Abstract
A tandem chain walking polymerization (CWP) and ATRP was developed for efficient synthesis of nanoparticles for bioconjugation. Using the chain walking palladium-α-diimine catalyst (catalyst 1), dendritic polymers bearing multiple initiation sites were synthesized and used as macro-initiators for subsequent Cu(I)-mediated ATRP. Control of molecular weight and size of the water soluble core-shell polymeric nanoparticles was achieved by tuning reaction conditions. Addition of N-acryloyloxysuccinamide (NAS) monomer at the end of the ATRP afforded NHS activated polymer nanoparticles. Conjugation with both small dye molecule and protein (ovalbumin) yielded nanoparticle conjugates with relatively high dye or protein per particle ratio. With the efficient synthesis and good biocompatibility, these nanoparticles may find many potential applications in bioconjugation.
INTRODUCTION
Nanoparticle-biomolecule conjugates are actively investigated for various nanobiotechnology applications including catalysis, sensors, bioanalysis, drug delivery, and bioelectronics.1 In parallel to the development of inorganic nanoparticles for these applications, organic nanostructures have received increasing attention recently because in principle organic synthesis can provide more precise control over molecular structure, size and functionality of nanostructures.2–5 Among others, nanoparticles based on dendritic macromolecules are especially attractive because they have globular shape in solution with molecular dimensions right in the nanometer range.6,7 However, the difficulty of preparing dendrimers through step-wise synthesis and the ultimate size limit for regular dendrimers warrant the search of more efficient methodologies for constructing dendritic nanoparticles. In the current study, we combined two powerful transition-metal catalyzed polymerization methods: chain walking polymerization (CWP) followed by atom transfer radical polymerization (ATRP), to efficiently synthesize dendritic nanoparticles with tunable sizes and reactive surface functionalities. To the best of our knowledge, this is the first example of tandem catalytic coordination/living radical polymerization for constructing polymer nanoparticles.8,9
Our previous studies have shown that CWP provides efficient control to polymer topology.10–13 Linear, hyperbranched, and dendritic copolymers containing a range of functionalities were obtained by CWP of simple olefinic monomers. In addition, we recently succeeded in a facile synthesis of core-shell dendritic polymeric amphiphiles that behave as unimolecular micelles in water.13 Despite of its efficiency, this approach has a few limitations: (1) the size of the nanoparticle is relatively small as limited by the length of available hetero-difunctionalized poly(ethyelene glycol) (PEG); (2) multi-step functionalization of PEG chain ends is tedious and inefficient