rate of signal decay (k−1), and numbers of glutamate transients per 5 min interval. For tonic levels, a 10 s average was taken after the electrode reached a stable baseline for at least 20 min. The background current from the sentinel site was subtracted from the current of the glutamate-oxidase site for a more selective glutamate measure. We have performed this type of analysis previously as more fully described in Stephens et al. (2011).
Results
The kindling-associated asymmetry of 7S SNARE complexes in hippocampal synaptosomes becomes permanent coincident with Racine stage 3
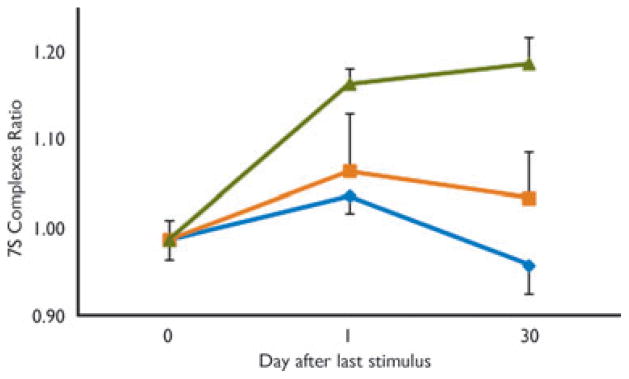
A time course study using seven surgical controls (day 0, Fig. 1) and 59 stimulated animals was undertaken to determine at what stage during amygdalar kindling 7SC asymmetric accumulation becomes persistent, independent of further stimulation. Animals were stimulated at their AD threshold (228 ± 19 μA) until reaching a specific Racine behavioral stage. They received no further stimuli and were euthanatized after 1 or 30 days for measurement of hippocampal synaptosomal 7SC. For 7SC asymmetry to persist, it appears animals must be stimulated past Racine behavioral stage 3 (Fig. 1).
Figure 1.
7SC asymmetry persists only if animals are kindled to ≥stage 4. Animals were amygdala-kindled to a specific behavioral stage and then left untreated for 1 or 30 days at which time they were euthanatized, synaptosomes were prepared, and 7SC measurements were made as described in Methods. Animals kindled to early (
 = 1 + 2) and late (
= 1 + 2) and late (
 = 4 + 5) stages were grouped; Stage 3 =
= 4 + 5) stages were grouped; Stage 3 =
 . Each point represents an average of at least nine animals (mean ± SEM).
. Each point represents an average of at least nine animals (mean ± SEM).
Epilepsia © ILAE
The kindling-associated asymmetry of 7S SNARE complexes in hippocampal synaptosomes does not increase with repeated stimulus-evoked seizures
The 7SC ratio observed in hippocampal tissue of animals analyzed 6 months after experiencing 10 consecutive stage 5 seizures was no different from that of animals experiencing two consecutive stage 5 seizures, but the 7SC ratio of both was significantly greater than surgical controls: control ratio = 0.98 ± 0.02 (n = 10), two stage 5 seizures = 1.09 ± 0.02 (n = 14), 10 stage 5 seizures = 1.10 ± 0.04 (n = 12); F = 4.68, p = 0.02. These data coupled with the time-course study indicate that development and maintenance of 7SC asymmetry is associated with evolution through behavioral stages and thereby only indirectly with number of stimulus-evoked seizures.
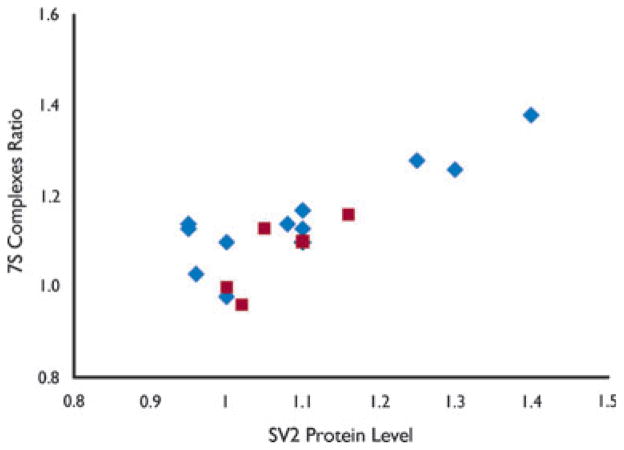
Six months after receiving no further stimuli, animals experiencing 10 consecutive stage 5 seizures showed a total hippocampal elevation of SV2 that correlated with the increase of 7SC (Pearson’s correlation coefficient r = 0.87); the elevation of SV2 (1.14 ± 0.03, n = 12) was no different than that measured in animals 6 months after experiencing two consecutive stage 5 seizures (1.09 ± 0.03, n = 7), but greater than in surgical controls (1.00 ± 0.04, n = 4; F = 4.22, p = 0.03). There was a strong correlation between asymmetric 7SC accumulation and increased total hippocampal SV2 at 6 months in all kindled animals in which it was measured (Fig. 2). NSF was nonsignificantly diminished compared to nonkindled controls (data not shown).
Figure 2.
Rise in total SV2 protein levels correlates with increasing 7SC asymmetry in kindled hippocampus. The 7SC ratio and SV2 protein levels were measured in hippocampal synaptosomes prepared from animals following a 30-day latent period after receiving amygdalar stimulation culminating in either 2 (n = 5,
 ) or 10 (n = 12,
) or 10 (n = 12,
 ) consecutive stage 5 seizures. Pearson’s correlation coefficient r = 0.833.
