Abstract
Renal dopamine receptors participate in the regulation of blood pressure. Genetic factors, including polymorphisms of the dopamine D2 receptor gene (DRD2) are associated with essential hypertension, but the mechanisms of their contribution are incompletely understood. Mice lacking Drd2 (D2−/−) have elevated blood pressure, increased renal expression of inflammatory factors, and renal injury. We tested the hypothesis that decreased dopamine D2 receptor (D2R) function increases vulnerability to renal inflammation independently of blood pressure, is an immediate cause of renal injury, and contributes to the subsequent development of hypertension. In D2−/− mice, treatment with apocynin normalized blood pressure and decreased oxidative stress, but did not affect the expression of inflammatory factors. In mouse RPTCs Drd2 silencing increased the expression of TNFα and MCP-1, while treatment with a D2R agonist abolished the angiotensin II-induced increase in TNF-α and MCP-1. In uni-nephrectomized wild-type mice, selective Drd2 silencing by subcapsular infusion of Drd2 siRNA into the remaining kidney produced the same increase in renal cytokines/chemokines that occurs after Drd2 deletion, increased the expression of markers of renal injury, and increased blood pressure. Moreover, in mice with two intact kidneys, short-term Drd2 silencing in one kidney, leaving the other kidney undisturbed, induced inflammatory factors and markers of renal injury in the treated kidney without increasing blood pressure. Our results demonstrate that the impact of decreased D2R function on renal inflammation is a primary effect, not necessarily associated with enhanced oxidant activity, or blood pressure; renal damage is the cause, not the result, of hypertension. Deficient renal D2R function may be of clinical relevance since common polymorphisms of the human DRD2 gene result in decreased D2R expression and function.
Introduction
Dopamine synthesized in the kidney is necessary for the maintenance of normal blood pressure and renal function [1]. The disruption of any of the dopamine receptor subtype genes in mice produces receptor subtype-specific hypertension [2]. In particular, the hypertension in mice with disruption of the dopamine D2 receptor (Drd2) gene (D2−/−) is associated with increased production of reactive oxygen species (ROS) [3], [4].
Infiltration of inflammatory cells and oxidative stress in the kidney are involved in the development of renal injury and the induction and maintenance of hypertension [5]. Renal tubule cells produce both pro- and anti-inflammatory cytokines and chemokines [6], which are secreted across their apical and basolateral membranes [7], and contribute to the development and progression of glomerular and tubular injury. However, the factors that regulate cytokine production in these cells are incompletely understood. Dopamine and dopaminergic drugs have been shown to regulate the immune response and the inflammatory reaction [8]. Dopamine inhibits the release of IFNγ, IL-2, and IL-4 [9] and the lipopolysaccharide-stimulated production of IL-12p40 [10] in immune cells. Administration of dopamine or dopaminergic agonists in vivo reduces the TNFα response to endotoxin [11] and the activation of leukocytes in experimental sepsis [12]. Conversely, treatment with a dopaminergic antagonist stimulates constitutive and inducible gene expression of IL-1β, IL-6, and TNFα in macrophages [13]. In brain-dead rats, a condition that is associated with profound inflammation in end-organs, dopamine reduces renal monocyte infiltration [14], expression of IL-6, and improves renal function after transplantation [15]. Furthermore, mice with intrarenal dopamine deficiency have increased oxidative stress and infiltration of inflammatory cells [16] and decreased renal dopamine production is associated with increased detrimental effects of Ang II on renal injury [17].
The anti-inflammatory effects of dopamine and dopaminergic agonists are mediated, at least in part, by the D2R. D2Rs are expressed in lymphocytes, monocytes, neutrophils, macrophages, and other immuno-competent cells [18]. The D2R/D3R agonist, bromocriptine, inhibits lymphocyte proliferation [19] and decreases antigen-induced macrophage activation and secretion of IL-2, IL-4, and IFNγ [11]. In normal human lymphocytes, D2R agonists increase the secretion of anti-inflammatory cytokines by de novo gene expression [20]. GLC756, a novel mixed dopamine D1R antagonist and D2R agonist, inhibits the release of TNFα from activated mast cells [21].
We hypothesized that the D2R decreases renal inflammation and prevents renal injury by regulating the inflammatory response in renal proximal tubule cells (RPTCs). To test this hypothesis, we studied parameters of inflammation and injury in the renal cortex of D2−/− mice and the effect of D2 R silencing on the expression of inflammatory factors in mouse RPTCs. Because angiotensin (Ang) II and dopamine receptors counter-regulate each other and Ang II, via the AT1R, promotes inflammation and renal injury [17], [18], [22], we also determined if stimulation of D2R opposes the effects of Ang II in these cells. Because D2R deficiency increases blood pressure and oxidative stress, we studied the effects of normalizing blood pressure and decreasing oxidative stress on the renal expression of cytokines/chemokines in D2−/− mice. Finally, we studied renal expression of inflammatory factors and markers of renal injury in two mouse models of selective Drd2 silencing in the kidney.
Methods
D2 Receptor-deficient Mice
The original F2 hybrid strain (129/SvXC57BL/6J, Oregon Health Sciences University) that contained the mutated Drd2 allele (D2−/−) was bred onto the C57BL/6J background for >20 generations [3]. All animal-related studies were approved by the Institutional Animal Care and Use Committee. D2−/− mice and wild-type littermates (D2+/+) were studied at 6 to 8 months of age. Mice were housed in metabolic cages for 24 h urine collection and then anesthetized for blood pressure measurement via the femoral artery, as reported previously [4]. The organs were harvested and flash-frozen. As we have reported previously [4], both systolic (121±3 (D2−/−) vs. 89 (D2+/+) mm Hg; n = 9; P<0.01) and diastolic blood pressures (87±2 (D2−/−) vs. 63±5 (D2+/+) mm Hg; n = 9; P<0.02), were increased in D2−/− mice, relative to D2+/+ littermates. A group of mice was treated for 10 days with apocynin (3 mg/kg/day, Sigma, St. Louis, MO), which inhibits NADPH oxidase activity, or vehicle, via a subcutaneously implanted osmotic mini-pump (Alzet®, Cupertino, CA). Urine collection, blood pressure measurement and tissue harvesting were performed as described above.
Acute Renal Specific Down-regulation of D2R
Renal cortical Drd2 was silenced by the subcapsular infusion of Drd2-specific siRNA via an osmotic minipump. Adult male C57BL/6J mice were uni-nephrectomized one week prior to the implantation of the minipump. For the implantation, the mice were anesthetized with pentobarbital (50 mg/kg body weight, intraperitoneally). The osmotic minipumps (100 µl; flow rate: 0.5 µl/hr for 7 days) were filled with validated Drd2-specific siRNA (delivery rate 3 µg/day) or non-silencing siRNA as control. The siRNAs were dissolved in an in vivo transfection reagent (TransIT® In Vivo Gene Delivery System, Mirus) under sterile conditions. The minipumps were fitted with a polyethylene delivery tubing (Alzet #0007701) and the tip of the tubing was inserted within the subcapsular space of the remaining kidney. Surgical glue was applied at the puncture site to hold the tubing in place and prevent extra-renal leakage. The osmotic pump was sutured to the abdominal wall to prevent excessive movement of the pump for the duration of the study.
Silencing of Drd2 was also performed in mice that did not undergo unilateral nephrectomy. Drd2-specific siRNA was infused, as described above, under the capsule of the left kidney of C57BL/6J mice while the right kidney was left undisturbed. In both groups, blood pressure was measured, as above, before and after the 7-day siRNA infusion. Tissues were harvested after the last blood pressure determination.
Urine Measurements
Urinary levels of IL-6 and IL-10 (SABiosciences-Qiagen, Frederick, MD) and albumin (Albuwell M, Exocell, Philadelphia, PA) were determined by ELISA, the latter using an antibody specific for murine albumin. Values were corrected for urinary creatinine.