and (3) the approach is not general for other type of shell structures. ATRP has evolved into a major living/controlled radical polymerization method for precision polymer synthesis.14 To overcome the limitations described above, herein we combined two powerful transition-metal catalyzed polymerization methods to develop a tandem CWP3 ATRP approach as a general and efficient methodology for constructing dendritic nanoparticles with controllable size and reactive surface functionalities that are ready for bioconjugation.
RESULTS AND DISCUSSION
Macro-initiators Synthesis
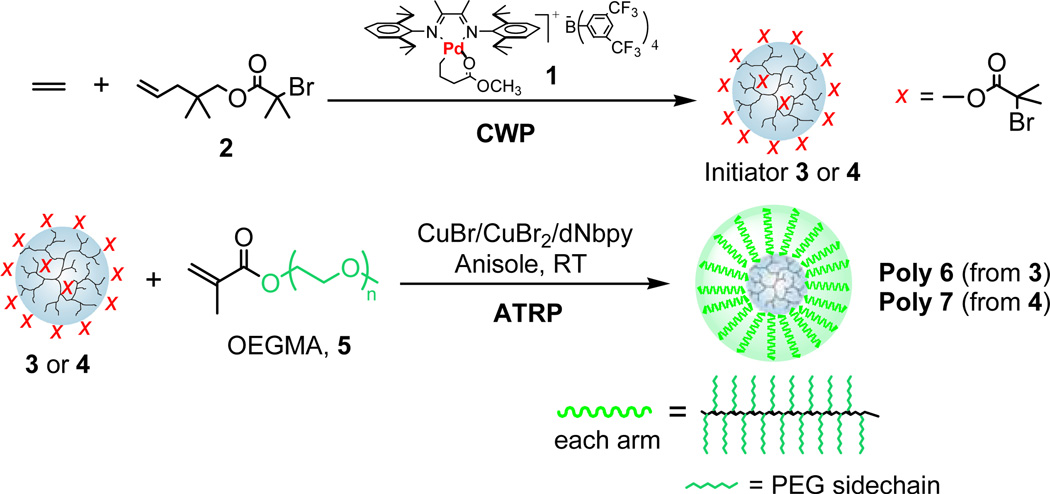
As shown in Scheme 1, the tandem polymerizations were carried out in two steps. In the first step, dendritic macro-initiators bearing many radical initiation sites on chain ends were synthesized through copolymerization of ethylene and comonomer 2 using the chain walking palladium-α-diimine catalyst 15 under conditions reported previously.13 By varying CWP conditions, two dendritic macro-initiators were prepared for this study: initiator 3 with a number-averaged molecular weight (Mn, measured by size exclusion chromatography using a multi-angle light scattering detector, SEC-MALS) of 206,000 g/mol and 538 initiation sites (x groups), and initiator 4 with Mn of 124,000 g/mol and 357 initiation sites. The relative molecular weight data for 3 and 4 were obtained by regular SEC using polystyrene standards. The absolute molecular weight and molecular size (radius of gyration, Rg) were measured by SEC-MALS (entry 1–2 in Table 1). Consistent with the dendritic topology, the relative molecular weight values obtained by regular SEC are significantly smaller than the absolute values measured by MALS. The number of initiation groups in the initiators was calculated from their corresponding NMR spectra (See Supporting Information for details).
Scheme 1.
Tandem CWP-ATRP for nanoparticle synthesis
Table 1.
SEC and SEC-MALS characterizations of Initiators and ATRP Polymers.
| entry | Polymer | Initiator | Time (h) |
Mn from SEC d (103 g/mol) |
Mw from SEC d (103 g/mol)) |
PDI from SEC |
Mn from MALS e (103 g/mol) |
Mw from MALS e (103 g/mol) |
PDI From MALS |
Rgf (nm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | - | - | 88 | 148 | 1.6 | 206 | 314 | 1.5 | 14 ± 2.2 |
| 2 | 4 | - | - | 53 | 88 | 1.6 | 124 | 192 | 1.5 | 11 ± 3.0 |
| 3 a | 6-1 | 3 | 1 | 149 | 255 | 1.6 | 610 | 940 | 1.5 | 19.2 ± 2.0 |
| 4 a | 6-2 | 3 | 2 | 198 | 324 | 1.6 | 1,023 | 1,407 | 1.4 | 21.5 ± 1.9 |
| 5 a | 6-3 | 3 | 4 | 279 | 431 | 1.5 | 1,894 | 2,646 | 1.4 | 27.7 ± 1.7 |
| 6 a | 6-4 | 3 | 6 | 328 | 505 | 1.5 | 2,669 | 3,870 | 1.4 | 32.9 ± 1.6 |
| 7 a | 6-5 | 3 | 8 | 346 | 558 | 1.6 | 3,449 | 4,961 | 1.4 | 37.3 ± 1.3 |
| 8 a | 6-6 | 3 | 13 | 391 | 606 | 1.5 | 5,096 | 7,302 | 1.4 | 45.5 ± 1.2 |
| 9 a | 6-7 | 3 | 24 | 435 | 669 | 1.5 | 7,040 | 9,430 | 1.3 | 55.3 ± 1.1 |
