Abstract
Reports have recently suggested that eosinophils have the potential to modulate allergen-dependent pulmonary immune responses. The studies presented expand these reports demonstrating in the mouse that eosinophils are required for the allergen dependent Th2 pulmonary immune responses mediated by dendritic cells (DC) and T lymphocytes. Specifically, the recruitment of peripheral eosinophils to the pulmonary lymphatic compartment(s) was required for the accumulation of myeloid DCs in draining lymph nodes and, in turn, antigen-specific T effector cell production. These effects on DCs and antigen-specific T cells did not require MHC II expression on eosinophils, suggesting that these granulocytes have an accessory role as opposed to direct T cell stimulation. The data also showed that eosinophils uniquely suppress the DC-mediated production of Th17, and to smaller degree Th1 responses. The cumulative effect of these eosinophil-dependent immune mechanisms is to promote the Th2 polarization characteristic of the pulmonary microenvironment following allergen challenge.
INTRODUCTION
Allergic asthma is a chronic inflammatory disease thought to be initiated by innate pulmonary immune responses that subsequently activate the adaptive immune system against specific environmental assaults. This adaptive immunity and the characteristically persistent Th2 inflammation are often dominated by the presence of activated T cells (1) and eosinophils (2) in the lung even in mild cases of asthma. In this paradigm, eosinophils and T cells are generally considered disparate mediators of the inflammation, with T cells acting as immune regulators and eosinophils as end-stage destructive effector cells. However, recent studies suggest that this perspective of eosinophil effector functions is too narrow. Specifically, in mouse models of allergic respiratory inflammation eosinophils appear to play a role during the secondary immune responses leading to the activation and proliferation of antigen-specific memory T cells (3, 4) and the subsequent recruitment of newly formed T effector cell populations to the lung (5, 6).
Dendritic cells (DCs) have long been classified as "professional" antigen presenting cells (APC) fundamental for both T cell activation as well as immune tolerance. Specifically, several elegant studies have demonstrated that lung DCs have a unique capacity to engulf exogenous antigen and migrate to lung draining lymph nodes (LDLNs) to present antigens to T cells (7). These DCs are uniquely necessary for the induction of allergic pulmonary pathologies in mouse models of allergic respiratory inflammation (8) and are increased in the lungs of asthmatic patients (9). Mouse models that conditionally deplete DCs by injection of targeted toxins during allergen provocation completely abolished activation of T cells and pulmonary inflammation, indicating DCs are essential for induced allergen-specific pathologies (10, 11). This dependency is highlighted by adoptive transfer studies of myeloid DCs into the lungs of sensitized mice and their restoration of allergic pulmonary inflammation, including eosinophil recruitment to the lung (8, 10).
Leukocytes other than DCs have also been shown to have the ability to promote and/or modulate the activation and polarization of T cells (12) and thus represent particularly problematic observations for paradigms suggesting the singular importance of DC-mediated events. In particular, two independent studies using genetically engineered mouse models deficient in eosinophils demonstrated that it was possible to correlate the loss of eosinophils with significant reductions in Th2 pulmonary pathologies (e.g., PHIL (5) and Δdbl-GATA1 (6)). Mice with a partial eosinophil deficiency, such as IL-5 knockout (13) and IL-5/eotaxin double knockout (4) mice have also been described to have impaired Th2 responses to allergen. In addition, eosinophils have been shown to have dendritic cell-like functions, such as the expression of MHC II and co-stimulatory receptors (14–16), a capacity to traffic to LDLNs (14, 17), and the ability to stimulate Th2 cytokine production from antigen-specific CD4+ T cells (3, 18). Eosinophils are also capable of releasing immune modulators such as Th2 cytokines (e.g., IL-4 and IL-25), Th1 cytokines (e.g., IL-12), or suppressive mediators (e.g., indoleamine 2, 3-dioxygenase (IDO) and TGF-β (19, 20). Collectively, these data suggest that the presence of these granulocytes may elicit underappreciated immune regulatory functions in the lung.
