Abstract
Osteoporosis (OP) is characterized by low bone mineral density (BMD) and has strong genetic determination. However, specific genetic variants influencing BMD and contributing to pathogenesis of osteoporosis are largely uncharacterized. Current genetic studies in bone filed, which aimed at identification of OP risk genes, are mostly focused on DNA, RNA, or protein level individually, lacking integrative evidences from the three levels of genetic information flow to confidently ascertain the significance of genes for osteoporosis. Our previous proteomics study discovered that superoxide dismutase 2 (SOD2) in circulating monocytes (CMCs, i.e., potential osteoclast precursors) was significantly up-regulated at protein level in vivo in Chinese with low vs. high hip BMD. Herein, at mRNA level, we found that SOD2 gene expression was also up-regulated in CMC (p < 0.05) in Chinese with low vs. high hip BMD. At DNA level, in 1,627 unrelated Chinese subjects, we identified eight SNPs at SOD2 gene locus that were suggestively associated with hip BMD (peak signal at rs11968525, p = 0.048). Among the eight SNPs, three SNPs (rs7754103, rs7754295, and rs2053949) were associated with SOD2 mRNA expression level (p < 0.05), suggesting that they are expression quantitative trait locus (eQTL) regulating SOD2 gene expression. In conclusion, the present integrative evidences from DNA, RNA, and protein levels supported SOD2 as a susceptibility gene for osteoporosis.
Keywords: Osteoporosis, SOD2, eQTL, BMD
Introduction
Osteoporosis (OP), characterized by low bone mineral density (BMD)(1), is a major public health problem over the world (2). Genetic factors play an important role in the pathogenesis of OP, as evidenced by high heritability (≥ 50%) of BMD(3–5). However, specific genetic variants influencing BMD and contributing to development of OP are largely uncharacterized.
Identifying genes for osteoporosis is challenging because of its nature of complex genetic determination, including polygenic determinations, multiple gene-gene interactions, and multiple gene-environment interactions. So far, great attempts have been made to identify OP risk genes, however, most of which were focused on DNA, RNA, or protein levels individually and rarely integrates evidences at multiple molecule levels to ascertain the importance of certain gene(s) for bone phenotypes. For example, traditional genetic and genomics studies established relationship of gene(s) and phenotypes (e.g., BMD) at DNA level only without considering RNA or protein expression, and such established relevance usually does not provide an immediate insight into functions of gene nor gene expression regulation, which bridge gene code information and disease phenotype directly.
Our previous comparative expression proteomic study on circulating monocytes (CMCs, potential osteoclast precursors) identified five proteins differentially expressed in Chinese premenopausal women with extremely discordant hip BMD(6). Such differential expression was further validated by western blotting. These five proteins (RSU1, GSN, SOD2, GPX1, and P4HB) might affect CMCs’ trans-endothelium, differentiation, and/or downstream osteoclast functions, thus contribute to differential osteoclastogenesis and lead to BMD variation. The present study represents our pursuant effort to ascertain the significance of these genes to osteoporosis, with integrative evidences from three molecule levels (i.e., DNA, RNA, protein) of the gene information flow. This study highlights superoxide dismutase 2 (SOD2) as an important susceptibility gene for osteoporosis.
Materials and Methods
Samples
The study was approved by Research Administration of involved institutions. Signed informed consent documents were obtained from all study participants before entering the study. All the study subjects belong to Chinese Han ethnic group. Strict exclusion criteria (7) were adopted to minimize any known and potential confounding effects on variation of bone phenotype. Briefly, patients with chronic diseases/conditions that may potentially affect bone mass or bone metabolism were excluded. These diseases/conditions included chronic disorders involving vital organs (heart, lung, liver, kidney, brain), serious metabolic diseases (diabetes, hypo- or hyperparathyroidism, hyperthyroidism), other skeletal diseases (Paget’s disease, osteogenesis imperfecta, rheumatoid arthritis), chronic use of drugs affecting bone metabolism (corticosteroid therapy, anticonvulsant drugs), and malnutrition conditions (chronic diarrhea, chronic ulcerative colitis). For subjects recruited for gene expression studies, we also excluded diseases or conditions affecting immune system, such as influenza (within one week of recruitment), autoimmune or autoimmune-related diseases such as systemic lupus erythematosus, and immune-deficiency conditions such as AIDS, hematopoietic and lymphoreticular malignancies such as leukemia etc.