| 10 a | 6-8 | 3 | 48 | 463 | 708 | 1.5 | 8,500 | 10,440 | 1.2 | 61.8 ± 0.6 |
| 11 a | 6-9 | 3 | 72 | 478 | 737 | 1.5 | 9,180 | 10,980 | 1.2 | 64.0 ± 0.5 |
| 12 b | 7-1 | 4 | 1 | 97 | 157 | 1.6 | 344 | 533 | 1.5 | 15.2 ± 2.3 |
| 13 b | 7-2 | 4 | 2 | 135 | 213 | 1.6 | 543 | 798 | 1.4 | 17.0 ± 2.1 |
| 14 b | 7-3 | 4 | 4 | 195 | 310 | 1.6 | 995 | 1,373 | 1.4 | 20.5 ± 2.0 |
| 15 b | 7-4 | 4 | 6 | 238 | 368 | 1.5 | 1,350 | 1,957 | 1.4 | 25.4 ± 1.8 |
| 16 b | 7-5 | 4 | 8 | 271 | 417 | 1.5 | 1,789 | 2,558 | 1.4 | 28.1 ± 1.7 |
| 17 b | 7-6 | 4 | 12 | 321 | 519 | 1.5 | 2,560 | 3,609 | 1.4 | 33.1 ± 1.4 |
| 18 c | 9 | 3 | 36 g | 289 | 465 | 1.5 | 2,078 | 2,634 | 1.3 | 27.9 ± 1.5 |
Note:
reaction conditions: initiator 3, [COC(CH3)Br]=8mM, [Cu(I)]=16mM, [Cu(II)]=1.6mM, [dNbpy]=35.2mM, [M]=0.5M, solvent: anisole, room temperature;
reaction conditions: initiator 4, [COC(CH3)Br]=8mM, [Cu(I)]=16mM, [Cu(II)]=1.6mM, [dNbpy]=35.2mM, [M]=0.5M, solvent: anisole, room temperature;
reaction conditions: initiator 3, [COC(CH3)Br]=8mM, [Cu(I)]=16mM, [Cu(II)]=1.6mM, [dNbpy]=35.2mM, [M]=0.5M, [NAS]=0.1M, solvent: anisole, room temperature;
data based on SEC calibration using linear polystryrene standards (Aldrich);
data based on SEC-MALS;
weight average radius of gyration;
reaction time is 12 hours + additional 24 hours after addition of NAS.
ATRP with the Macro-initiators
In the second step, the dendritic macro-initiators (3 or 4) were used to initiate ATRP polymerizations to form core-shell polymers. We chose an oligo(ethylenegylcol) methacrylate (OEGMA, Mn=300) as the comonomer for the ATRP to provide both water solubility and biocompatibility for the final nanoparticles. Following reported conditions,16,17 ATRP was carried out at room temperature with a CuBr/CuBr2/dNbpy (molar ratio of 1:0.1:2.2) catalyst system. The low polymerization temperature and the addition of small amount of CuBr2 were to ensure very low stationary radical concentration on each dendritic polymer, and hence, suppress intra- and intermolecular radical coupling reactions.16,17 Our ATRP condition optimization indicated that a ratio of CuBr/initiator = 2:1 afforded the best control to the polymerization. Presumably the excess amount of the catalyst could compensate partial loss of Cu(I) during the relatively long polymerization period.
A series of amphiphilic core-shell copolymers were prepared by ATRP of OEGMA using dendritic macro-initiator 3 (entry 3–11 in Table 1) and 4 (entry 12–17 in Table 1). The molecular weight and molecular size data for the core-shell copolymers increase with the ATRP polymerization time. (Table 1, entry 3–17). Again, consistent with the dendritic core-shell topology, the relative molecular weight values obtained by regular SEC are much smaller than the absolute values measured by MALS. As expected from the radial growth, this discrepancy increases with the growth of the final polymer size.