In this report, we suggest a solution to the paradox of the dual importance of both eosinophils and DCs in allergen-specific T cell responses. The studies presented capitalize on unique adoptive cell transfer approaches possible in the mouse as part of reductionist strategies defining mechanisms underlying immune responses. Specifically, we present data demonstrating that rather than mutually exclusive activities, eosinophils and DCs act in concert, particularly in the lung draining lymph nodes (LDLN) where T cell activation and polarization primarily occur during allergen challenge in mice (21). Specifically, our studies demonstrate that eosinophils induce the accumulation of mature myeloid DCs to lung draining lymph nodes during allergen challenge. The activation of T cells in response to this DC accumulation was independent of MHC II expression on eosinophils, suggesting eosinophils are not required APCs in the secondary immune responses. Instead, we demonstrate through adoptive transfer of activated myeloid DCs that the ability of these transferred cells to polarize pulmonary immune responses was a function of the presence of eosinophils. That is, in the presence of eosinophils (i.e., wild type recipient mice) transfer of myeloid DCs leads to characteristically Th2 polarized immune responses in the lung. In contrast, in the absence of eosinophils (i.e., PHIL recipient mice) the transferred myeloid DCs promote a mixed Th2/Th17/Th1 phenotype and the accumulation of pulmonary neutrophils. Significantly, the Th17 component and the induced neutrophilia of this response in PHIL recipient mice were suppressed by co-transfer of eosinophils during allergen challenge. These results imply an important function for eosinophils in modulating the balance between Th2 and Th17 pathways generated by DCs during allergen challenge and, in turn, the development of allergic respiratory inflammation.
MATERIALS AND METHODS
Mice
All studies were performed with mice on the C57BL/6J background. Eosinophil-deficient PHIL mice (22) and IL-5−/− mice (13) were generated from established institutional colonies. MHC II knockout mice (B6.129S2-H2dlAb1-Ea/J) were purchased from the Jackson Laboratory (Jackson Research Laboratories, Bar Harbor, ME). MHC II−/− mice were crossed with IL-5–expressing transgenic mice (NJ.1638 (23)) mice to generate MHC II−/− IL-5 Tg mice to isolate MHC II −/− donor eosinophils. Mice were maintained in ventilated micro-isolator cages housed in the specific pathogen-free animal facility at the Mayo Clinic Arizona. Protocols and studies involving animals were performed in accordance with National Institutes of Health and Mayo Foundation institutional guidelines.
OVA sensitization/challenge protocols and adoptive cell transfer strategies
Unless indicated otherwise, mice were sensitized with 100µl injections (i.p.) of 400µg/ml OVA grade VI (Sigma-Aldrich) and 2.25mg of Imject Alum (Thermo Scientific) on days 0 and 14 of the protocols. In studies involving no adoptive transfers or only eosinophil adoptive transfers, mice were challenged with aerosolized OVA (grade VI) at a concentration of 1% (w/vol) for 25 minutes on days 24–26 and assessed on day 28. Time course studies are indicated as hours after first OVA challenge. Intraperitoneal transfer (i.p.) of eosinophils was completed by adoptively transferring 4 × 107 eosinophils on day 24 prior to OVA challenge. Experiments including DC adoptive transfer were completed as described in previously published studies (8, 24). Briefly, intratracheal transfer of 2–3 × 106 million bone marrow-derived OVA-pulsed myeloid DCs (30µl of saline) into OVA-sensitized mice (or non-sensitized where indicated) were performed immediately prior to 1% OVA challenge on day 24. Continued OVA challenges of these mice occurred on days 25–27 (20 minutes each day) and endpoint assessments were performed on day 29. Where indicated, some mice received both 4 × 107 eosinophils by i.p. transfer and 2–3 × 106 DCs by i.t. transfer on day 24, with 1% OVA challenges on days 24–27; endpoint assessments were performed on day 29. Controls include saline sensitized mice, saline challenged mice, and mice receiving saline transfers rather than cell transfers. Each of these OVA-protocols and adoptive cell transfer strategies are outlined in Supplementary Fig 1.
In vivo lymph node proliferation assay
Four hours prior to harvesting lung draining lymph nodes (i.e., LDLNs) of OVA-treated (i.e., OVA-sensitized/challenged) mice on day 28, the animals were injected (i.p.) with 5-bromo-2’deozyuridine (BrdU, 3mg/mouse) (Sigma-Aldrich). BrdU incorporation into T cells was assessed by co-labeling cells with antibodies to TCR-β and following manufacturer’s recommendations for the FITC BrdU Flow Kit (BD Biosciences).
Collection and cell differentials of BAL fluid-derived leukocytes
BAL assessments were completed as described previously (5). Cell differentials of each sample were performed on ≥300 cells.
Histology
Histopathologic changes of the airways were assessed as described previously (5). Formalin-fixed and paraffin-embedded sections of mouse lungs were stained with hematoxylin and eosin or periodic acid Schiff.