Sample 1: for mRNA expression analyses
Based on an archived dataset for a population of 875 Chinese females aged 20–45 years when peak bone mass (PBM) is attained (8;9), we selected top and bottom 100 subjects according to distribution of Z-score of hip BMD. Herein, Z-score is defined as the number of standard deviations a subject’s BMD differs from the average BMD of their age-gender- and ethnicity- matched population. Out of the 100 subjects, 14 subjects with extremely high PBM and 12 subjects with extremely low PBM (1.57±0.57vs. - 1.72±0.60; mean ± S.D., Z-score) were recruited to quantify mRNA expression level.
Sample 2: for SNP association analyses
A total of 1,627 unrelated adult Chinese (age: 34.5 ± 13.2 years), including 802 males and 825 females, were recruited from the cities of Xi’an and Changsha and their surrounding areas. Such sample was originally recruited for genetic studies on complex diseases, including osteoporosis and obesity.
Sample 3: for expression quantitative trait locus (eQTL) analyses
Sample 3 includes 22 subjects that are shared by the above mRNA expression study and SNP association study. Therefore, both SNP data and mRNA expression data were available for those subjects for expression QTL analyses to identify eQTLs which may regulate expression of genes of interest (herein, RSU1, GSN, SOD2, GPX1, and P4HB).
BMD Measurement
For each subject, areal BMD (g/cm2) at the total hip (femoral neck, trochanter, and intertrochanter region) was measured with a Hologic 4500 W dual energy X ray absorptiometer (DEXA) scanner (Hologic Corporation, Waltham, MA, USA). The machines used in this study (one at site of Xi’an, one at site of Changsha) were calibrated daily, and the coefficient of variation for repeated measurements of hip BMD was less than 1.5%.
Monocyte Isolation
For sample 1, peripheral blood mononuclear cells (PBMC) were isolated from 50 ml fresh anti-coagulated peripheral blood using lymphoprep solution (Axis-Shield PoC AS, Oslo, Norway). Then, monocytes were isolated from PBMC using a monocyte negative isolation kit (Dynal Biotech Inc., Lake Success, NY) following the manufacturer’s recommendation. The kit depleted T cells, B cells, and NK cells from mononuclear cells, leaving monocytes untouched and free of the surface-bound antibody and beads. Without antibody-coagulated beads binding to the target cell surface, the isolation procedure avoided activation of the cells and change of gene expression profiles. The purity of the isolated monocyte sample was monitored by BD-FACScalibur flow cytometry (BD Biosciences, San Jose, CA USA) with fluorescence labeled antibodies PE-CD14 and FITC-CD45. The purity was 70%–90% in our subjects (10).
Total RNA Extraction and mRNA Expression Assay
For sample 1, total RNA in monocytes was extracted using Qiagen RNeasy Mini Kit (Qiagen, Inc., Valencia, CA) following the procedures recommended by the manufacturer. The mRNA expression levels for five target genes (RSU1, GSN, SOD2, GPX1, and P4HB) represented by a total of 18 probes were acquired with Affymetrix Human Genome U133 Plus 2.0 Arrays. Brief experimental procedures include: RNA was converted to double-stranded cDNA. In vitro transcription was performed to produce biotin-labeled cRNA (BioArray HighYield RNA Transcription Labeling Kit; Enzo Diagnostics). Biotinylated cRNA was cleaned, fragmented, and hybridized (Affymetrix Genechip Hybridization Oven 640) to the array. Then, microarrays were washed (Affymetrix Fluidics Station 450), stained with phycoerythrin-streptavidin, and scanned using an Affymetrix scanner (Gene Array Scanner 3000). GeneChip Operating Software (GCOS 1.2) was used to process and generate intensity data at probe level (raw data). Robust Multiarray Average (RMA) algorithm(11) was used to transform the probe-level data into gene expression data. Compared with other algorithms, RMA provides the most reproducible results and shows the highest correlation coefficients with RT-PCR data(12).