Cell Culture
Undifferentiated mouse cells were cultured from progenitor kidney cells, kindly supplied by Dr. Ulrich Hopfer (Case Western Reserve University, School of Medicine), isolated from mouse embryo kidneys following the procedure described by Woost et al. [23]. Differentiated mouse RPTCs were cultured to 60–70% confluence and transfected (Hyperfect, Qiagen, Valencia, CA) with vehicle, non-silencing siRNA (30 nmol/l; All stars, Qiagen) or Drd2 siRNA (30 nmol/l, Qiagen). Cells were studied after 72 h. For other experiments cells were cultured to 90–95% confluence, serum starved for 2 h and treated for 24 h in serum-free medium with vehicle (PBS) or 100 nmol/l Ang II in the presence or absence of 1 μmol/l quinpirole (D2R/D3R agonist), or 1 μmol/l quinpirole plus 1 μmol/l L-741,262 (D2R antagonist) [24].
RNA Extraction and cDNA Preparation
Kidney samples were homogenized, and total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA) and further purified using the RNeasy RNA Extraction Mini kit (Qiagen). RNA samples were converted into first strand cDNA using an RT2 First Strand kit, following the manufacturer’s protocol (SABiosciences-Qiagen).
Gene Expression Profiling of Inflammatory Cytokines and Receptors
Gene expression analysis was carried out in groups of four mice using an RT2 Profiler PCR array system (SABiosciences-Qiagen) that contained a panel of 84 genes. Real-time PCR was performed following the manufacturer’s protocol. Quality controls were all within the recommended range. Data were analyzed by the Δ Ct method [25].
Quantitative Real-time PCR
Quantitative gene expression was analyzed by real-time PCR, performed on an ABI Prism 7900 HT (Applied Biosystems, Foster City, CA). The assay used gene specific primers (SABiosciences-Qiagen) and SYBR Green real-time PCR detection method and was performed as described in the manufacturer’s manual. Primers used were as follows: MCP-1: PPM03151F; MCP-2: PPM03165A; TNFα: PPM03113F; Ltα: PPM03114A; IL-4: PPM03013E; IL-5αr: PPM03026E; IL-11: PPM03018E; IL-13: PPM03021A; collagen, type 1, α1 (Col 1α1): PPM-3845F; NFkB1: PPM02930E; osteopontin: PPM03648C; Actin: PPM0294A; GAPDH: PPM02946E. Data were analyzed using the Δ Δ Ct method [25].
Immunoblotting
Mouse kidney homogenates and cell lysates were subjected to immunoblotting, as reported previously [3], [4]. The primary antibodies used were rat anti-mouse TNFα (BioLegend, San Diego, CA), rabbit polyclonal MCP-1 (Millipore, Billerica, CA), rabbit polyclonal IL-6 (Abcam, Cambridge, MA); rabbit polyclonal D2R (Millipore), and polyclonal anti-actin (Sigma). The densitometry values were corrected by the expression of GAPDH and are shown as percentage of the mean density of the control group.
Reporter Assay
NFkB activation was analyzed via the transient expression of an NFkB luciferase reporter system by reverse transfection (Cignal Reporter Assay, SABiosciences-Qiagen). Cells were treated with Drd2-specific siRNA or non-silencing siRNA, as described above. After 48 h, the cells were trypsinized and seeded for reverse transfection. The assay was performed following the manufacturer’s procedures.
Histochemistry and Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues of D2+/+ and D2−/− mice were stained with Masson trichrome to evaluate glomerular fibrosis and with hematoxylin eosin (H–E) to evaluate tubular damage. The pathological abnormalities were graded in a blinded manner. Sclerosis was defined as collapse or obliteration of the glomerular capillary tuft associated with increased hyaline matrix [26]. Glomerular sclerosis was expressed as the percentage of glomeruli showing more than 25% sclerosis.
Tissue sections were immunostained for the presence of macrophages and monocytes using a specific rat anti-mouse macrophage/monocyte monoclonal antibody (Millipore) and an avidin–biotin immunoperoxidase kit (Vectastain Elite, Vector Laboratories, Burlingame, CA). The kidneys were lightly counterstained with hematoxylin. The total number of positive cells in 10 randomly selected fields was counted.
Statistical Analysis
Data are mean ± SEM. Comparisons between 2 groups used the Student’s t test. One-way ANOVA followed by post-hoc analysis using the Newman–Keuls multiple comparison test was used to assess significant differences among three or more groups. P<0.05 was considered statistically significant.
Results
Renal Injury and Inflammation Occurs in D2−/− Mice
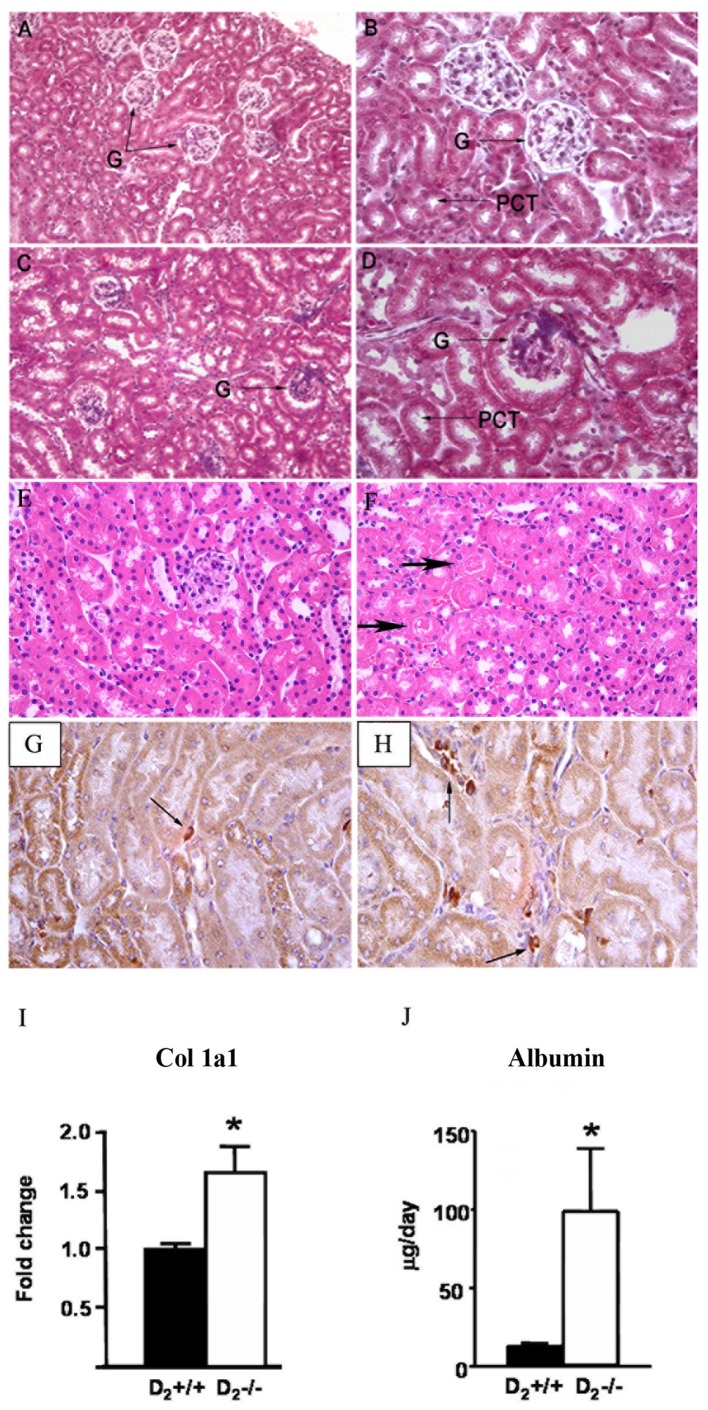
Masson staining of D2−/− mouse kidney sections showed glomerulosclerosis and dilation of renal tubules (Fig 1C–D). H-E staining showed the presence of tubular proteinaceous casts (Figure 1F). These lesions were not observed in D2+/+ mice ( Figure 1A, B,E ). The percentage of glomeruli showing more than 25% sclerosis was greater in D2−/− than D2+/+ mice (35±9% vs. 5±6%, P<0.01). There were more infiltrating macrophages/monocytes in kidney sections from D2−/− mice ( Figure 1H ) than D2+/+ mice ( Figure 1G (68±3 vs.15±1 positive cells/10 fields, P<0.01). The level of mRNA expression of Col 1α1 was about 60% higher in renal cortex of D2−/− than D2+/+ mice ( Figure 1I ). Microalbuminuria, a functional parameter of renal damage, was 9-fold higher in D2−/− mice than in D2+/+ littermates ( Figure 1J ).