Lymph node and lung cell isolation
Total pulmonary leukocytes were recovered from lungs perfused with PBS-EDTA before removal, placed in complete RPMI media with 175 U/ml collagenase type I (Invitrogen), 2mMCaCl2, and digested for 45 minutes at 37° C. Single-cell suspensions of lung and lymph nodes were obtained by homogenization with frosted glass slides followed by passing through a 40µm nylon filter to remove larger aggregates of cells per tissue. Red blood cells were lysed by brief exposure to PharmLyse (BD Biosciences). Cell counts were completed after the last wash before staining for flow cytometry or lymph node culture assays.
Flow cytometry analysis
Single-cell suspensions were stained for 25 min on ice with cell type-specific antibodies after blockade of Fc receptors using 1µg/µl of Fc blocker (CD16/32; BD Biosciences). Antibodies used for staining unique cell types were as follows: Antibodies used to characterize T cell populations - CD4 (RM4–5; eBiosciences), TCR-β (H57–597; BD Biosciences), and CD44 (IM27; eBiosciences). Eosinophils were identified by antibodies to CCR3 (83101; R&D Systems) and Siglec-F (E50–2440; BD Biosciences). Macrophages were stained with F4/80 (BM8; BD Biosciences). Dendritic cell subtypes and activation status were stained with CD11c (HL3; BD Biosciences), Gr1 (RB6–8C5; eBiosciences), B220 (RA3–6B2; eBiosciences), MHC II (AF6–120; BD Biosciences), and CD11b (M1/70; eBiosciences). Flow cytometry was performed on a cytofluorimeter (Cyan; DAKO). Data acquisition and analysis were performed using Summit (version 4.3; Dako) software. Calculations of cell numbers are completed by counting total cellularity of whole lung or LDLN single cell suspensions and multiplying with the percent of the population as determined by flow cytometry.
Cytokine assays
Mouse IL-17, IL-13, and IFN-γ levels were assessed using immunoassay kits (R&D Systems) according to the manufacturer’s instructions. The limits of detection for each ELISA assay were 5–10pg/ml.
Lymph node culture
Single-cell suspensions of LDLNs were plated for 72 hours on a 96-well plate at 5 million cells per well with either complete RPMI media alone or OVA (grade VI (Sigma-Aldrich)) that had been previously treated with 200ug/ml Detoxi-Gel™ endotoxin removing gel (Thermo Scientific). Supernatants were analyzed by ELISA.
Culture and selection of bone marrow-derived myeloid OVA-pulsed dendritic cells
Bone marrow cells were isolated from naïve 6–12 week old C57BL/6J wild-type mice and red blood cells were lysed by PharmLyse treatment followed by washing with cold MACS buffer (PBS, 0.5% (w/vol) BSA, 2mM EDTA). Lymphocytes were removed from the bone marrow by negative selection with magnetic beads conjugated with antibodies to CD45R/B220 and CD90/Thy1.2 as per the manufacturer’s recommendations (Miltenyi Biotech). The negative fraction was plated (2 × 105 cells/ml) in complete RPMI media supplemented with GM-CSF (30ng/ml; R&D Systems) and IL-4 (10ng/ml; R&D Systems) for 2–3 days using 100mm tissue culture dishes. Sequentially lower doses of GM-CSF were used with 20ng/ml on day 3 and 10 ng/ml on day 6. as described previously (25). On day 8 or 9 cells were re-suspended in fresh complete RPMI media containing 10ng/mL GM-CSF and 200ug/ml of Detoxi-Gel™ treated OVA grade VI (Sigma-Aldrich). Sixteen-eighteen hours later the cells were collected and stained with antibodies to select myeloid dendritic cells (F4/80−CD11c+B220−) for adoptive transfers. Cells were sorted by FACSAria (BD Biosciences), washed in cold PBS twice and re-suspended for intratracheal transfer (i.t.) into mice. No significant differences in results were seen between sorted and unsorted bone marrow-derived OVA-pulsed DCs (data not shown).
Isolation of mouse peripheral blood eosinophils
Eosinophils were isolated from IL-5 expressing transgenic mice (NJ.1638) or NJ.1638/MHC II−/− as previously described (5). In brief, peripheral blood pooled from mice was layered onto single-step Histopaque 1119 gradient (Sigma-Aldrich). The eosinophil containing interface was then treated briefly (<10 seconds) with ice cold distilled water to lyse any remaining red blood cells prior to adding 1/10 volume of 10× PBS (i.e., cells are returned to a final concentration of 1× PBS). No differences in T cell proliferation were found when cells were lysed with the ammonium chloride-based PharmLyse solution. Eosinophils of greater than 99% purity were isolated according to the manufacturer’s recommendations (Miltenyi Biotech) by negative selection with magnetic beads conjugated with antibodies to CD45R/B220 and CD90/Thy1.2.