SNP Genotyping
For sample 2, a total of 199 SNPs (Chr6: 159,613 Kb-160, 061Kb) (Build 36) at the SOD2 gene locus were covered by and genotyped using the Affymetrix Genome-Wide Human SNP Array 6.0. Experimental procedures for the microarray assays were performed using the standard protocol recommended by the manufacturer. Briefly, fluorescence intensities were quantified using an Affymetrix array scanner 3000 7G. Data management and analyses were performed using the Affymetrix® GeneChip® Command Console® Software (AGCC). Contrast QC threshold was set at the default value of greater than 0.4 for quality control. The average ‘contrast QC’ across the entire sample is 2.62. Out of the 199 SNPs, 50 SNPs with allele frequencies deviating from Hardy-Weinberg equilibrium (p < 0.01) and/or with minor allele frequency (MAF) <1.0% were excluded. Thus, 149SNPs were tested in subsequent association analyses.
Statistical Analysis
mRNA differential expression analyses in sample 1
Based on the expression data transformed by the RMA algorithm, Student’s t-tests were conducted to compare the mean expression signals in the low vs. high BMD subjects for probes of the five genes RSU1, GSN, SOD2, GPX1, and P4HB.
Association analyses and linkage disequilibrium (LD) analysis in sample 2
Age, gender, height, and weight, as significant covariates, were used to adjust the raw BMD values for subsequent association analyses. PLINK (http://pngu.mgh.harvard.edu/~purcell/plink/) was used to perform genotypic association analyses, to compare the difference of mean BMD values among carriers of different genotypes at each SNP site. The LD [standardized D′ (D/Dmax)] patterns for SNPs of interest were analyzed and plotted using the Haploview program(13) (http://www.broad.mit.edu/mpg/haploview/).
Expression QTL analyses in sample 3
Based on SNP genotype data and mRNA expression data in sample 3, genotypic association analyses were performed to compare the mean mRNA expression levels among carriers of different genotypes at each SNP sites. This is to identify SNPs that potentially regulate gene expression, i.e., eQTLs.
Results
Table 1 list all the basic characteristics of the studied samples. Our previous comparative expression proteomic study(6) on CMCs, in a sample of 30 unrelated premenopausal Chinese Han females with discordant hip BMD, identified five differentially expressed proteins (RSU1, GSN, SOD2, GPX1, and P4HB), which may play an important role in pathogenesis of osteoporosis.
Table 1.
Basic Characteristics of the Studied Samples
|
* Protein Expression Analyses
|
mRNA Expression Analyses
|
SNP-BMD Association Analyses | eQTL Analyses | |||
|---|---|---|---|---|---|---|
| Low BMD | High BMD | Low BMD | High BMD | |||
| Sample Size | 15 | 15 | 12 | 14 | 1627 | 22 |
| Age (year) | 26.4(4.2) | 29.0(6.4) | 25.3(3.1) | 28.7(4.7) | 34.5(13.2) | 26.9(4.4) |
| Height (cm) | 156.8(4.5)159.4(5.1) | 158.9(4.4) | 158.9(5.3) | 164.3(8.2) | 159.5(4.7) | |
| Weight (kg) | 50.1(6.7) | 54.6(5.7) | 51.5(7.3) | 55.8(5.7) | 60.1(10.5) | 53.4(7.0) |
| Hip BMD (g/cm2) | 0.71(0.05)1.03(0.05) | 0.70(0.06) | 1.03(0.05) | 0.92 (0.13) | 0.84(0.17) | |
Notes: Presented are mean (SD); eQTL: expression quantitative trait locus.
Presented are samples used for discovery in our previous proteomics study (6).