Figure 1. Renal inflammation and injury in D2−/− mice.
Masson stained sections of D2+/+ mouse kidney (A and B) and D2−/− mouse kidney (C and D). H-E stained sections of D2+/+ mouse kidney (E) and D2−/− mouse kidney (F). G: glomerulus. PCT: proximal convoluted tubule. Proteinaceous casts are marked with arrows (F). Sections from 3 mouse kidneys per group were studied. G and H: Inflammatory cell infiltration. Kidney sections from D2+/+ (G) and D2−/− (H) mice were immunostained for the presence of macrophages and monocytes (arrows). The number of positive cells in 10 randomly selected fields was greater in D2−/− (68±3) than in D2+/+ (15±1, P<0.01) mice. Sections from 3 mouse kidneys per group were studied. I. Renal cortical expression of Col 1α1 mRNA determined by qRT-PCR. Results were corrected for expression of GAPDH mRNA and expressed as fold change in comparison to their expression in D2+/+ mice. *P<0.05 vs D2+/+; n = 5/group. J. Urinary microalbuminuria. Urine samples were collected for 24 h from mice in metabolic cages. Albumin was measured by ELISA. *P<0.04 vs. D2+/+; n = 5/group. Magnification: A and C: 100X; B, D, G and H: 400X; E-F: 200X.
The Expression of Chemokines and Cytokines Involved in Macrophage Recruitment and Inflammation is Increased in the Renal Cortex but not in the Left Ventricle of the Heart of D2−/− Mice
Expression of 84 cytokines and chemokines was analyzed in the renal cortex of D2−/− and D2+/+ mice using a quantitative RT- PCR (qRT-PCR) array. Twenty one genes were up-regulated and 15 were down-regulated in D2−/− mice ( Table 1 ). Of the genes that were up-regulated, 10 belong to the C-C subfamily of chemokines, including four of the macrophage chemoattractant group and three of the TNF superfamily. IL-10 and IL-18 genes were also up-regulated. Seven of the 15 down-regulated genes were interleukins ( Table 1 ). Most of the up-regulated chemokines are inflammatory and belong to the CCL subfamily, involved in macrophage (MCP-1, MIP-1α, RANTES, MCP-2, MCP-5) and/or T cell (Eotaxin-1, TARC, MIP-3α, CCL-25) recruitment, as opposed to homeostatic [27]. Some of the chemokines, belonging to the CXCL superfamily that attract neutrophils, were also up-regulated (MIG, IP-10, I-TAC) [28]. Three of the four members of the TNF superfamily of inflammatory cytokines were up-regulated, namely TNFα, lymphotoxin-α (Ltα), and lymphotoxin-β (TNFβ). CD40L, the other member of the superfamily included in the array, was decreased. In contrast to the increased expression of pro-inflammatory chemokines, several anti-inflammatory interleukins (IL-4, IL-11, IL13, and IL-17B which stimulates IL-11) were decreased, except for IL-10 which was increased ( Table 1 ).
Table 1. Gene expression profiling of cytokines, chemokines and receptors in the kidney of D2+/+ and D2−/− mice.
| Genes | Fold change | |
| Up-regulated | ||
| Ccl2 | Chemokine (C-C motiv) ligand 2 (MCP-1) | 1.87 |
| Ccl8 | Chemokine (C-C motiv) ligand 8 (MCP-2) | 1.95 |
| Ccl7 | Chemokine (C-C motiv) ligand 7 (MCP-3) | 2.19 |
| Ccl12 | Chemokine (C-C motiv) ligand 12 (MCP-4) | 2.78 |
| Tnfα | Tumor necrosis factor alpha | 1.65 |
| Ltα | Lymphotoxin α (Lta/TNF β) | 1.41 |
| Ltβ | Lymphotoxin β (Ltb/TNF C) | 2.02 |
| Cxcr5 | Chemokine (C-X-C motif) receptor 5 | 2.19 |
| Ccl11 | Chemokine (C-C motif) ligand 11 (eotaxin-1) | 2.68 |
| Ccl17 | Chemokine (C-C motif) ligand 17 | 2.37 |
| Ccl20 | Chemokine (C-C motif) ligand 20 | 3.09 |
| Ccl25 | Chemokine (C-C motif) ligand 25 | 2.75 |
| Ccr7 | Chemokine (C-C motif) receptor 7 | 2.61 |
| Cxcl9 | Chemokine (C-X-C motif) ligand 9 (MIG) | 2.16 |
| Ccl5 | Chemokine (C-C motif) ligand 5 (RANTES) | 1.59 |
| Ccl4 | Chemokine (C-C motif) ligand 4 (MIP-α) | 1.64 |
| Cxcl10 | Chemokine (C-X-C motif) ligand 10 | 1.72 |
| Cxcl11 | Chemokine (C-X-C motif) ligand 11 | 1.71 |
| Il-10 | Interleukin 10 | 1.78 |
| Il-18 | Interleukin 18 | 2.07 |
| Il-5 rα | Interleukin 5 receptor, α | 3.13 |
| Down-regulated | ||
| Ccl1 | Chemokine (C-C motif) ligand 1 | −2.46 |
| Ccl24 | Chemokine (C-C motif) ligand 24 | −2.00 |
| Ccr1 | Chemokine (C-C motif) receptor 1 | −2.27 |
| Crp | C-reactive protein, pentraxin-related | −2.49 |
| Pf4 | Platelet factor 4 | −2.03 |
| Cxcl12 | Chemokine (C-X-C motif) ligand 12 | −1.75 |
| Il-11 | Interleukin 11 | −2.02 |
| Il-13 | Interleukin 13 | −3.20 |
| Il-17B | Interleukin 17B | −3.57 |
| Il-20 | Interleukin 20 | −5.70 |
| Il-3 | Interleukin 3 | −2.36 |
| Il-4 | Interleukin 4 | −1.71 |
| Il-1f6 | Interleukin 1 family, member 6 | −2.43 |
| Il-8rβ | Interleukin 8 receptor, | −3.72 |
| Cd40lg | CD40 ligand | −2.90 |
Fold-change was calculated by the Δ Ct method. n = 3/group.