Statistics
All data are derived from at least three independent experiments each with cohort sizes of 1–4 mice (error bars, SEM). *p<0.05, **p<0.01, and ***p<0.001. (unpaired two-tailed student’s t-test).
RESULTS
Peripheral eosinophils are required for T cell activation
We utilized mice that are eosinophil-sufficient (wild type), eosinophil-low (IL-5−/− mice ((13)) and eosinophil-null (PHIL mice (5, 22)) to test the hypothesis that eosinophil levels and their specific tissue localization effect T cell accumulation/activation during allergen provocation. Wild type, IL-5−/−, and PHIL mice were sensitized with intraperitoneal (i.p.) injections of OVA/Alum (days 0 and 14) and challenged via the airways with a 1% OVA aerosol (OVA-treated) on days 24, 25, and 26 (control animals received saline alone) (Supplementary Figure 1(A)). The partial (IL-5−/−) and complete (PHIL) absence of eosinophils in these mice were each accompanied by a loss of the OVA-induced CD4+ T cell accumulation in the lung as compared to OVA-treated wild type animals (Figure 1(A))). However, injection of these OVA-treated mice with BrdU (four hours prior to endpoint assessments on day 28) to measure T cell proliferation in LDLNs showed that only the complete loss of eosinophils (i.e., PHIL mice) and not the partial (85%) loss (i.e., IL-5−/− mice (data not shown)), blocked T cell proliferation in the lymphatic compartment (Figure 1(B)). Moreover, the failure to induce proliferation in the LDLNs of PHIL mice was not unique to the OVA allergen as sensitization and challenge with a more environmentally relevant allergen, ragweed, produced similar results (Supplementary Figure 2). In order to bypass the complete absence of peripheral eosinophils in PHIL mice and promote their recruitment to LDLNs following OVA provocation, eosinophils were adoptively transferred by i.p. injection; this strategy has been shown to promote leukocyte recruitment promptly to LDLN within hours of injection (3, 11). PHIL mice received 4 × 107 eosinophils by i.p. transfer one hour prior to the first OVA challenge (Supplementary Figure 1(B)). This was sufficient to return eosinophil percentages in the LDLNs to levels similar to those observed in OVA-treated wild type mice (Figure 1(C)). It is noteworthy that as previously reported (3), transfer of eosinophils via the peritoneal cavity restored eosinophil accumulation only to the lymphatic compartments of PHIL mice and not in the lung itself, where eosinophil levels remained >98% lower relative to wild type animals. In addition, unlike the trafficking of mononuclear cells such as dendritic cells (26) or T cells (27), eosinophil movement through lymphatic circulation occurred independently of CCR7 expression (Supplementary Figure 3). Concomitant with eosinophil accumulation to the LDLN of OVA-treated PHIL mice, CD4+ T cell numbers in the LDLN increased to levels equivalent to that observed in OVA-treated wild type animals (Figure 1(D)). Similar to OVA-treated IL-5−/− mice, the accumulation was limited to the LDLN; that is, i.p. transfer of eosinophils into OVA-treated PHIL mice did not induced the accumulation of CD4+ T cells into the lungs (Figure 1(E)). These data demonstrate that the localization of eosinophils to the LDLN was necessary for, and limited to, T cell accumulation in LDLN in response to airway allergen challenge of OVA-treated PHIL mice.
Figure 1. Peripheral eosinophils are required for T cell activation in LDLNs of OVA-treated mice.
(A) Wild type, IL-5 knockout (IL-5−/−), and PHIL mice were subjected to OVA sensitization on day 0 and 14, acute OVA challenge on days 24–26 (OVA-treated), and assessed on day 28. Control animals were treated with saline alone. CD4+/TCR-β+ T cell numbers were determined by flow cytometry of total cells in the lungs of OVA-treated mice. (B) Mice treated as in (A) were injected with BrdU (4 hours prior to sacrifice) and assessed for total TCR-β+/BrdU+ T cells by flow cytometry (i.e., cell proliferation). (C) Eosinophil and CD4+ T cell numbers in mice were determined (day 28) in OVA-treated wild type, PHIL, and PHIL mice that were also transferred (i.p.) with 4 × 107 eosinophils (Eos (i.p.)) one hour prior to the first OVA challenge). Representative FACS plots are shown of eosinophils (Siglec-F+/CCR3+) in the lung, LDLNs, spleen, and a non-pulmonary lymphoid compartment (mesenteric lymph node (MLN)). Numbers above boxes indicate percent of eosinophils out of the total live population. CD4+/TCR-β+ T cell numbers were assessed by flow cytometry of total cells in the LDLNs (D) and lungs (E) of OVA-treated mice following eosinophil adoptive transfer. All data are derived from at least three independent experiments each with cohort sizes of 2–6 mice (Mean ± SEM.). *p<0.05, **p<0.01, and ***p<0.001. (unpaired two-tailed student’s t-test).