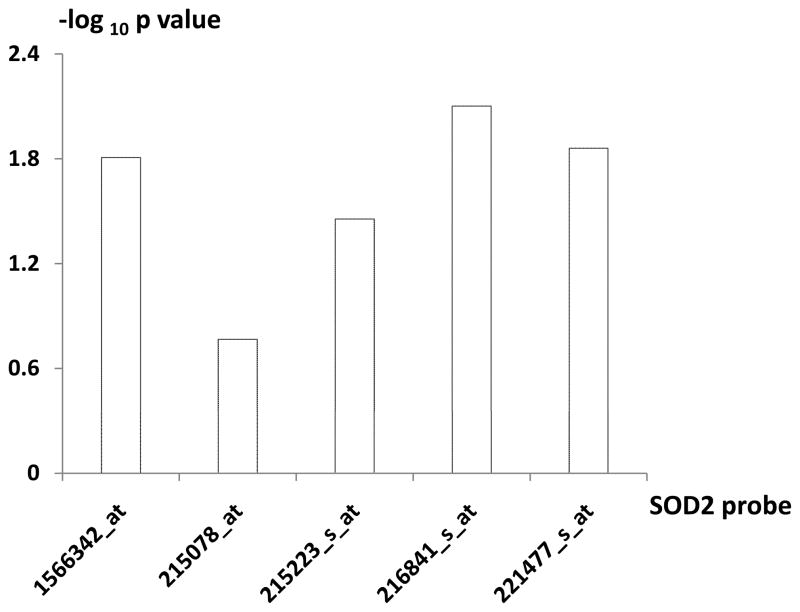
Herein, based on the mRNA expression data in 26 unrelated premenopausal Chinese Han females with discordant hip BMD(10;14), we analyzed expression levels of the five genes (RSU1, GSN, SOD2, GPX1, and P4HB) using a total of 18 expression probes covered by the Affymetrix Human Genome U133 Plus 2.0 Arrays. No evidence of differential expression at mRNA level was found for 13 probes representing genes RSU1, GSN, GPX1, and P4HB (data not shown). However, as shown in Table 2, four of the 5 probes, representing SOD2 gene, consistently showed elevated expression in the low vs. high BMD subjects (p < 0.05) (Figure 1). Notably, the up-regulation of SOD2 gene at mRNA level in low BMD subjects is consistent with that discovered at protein level (6). Therefore, the SOD2 gene was followed-up subsequently.
Table 2.
Expression of SOD2 Gene in the Low vs. HIgh Hip BMD Groups
| SOD2 | Gene Expression | P-value | Expression in high BMD group | Expression in low BMD group | L/H |
|---|---|---|---|---|---|
| mRNA | 1566342_at | 1.56E-02 | 5.02(0.10) | 5.39(0.10) | 1.08 |
| 215078_at | 1.71E-01 | 5.65(0.43) | 6.63(0.56) | 1.18 | |
| 215223_s_at | 3.51E-02 | 9.17(0.18) | 9.95(0.32) | 1.09 | |
| 216841_s_at | 7.92E-03 | 7.77(0.13) | 8.40(0.17) | 1.08 | |
| 221477_s_at | 1.38E-02 | 9.17(0.11) | 9.73(0.19) | 1.06 | |
| Protein | 3.00E-02 | 19807.2 | 28810.2 | 1.45 | |
Notes:
The mRNA expression data were detected with Affymetrix Human Genome U133 Plus 2.0 arrays. RMA algorithm was used to transform the probe-level intensity data into gene expression data.
The values in the “()” are standard error.
The protein expression levels were quantified using western blotting as reported previously (6).
Student’s t-test was performed to compare the expression levels in the two groups of samples.
L/H is the ratio of mean expression levels in the low vs. high BMD groups of samples.
Figure 1.
log Plot of p Value for RNA Differential Expression of SOD2 Five Probes in High vs. Low BMD Groups.
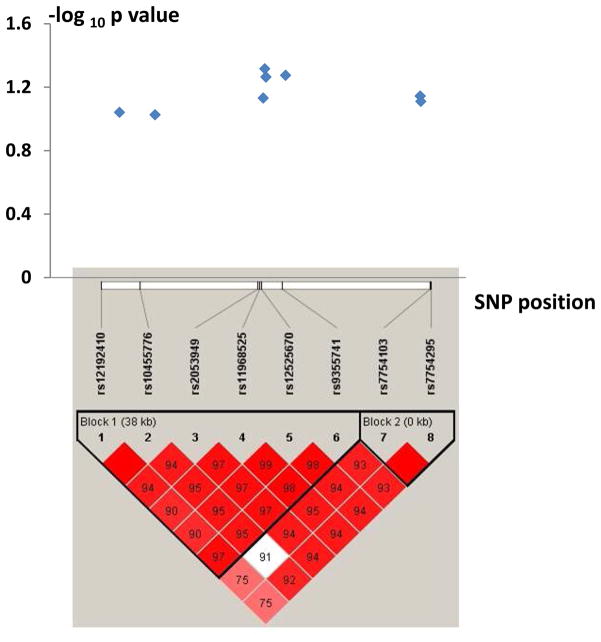
To test the importance of SOD2 gene to osteoporosis at DNA level, we performed a population association study for 149 QC eligible SNPs at SOD2 locus in 1,627 unrelated Chinese Han adults. Genotypic association analyses detected that eight SNPs were suggestively associated with hip BMD (p < 0.1) (Figure 2), with strongest association signals observed at SNP rs11968525 (p = 0.048) (Table 3). Moreover, the eight SNPs cluster into two haplotype blocks, with strong LD among SNPs within a block (Figure 2).