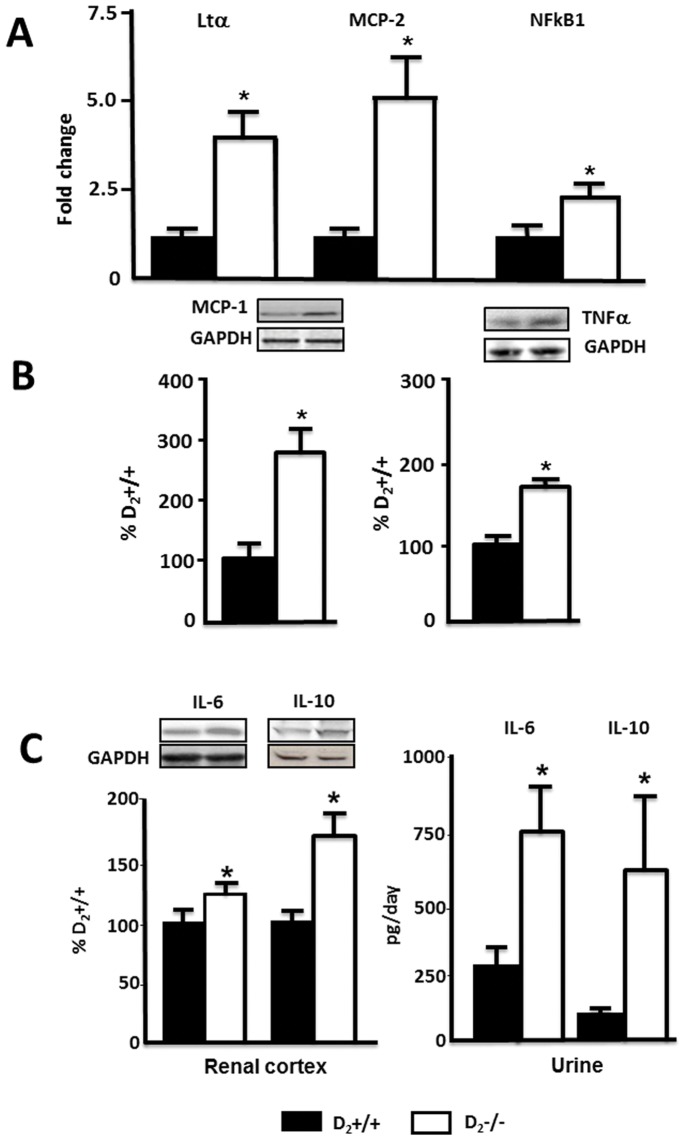
Further experiments were focused on the TNF and MCP families and on IL-6 and IL-10, both of which are downstream TNFα, and on NFkB, which is activated and increased by TNFα transcription [29], [30]. IL-6 is involved in the development of renal inflammation and injury [31], and IL-10 has potent anti-inflammatory properties, repressing the expression of TNFα, IL-6, and IL-1 [32]. We also quantified the expression of p50, the DNA binding subunit of NFkB protein complex, a parameter of NFkB activation [33]. Increased renal cortex expression of Ltα, MCP-2, and NFkB1 (p50) in D2−/− mice was confirmed by qRT-PCR and found to be four-, five-, and two -fold higher, respectively, than in D2+/+ ( Figure 2A ). Increased protein expression of MCP-1 (270±30 vs 100±15%) and TNFα (163±7 vs 100±3%) was confirmed by western blot ( Figure 2B ). Protein expressions of IL-6 and IL-10 in renal cortex were also increased by about 30% and 60% respectively, and urinary excretion of IL-6 was about three-fold higher while that of IL-10 was about five-fold higher in D2−/− than in D2+/+ mice ( Figure 2C ). Decreased renal cortical mRNA expression of IL-4, IL-11, and IL-13 was also confirmed by qRT-PCR (data not shown).
Figure 2. Expression of chemokines/cytokines in renal cortex and urine of D2−/− mice. A.
. Expression of Ltα, MCP-2, and NFkB1 mRNA was quantified by qRT-PCR; results were corrected for expression of GAPDH mRNA and expressed as fold change in comparison to their expression in D2+/+ mice. *P<0.03 vs. D2+/+ mice. B. Protein expression of MCP-1 (17 kDa) and TNFα protein (25 kDa) was semi-quantified by immunoblotting. Inset shows one set of immunoblots. Results were corrected for expression of actin and expressed as percentage of the expression in D2+/+ mice, *P<0.02 vs. D2+/+ mice, n = 5/group. C. Protein expression of IL-6 (25 kDa) and IL-10 (20 kDa) protein semi-quantified by immunoblotting. Results were corrected for expression of actin and expressed as percentage of the expression in D2+/+, * P<0.05 vs. D2+/+ mice, n = 5/group Urinary excretion of IL-6 and IL-10 was quantified by ELISA. *P<0.02 vs. D2+/+ mice, n = 5/group.
The gene expression of chemokines/cytokines in the heart left ventricle was also determined by qRT-PCR. The expressions of MCP-1, MCP-2, TNFα, and Ltα, as well as IL-11, IL-13, and IL-5 receptor α, were similar in D2−/− and D2+/+ mice ( Table 2 ). This indicated that renal alterations in pro- and anti-inflammatory factors in D2−/− mice were organ specific and not caused by systemic perturbations.
Table 2. Expression of cytokines and chemokines in the heart left ventricle of D2+/+ and D2−/− mice determined by qRT-PCR.
| ΔCt D2+/+ | ΔCt D2−/− | Fold change | P | |
| MCP-1 | 5.6±0.3 | 6.6±0.7 | 0.49 | NS |
| MCP-2 | 8.6±1.3 | 8.8±0.3 | 0.83 | NS |
| Tnfα | 10.5±0.5 | 10.7±1.8 | 0.86 | NS |
| Ltα | 11.7±1.2 | 12.0±0.3 | 0.79 | NS |
| Il-5 ra | 13.6±0.4 | 13.9±1.8 | 0.83 | NS |
| IL-11 | 13.4±0.8 | 13.3±1.9 | 1.05 | NS |
| IL-13 | 14.0±0.4 | 14.2±1.4 | 0.90 | NS |
Fold-change was calculated by the ΔΔCt method. Abbreviations as in Table 1. NS = not significant; n = 5/group.
Decreasing Blood Pressure and ROS does not Normalize the Expression of Inflammatory Factors in Renal Cortex of D2−/− Mice
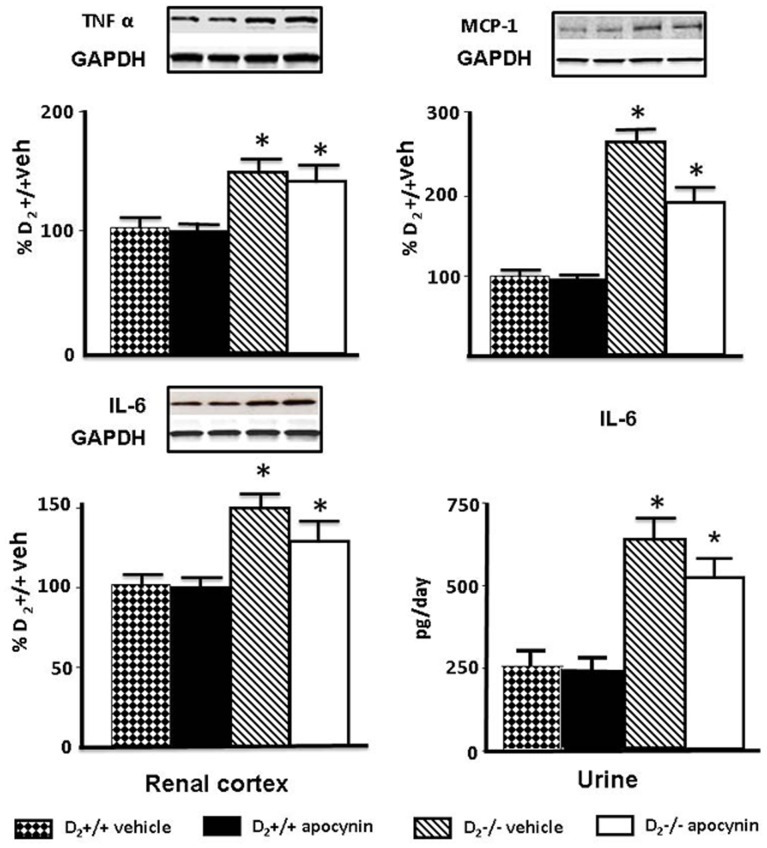
Treatment with apocynin decreased systolic blood pressure in D2−/− mice (vehicle: 121±5; apocynin: 96±2 mm Hg; n = 5; P<0.05) but not in D2+/+ mice (vehicle: 98±3; apocynin 95±5 mmHg; n = 5). Apocynin also decreased the urinary excretion of the oxidative stress marker 8-isoprostane in D2−/− mice (vehicle: 3166±456; apocynin: 1874±553 pg/mg creatinine; n = 5, P<0.04) to levels similar to those in wild-type mice (vehicle: 1344±365; apocynin: 1542±280 pg/mg creatinine; n = 5). Treatment with apocynin, however, did not normalize the expression of TNFα, MCP-1, or IL-6 in D2−/− mice. TNFα expression in renal cortex was higher in vehicle-treated D2−/− than vehicle- treated D2+/+ mice; apocynin had no effect on TNFα expression in D2+/+ or D2−/− mice. MCP-1 protein expression was also higher in vehicle-treated D2−/− than in vehicle-treated D2+/+ mice; apocynin had no effect on MCP-1 expression in D2+/+ mice but decreased it in D2−/− mice although not to the level observed in D2+/+ mice ( Figure 3 ). Renal cortical IL-6 protein expression and urinary excretion of IL-6 were also higher in vehicle-treated D2−/− than in vehicle-treated D2+/+ mice; apocynin had no effect on IL-6 in D2+/+ mice but modestly decreased its levels in D2−/− mice although they remained higher than D2+/+ mice ( Figure 3 ).