Eosinophils do not require MHC II to activate T cells
Eosinophils have been demonstrated to express MHC II molecules and potentially mediate antigen presentation in vivo as part of immune responses leading to the T cell activation (14, 15, 18). We adoptively transferred (i.p.) MHC II-deficient eosinophils into OVA-treated PHIL mice to determine if the T cell activation/proliferation occurring in the LDLNs of recipient animals was a consequence of MHC II-dependent antigen presentation by eosinophils. These studies showed that migration of MHC II+/+ or MHC II−/− eosinophils to LDLNs was equivalent (Figure 2(A)). More importantly, transfer of either MHC II+/+ or MHC II−/− eosinophils into OVA-treated PHIL recipients promoted comparable levels of CD4+ effector T cell accumulation in LDLNs (Figure 2(B)), showing that eosinophils do not require expression of MHC II to promote the activation of T cells in the LDLN following allergen challenge.
Figure 2. MHC II expression on eosinophils is not necessary for T cell activation.
OVA-treated wild type and PHIL mice as well as OVA-treated PHIL mice following adoptive transfer (i.p.) of either 4 × 107 MHC II+/+ or MHC II−/− eosinophils (Eos (i.p.)) one hour prior to the first OVA challenge (day24) were assessed on day 28 of the protocol; control animals were challenged with saline alone. (A) Representative FACS plots of LDLN cells demonstrate the presence of eosinophils (Siglec-F+/CCR3+). Numbers above boxes indicate percent of eosinophils out of the total live population. (B) Effector T cell (CD4+/TCR-β+/CD44hi) numbers were determined in LDLNs from OVA-treated mice following eosinophil adoptive transfer. All data are derived from at least three independent experiments each with cohort sizes of 2–4 mice (Mean ± SEM.). *p<0.05; **p<0.01. (unpaired two-tailed student’s t-test).
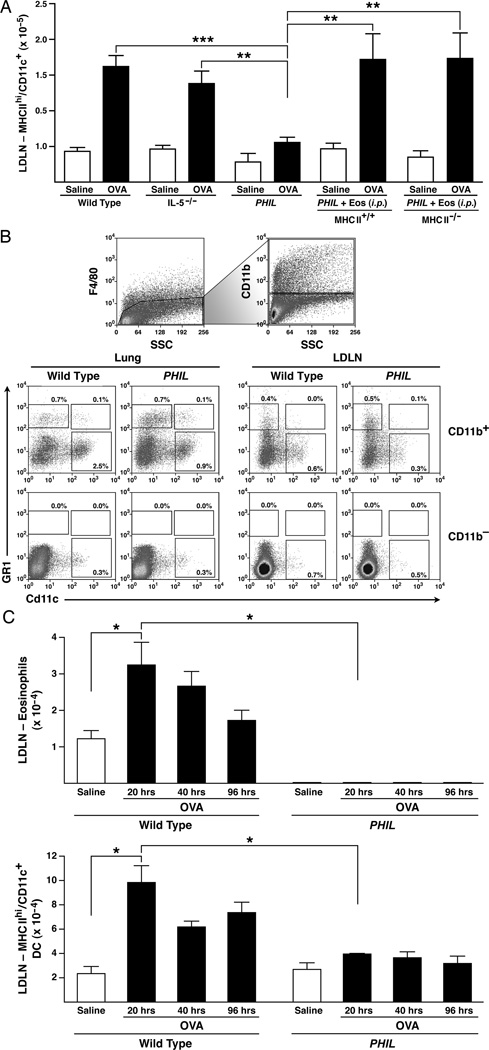
The accumulation of myeloid dendritic cells is dependent on eosinophils
Commonly accepted paradigms on DC functions suggest an independence between eosinophils and LDLN T cell activation. However, our results demonstrating that eosinophils are required for T cell proliferation, as well as studies describing eosinophil-mediated effects on DCs (28, 29), suggested a possible eosinophil-dependence of DC activities in secondary immune responses following allergen challenge. To test this hypothesis, LDLNs in OVA-treated wild type vs. OVA-treated PHIL mice were examined with and without co-transfer of eosinophils to determine the relative effects of eosinophils on activated MHC IIhi DCs accumulation in the LDLN. Significantly, OVA-treated PHIL mice have reduced numbers of DCs in the LDLNs as compared to OVA-treated wild type mice (Figure 3(A)). This reduction occurred without changes in basophil levels in the LDLN, thus suggesting that eosinophils alone and not basophils are correlated with DC levels (Supplementary Figure 4). Furthermore, the induced DC accumulation in the LDLN of OVA-treated PHIL mice was restored by i.p. adoptive transfer of MHC II+/+ or MHC II−/− eosinophils (Figure 3(A)). Significantly, the presence, although reduced, of eosinophils in the LDLNs of OVA-treated IL-5−/− mice was also sufficient to induce accumulation of activated MHC IIhi DCs.