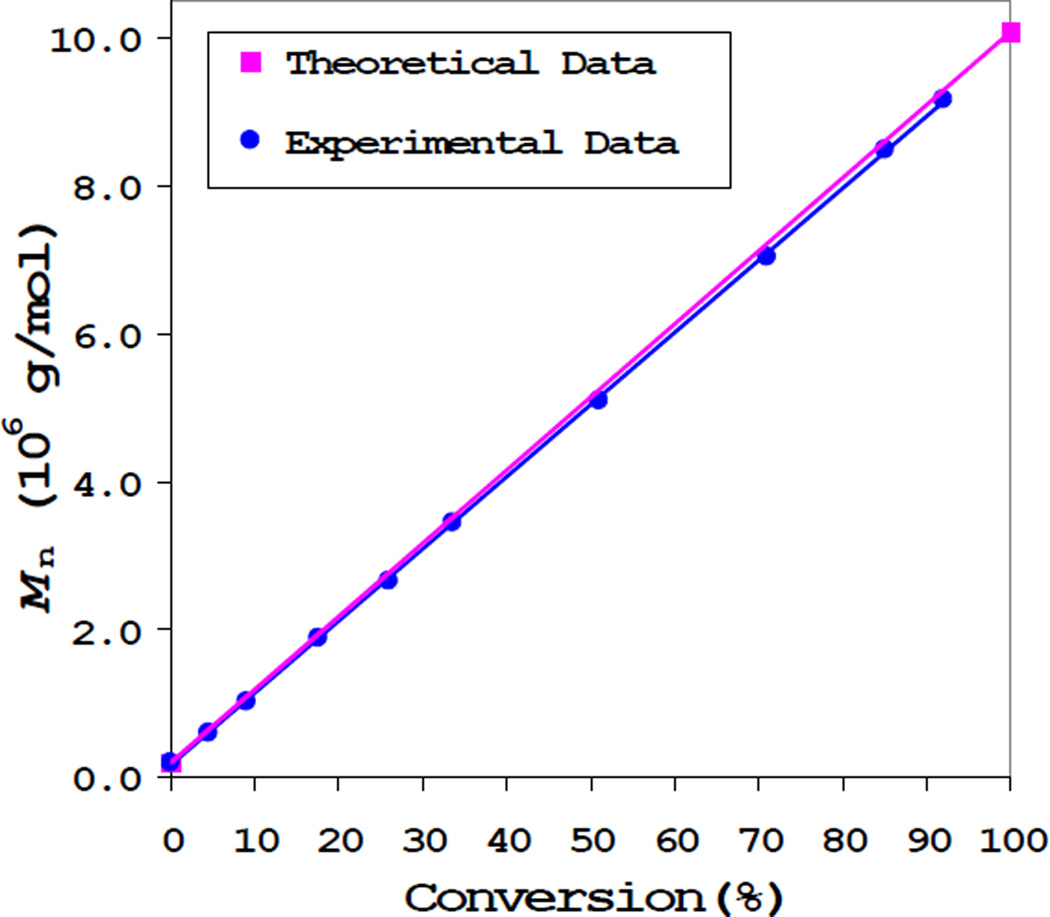
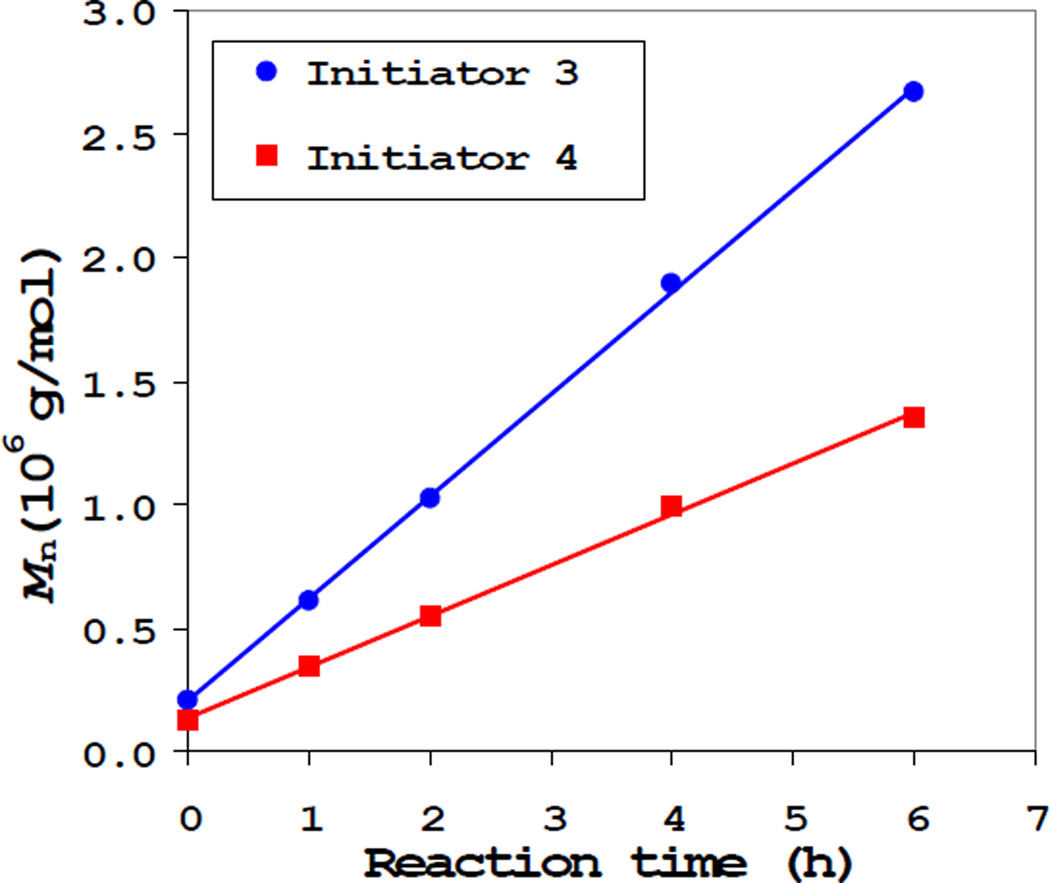
ATRP proceeded in a controlled fashion as evidenced by the linear growth of Mn of the dendritic polymers with the monomer conversion (Figure 1). Simply by controlling the polymerization time, a series of polymer (6-1 to 6-9 in Table 1) was obtained in gram quantities with a broad range of molecular weights and sizes (from Mn=610,000 g/mol and radius of gyration Rg=19.2 nm to Mn=9,180,000 g/mol and Rg=64 nm). Based on the conversions, theoretical values of the number average molecular weight are also shown in Figure 1, which correlate well with the experimental data. At relatively low monomer conversions, the polymerization follows pseudo-zero order kinetics and the Mn of the polymers increases linearly with reaction time (up to 8 hours, Figure 2), which provided us a very convenient method to control the molecular weight and size for the final polymer nanoparticle.
Figure 1.
Mn vs. conversion of polymerization using macro-initiator 3; Mn was obtained from SEC-MALS; Reaction conditions: [COC(CH3)Br]=8 mM, [Cu(I)]=16 mM, [Cu(II)]=1.6 mM, [dNbpy]=35.2 mM, [M]=0.5 M, solvent: anisole, reaction temperature: 25°C.
Figure 2.
Mn vs. reaction time in two polymerization reactions with initiator 3 (Mn=206,000 g/mol) and initiator 4 (Mn=124,000 g/mol). Mn was obtained from SEC-MALS; Reaction conditions for both polymerizations: [COC(CH3)Br]=8 mM, [Cu(I)]=16 mM, [Cu(II)]=1.6 mM, [dNbpy]=35.2 mM, [M]=0.5 M, solvent: anisole, reaction temperature: 25°C.