Figure 3. Effect of apocynin on renal cortical expression of TNFα, MCP-1, and IL-6, and urinary excretion of IL-6.
Expression of TNFα (25 kDa) and MCP-1 (17 kDa) protein in renal cortex was semi-quantified by immunoblotting. Inset shows one set of immunoblots. Results were corrected for expression of GAPDH and expressed as percentage of D2+/+ mice treated with vehicle, *P<0.05 vs. vehicle or apocynin treated D2+/+; n = 5/group. Renal expression, semi-quantified by immunoblotting (25 kDa), and urinary excretion of IL-6 quantified by ELISA in 24 h urine samples. Results are expressed as percentage of D2+/+ mice treated with vehicle. *P<0.05 vs. vehicle or apocynin treated D2+/+; n = 5/group.
Drd2 Silencing in Mouse RPTCs Results in Increased NFκB Transcriptional Activity and TNFα and MCP-1 Expression
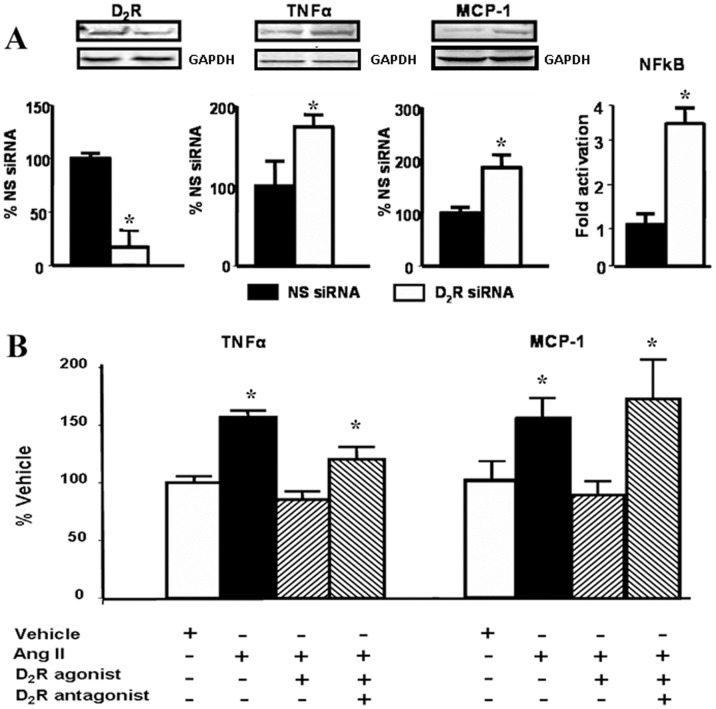
Mouse RPTCs in culture endogenously express D2R, TNFα, and MCP-1. Forty-eight hour-treatment with Drd2 siRNA decreased D2R protein expression by about 85%. The treatment increased NFkB transcriptional activity (3.5-fold) and about two-fold the expression of both TNFα, and MCP-1 which are downstream of NFkB ( Figure 4A ).
Figure 4. D2R function in moue renal proximal tubule cells A.
Effect of silencing of D2R on the expression of pro-inflammatory cytokines/chemokines in mouse RPTCs. Cells were cultured to 60–70% confluence and transfected with non-silencing (NS siRNA) or Drd2 siRNA. After 48 h the cells were washed and lysed. Protein expression of D2R (55 kDa), TNFα (25 kDa), and MCP-1(17 kDa) was semi-quantified by immunoblotting. Inset shows one set of immunoblots. NFkB activation was analyzed via the transient expression of a NFkB-luciferase reporter system by reverse transfection Results are expressed as percentage of NS siRNA or fold activation compared to NS siRNA. *P<0.05 vs. NS (non-silencing) siRNA, n = 4/group. B. Effects of Ang II and D2R stimulation on TNFα and MCP-1 in mouse RPTCs. Cells were serum starved for 2 h before treatment for 24 h in serum-free medium with vehicle (PBS) or 100 nM Ang II, in the presence or absence of 1 μM quinpirole (D2R/D3R agonist) or 1 μM quinpirole plus 1 μM L-741,262 (D2R antagonist). Expression of TNFα (25 kDa) and MCP-1 (17 kDa) protein was semi-quantified by immunoblotting. Inset shows one set of immunoblots. Results were corrected for actin and expressed as % of vehicle. * P<0.05 vs. vehicle; n = 6/group.
Stimulation of D2R Counteracts the Effects of Ang II in Mouse RPTCs
Treatment with Ang II (100 nmol/l) increased the expression of TNFα by about 50% and that of MCP-1 about 60% in mouse RPTCs. Treatment with quinpirole (1 μmol/l), a D2R/D3R agonist, prevented the stimulatory effect of Ang II on the expression of TNFα and MCP-1. The effect of quinpirole was blocked by the addition of L-741,262, a selective D2R antagonist ( Figure 4B ).