Figure 3. Eosinophils are required for activation/accumulation of myeloid DCs.
(A) OVA-treated wild type and PHIL mice as well as OVA-treated PHIL mice following adoptive transfer (i.p.) of either 4 × 107 MHC II+/+ or MHC II−/− (Eos (i.p.)) one hour prior to the first OVA challenge (day24) were assessed on day 28 of the protocol; control animals were challenged with saline alone. OVA-sensitized wild type, IL5−/−, and PHIL mice challenged with either OVA or saline are shown for comparison. LDLNs of OVA-treated recipient PHIL mice were assessed for the presence of MHC IIhiCD11c+DCs by flow cytometry as calculated from total single cell suspensions of LDLNs. (B) Lungs and LDLN from OVA-treated wild type and PHIL mice that did not undergo eosinophil adoptive transfer were assessed for various DC populations. Total cell suspensions were gated on the F4/80-negative (non-macrophage) population and then gated into CD11b+ and CD11b− groups. Percents out of total populations of these were determined for lymphocytic DCs (F4/80−/CD11b+/Gr1+/CD11c+), plasmacytoid DCs (F4/80−/CD11b−/Gr1+/CD11c+) and monocytic inflammatory DCs (F4/80−/CD11b+/Gr1+/Cd11c−), and myeloid DCs (F4/80−/CD11b+/Gr1−/CD11c+) populations. (C) Kinetic assessment of eosinophils and activated DCs (MHC IIhiCD11c+) accumulation in the LDLNs of OVA-treated wild type vs. PHIL mice. (B). DC and eosinophil numbers were determined by collecting LDLN for flow cytometry analysis at 20, 40, and 96 hours after the first challenge of the three OVA challenges. All data are derived from at least three independent experiments each with cohort sizes of 2–5 mice (Mean ± SEM.). *p<0.05, **p<0.01, and ***p<0.001. (unpaired two-tailed student’s t-test).
Myeloid dendritic cells in particular have been suggested to be key agonists of pathways linked with pulmonary allergic immune responses (7). Assessments of the DC subtypes present in the lungs and LDLNs of OVA-treated wild type and PHIL mice five days after the first OVA challenge revealed that OVA-treated PHIL mice have equivalent (i.e., relative to wild type) percentages of lymphocytic DCs (F4/80−/CD11b+/Gr1+/CD11c+), plasmacytoid DCs (F4/80−/CD11b−/Gr1+/CD11c+) and monocytic inflammatory DCs (F4/80−/CD11b+/Gr1+/Cd11c−) in both the lungs and LDLNs (Figure 3(B)). However, myeloid DC (F4/80−/CD11b+/Gr1−/CD11c+) levels were lower in both the lung and LDLN of OVA-treated PHIL mice relative to control OVA-treated wild type animals (Figure 3(B)). Significantly, the total number of myeloid DCs were significantly reduced in the lungs of OVA-treated PHIL mice as compared to wild type mice (0.47 ×106 ±0.7×105 vs 1.02×106 ±1.5×105, respectively, p=0.007). In addition, the number of myeloid DCs in the LDLN (2.56 ×105 ±0.3×105 vs 5.90×104 ±0.8×105, OVA-treated PHIL vs wild type, respectively, (p=0.0007) mirrored that of LDLN MHC IIhi DCs. Thus, myeloid DC accumulation in both the lungs and LDLNs of mice following allergen provocation appears to be a function of one or more eosinophil activities.
Several studies have demonstrated that myeloid DCs migrate from the lung to the LDLNs within ~10 hours after aerosolized allergen challenge, peaking at ~40 hours (30). Thus, we assessed DC levels at various time points after allergen challenge to determine if OVA-treated PHIL mice had recruited DCs at a rate similar to OVA-wild type mice and simply failed to accumulate these cells in draining pulmonary lymphoid compartments because of a change(s) in their survival (i.e., LDLN half-life). The LDLNs of OVA-treated wild type mice accumulated eosinophils and MHC IIhi activated DCs within 20 hours of OVA challenge (Figure 3(C)); myeloid DCs (F4/80−/CD11b+/Gr1−/CD11c+) populations mirrored MHC IIhi DCs (MHC IIhi/CD11c+). In contrast, OVA-treated PHIL mice fail to accumulate DCs within 20 hours of allergen challenge, suggesting a deficiency in recruitment of these lung DCs to the LDLN. These data demonstrated that the presence of eosinophils in the LDLN is necessary to promote myeloid DC recruitment to LDLNs and in turn, T cell activation during OVA provocation.