Figure 2.
Association with Hip BMD and Haplotype Blocks for the Eight Interesting SNPs at the SOD2 Locus. Note: (1) The numbers in the boxes are standardized D′ (D/Dmax) between two SNPs, and the empty boxes mean the D′ levels are 1.0.
Table 3.
Associations of SNPs at SOD2 Locus with Hip BMD and with SOD2 mRNA Expression Level
| rs12192410 | rs10455776 | rs2053949 | rs11968525 | rs12525670 | rs9355741 | rs7754103 | rs7754295 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1Allele A/B | G/A | A/C | C/T | T/C | T/C | A/G | A/G | A/G | ||
| 2MAF | 0.18 | 0.07 | 0.41 | 0.19 | 0.18 | 0.29 | 0.10 | 0.10 | ||
| β Values of Association with Hip BMD | 8.23E-03 | 1.26E-02 | −6.94E-03 | 9.50E-03 | 9.31E-03 | −8.03E-03 | 1.14E-02 | 1.11E-02 | ||
| P Values of Association with Hip BMD | 9.10E-02 | 9.42E-02 | 7.39E-02 | 4.83E-02 | 5.44E-02 | 5.31E-02 | 7.18E-02 | 7.76E-02 | ||
| Mean BMD (g/cm2) | AA | 0.931 | 0.991 | 0.914 | 0.942 | 0.937 | 0.903 | 1.005 | 1.005 | |
| Stratified by Genotypes | AB | 0.923 | 0.917 | 0.920 | 0.920 | 0.922 | 0.920 | 0.920 | 0.919 | |
| BB | 0.919 | 0.920 | 0.922 | 0.919 | 0.918 | 0.923 | 0.920 | 0.919 | ||
| Genotypic Effect of SNP on BMD (BB vs. AA)3 | ↓ | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ↓ | ||
| P Value of eQTL Analyses | 215078_at | NS | NS | NS | 8.47E-02 | 8.47E-02 | NS | NS | NS | |
| 215223_s_at | NS | NS | 2.97E-02 | NS | NS | 6.47E-02 | NS | NS | ||
| 216841_s_at | NS | NS | 3.35E-02 | NS | NS | NS | NS | NS | ||
| 221477_s_at | NS | NS | 8.70E-02 | NS | NS | NS | NS | NS | ||
| 1566342_at | NS | NS | 2.18E-01 | NS | NS | NS | 2.60E-02 | 2.60E-02 | ||
| Mean Expression Levels Stratified by Genotypes | 1566342_at | AB/BB | 5.14/5.21 | 5.31/5.15 | 5.17/5.05 | 5.10/5.24 | 5.10/5.24 | 5.28/5.02 | 4.83/5.26 | 4.83/5.26 |
| 221477_s_at | AB/BB | 9.25/9.60 | 9.42/9.49 | 9.40/9.29 | 9.25/9.63 | 9.25/9.63 | 9.61/9.23 | 9.04/9.57 | 9.04/9.57 | |
| 216841_s_at | AB/BB | 7.82/8.24 | 7.83/8.16 | 8.09/7.64 | 7.83/8.26 | 7.83/8.26 | 8.25/7.80 | 7.83/8.14 | 7.83/8.14 | |
| 215223_s_at | AB/BB | 9.17/9.81 | 9.32/9.65 | 9.49/9.03 | 9.16/9.86 | 9.16/9.86 | 9.87/9.06 | 8.95/9.71 | 8.95/9.71 | |
| 215078_at | AB/BB | 5.33/6.47 | 5.56/6.20 | 5.96/5.49 | 5.26/6.60 | 5.26/6.60 | 6.53/5.23 | 4.90/6.31 | 4.90/6.31 | |
| Genotypic Effect of SNP on Expression (BB vs. AB)4 | ↑ | ↑ | ↓ | ↑ | ↑ | ↓ | ↑ | ↑ | ||
Alleles A and B represent minor and major alleles, respectively;
MAF: minor allele frequency in the study sample;
↑ & ↓ increased & decreased BMD in carriers of genotype BB vs. AA;
↑ & ↓ increased & decreased mRNA expression in carriers of genotype BB vs. AB. In the sample for eQTL analyses (N=22), no homozygous minor allele was observed for the SNPs, except SNP rs2053949 with two AA carriers). For all the eight SNPs, the trend of association for SNP and mRNA expression is reverse to that for SNP and BMD. Such findings are consistent with the negative correlation between SOD2 gene expression and BMD observed at both mRNA and protein levels, i.e., higher SOD2 gene expression, lower BMD (Table 2). NS: not significant.