) consecutive stage 5 seizures. Pearson’s correlation coefficient r = 0.833.
Epilepsia © ILAE
The asymmetry of 7S SNARE complexes occurs in some but not all nonhippocampal limbic sites and hippocampal subfields
Olfactory bulb, frontal cortex, and entorhinal cortex were analyzed in amygdala-kindled (n = 10, AD = 320 ± 47 μA, 6.6 ± 0.4 stimuli) animals following a 30-day latent period subsequent to experiencing two consecutive stage 5 seizures and compared to four controls. There was no 7SC asymmetry in olfactory bulbs or frontal cortexes of kindled animals. Both hippocampus and entorhinal cortex demonstrated a persistent ipsilateral increase of 7SC in kindled animals (Table 1). To determine whether the hippocampal 7SC asymmetry associated with kindling was confined to a subregion or diffusely distributed throughout the hippocampus, animals were kindled (n = 16, AD = 267 ± 28 μA, 6.5 ± 0.6 stimuli) to two consecutive stage 5 seizures and then experienced a 30-day latent period, after which hippocampi were removed, dissected, and pooled as described in Methods. A significant difference (F = 3.40, p = 0.02) in the ipsilateral/contralateral ratio of 7SC was observed between the groups: the ratio was increased in kindled DG (p = 0.01, post hoc Student’s t-test) and CA1 (p = 0.03), but not CA3 (p = 0.9), compared to controls. Among the subregions in the kindled animals, the DG (p = 0.02) and CA1 (p = 0.03) 7SC ratios were equally elevated over CA3 (Table 2).
Table 1.
7S complex asymmetry in limbic areas after a 30-day latent period following amygdalar kindling
| 7S SNARE complex ratios
|
||||
|---|---|---|---|---|
| Olfactory bulb | Entorhinal cortex | Frontal cortex | Hippocampus | |
| Kindled | 1.00 ± 0.01 | 1.12 ± 0.03a | 1.00 ± 0.03 | 1.14 ± 0.02a |
| Mean ± SEM (10) | ||||
| Control | 0.98 ± 0.03 | 0.96 ± 0.03 | 0.99 ± 0.02 | 1.00 ± 0.01 |
| Mean ± SEM (4) | ||||
The ipsilateral/contralateral ratio of 7S complex in the rat hippocampus expressed as Mean ± SEM with sample size (n) indicated. Animals were euthanitized after a 30-day latent period following two Racine stage 5 seizures occurring on two consecutive days. ANOVA with post hoc t-test:
p < 0.001 kindled versus control for region indicated.
Table 2.
7S Complex asymmetry and SV2 in hippocampal subfields after a 30-day latent period following amygdalar kindling
| Region | 7S SNARE complex ratiosa |
SV2b |
||
|---|---|---|---|---|
| Kindled Mean ± SEM (8) |
Control Mean ± SEM (3) |
Kindled Mean ± SEM (13) |
Control Mean ± SEM (7) |
|
| DG | 1.15 ± 0.04c | 1.00 ± 0.02 | 1.77 ± 0.08 | 1.00 ± 0.01 |
| CA3 | 1.00 ± 0.04 | 1.00 ± 0.03 | 1.81 ± 0.08 | 1.00 ± 0.01 |
| CA1 | 1.15 ± 0.05c | 0.99 ± 0.03 | 2.10 ± 0.08d | 1.00 ± 0.01 |
Ratio = [7SC ipsilateral/syntaxin 1]/[7SC contralateral/syntaxin 1], where unity is expected.
Total SV2 protein = [(contralateral/syntaxin 1 + ipsilateral/syntaxin 1)/2]/(mean contralateral control/syntaxin 1)]. If no changes in protein level occur, then the ratio should be unity.
7SC Ratio: Kindled DG = Kindled CA1 ≠ Kindled CA3 = Control DG, CA3, CA1.
Total SV2 protein: kindled CA1 > CA3 = DG. Kindled DG/CA3/CA1 > control DG/CA3/CA1 (see Results section for discussion of analysis).
Fluorescence intensity of the protein band of interest was normalized to the intensity of monomeric syntaxin 1 band in the same lane, hence quantities have no units. Numbers in parentheses refer to replicates of synaptosomes prepared from two (=2n) animals.
There were no differences in the quantity of synaptosomal syntaxin and NSF, either among hippocampal subregions, or between kindled and control animals, or between right and left sides. Total SV2 protein (left + right homologous regions) among subregions between kindled (n = 26, AD = 276 ± 30 μA, 6.8 ± 0.5 stimuli) and control animals differed (F = 2.83, p = 0.02). As illustrated in Table 2, post hoc comparisons of means shows that total SV2 protein was greater in kindled than control DG (p = 0.02), CA3 (p = 0.02), and CA1 (p = 0.01). In kindled animals, total SV2 protein was significantly greater in CA1 than DG (p = 0.01) and CA3 (p = 0.02); there was no difference between DG and CA3 total SV2 protein (p = 0.78). There was no difference in total SV2 protein (right + left) among hippocampal subfields of controls (Table 2). There was no difference in SV2 protein levels either between ipsilateral and contralateral homologous hippocampal subfields of kindled animals or between right and left homologous hippocampal subfields of controls (data not shown).