Adoptive transfer of OVA-pulsed myeloid DCs is sufficient for DC accumulation and T cell activation in the LDLNs of eosinophil-deficient mice
We performed adoptive transfer experiments of activated (i.e., OVA-pulsed) myeloid DCs similar in design of other studies (8, 10, 31) to circumvent the limitation of eosinophil-dependent DC activation/recruitment. This allowed for the assessment of activated myeloid DCs to induce immune responses in the absence of eosinophils. Specifically, bone marrow-derived OVA-pulsed myeloid DCs were adoptively transferred i.t. on the first day of allergen challenge into either OVA-sensitized PHIL or non-sensitized PHIL mice (Supplementary Figure 1(C)); OVA-sensitized wild type animals served as controls. These adoptively transferred OVA-pulsed DCs accumulated in the LDLNs in all mice receiving DCs, including non-sensitized PHIL mice, demonstrating that recruitment of activated DCs to LDLNs is eosinophil-independent in this system (Figure 4(A)). Despite the presence of DCs in the LDLN of non-sensitized PHIL mice following adoptive transfer, production of effector T cells is deficient (Figure 4(B)), demonstrating that OVA-specific memory T cells are required for their activation into effector T cells. Furthermore, the increase in effector T cells that occurred in OVA-treated PHIL mice receiving activated DCs was equivalent to the levels observed in OVA-treated wild type mice receiving DCs.
Figure 4. OVA-pulsed myeloid DCs are sufficient for DC accumulation and T cell activation in the LDLNs of eosinophil-deficient mice.
Bone marrow-derived and OVA-pulsed DCs (2–3 million cells) were transferred into the lungs of OVA-treated (OVA-sensitized/challenged) wild type or PHIL mice (i.t.) on day 24 of the acute OVA protocol and assessed on day 29. Mice receiving DC via i.t. transfer are indicated by (DC) and vehicle i.t. control is indicated by (Control). An additional cohort of OVA-treated PHIL mice also were adoptively transferred with 4 × 107 eosinophils i.p. on the same day (i.e., day 24) as the DC transfer (DC + Eos). Non-sensitized but OVA-challenged PHIL recipients adoptively transferred (i.t.) with DCs (NS-PHIL) were included as controls to determine the necessity of antigen-dependent memory T cell activation. OVA sensitized/challenged wild type and PHIL mice subjected to the acute OVA protocol without cell transfer were performed as additional controls. (A) DCs (Gr1−CD11c+) and (B) Effector (CD4+/TCRβ+/CD44hi) T cell populations in LDLNs were assessed in each group of mice by flow cytometry. All data are derived from at least four independent experiments each with cohort sizes of 2–4 mice (Mean ± SEM). *p<0.05 and **p<0.01 (unpaired two-tailed student’s t-test).
Adoptive transfer of OVA-pulsed myeloid DCs into PHIL leads to neutrophilic inflammation that is reduced by restoration of peripheral eosinophils
OVA-treated PHIL mice receiving OVA-pulsed DCs (i.t.) displayed histopathologic changes, including goblet cell metaplasia/airway epithelial cell mucin accumulation that was indistinguishable from OVA-treated wild type control animals (Figure 5(A)). OVA-treated PHIL mice transferred with DCs (i.t.) and eosinophils (i.p.) were comparable in their histopathology to OVA-treated PHIL mice transferred only with DCs. Marked elevations in BAL total cellularity also occurred in OVA-treated (i.e., OVA-sensitized/challenged) PHIL mice receiving OVA-pulsed DCs relative to non-sensitized PHIL animals (Figure 5(B)). However, a significant change occurred in the composition of the BAL cellularity. In OVA-treated PHIL DC-recipient mice, the OVA-induced BAL cellularity was predominantly a neutrophilic and lymphocytic infiltrate. The significance of this airway neutrophilia is that it was antigen-dependent (i.e., memory T cell dependent) as non-sensitized PHIL DC-recipient mice (i.e., mice with no OVA-specific memory T cells), failed to develop airways inflammation (Figure 5(A)). In comparison, OVA-treated wild type mice receiving OVA-pulsed DCs developed both airways inflammation and a significant eosinophilic infiltrate in the airways with no increase in neutrophil levels (Figure 5(B)). Surprisingly, despite the induced neutrophilia in the OVA-treated DC-recipient PHIL mice, the BAL cytokine milieu was Th2 polarized with elevated expression levels of cytokines such as IL-13 and undetectable BAL levels of either IFN-γ or IL-17 (Figure 5(C)). Thus, in the absence of eosinophils, OVA-mediated immune responses were mixed with a decidedly Th2 polarized cytokine response in the lung that nonetheless was accompanied by an induced airway neutrophilia. Significantly, OVA-treated PHIL mice receiving co-transfer of eosinophils with the OVA-pulsed DCs resulted in a significant reduction in the percent of neutrophils in the BAL as compared to PHIL mice transferred with OVA-pulsed DCs alone (Figure 5(B)), suggesting eosinophils are key mediators of modulating the pulmonary cellular infiltrate after OVA challenge.