Renal Specific Drd2 Down-regulation Recapitulates the Effects of Germline Drd2 Knockout on Inflammatory Factors Independently of Changes in Blood Pressure
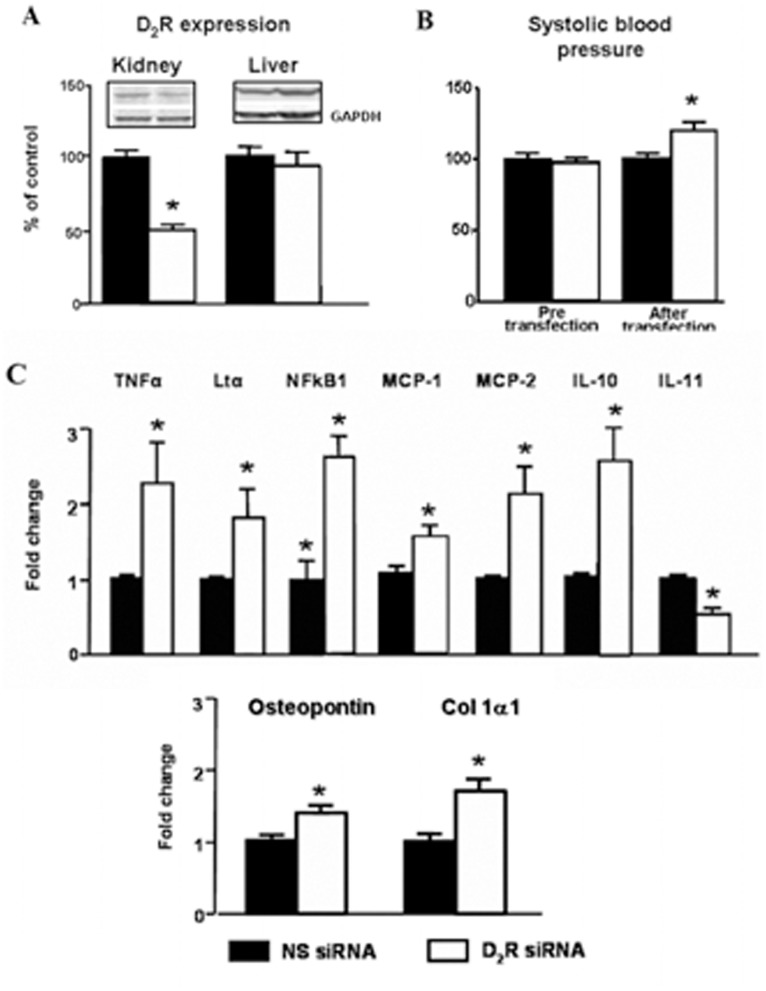
To determine further the role of D2R in the renal inflammatory reaction, we acutely and selectively silenced renal Drd2s in mice in order to avoid the confounding effects of systemic D2R deletion. Infusion of Drd2 siRNA for seven days in uni-nephrectomized mice decreased renal cortical expression of D2R by 50% but did not affect the expression of the receptor in the liver, indicating renal selectivity of the down-regulation ( Figure 5A ). As with systemic Drd2 deletion, treatment with Drd2 siRNA increased systolic blood pressure by about 20 mmHg ( Figure 5B ), an increase of the same magnitude of that observed in mice with systemic Drd2 deletion [3], [4]. This highlights the role of D2R in the regulation of blood pressure via the kidney. Subcapsular renal Drd2 silencing in uni-nephrectomized mice increased renal cortical mRNA expression of TNFα, Ltα, NFkB1, MCP-2 and IL-10, and simultaneously decreased the expression of IL-11. These results are similar to those found in mice with systemic Drd2 deletion, confirming the role of renal D2R in the regulation of the expression of inflammatory factors. Furthermore, the expression of osteopontin and Col 1α1, markers of tissue damage [34], was also increased in the kidneys with silenced D2Rs ( Figure 5C ).
Figure 5. Effect of selective renal silencing of D2R in the remaining kidney of uni-nephrectomized mice on blood pressure and expression on inflammatory factors in the kidney and liver.
Renal cortical Drd2 was silenced by the renal subcapsular infusion for seven days of Drd2 siRNA, via an osmotic minipump in uni-nephrectomized adult male C57BL/6J mice (see Methods). A. Expression of D2R protein (55 kDa band) in renal cortex and liver was semi-quantified by immunoblotting. Results were corrected for GAPDH and expressed as % of non-silencing siRNA treated kidneys. * P<0.05 vs non-silencing (NS) siRNA; n = 5/group. B. Systolic blood pressure measured under anesthesia in mice before and seven days after Drd2 siRNA infusion. * P<0.05 vs, NS siRNA; n = 5/group. C. Renal cortical expression of TNFα, Ltα, NFkB1, MCP-1, MCP-2, IL-10, IL-11 osteopontin, and Col 1α1 mRNA was quantified by qRT-PCR, results corrected for expression of GAPDH mRNA, and expressed as fold change in comparison to their expression in mice treated with NS siRNA. *P<0.05 vs. NS; n = 5/group.
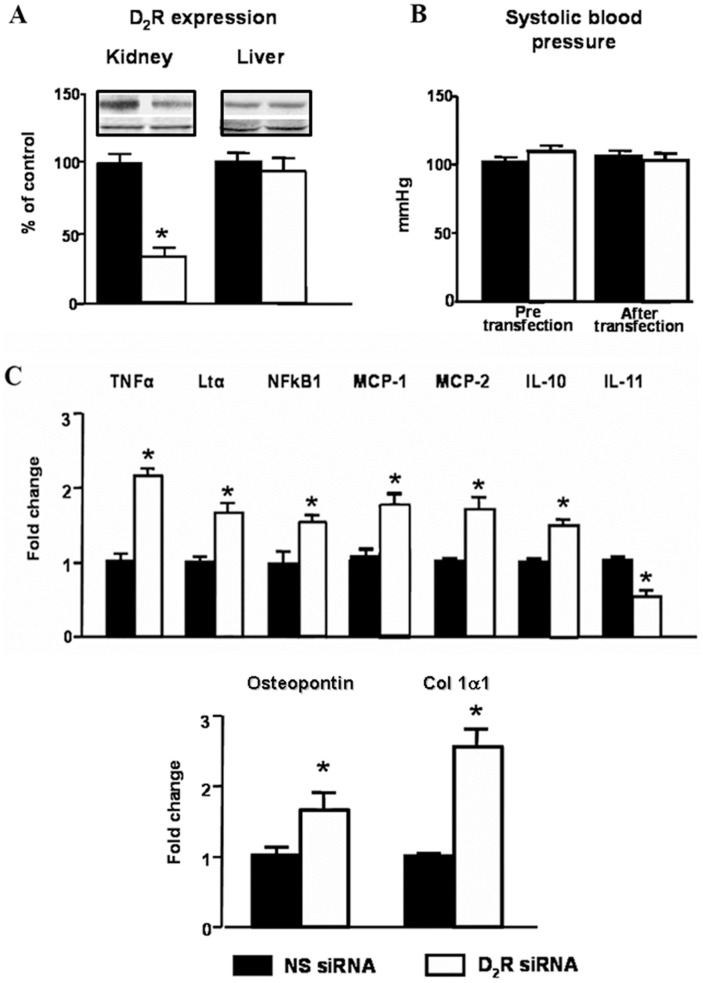
In order to eliminate the confounding effect of uni-nephrectomy and the increase in blood pressure in the above experiments, we also studied the effect of chronic unilateral renal subcapsular infusion of Drd2 siRNA in mice with two intact kidneys. Selective down-regulation of Drd2 in one kidney ( Figure 6A ) had no effect on systolic blood pressure ( Figure 6B ), suggesting that the intact kidney, in the short-term, is able to compensate for the effects of decreased Drd2 expression in the treated kidney. The mRNA expression of TNFα, Ltα, NFκB1, MCP-1 and MCP-2 was increased in the treated kidney to the same extent as in treated uni-nephrectomized mice; NFkB1 and IL-10 were increased but to a lesser extent than in uni-nephrectomized mice. The mRNA expression of IL-11 was similarly decreased. In contrast the expression of the injury markers osteopontin and Col 1α1 was increased to a greater extent than in infused remnant kidney of uni-nephrectomized mice ( Figure 6C ).
Figure 6. Effect of selective renal silencing of D2R in one kidney of mice without uni-nephrectomy on blood pressure and expression of inflammatory factors in the kidney and liver.
Renal cortical D2R was silenced by the renal subcapsular infusion in the left kidney for seven days of Drd2 siRNA, via an osmotic minipump in adult male C57BL/6J mice (see Methods). A. Expression of D2R protein (55 kDa band) in renal cortex and liver was semi-quantified by immunoblotting. Results were corrected for GAPDH and expressed as % of NS siRNA treated kidneys. * P<0.05 vs non-silencing NS siRNA; n = 4/group. B. Systolic blood pressure measured under anesthesia in mice before and seven days after Drd2 siRNA infusion; n = 5/group. C. Renal cortical expression of TNFα, Ltα, NFkB1, MCP-1, MCP-2, IL-10, IL-11, osteopontin and collagen 1α1 mRNA was quantified by qRT-PCR, results corrected for expression of GAPDH mRNA, and expressed as fold change in comparison to their expression in mice treated with NS siRNA. *P<0.05 vs. NS; n = 5/group.
Discussion
Our results show increased renal expression of pro-inflammatory and decreased expression of anti-inflammatory cytokines/chemokines, as well as histological and functional evidence of renal inflammation and injury in mice lacking D2Rs. These alterations are renal-specific and are mimicked in mouse RPTCs in which the Drd2 is silenced. Moreover, selective unilateral renal D2R down-regulation in mice with two kidneys, in the absence of elevated blood pressure, reproduced the alterations in inflammatory factors and renal injury observed in D2−/− mice. Thus, our findings indicate that D2Rs in the kidney have a direct and significant role in regulating the mechanisms involved in the development of renal inflammation and injury, as well as in blood pressure control.