Functional Nanoparticle Synthesis
To introduce reactive groups to the dendritic nanoparticles for further bioconjugation, an acrylate comonomer containing N-hydroxysuccinamide (NHS) group, N-acryloyloxysuccinamide (NAS, 8), was used to cap the end of polymer arms. This methodology is based on the fact that the addition of an acrylate monomer will generate a more stable secondary C-Br bond which is significantly less reactive for further polymerization. This essentially “caps” each methacrylate chain end with an acrylate unit (NAS).18 For example, after 12 hours of OEGMA polymerization using macro-initiator 3, NAS comonomer was added and the polymerization was allowed to continue for another 24 hours (Scheme 2). Scaffold 9 was obtained after dialysis which has a Mn of 2,078,000 g/mol and Rg of 27.9 nm (entry 18 in Table 1). From the 1H NMR spectrum it was calculated that 49% of the chain ends in scaffold 9 are converted to NHS activated ester groups.
Scheme 2.
Synthesis of functional nanoparticles.
Hydrodynamic Characterization of Scaffold 9 in Aqueous Solution
Dynamic light scattering measurements of scaffold 9 in PBS buffer solution were performed using a Dawn EOS 18-angle light scattering detector (laser wavelength λ=690nm) coupled with a QELS detector (Wyatt Technology Corporation, Santa Barbara, CA). The measurements were done using batch mode at scaffold concentration of 1 mg/mL in PBS buffer solutions at 25°C. The average hydrodynamic radius, Rh, of scaffold 9 in PBS buffer solution was measured to be 52 ± 2 nm by dynamic light scattering.