Chronic amygdalar kindling is associated with changes in spontaneous glutamate release in the hippocampal trisynaptic pathway
Studies were carried out with the MEA glutamate recording technology to directly investigate tonic (basal) and phasic (potassium-evoked and rapid, spontaneous transients) glutamate release in the DG, CA3, and CA1 in isoflurane anesthetized rats. Animals had surgical implantation of kindling electrodes and then either received no electrical stimulation (n = 13 controls) or were kindled to two consecutive stage 5 seizures (n = 12, AD = 200 ± 19 μA, 6.9 ± 0.3 stimuli). After a 30-day latent period, the kindling electrodes were removed and MEAs were implanted (Fig. 3). No differences in tonic glutamate signaling were observed among the DG, CA3, or CA1 regions in either hippocampus of kindled or control animals (Fig. 4A, Table 3). Additional studies were performed using locally applied potassium to produce a maximal phasic change in glutamate release (Stephens et al., 2011). Much like the tonic studies, K+-evoked glutamate release was not significantly different between kindled and control animals in any subregions of the bilateral hippocampi (Table 3).
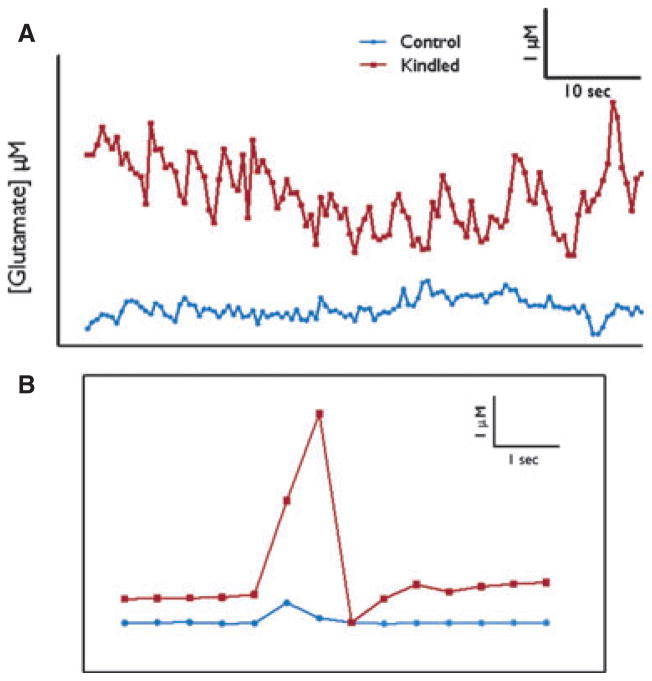
Figure 4.
Real-time measures of extracellular glutamate can be recorded using MEAs. (A) Typical traces measuring resting glutamate levels in kindled (
 ) and control (
) and control (
 ) animals. (B) Spontaneous transient glutamate peaks in DG of kindled (
) animals. (B) Spontaneous transient glutamate peaks in DG of kindled (
 ) and control (
) and control (
 ) rats with amplitude and time indicated. Concentration of glutamate is calculated by multiplying the recorded current by the slope obtained during calibration of the electrode. Calibrations are performed as described previously (Burmeister & Gerhardt, 2001).
) rats with amplitude and time indicated. Concentration of glutamate is calculated by multiplying the recorded current by the slope obtained during calibration of the electrode. Calibrations are performed as described previously (Burmeister & Gerhardt, 2001).
Epilepsia © ILAE
Table 3.
Electrochemistry comparisons between kindled animals and surgical controls measured in hippocampal subfields after a 30-day latent period following amygdalar kindling
| Measure | Region | Ipsilateral
|
Contralateral
|
||
|---|---|---|---|---|---|
| Kindled | Control | Kindled | Control | ||
| Tonic glutamate level | DG | 5.77 ± 1.80 (12) | 2.26 ± 0.60 (11) | 1.84 ± 0.27 (8) | 2.81 ± 0.91 (5) |
| CA3 | 3.48 ± 0.94 (10) | 2.24 ± 0.55 (6) | 1.57 ± 0.41 (4) | 1.87 ± 0.72 (5) | |
| CA1 | 3.20 ± 0.94 (9) | 3.07 ± 1.18 (6) | 3.20 ± 0.94 (9) | 2.87 ± 1.24 (6) | |
| K+-evoked peak amplitude | DG | 5.40 ± 1.85 (11) | 6.22 ± 0.94 (11) | 4.70 ± 1.10 (8) | 4.11 ± 0.97 (8) |
| CA3 | 5.09 ± 1.13 (10) | 6.60 ± 1.73 (10) | 4.38 ± 1.58 (6) | 8.51 ± 3.44 (6) | |
| CA1 | 5.97 ± 1.38 (9) | 9.91 ± 3.37 (9) | 5.67 ± 1.63 (7) | 4.72 ± 1.36 (6) | |
| K+-evoked peak area | DG | 22.11 ± 9.62 (12) | 28.22 ± 10.62 (11) | 25.21 ± 7.25 (7) | 11.93 ± 5.71 (5) |
| CA3 | 18.20 ± 4.01 (10) | 61.35 ± 21.09 (10) | 29.26 ± 9.08 (6) | 10.36 ± 2.80 (5) | |
| CA1 | 25.75 ± 4.30 (9) | 30.69 ± 8.57 (7) | 16.93 ± 6.58 (7) | 21.18 ± 5.89 (6) | |
Microelectrode arrays were used to detect resting (tonic) glutamate levels and (phasic) K+-evoked release of glutamate in hippocampal subfields of kindled and control animals as described in Methods. Quantities expressed are micromolar; (n) = number of animals. There were no differences in a region across hemispheres and between kindled and control.