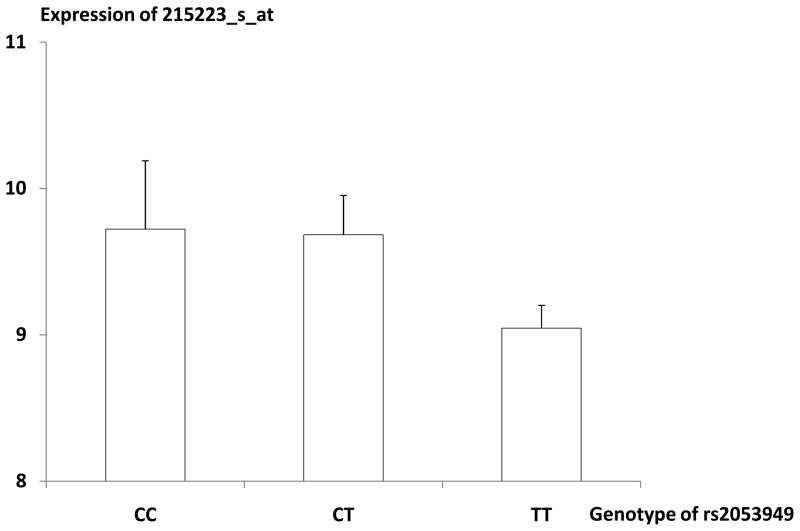
To explore the preliminary mechanisms underlying the associations between SNPs and BMD, we conducted expression QTL analyses to find out whether these detected SNPs at SOD2 genes exert effect on SOD2 gene transcription. As shown in Table 2, in 22 subjects with both SNP data and mRNA expression data for SOD2 gene, three SNPs (rs7754103, rs7754295, and rs2053949) were significantly associated with expression level of SOD2 gene represented by at least one probe (p < 0.05), indicating that these SNPs are potential eQTLs influencing expression of SOD2 gene. Notably, each SNP showed consistent genotype-specific effect, across all the five representative probes, on SOD2 mRNA expression. Take SNP rs2053949 for an example, the five probes consistently showed lower expression in TT carriers than in CT carriers. Interestingly, the trend of genotypic effect on mRNA expression was reverse to that on the phenotype level. Take SNP rs2053949 for an example, compared to CT carriers, TT carriers were associated with lower level of mRNA expression but higher level of BMD (Figure 3). Such observation coincides with the negative association between SOD2 mRNA expression level and BMD level as observed in the Sample 2, i.e., higher expression associated with lower BMD.
Figure 3.
Distribution of Expression Level of SOD2 Probe 215223_s_atin the Different Genotype Groups of rs2053949. Note: mean (SE)
Discussion
The present study, with integrative evidences at DNA and mRNA levels, warrants our previous findings at protein level, and strongly supports the functional relevance of SOD2 gene to osteoporosis in Chinese. Our data highlight a significant role of the SOD2 gene in BMD variation and pathogenesis of osteoporosis. In bone filed, this study represents our first attempt to employ an integrative method to ascertain the significance of a gene to BMD variation and osteoporosis.
SOD2 gene encodes a free radical scavenging enzyme, which removes superoxidate and catalyzes the production of hydrogen peroxide (H2O2). Oxidative stress plays an important role in the pathogenesis of osteoporosis. Reactive oxygen species (ROS) such as H2O2 may contribute to differentiation and formation of osteoclast and activity of mature osteoclasts(15–19). For example, ROS may increase osteoclast differentiation and bone resorption by stimulating the expression of receptor activator of NF-kappaB ligand (RANKL) in human osteoblast-like MG63 cell line(17). Mouse calvarias showed a significant increase in bone resorption after exposure to H2O2 (19). Evidence showed that there was a negative correlation between serum SOD activity and lumbar BMD in osteoporotic patients(20), and osteoporotic women had significantly higher SOD activity in plasma(21). Our previous proteomics study(6) and the present study consistently showed that SOD2 expression in CMCs are inversely associated with hip BMD at both protein and mRNA levels, i.e., the higher the expression level of SOD2 gene in CMCs, the lower the BMD. Collectively, all these evidence strongly highlights the functional significance of SOD2 gene in regulating BMD. Furthermore, this study showed that SNPs of the SOD2 gene, via regulating SOD2 mRNA transcription, are suggestively associated with BMD variation in population.