Conjugation of Scaffold 9 with Fluorescein Dye
To investigate the reactivity and availability of the NHS group on the prepared nanoparticles, a derivative (10) of a common dye molecule fluorescein was used in our initial conjugation studies. The protein mimic dye molecule 10 was prepared by following a literature reported procedure (Scheme 3). 21 The molar absorptivity (ε) of 10 was determined from Beer’s law to be 51,300 M−1cm−1 (See Supporting Information Figure S1 and S2).
Scheme 3.
Synthesis of Fluorescein derivative 10.
Scaffold 9 (1µM) was incubated in a PBS buffer solution of 10 (pH 8.5, 1mM) at 4 °C for 12 hours, followed by dialysis against water (molecular weight cut-off (MWCO) of dialysis tube is 10,000 g/mol). Conjugate 11 was obtained as bright yellow oil (UV/Vis and fluorescence spectra of 11 are in the Supporting Information, Figure S3 and S4). Calculated from its absorbance, the average number of dye molecules per polymer scaffold in F-conjugate 11 is 136, indicating that about 50% of the NHS groups were conjugated to the dye.
Conjugation of Scaffold 9 with Ovalbumin
Ovalbumin, one of the most commonly used proteins, was chosen for our protein conjugation studies. Ovalbumin is relatively small (MW=44,287 g/mol) compared to scaffold 9 (Mn=2,078,000 g/mol). Conjugation of ovalbumin to 9 was conducted at similar conditions as for the preparation of 11 (ratio of protein to scaffold: weight ratio=1:1, molar ratio=47:1). At the end of the reaction, an excess amount (10 eq.) of amino-diethyleneglycol was added to quench any remaining NHS activated chain ends (Scheme 5).
Scheme 5.
Synthesis of OB-conjugate 12.
The crude reaction solution was subjected to SEC to confirm the formation of OB-conjugate 12. Figure 3 compares the SEC traces of the conjugation solution, free ovalbumin, and free scaffold 9, respectively. The shift of UV absorbance at 280 nm indicated that the majority of ovalbumin was conjugated to the nanoparticle scaffold. Integration of the SEC curve shows that 84% of the ovalbumin exists as conjugated form and 16% as free ovalbumin form. Bradford assay 19 was also used to obtain protein concentration of each fraction, from which the amount of protein conjugated to the scaffold was estimated to be 83%, a value agreeing with the SEC result. This relatively high conjugation efficiency indicates the effectiveness of using our core-shell nanoparticles as scaffolds for bioconjugation applications. Based on the quantity of scaffold and the amount of conjugated proteins, the number of proteins per scaffold was determined to be 40 in OB-conjugate 12.
Figure 3.
Overlay of SEC chromatograms of OB-conjugate 12 crude solution ( ), scaffold 9 (
), scaffold 9 ( ) and ovalbumin alone (
) and ovalbumin alone ( ). Mobile phase: 1xPBS buffer pH 7.4 at 1 ml/min. Absorbance was monitored at wavelength 280nm.
). Mobile phase: 1xPBS buffer pH 7.4 at 1 ml/min. Absorbance was monitored at wavelength 280nm.
We also run ovalbumin conjugation reactions at increased protein/scaffold ratio. At higher protein/scaffold ratio, while the total number of protein conjugated to one scaffold increased, the overall protein conjugation efficiency decreased. For example, when the protein/scaffold ratio was doubled (i.e., 94:1), the number of proteins conjugated per scaffold was increased from 40 to 49, which means only 52% of the protein was conjugated to the scaffold. A number of factors may limit the maximum number of proteins that can be conjugated to one scaffold. First, the steric repulsion between conjugated proteins may prevent further conjugation reaction. This effect was observed in a recent study of a different system in which much higher number of proteins per scaffold was obtained for a significantly smaller protein.20 Second, proteins usually have multiple nucleophilic functionalities on their surfaces (e.g., ovalbumin carries about 20 surface exposed lysines) which may result in consumption of multiple NHS groups per protein conjugation. Third, both the conformational flexibility and the structural non-uniformity of the core-shell polymers will make a portion of the NHS groups not accessible for protein conjugation. Regardless of these factors, our results have clearly demonstrated that our core-shell nanoparticles are excellent scaffolds for effective multivalent bioconjugation.