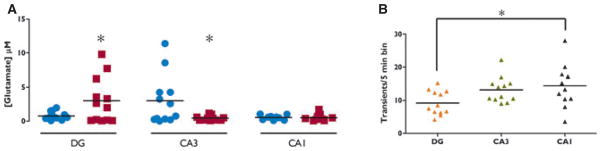
Spontaneous transient glutamate peaks that we term “transients of glutamate activity” (Hascup et al., 2011) were recorded during the tonic recording sessions (see Fig. 4B). In the ipsilateral DG of kindled animals, the amplitude of these transients was increased by nearly 400% as compared to the DG of controls (3.01 μM ± 0.96, n = 12 vs. 0.77 μM ± 0.16, n = 13; p = 0.02; Fig. 5A). In contrast, the amplitude of bursts was significantly decreased in the CA3 ipsilateral to the kindling electrode in kindled animals (0.46 μM ± 0.11; n = 12) versus controls (3.02 μM ± 1.06; n = 12; p = 0.03; Fig. 5A). No significant differences were seen in the amplitudes of the glutamate transients from data collected in ipsilateral CA1. In addition, there were no differences in the amplitudes of the glutamate transients in the contralateral DG, CA3, or CA1 in the kindled rats as compared to data collected from the contralateral side in controls (data not shown). Interestingly, the exponential rate of glutamate signal decay (“down slope” or k−1) is a good measure of reuptake activity by glutamate transporters (Nickell et al., 2007). There was no difference in this measure of the KCl-evoked glutamate potentials between ipsilateral DG (0.26 ± 0.06, n = 12 vs. 0.31 ± 0.09, n = 11) or CA3 (0.24 ± 0.05, n = 10 vs. 0.15 ± 0.05, n = 11) of kindled and control animals, indicating reuptake of glutamate into glia and postsynaptic membranes was likely unaffected and not an explanation for the amplitude differences observed (Danbolt, 2001).
Figure 5.
Spontaneous glutamate release in ipsilateral rat hippocampus 30 days after stage 5 kindling is altered. (A) The amplitude of glutamate transients was significantly higher (p = 0.026, Student’s t-test) in kindled DG (3.01 μM ± 0.96) compared to control DG (0.77 μM ± 0.16). The amplitude of transients was significantly decreased (p = 0.032, t-test) in kindled CA3 (0.46 μM ± 0.11) compared to control CA3 (3.02 μM ± 1.06). No difference was seen in CA1 (
 = kindled,
= kindled,
 = control). (B) Measured as number of transient peaks/5 min interval, MEAs recorded a significantly greater number of events in CA1 compared to DG ipsilateral to the kindling stimulation site (one-way ANOVA with Bonferroni post hoc comparisons, p = 0.03). No such differences were detected in contralateral hippocampus of kindled animals or in either hippocampus of surgical controls (
= control). (B) Measured as number of transient peaks/5 min interval, MEAs recorded a significantly greater number of events in CA1 compared to DG ipsilateral to the kindling stimulation site (one-way ANOVA with Bonferroni post hoc comparisons, p = 0.03). No such differences were detected in contralateral hippocampus of kindled animals or in either hippocampus of surgical controls (
 = DG,
= DG,
 = CA3, ▲= CA1).
= CA3, ▲= CA1).
Epilepsia © ILAE
The number of glutamate transients per 5 min was increased in CA1 versus the DG in ipsilateral kindled hippocampus [F = 3.90, p < 0.05; post hoc comparison using Bonferroni multiple comparison test: mean average transients DG = 9.21 ± 1.06 (n = 12), CA1 = 14.42 ± 1.83 (n = 12); Fig. 5B]. No difference in the number of glutamate transients per 5 min was observed across regions in the bilateral hippocampi of controls or in the hippocampus of kindled animals contralateral to the stimulated amygdala. Taken together, the data support the conclusion that the stability and/or flow of glutamate signaling from the perforant path though the hippocampal trisynaptic glutamatergic pathway is affected in kindled rats compared to nonkindled surgical controls.
Discussion
From its discovery, kindling has been understood to model focal onset epilepsy with secondary generalization, epileptogenesis, and central nervous system plasticity (McNamara et al., 1980; Bertram, 2007). Although it is believed to be a behavioral expression of changes occurring at the individual neuronal and network levels, our ken of the phenomenon has not extended to its molecular underpinnings. The behavioral progression during the kindling process suggests that increasing numbers of neuronal circuits are recruited and that intrinsic mechanisms to prevent progression to convulsions are ineffective, making it a good model for studying the biology of epileptogenesis. Transient changes of diverse molecular systems have been demonstrated but no minimal yet requisite specific drivers of the process have been identified. We have previously reported that 7SC, the minimal molecular component needed for vesicle fusion to the synaptic membrane, is increased in the ipsilateral hippocampus after a 30-day latent period following induction to a minimally kindled state of two Racine stage 5 seizures (Matveeva et al., 2003). Past studies have shown that 7SC accumulation is not due to electrode implantation or electroshock-induced seizure, occurs in ipsilateral hippocampus regardless of stimulation in left or right hemisphere, and happens only when a kindling-inducing frequency is used for stimulation, indicating that it is not merely an effect of current or an applied potential difference (Matveeva et al., 2007, 2008). 7SC accumulation correlates with behavioral stage (Racine staging) and evolves whether stimulations occurred in amygdalar, entorhinal, or septal regions; accumulation is long-lasting, perhaps permanent, persisting out to ≥12 months poststimulation (Matveeva et al., 2008). The present study showed that 7SC accumulation not only does not occur outside the limbic system (Matveeva et al., 2003), but does not occur diffusely within the limbic system. Robust changes were seen in entorhinal cortex and hippocampus, where maintenance required behavioral evolution to at least Racine stage 4. As observed with electroshock seizures, additional kindling stimulus-evoked seizures, presumed insufficient to induce sprouting (see below), do not further alter 7SC or SNARE regulators once an animal has experienced a Racine stage 5 seizure. These data underscore our original contention that 7SC accumulation is a product of some permanent neural change that occurs early during kindling epileptogenesis.