Chemokines that play an essential role in the direct migration of various types of immune cells were up-regulated in kidneys of D2−/− mice, Drd2-silenced kidneys and RPTCs. In several models of renal injury, MCP-1 and RANTES are expressed in damaged renal tissues and precede the recruitment of inflammatory cells that is a characteristic of many kidney diseases [7]. The infiltrating cells mediate the initiation and progression of injury by direct cytotoxicity, secretion of pro-inflammatory cytokines, and the induction of other pro-inflammatory mediators in renal tubule cells.
The increased gene transcription/protein expression of inflammatory factors with Drd2 silencing may be caused by decreased D2R-dependent inhibition leading to increased production of TNFα, a major regulator of cytokine/chemokine expression. Experimental and clinical studies have demonstrated the role of TNFα as a mediator of inflammatory tissue damage in the pathogenesis of acute and chronic renal disease. TNFα is released from renal cells in response to injury and induces glomerular fibrin deposition, cellular infiltration, and vasoconstriction [35] but causes marked natriuresis [36]. TNFα stimulation increases the expression of IL-6, IL-10, and MCP-1 [22]. In immune cells, TNFα production is decreased by dopamine and D2R agonists [21] and in adrenal cortical cells, dopamine, through the D2R, inhibits basal and secretagogue-stimulated TNFα. Our results in mouse RPTCs showing increased basal TNFα expression in response to Drd2 silencing and inhibition of Ang II-induced TNFα stimulation by D2R activation, indicate that in RPTCs the D2R negatively regulates both basal and Ang II-stimulated TNFα production.
TNFα and other members of the TNF superfamily regulate the expression of a large number of cytokines and chemokines by several mechanisms [37], one of which is the activation and nuclear translocation of NFkB [38]. NFκB, which is activated by TNFα, mediates the inflammatory response to TNFα, IL-1β, and other inflammatory factors in renal cells [33]. In turn, the transcription of TNFα and TNF superfamily members is increased by NFkB activation, generating a positive-feedback loop of activation [39]. Our data show that deficient D2R expression results in NFkB activation, as indicated by the increased renal expression of NFkB1 (p50) and NFkB transcriptional activity in mouse RPTCs. NFkB has been implicated as a factor in diabetic nephropathy [40]. Because the D2R has been shown to positively regulate NFkB activation in neural-derived cell lines [41], [42] it is likely that the negative regulation observed in the current studies is mediated by its direct effects on TNFα expression and function. Most of the down-regulated cytokines in the renal cortex of D2−/− mice are Th2-type cytokines (e.g., IL-4 and IL-13); the transcription of these cytokines is mainly dependent on factors other than TNFα or NFkB [43] and is negatively regulated by Th1-type cytokines [44].
The hypertension noted in D2−/− mice is at least partially related to increased renal production of ROS [4]. To evaluate the potentially confounding effect of high blood pressure and ROS on renal inflammation, we treated D2−/− mice with apocynin, which normalized both blood pressure and ROS production [4] as it does in several experimental models of hypertension [45]. Apocynin had no significant effect on the expression of TNFα, and IL-6, although it decreased MCP-1 expression. These results suggest that, in D2−/− mice, high blood pressure or increased ROS may contribute but neither is the major cause of the increased expression of pro-inflammatory factors. However, an effect of persistent inflammation due to preexisting hypertension cannot be ruled out.
The selective unilateral renal silencing of D2R for seven days, in mice with two kidneys, did not increase blood pressure but nonetheless increased renal expression of pro-inflammatory chemokines/cytokines and decreased expression of the anti-inflammatory, IL-11. This indicates that hypertension, per se, is not necessary for the development of renal inflammation but may be a contributing factor. Moreover, the expression of the anti-inflammatory, IL-10, was increased, indicating some compensatory feed-back mechanism. Nevertheless, our results show that impaired D2R function (due to decreased D2R expression) results in a defective balance of pro-inflammatory and anti-inflammatory factors that contribute to renal inflammation and injury.
As mentioned above, intrarenal dopamine buffers the deleterious effects of Ang II on renal inflammation and injury [16], [17]. Our results suggest that these effects are mediated by the D2R. Infusion of Ang II in rats increases TNFα production in renal glomerular endothelial cells, tubules, and vessels, and enhances expression of MCP-1 [22]. Stimulation of the D2R reversed the increased expression of TNFα and MCP-1 elicited by Ang II in mouse RPTCs, indicating that D2R may counterbalance the damaging effect of Ang II in the kidney.
The current studies contribute to the understanding of the mechanisms that cause the development of renal inflammation, as well as the development and maintenance of hypertension [5] and suggest that decreased D2R function may play a significant role in these processes. Deficient renal D2R function may be of clinical relevance since polymorphisms of the Drd2 gene, that are commonly observed in humans, result in decreased D2R expression and function as a consequence of decreased D2R mRNA stability and decreased synthesis of the receptor or decreased receptor affinity [46]–[50]. Some of the D2R polymorphisms are associated with elevated blood pressure and essential hypertension [51]–[53]. Moreover, a recent study in an Asian Indian population with type 2 diabetes found that a D2R polymorphism, resulting in decreased expression of the receptor, confers susceptibility to chronic diabetic nephropathy [54]. Further studies are needed to establish the role of D2R polymorphisms in conferring susceptibility to chronic renal disease and to determine whether or not modulation of renal D2R function may be an option in the treatment of hypertension and renal injury.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by grants from the National Institutes of Health, HL068686, HL023081, HL074940, HL092196, DK039308 and DK090918 (www.NIH.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jose PA, Soares-da-Silva P, Eisner GM, Felder RA. Dopamine and G protein-coupled receptor kinase 4 in the kidney: Role in blood pressure regulation. Biochim Biophys Acta. 2010;802:1259–1267. doi: 10.1016/j.bbadis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, et al. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–H569. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li XX, Bek M, Asico LD, Yang Z, Grandy DK, et al. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension. 2001;38:303–308. doi: 10.1161/01.hyp.38.3.303. [DOI] [PubMed] [Google Scholar]
- 4.Armando I, Wang X, Villar VA, Jones JE, Asico LD, et al. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension. 2007;49:672–678. doi: 10.1161/01.HYP.0000254486.00883.3d. [DOI] [PubMed] [Google Scholar]
- 5.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140. doi: 10.1161/HYPERTENSIONAHA.110.163576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segerer S, Schlöndorff D. Role of Chemokines for the Localization of Leukocyte Subsets in the Kidney. Semin Nephrol. 2007;27:260–274. doi: 10.1016/j.semnephrol.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Tay YC, Harris DC. Proximal tubule cells stimulated by lipopolysaccharide inhibit macrophage activation. Kidney Int. 2004;66:655–662. doi: 10.1111/j.1523-1755.2004.00786.x. [DOI] [PubMed] [Google Scholar]
- 8.Bendele AM, Spaethe SM, Benslay DN, Bryant HU. Anti-inflammatory activity of pergolide, a dopamine receptor agonist. J Pharmacol Exp Ther. 1991;259:169–175. [PubMed] [Google Scholar]
- 9.Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, et al. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. Int Immunopharmacol. 2003;3:1019–1026. doi: 10.1016/S1567-5769(03)00100-0. [DOI] [PubMed] [Google Scholar]