Conclusion
In summary, we have shown the first example of tandem catalytic coordination/living radical polymerization for efficient synthesis of nanoparticles for bioconjugation. Using a chain walking palladium catalyst, dendritic macro-initiators bearing multiple radical initiation sites were prepared which were used subsequently in a Cu(I)-mediated ATRP for synthesizing dendritic polymer nanoparticles. The size for both the core and the shell can be controlled precisely. Addition of NAS monomer at the end of the ATRP afforded NHS activated polymer nanoparticles as convenient scaffolds for bioconjugation. Conjugation with both small dye molecule and ovalbumin demonstrated that these nanoparticles are effective for multivalent bioconjugation. Currently we are preparing nanoparticle conjugates with biologically active proteins for specific biological functions. We are also applying this general methodology to prepare dendritic polymer nanoparticles having other kinds of shell structures.
Supplementary Material
Scheme 4.
Synthesis of Fluorecein-conjugate 11.
ACKNOWLEDGMENT
We thank the National Science Foundation (DMR-0135233, ZG: CAREER) and NIH/NIAID (AI056464 & AI061363) for partial financial support. ZG gratefully acknowledges a Camille Dreyfus Teacher-Scholar Award.
Footnotes
Supporting Information Available: Experimental for the synthesis, polymerization, spectroscopic studies (PDF). This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Katz E, Willner I. Angew. Chem. Int. Ed. 2004;43:6042–6108. doi: 10.1002/anie.200400651. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 2.Grimsdale AC, Müllen K. Angew. Chem. Int. Ed. 2005;44:5592–5629. doi: 10.1002/anie.200500805. [DOI] [PubMed] [Google Scholar]
- 3.Moore JS. Acc. Chem. Res. 1997;30:402–413. [Google Scholar]
- 4.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, DeSimone JM. J. Am. Chem. Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 5.Qi K, Ma Q, Remsen EE, Clark GC, Jr., Wooley LK. J. Am. Chem. Soc. 2004;126:6599–6607. doi: 10.1021/ja039647k. [DOI] [PubMed] [Google Scholar]
- 6.Frechet JMJ. In: Dendrimers and Other Dendritic Polymers. Tomalia D, editor. Hoboken, N. J.: John Wiley & Sons Ltd; 2001. [Google Scholar]
- 7.Kramer M, Stumbe J-F, Turk H, Krause S, Komp A, Delineau L, Prokhorova S, Kautz H, Haag R. Angew. Chem., Int. Ed. 2002;41:4252–4256. doi: 10.1002/1521-3773(20021115)41:22<4252::AID-ANIE4252>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Wasilke J-C, Obrey SJ, Baker RT, Bazan GC. Chem. Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]
- 9.Kolb L, Monteil V, Thomann R, Mecking S. Angew. Chem., Int. Ed. 2005;44:429–432. doi: 10.1002/anie.200460455. [DOI] [PubMed] [Google Scholar]
- 10.Guan Z, Cotts PM, McCord EF, McLain SJ. Science. 1999;283:2059–2062. doi: 10.1126/science.283.5410.2059. [DOI] [PubMed] [Google Scholar]
- 11.Guan Z. J. Polym. Sci., Part A: Polym. Chem. 2003;41:3680–3692. [Google Scholar]
- 12.Chen G, Ma XS, Guan Z. J. Am. Chem. Soc. 2003;125:6697–6704. doi: 10.1021/ja028921s. [DOI] [PubMed] [Google Scholar]
- 13.Chen G, Guan Z. J. Am. Chem. Soc. 2004;126:2662–2663. doi: 10.1021/ja039829e. [DOI] [PubMed] [Google Scholar]
- 14.Matyjaszewski K, Xia J. Chem. Rev. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 15.Johnson LK, Killian CM, Brookhart M. J. Am. Chem. Soc. 1995;117:6414–6415. [Google Scholar]
- 16.Sumerlin BS, Neugebauer D, Matyjaszewski K. Macromolecules. 2005;38:702–708. [Google Scholar]
- 17.von Werne T, Patten TE. J. Am. Chem. Soc. 2001;123:7497–7505. doi: 10.1021/ja010235q. [DOI] [PubMed] [Google Scholar]
- 18.Bon AFS, Steward AG, Haddleton DM. J. Polym. Sci., Part A: Polym. Chem. 2000;38:2678–2686. [Google Scholar]
- 19.Bradford MM. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Huynh D, Guan Z, Felgner PL. unpublished results. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.