It is possible that this molecular change is related to the histologic phenomenon of sprouting, in which mossy fiber axons of DG granule cells extend recurrent collateral fibers to form new synapses on neurons in DG and CA3. Sprouting has been reported in animal models of epilepsy and in patients with temporal lobe epilepsy. Because hippocampal tissues were used for biochemical determinations, histologic evaluation for cell loss and mossy fiber sprouting could not be performed. However, Osawa et al. (2001) have demonstrated that in the case of amygdalar stimulation, kindling develops without evidence of mossy fiber sprouting until after repeated kindled convulsions and following death of DG granule cells. In their study, only animals that had experienced >29 AD-producing stimulations and >10 stage 5 convulsions showed histopathologic changes, including mossy fiber sprouting and granule cell loss, compared to surgical and electrical controls. In contrast, amygdala-kindled animals in the present study experienced at most 15 AD-producing stimuli and two stage 5 convulsions. Furthermore, previous studies of amygdalar and entorhinal kindling indicated that 7SC begin to accumulate as early as behavioral stage 1 (Matveeva et al., 2003). Finally, van Vliet et al. (2009) have shown decreased expression of hippocampal SV2A in animals following status epilepticus, due at least in part to neuronal cell loss; in the present study, levels of SV2A/B were increased. Therefore, it appears unlikely that the animals in this study had either granule cell loss or mossy fiber sprouting, and the alterations in 7SC are not associated with or a consequence of sprouting or neuronal loss.
Ipsilateral accumulation of 7SC begins early in the kindling process and continues to increase, reaching a near maximum by onset of the first-experienced stage 5 seizure (Matveeva et al., 2003, 2008). Therefore, this unilateral change begins when electrographic and behavioral changes are also still unilateral. What is remarkable is that this specific presynaptic molecular change remains unilateral, despite further progression and generalization of electrical and behavioral phenomena. This does not appear to be the case for the several SNARE effectors that have been examined, protein levels of which appear to change bilaterally in hippocampus. Tomosyn, a postulated inhibitor of 7SC formation (McEwen et al., 2006), transiently rises early in the kindling process, and NSF gradually decreases over months after completion (Matveeva et al., 2008), perhaps again suggested by its nonsignificant decrease measured in the current study at 1 month after cessation of kindling stimuli. Bilateral hippocampal SV2 protein levels increase coincident with asymmetric 7SC accumulation (Matveeva et al., 2008); this study indicates a strong linear correlation of the two measures. SV2A knockout mice develop epilepsy (Crowder et al., 1999; Janz et al., 1999) and reduced action potential-dependent γ-aminobutyric acid (GABA)ergic neurotransmission in CA3 (Crowder et al., 1999), and cultured hippocampal pyramidal neurons from SV2A/B knockouts show a sustained increase in Ca2+-dependent excitatory synaptic neurotransmission (Janz et al., 1999). At the very least, SV2 regulates the expression and trafficking of the vesicle-associated calcium sensor protein synaptotagmin; studies with SV2A mutants indicate that this activity can be dissociated from its action on the maintenance of normal synaptic depression (Nowack et al., 2010). These and our observations are consistent with an emerging picture of multiple interweaving roles for SV2 in the regulation of neurotransmitter release and in maintaining neuronal network stability.
At the molecular level, the accumulation of 7SC is indicative of either increased synaptic vesicle–plasma membrane fusion or increased SNARE complex formation just prior to fusion. Either scenario could be associated with increased or aberrant neurotransmitter secretion. If this process were predominating at excitatory synapses, one would expect it to be proconvulsant and a driver of epileptogenesis; if it occurred primarily at inhibitory synapses, then it would be anticonvulsant and a reactive response. Increased excitatory L-glutamate neurotransmitter release from presynaptic nerve terminals has been demonstrated in the hippocampus of amygdalar (Minamoto et al., 1992) and entorhinal cortical (Geula et al., 1988; Jarvie et al., 1990) kindled rats. Similar results were seen in rat hippocampal slices 1 month after Schaffer collateral–commissural kindling (Kamphuis et al., 1991) and in cerebral cortical synaptosomes 2 weeks after septal kindling (Yamagata et al., 1995). Extracellular hippocampal glutamate overflow induced by repeated short-term high potassium stimuli and measured by in vivo microdialysis has been correlated with kindling phenomena (Ueda et al., 2000). These prior reports suggest that the high frequency network discharges passing through the hippocampus and associated with kindled epilepsy involve changes in the regulation of elements of the presynaptic secretory machinery at excitatory synapses, which are extensively glutamatergic in the hippocampal trisynaptic pathway.