- 10.Haskó G, Szabó C, Németh ZH, Deitch EA. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a β-adrenoceptor-mediated mechanism. J Neuroimmunol. 2002;122:34–39. doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- 11.Bach F, Grundmann U, Bauer M, Buchinger H, Soltész S, et al. Modulation of the inflammatory response to cardiopulmonary bypass by dopexamine and epidural anesthesia. Acta Anesthesiol Scand. 2002;46:1227–1235. doi: 10.1034/j.1399-6576.2002.461010.x. [DOI] [PubMed] [Google Scholar]
- 12.Birnbaum J, Klotz E, Spies CD, Lorenz B, Stuebs P, et al. Effects of dopexamine on the intestinal microvascular blood flow and leukocyte activation in a sepsis model in rats. Crit Care. 2006;10:R117–124. doi: 10.1186/cc5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu XH, Zellweger R, Wichmann MW, Ayala A, Chaudry IH. Effects of prolactin and metoclopramide on macrophage cytokine gene expression in late sepsis. Cytokine. 2007;9:437–446. doi: 10.1006/cyto.1996.0186. [DOI] [PubMed] [Google Scholar]
- 14.Hoeger S, Gottmann U, Liu Z, Schnuelle P, Birck R, et al. Dopamine treatment in brain-dead rats mediates anti-inflammatory effects: the role of hemodynamic stabilization and D-receptor stimulation. Transpl Int. 2007;20:790–799. doi: 10.1111/j.1432-2277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoeger S, Reisenbuechler A, Gottmann U, Doyon F, Braun C, et al. Donor dopamine treatment in brain dead rats is associated with an improvement in renal function early after transplantation and a reduction in renal inflammation. Transpl Int. 2008;21:1072–1080. doi: 10.1111/j.1432-2277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang MZ, Yao B, Wang S, Fan X, Wu G, et al. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest. 2011;121:2845–2854. doi: 10.1172/JCI57324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S, Yao B, Zhou Y, Yin H, Zhang MZ, et al. Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury. Am J Physiol Renal Physiol. 2012;302:F742–F749. doi: 10.1152/ajprenal.00583.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levite M. Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Curr Opin Pharmacol. 2008;l8:460–471. doi: 10.1016/j.coph.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Morikawa K, Oseko F, Morikawa S. Immunosuppressive activity of bromocriptine on human T lymphocyte function in vitro. Clin Exp Immunol. 1994;95:514–518. doi: 10.1111/j.1365-2249.1994.tb07028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human t-cells and triggers the selective secretion of either IL-10, TNFα or both. J Neuroimmunol. 2005;169:161–171. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Laengle UW, Markstein R, Pralet D, Seewald W, Roman D. Effect of GLC756, a novel mixed dopamine D1 receptor antagonist and dopamine D2 receptor agonist, on TNF-α release in vitro from activated rat mast cells. Exp Eye Res. 2006;83:1335–1339. doi: 10.1016/j.exer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int. 2002;82:S12–22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- 23.Woost PG, Kolb RJ, Finesilver M, Mackraj I, Imboden H, et al. Strategy for the development of a matched set of transport-competent, angiotensin receptor-deficient proximal tubule cell lines. In Vitro Cell Dev Biol Anim. 2006;42:189–200. doi: 10.1290/0511076.1. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson SM, Norton CS, Watson SJ, Akil H, Robinson TE. Amphetamine-evoked c-fos mRNA expression in the caudate-putamen: the effects of DA and NMDA receptor antagonists vary as a function of neuronal phenotype and environmental context. J Neurochem. 2003;86:33–44. doi: 10.1046/j.1471-4159.2003.01815.x. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔ CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Yoneda M, Sanada H, Yatabe J, Midorikawa S, Hashimoto S, et al. Differential effects of angiotensin II type-1 receptor antisense oligonucleotides on renal function in spontaneously hypertensive rats. Hypertension. 2005;48:58–65. doi: 10.1161/01.HYP.0000171587.44736.ba. [DOI] [PubMed] [Google Scholar]
- 27.Bonecchi R, Galliera E, Borroni EM, Corsi M, Locati M, et al. Chemokines and chemokine receptors: an overview. Front Biosci. 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 28.Romagnani P, Beltrame C, Annunziato F, Lasagni L, Luconi M, et al. Role for interactions between IP-10/Mig and CXCR3 in proliferative glomerulonephritis. J Am Soc Nephrol. 1999;10:2518–2526. doi: 10.1681/ASN.V10122518. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L, Sinniah R, I-Hong Hsu S. Pathogenic Role of NFkB activation in tubulointerstitial inflammatory lesions in human lupus nephritis. J Histochem Cytochem. 2008;56:517–529. doi: 10.1369/jhc.7A7368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 31.Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. 2005;312:1170–1178. doi: 10.1124/jpet.104.078659. [DOI] [PubMed] [Google Scholar]
- 32.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, et al. NFkB in renal inflammation. J Am Soc Nephrol. 2010;21:1254–1262. doi: 10.1681/ASN.2010020218. [DOI] [PubMed] [Google Scholar]
- 34.Lea WB, Kwak ES, Luther JM, Fowler SM, Wang Z, et al. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 2009;75:936–944. doi: 10.1038/ki.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- 36.Shahid M, Francis J, Matrougui K, Majid DS. Involvement of tumor necrosis factor-α in natriuretic response to systemic infusion of nitric oxide synthase inhibitor in anesthetized mice. Am J Physiol Renal Physiol. 2010;299:F217–F224. doi: 10.1152/ajprenal.00611.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Verma IM. NFkB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 39.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolycacharide-induced NFkB activation. Science. 2005;309:1954–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 40.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 41.Yang M, Zhang H, Voyno-Yasenetskaya T, Ye RD. Requirement of Gβγ and c-Src in D2 dopamine receptor-mediated nuclear factor-κB activation. Mol Pharmacol. 2003;64:447–455. doi: 10.1124/mol.64.2.447. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi Y, Fukunaga K. Differential regulation of NF-κB, SRE and CRE by dopamine D1 and D2 receptors in transfected NG108-15 cells. J Neurochem. 2003;85:729–739. doi: 10.1046/j.1471-4159.2003.01711.x. [DOI] [PubMed] [Google Scholar]
- 43.Lavender P, Cousins D, Lee T. Regulation of Th2 cytokine gene transcription. Chem Immunol. 2000;78:16–29. doi: 10.1159/000058813. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J. Transcriptional regulation of Th2 cell differentiation. Immunol Cell Biol. 2010;88:244–249. doi: 10.1038/icb.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, et al. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–R1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- 47.Thompson J, Thomas N, Singleton A, Piggott M, Lloyd S, et al. D2 dopamine receptor gene (DRD2) Taq1A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Pohjalainen T, Rinne JO, Någren K, Lehikoinen P, Anttila K, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 49.Jönsson EG, Nöthen MM, Grünhage F, Farde L, Nakashima Y, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 50.Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- 51.Fang YJ, Thomas GN, Xu ZL, Fang JQ, Critchley JA, et al. An affected pedigree member analysis of linkage between the dopamine D2 receptor gene TaqI polymorphism and obesity and hypertension. Int J Cardiol. 2005;102:111–116. doi: 10.1016/j.ijcard.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Rosmond R, Rankinen T, Chagnon M, Pérusse L, Chagnon YC, et al. Polymorphism in exon 6 of the dopamine D(2) receptor gene (DRD2) is associated with elevated blood pressure and personality disorders in men. J Hum Hypertens. 2001;15:553–558. doi: 10.1038/sj.jhh.1001231. [DOI] [PubMed] [Google Scholar]
- 53.Thomas GN, Critchley JA, Tomlinson B, Cockram CS, Chan JC. Relationships between the taqI polymorphism of the dopamine D2 receptor and blood pressure in hyperglycaemic and normoglycaemic Chinese subjects. Clin Endocrinol (Oxf) 2001;55:605–611. doi: 10.1046/j.1365-2265.2001.01404.x. [DOI] [PubMed] [Google Scholar]
- 54.Prasad P, Kumar KM, Ammini AC, Gupta A, Gupta R, et al. Association of dopaminergic pathway gene polymorphisms with chronic renal insufficiency among Asian Indians with type-2 diabetes. BMC Genet. 2008;9:26. doi: 10.1186/1471-2156-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]