As a whole, the present work attested successful application of the “integrative” study strategy to ascertain functional relevance of certain gene(s) to bone phenotype variation. As is commonly recognized, in biological systems, genetic information carried by DNA is passed on to RNA molecules via transcription and then to protein molecules through translation. Sequence variants at DNA level represent a class of heritable molecules and contribute to variability of complex traits in population. Such variants at DNA level may lead to variation in quantifiable intermediate phenotypes (such as RNA and protein expression levels), which subsequently lead to variation of end phenotypes, herein BMD. Therefore, integrating substantial evidences from the three levels (i.e., DNA, RNA and protein) could ascertain the potential functioning mechanism of genes and their contribution to variation in complex traits and susceptibility to diseases. From the genetic-information-flow point of view, the findings of the present and previous proteomics studies(6), suggested a possible regulatory mechanism explaining how SOD2 gene contribute to BMD variation in humans. Briefly, different genotypes of SNPs in SOD2 gene, via regulating differential transcription of SOD2 mRNA thus differential protein expression and enzyme activity, consequently effect on osteoclast differentiation, and contribute to differential bone resorption and BMD variation in population.
It is not surprising that no significant differential expression was detected at mRNA level for the other four studied genes (RSU1, GSN, GPX1, and P4HB) that showed significant differential expression at protein level in low vs. high BMD subjects(6). Such low correlation between mRNA and protein expression levels may be due to the complicated regulations when genetic information was transferred from mRNA to protein, which was detailed by Guo et al.(22). Coincidently, our previous study found that correlation between mRNA expression and protein expression in CMCs is moderate and varies at the transcriptome-proteome scale (22).
The association signals at the population are relatively weak, which may be attributed to limited sample size (1627) and minor effect of causal loci. Large sample size is required to increase the statistical power for association analysis.
In conclusion, this study highlights SOD2 gene as a susceptibility gene for osteoporosis. Integration of evidences from genetics, functional genomics, and proteomics, should be fruitful in ascertaining function of genes and uncovering molecular regulatory mechanisms involved in pathogenesis of osteoporosis. Such a study strategy is also applicable to research on other complex traits or diseases.
Acknowledgments
The study was partially supported by Natural Science Foundation of China (NSFC) (30600364, 31071097, 30771222, and 30900810), NSFC-CIHR (Canadian Institutes of Health Research) Joint Health Research Initiative Proposal (30811120436), NSFC/RGC (Research Grants Council) Joint Research Scheme (30731160618), and Shanghai Leading Academic Discipline Project (S30501) and startup fund from Shanghai University of Science and Technology. HWD was partially supported by grants from NIH (R03TW008221, R01AR050496, R01AG026564, R01AR057049, and P50AR055081) and Edward G. Schlieder Endowed Chair.
Footnotes
All other authors have no conflicts of interest.
Contributor Information
Fei-Yan Deng, Email: fdeng@tulane.
Shu-Feng Lei, Email: lei@hunnu.edu.cn.
Xiang-Ding Chen, Email: xdchen2001@hunnu.edu.cn.
Li-Jun Tan, Email: lijuntan2004@yahoo.com.cn.
Xue-Zhen Zhu, Email: hshen4@tulane.edu, chezhenzhen2@gmail.com.
Hong-Wen Deng, Email: hdeng2@tulane.edu.