The present study utilized a novel MEA technology that is capable of recording both tonic (basal) and phasic (KCl-evoked and spontaneous transients) glutamate release at 1 Hz and with enhanced spatial resolution to measure glutamate signaling in the DG, CA3, and CA1 regions of the hippocampus of control and kindled rats (Stephens et al., 2011). Consistent with the asymmetric accumulation of 7SC, we saw no evidence of changes in glutamate release in the contralateral hippocampus. In addition, both resting and KCl-evoked glutamate release were not seen to be significantly affected in the DG, CA3, or CA1 of kindled rats as compared to controls. These data would suggest that neurons in these regions of kindled rodents behave normally when stimulated by externally mediated depolarization. However, significant changes were seen in the amplitude of spontaneous glutamate transients in the ipsilateral DG and CA3 of amygdala-kindled rats, thus supporting a change in the phasic properties of glutamate signaling in the trisynaptic pathway following kindling. In addition, we observed an increase in the number of spontaneous glutamate transients in the CA1 as compared to the DG in the ipsilateral hippocampus of kindled rats. Therefore, our data demonstrate that kindling produces ipsilateral changes in the spontaneous discharge of glutamate in all three subregions of the trisynaptic pathway following kindling. However, among the DG, CA3, and CA1 subregions the changes are distinctly different.
Of particular note is the complex nature of the change in glutamate signaling that occurs in the DG, CA3, and CA1 of the rat hippocampus following kindling. First, the DG has significantly enhanced levels of synaptic glutamate overflow, whereas the CA3 has a significant reduction compared to controls; CA1 in this regard appears to be unchanged. However, upon closer examination, the CA1 exhibits an increased number of spontaneous transients per 5 min that is significantly different from DG in the kindled rats. Interestingly, dentate seizure discharges in control animals are shorter as compared with those recorded from epileptic rats (Stringer & Lothman, 1989), lending support to the hypothesis that the DG may amplify seizures in chronic, animal models of epileptogenesis such as kindling. The DG may also gate the propagation of epileptiform discharge to the hippocampus (Collins et al., 1983; Walther et al., 1986; Jones & Lambert, 1990). Therefore, we observed that kindling produces distinct differences in glutamate signaling that were greatest in the DG.
These are the first studies to investigate rapid, spontaneous glutamate release in subregions of the rat hippocampus following kindling. We have previously seen changes in the amplitude, peak area, and number of glutamate transients in the CA1 region of the rat hippocampus following 4-amino-pyridine (4-AP) –induced seizures (Stephens et al., 2011) and changes in glutamate transients in the prefrontal cortex of an animal model of depression (Hascup et al., 2011). We opine that such signals likely represent synchronous firing of glutamate projections that result in transient glutamate overflow that can now be measured by the MEA technology. Such transients are inhibited by tetrodotoxin, a sodium channel blocker, in the CA1 regions during 4-AP–induced seizures (Stephens et al., 2011). Additional electrophysiologic and pharmacologic studies are needed to better understand these neurochemical events. However, they are consistent with the biochemical findings of alterations in 7S SNARE complexes and SNARE regulators, particularly SV2, seen in the kindled rats that would affect glutamate neurotransmission in the trisynaptic pathway. Of particular relevance, total SV2 protein levels (Table 2) and numbers of spontaneous glutamate transients (Fig. 5) both appear to increase with passage from DG through CA3 to CA1. The rise in total SV2 clearly appears to be yoked to increasing 7SC asymmetry (Fig. 2). It is possible that both the rise in SV2 and the progression of increasing glutamate transients through the trisynaptic circuit are driven by the asymmetric and regional changes in 7SC through multiple, daedal but as yet undetermined mechanisms.
SNARE regulators such as SV2, which influence SNARE complex assembly, are thought to affect the dynamics of the “ready release” pool (Ashery et al., 2000; Yizhar et al., 2004). Equally important is the disassembly of SNARE complexes for recycling, which is controlled by other SNARE regulators including N-ethylmaleimide sensitive Factor (NSF) and α-soluble NSF attachment protein (α-SNAP) (Whiteheart et al., 2001). Several of these assembly/disassembly regulators appear to be responsive to different factors (e.g., calcium, diacylglycerol, phosphorylation), thus accounting for the dynamic nature of neurotransmission. As kindling epileptogenesis evolves, synaptosomal levels of certain SNARE regulators (NSF, SV2, and tomosyn) are altered; concomitant treatment with the antiepileptic drug levetiracetam reverses kindling-induced 7SC accumulation and increased SV2 (Matveeva et al., 2008). The effects of levetiracetam on epileptogenesis and on 7SC accumulation suggest that the neuronal secretory machinery could be a viable therapeutic target. Consistently, in a separate set of studies using a VAMP-2 deficient mouse model (Matveeva et al., 2011), we have shown that alteration of this v-SNARE has a dramatic inhibitory effect on the course of kindling compared to wild-type control animals, reducing both resting and stimulated glutamate release as well as the levels of asymmetric SNARE complex accumulation in the fully kindled state. Our observations in the present and previous studies using a well-established kindling paradigm make a compelling case for the intimate and essential involvement of elements of the secretory pathway in the processes that drive the epileptogenic response in hippocampus. It is our contention that the asymmetric increase of 7S SNARE complexes is an important early component of kindling epileptogenesis that remains confined but is a permanent driver of epileptic behavior.
Acknowledgments
We acknowledge the technical assistance of Ramona Alcala, are indebted to Dr. Eric M. Blalock for instructing us at the bench in the hippocampal sub-dissection, and thank Dr. Richard Kryscio for help with statistical analyses. This work is supported by The Department of Veterans Affairs (JTS), DARPA N66001-09-C-2080 (GG), and HL56652 and HL091893 (SWW).
Footnotes
Disclosure
GG is the sole proprietor of Quanteon, LLC that make the FAST 16 mKII recording system used in these studies. None of the other authors has any conflicts to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Ashery U, Varoqueaux F, Voets T, Betz A, Thakur P, Koch H, Neher E, Brose N, Rettig J. Munc13-1 acts as a priming factor for large dense-core vesicles in bovine chromaffin cells. EMBO J. 2000;19:3586–3596. doi: 10.1093/emboj/19.14.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram E. The relevance of kindling for human epilepsy. Epilepsia. 2007;48(Suppl 2):65–74. doi: 10.1111/j.1528-1167.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2005;38:1–47. doi: 10.1017/S0033583505004051. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Gerhardt GA. Self-referencing ceramic-based multi-site microelectrodes for the detection and elimination of interferences from the measurement of L-glutamate and other analytes. Anal Chem. 2001;73:1037–1042. doi: 10.1021/ac0010429. [DOI] [PubMed] [Google Scholar]
- Collins RC, Tearse RG, Lothman EW. Functional anatomy of limbic seizures: focal discharges from medial entorhinal cortex in rat. Brain Res. 1983;280:25–40. doi: 10.1016/0006-8993(83)91170-8. [DOI] [PubMed] [Google Scholar]
- Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM. Abnormal neurotrans-mission in mice lacking synaptic vesicle protein 2A (SV2A) Proc Natl Acad Sci U S A. 1999;96:15268–15273. doi: 10.1073/pnas.96.26.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Day BK, Pomerleau F, Burmeister JJ, Huettl P, Gerhardt GA. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J Neurochem. 2006;96:1626–1635. doi: 10.1111/j.1471-4159.2006.03673.x. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Jarvie PE, Heath JW, Kidd GJ, Rostas JA. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986;372:115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Heath JW, Harrison SM, Jarvie PE, Glenfield PJ, Rostas JA. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: homogeneity and morphology of subcellular fractions. Brain Res. 1988;441:59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
- Friedemann MN, Gerhardt GA. Regional effects of aging on dopaminergic function in the Fischer-344 rat. Neurobiol Aging. 1992;13:325–332. doi: 10.1016/0197-4580(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Geula C, Jarvie PA, Logan TC, Slevin JT. Long-term enhancement of K+-evoked release of L-glutamate in entorhinal kindled rats. Brain Res. 1988;442:368–372. doi: 10.1016/0006-8993(88)91528-4. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Hascup KN, Hascup ER, Stephens ML, Glaser PE, Yoshitake T, Mathe AA, Gerhardt GA, Kehr J. Resting glutamate levels and rapid glutamate transients in the prefrontal cortex of the flinders sensitive line rat: a genetic rodent model of depression. Neuropsychopharmacology. 2011;36:1769–1777. doi: 10.1038/npp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Yamasaki S, Nauenburg S, Binz T, Niemann H. Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J. 1995;14:2317–2325. doi: 10.1002/j.1460-2075.1995.tb07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Akagawa K. Neuron-specific antigen HPC-1 from bovine brain reveals strong homology to epimorphin, an essential factor involved in epithelial morphogenesis: identification of a novel protein family. Biochem Biophys Res Commun. 1992;187:1144–1150. doi: 10.1016/0006-291x(92)91316-i. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs – engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Janz R, Goda Y, Geppert M, Missler M, Sudhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24:1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Jarvie PA, Logan TC, Geula C, Slevin JT. Entorhinal kindling permanently enhances Ca2(+)-dependent L-glutamate release in regio inferior of rat hippocampus. Brain Res. 1990;508:188–193. doi: 10.1016/0006-8993(90)90395-r. [DOI] [PubMed] [Google Scholar]
- Jones RS, Lambert JD. The role of excitatory amino acid receptors in the propagation of epileptiform discharges from the entorhinal cortex to the dentate gyrus in vitro. Exp Brain Res. 1990;80:310–322. doi: 10.1007/BF00228158. [DOI] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Land-field PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis W, Huisman E, Veerman MJ, Lopes da Silva FH. Development of changes in endogenous GABA release during kindling epileptogenesis in rat hippocampus. Brain Res. 1991;545:33–40. doi: 10.1016/0006-8993(91)91266-4. [DOI] [PubMed] [Google Scholar]
- Matveeva EA, Whiteheart SW, Slevin JT. Accumulation of 7S SNARE complexes in hippocampal synaptosomes from chronically kindled rats. J Neurochem. 2003;84:621–624. doi: 10.1046/j.1471-4159.2003.01589.x. [DOI] [PubMed] [Google Scholar]