Reference List
- 1.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ, III, Chrischilles EA, Cooper C, Lane AW, Riggs BL. How many women have osteoporosis? JBMR Anniversary Classic. J Bone Miner Res. 2005;20:886–892. doi: 10.1359/jbmr.2005.20.5.886. [DOI] [PubMed] [Google Scholar]
- 3.Deng HW, Mahaney MC, Williams JT, et al. Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet Epidemiol. 2002;22:12–25. doi: 10.1002/gepi.1040. [DOI] [PubMed] [Google Scholar]
- 4.Liu PY, Qin YJ, Recker RR, Deng HW. Evidence for a major gene underlying bone size variation in the Chinese. Am J Hum Biol. 2004;16:68–77. doi: 10.1002/ajhb.10240. [DOI] [PubMed] [Google Scholar]
- 5.Jian WX, Long JR, Li MX, Liu XH, Deng HW. Genetic determination of variation and covariation of bone mineral density at the hip and spine in a Chinese population. J Bone Miner Metab. 2005;23:181–185. doi: 10.1007/s00774-004-0558-3. [DOI] [PubMed] [Google Scholar]
- 6.Deng FY, Liu YZ, Li LM, et al. Proteomic analysis of circulating monocytes in Chinese premenopausal females with extremely discordant bone mineral density. Proteomics. 2008;8:4259–4272. doi: 10.1002/pmic.200700480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng HW, Shen H, Xu FH, et al. Tests of linkage and/or association of genes for vitamin D receptor, osteocalcin, and parathyroid hormone with bone mineral density. J Bone Miner Res. 2002;17:678–686. doi: 10.1359/jbmr.2002.17.4.678. [DOI] [PubMed] [Google Scholar]
- 8.Yao WJ, Wu CH, Wang ST, Chang CJ, Chiu NT, Yu CY. Differential changes in regional bone mineral density in healthy Chinese: age-related and sex-dependent. Calcif Tissue Int. 2001;68:330–336. doi: 10.1007/s002230001210. [DOI] [PubMed] [Google Scholar]
- 9.Qin M, Yu W, Meng X, Xing X, Xu L. Normal spinal changes of bone mineral density in 445 individuals: assessment by quantitative computed tomography. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1996;18:439–443. [PubMed] [Google Scholar]
- 10.Lei SF, Wu S, Li LM, et al. An in vivo genome wide gene expression study of circulating monocytes suggested GBP1, STAT1 and CXCL10 as novel risk genes for the differentiation of peak bone mass. Bone. 2009;44:1010–1014. doi: 10.1016/j.bone.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 12.Millenaar FF, Okyere J, May ST, van ZM, Voesenek LA, Peeters AJ. How to decide? Different methods of calculating gene expression from short oligonucleotide array data will give different results. BMC Bioinformatics. 2006;7:137. doi: 10.1186/1471-2105-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 14.Chen XD, Xiao P, Lei SF, et al. Gene expression profiling in monocytes and SNP association suggest the importance of the STAT1 gene for osteoporosis in both Chinese and Caucasians. J Bone Miner Res. 2010;25:339–355. doi: 10.1359/jbmr.090724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee NK, Choi YG, Baik JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Chang EJ, Kim HM, et al. Antioxidant alpha-lipoic acid inhibits osteoclast differentiation by reducing nuclear factor-kappaB DNA binding and prevents in vivo bone resorption induced by receptor activator of nuclear factor-kappaB ligand and tumor necrosis factor-alpha. Free Radic Biol Med. 2006;40:1483–1493. doi: 10.1016/j.freeradbiomed.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 17.Bai XC, Lu D, Liu AL, et al. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 18.Steinbeck MJ, Kim JK, Trudeau MJ, Hauschka PV, Karnovsky MJ. Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells. J Cell Physiol. 1998;176:574–587. doi: 10.1002/(SICI)1097-4652(199809)176:3<574::AID-JCP14>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser JH, Helfrich MH, Wallace HM, Ralston SH. Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone. 1996;19:223–226. doi: 10.1016/8756-3282(96)00177-9. [DOI] [PubMed] [Google Scholar]
- 20.Yalin S, Bagis S, Polat G, et al. Is there a role of free oxygen radicals in primary male osteoporosis? Clin Exp Rheumatol. 2005;23:689–692. [PubMed] [Google Scholar]
- 21.Ozgocmen S, Kaya H, Fadillioglu E, Aydogan R, Yilmaz Z. Role of antioxidant systems, lipid peroxidation, and nitric oxide in postmenopausal osteoporosis. Mol Cell Biochem. 2007;295:45–52. doi: 10.1007/s11010-006-9270-z. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Xiao P, Lei S, et al. How is mRNA expression predictive for protein expression? A correlation study on human circulating monocytes. Acta Biochim Biophys Sin (Shanghai) 2008;40:426–436. doi: 10.1111/j.1745-7270.2008.00418.x. [DOI] [PubMed] [Google Scholar]