- Matveeva EA, Vanaman TC, Whiteheart SW, Slevin JT. Asymmetric accumulation of hippocampal 7S SNARE complexes occurs regardless of kindling paradigm. Epilepsy Res. 2007;73:266–274. doi: 10.1016/j.eplepsyres.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveeva EA, Vanaman TC, Whiteheart SW, Slevin JT. Levetiracetam prevents kindling-induced asymmetric accumulation of hippocampal 7S SNARE complexes. Epilepsia. 2008;49:1749–1758. doi: 10.1111/j.1528-1167.2008.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveeva EA, Price DA, Whiteheart SW, Vanaman TC, Gerhardt GA, Slevin JT. Reduction of VAMP2 expression leads to a kindling-resistant phenotype in a murine model of epilepsy. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.11.055. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen JM, Madison JM, Dybbs M, Kaplan JM. Antagonistic regulation of synaptic vesicle priming by Tomosyn and UNC-13. Neuron. 2006;51:303–315. doi: 10.1016/j.neuron.2006.06.025. [DOI] [PubMed] [Google Scholar]
- McNamara JO. Analyses of the molecular basis of kindling development. Psychiatry Clin Neurosci. 1995;49:S175–S178. doi: 10.1111/j.1440-1819.1995.tb02167.x. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Byrne MC, Dasheiff RM, Fitz JG. The kindling model of epilepsy: a review. Prog Neurobiol. 1980;15:139–159. doi: 10.1016/0301-0082(80)90006-4. [DOI] [PubMed] [Google Scholar]
- Minamoto Y, Itano T, Tokuda M, Matsui H, Janjua NA, Hosokawa K, Okada Y, Murakami TH, Negi T, Hatase O. In vivo microdialysis of amino acid neurotransmitters in the hippocampus in amygdaloid kindled rat. Brain Res. 1992;573:345–348. doi: 10.1016/0006-8993(92)90786-9. [DOI] [PubMed] [Google Scholar]
- Mody I. The molecular basis of kindling. Brain Pathol. 1993;3:395–403. doi: 10.1111/j.1750-3639.1993.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Nickell J, Salvatore MF, Pomerleau F, Apparsundaram S, Gerhardt GA. Reduced plasma membrane surface expression of GLAST mediates decreased glutamate regulation in the aged striatum. Neurobiol Aging. 2007;28:1737–1748. doi: 10.1016/j.neurobiolaging.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Nowack A, Yao J, Custer KL, Bajjalieh SM. SV2 regulates neurotransmitter release via multiple mechanisms. Am J Physiol Cell Physiol. 2010;299:C960–C967. doi: 10.1152/ajpcell.00259.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Uemura S, Kimura H, Sato M. Amygdala kindling develops without mossy fiber sprouting and hippocampal neuronal degeneration in rats. Psychiatry Clin Neurosci. 2001;55:549–557. doi: 10.1046/j.1440-1819.2001.00905.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. Academic Press, Inc; San Diego: 2005. [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Ferrara LP. Lack of effect of entorhinal kindling on L-[3H]glutamic acid presynaptic uptake and postsynaptic binding in hippocampus. Exp Neurol. 1985;89:48–58. doi: 10.1016/0014-4886(85)90264-x. [DOI] [PubMed] [Google Scholar]
- Stephens ML, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA. Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus. Neurobiol Aging. 2011;32:811–820. doi: 10.1016/j.neurobiolaging.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer JL, Lothman EW. Maximal dentate gyrus activation: characteristics and alterations after repeated seizures. J Neurophysiol. 1989;62:136–143. doi: 10.1152/jn.1989.62.1.136. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Wilson DW, Brunner M, Arango N, Rothman JE. Domain structure of an N-ethylmaleimide-sensitive fusion protein involved in vesicular transport. J Biol Chem. 1993;268:2662–2666. [PubMed] [Google Scholar]
- Ueda Y, Doi T, Tokumaru J, Mitsuyama Y, Willmore LJ. Kindling phenomena induced by the repeated short-term high potassium stimuli in the ventral hippocampus of rats: on-line monitoring of extracellular glutamate overflow. Exp Brain Res. 2000;135:199–203. doi: 10.1007/s002210000509. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Aronica E, Redeker S, Boer K, Gorter JA. Decreased expression of synaptic vesicle protein 2A, the binding site for levetiracetam, during epileptogenesis and chronic epilepsy. Epilepsia. 2009;50:422–433. doi: 10.1111/j.1528-1167.2008.01727.x. [DOI] [PubMed] [Google Scholar]
- Walther H, Lambert JD, Jones RS, Heinemann U, Hamon B. Epileptiform activity in combined slices of the hippocampus, subiculum and entorhinal cortex during perfusion with low magnesium medium. Neurosci Lett. 1986;69:156–161. doi: 10.1016/0304-3940(86)90595-1. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Rossnagel K, Buhrow SA, Brunner M, Jaenicke R, Roth-man JE. N-ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteheart SW, Schraw T, Matveeva EA. N-ethylmaleimide sensitive factor (NSF) structure and function. Int Rev Cytol. 2001;207:71–112. doi: 10.1016/s0074-7696(01)07003-6. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Obata K, Greengard P, Czernik AJ. Increase in synapsin I phosphorylation implicates a presynaptic component in septal kindling. Neuroscience. 1995 doi: 10.1016/0306-4522(94)00492-n. In Press. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Matti U, Melamed R, Hagalili Y, Bruns D, Rettig J, Ashery U. Tomosyn inhibits priming of large dense-core vesicles in a calcium-dependent manner. Proc Natl Acad Sci U S A. 2004;101:2578–2583. doi: 10.1073/pnas.0308700100. [DOI] [PMC free article] [PubMed] [Google Scholar]