Figure 5. Transfer of OVA-pulsed myeloid DCs into PHIL leads to neutrophilic inflammation.
Bone marrow-derived and OVA-pulsed DCs (2–3 million cells) were transferred into the lungs of OVA-sensitized/challenged wild type and PHIL mice by intratracheal instillation (+ DC (i.t.)) on the first day of OVA challenge (day 24) and assessed on day 29. An additional cohort of OVA-treated PHIL mice also were adoptively transferred with 4 × 107 eosinophils i.p. on the same day (i.e., day 24) as the DC transfer (+ DC (i.t.) + Eos (i.p.)). In addition, bone-marrow derived OVA-pulsed myeloid DCs were transferred (i.t.) into a cohort of OVA-naïve recipient PHIL mice that were subsequently OVA-challenged (Non-sensitized/OVA-challenged (NS-PHIL)) to determine the necessity of antigen-dependent memory T cell activation. OVA sensitized/challenged wild type and PHIL mice subjected to the acute OVA protocol without cell transfer were performed as additional controls. (A) Representative images of lung sections assessing cellular inflammation (hematoxylin-eosin staining) and goblet cell metaplasia/airway epithelial cell mucin accumulation (PAS staining - dark purple cells). (B) Airway cellular infiltration was measured by calculating total bronchoalveolar fluid (BAL) cellularity and differentials of neutrophils, lymphocytes, and eosinophils. Mice receiving DC via (i.t.) transfer are indicated by (DC) and co-transfer of DCs (i.t.) and eosinophils (i.p.) is indicated by (DC + Eos) and vehicle transfer is indicated by (Control). Non-sensitized/OVA-challenged PHIL recipient mice + DC (i.t.) are indicated by (NS-PHIL). (C) BAL levels of IL-13 were measured from each group of mice. IL-17 and FN-γ levels were also performed these assessments and demonstrated that neither cytokine was detectable in the BALs of these mice. All data are derived from at least four independent experiments each with cohort sizes of 1–4 mice (Mean ± SEM). *p<0.05, **p<0.01, and ***p<0.001. (unpaired two-tailed student’s t-test).
Eosinophils establish the character of induced pulmonary immune responses to allergen by suppressing Th17 immunity in the LDLN following OVA challenge
The increased presence of neutrophils and lymphocytes as part of the BAL cellular infiltrate of OVA-treated PHIL mice following the transfer of OVA-pulsed DCs suggested that in the absence of eosinophils a change in the character of the OVA-induced memory T cell responses occurred. The role of eosinophils in modulating the unique phenotype of LDLN effector T cell populations was determined by co-culturing OVA with LDLN cells from each of these groups of mice and assessing their expression of the cytokines IL-17 (Th17), IFN-γ (Th1) and IL-13 (Th2). LDLN cells from OVA-treated wild type mice following adoptive transfer (i.t.) of OVA-pulsed DCs displayed a distinct Th2 polarized phenotype, expressing only IL-13 (Figure 6). In contrast, OVA-treated PHIL animals receiving OVA-pulsed DCs had a mixed effector T cell phenotype with elevated levels of IFN-γ (Th1), IL-13 (Th2), and IL-17 (Th17). Strikingly, adoptive transfer of eosinophils into OVA-treated PHIL recipients that also received OVA-pulsed DCs selectively resulted in the suppression of the induced Th17 responses (with a reduction of the airways neutrophilia (Figure (5B))) in these mice and to a lower, yet not significant degree, the Th1 responses; this leads to the emergence of a Th2 polarized immune response (Figure 6). These data demonstrate a unique role for eosinophils in suppressing Th17 and possibly Th1 events following transfer of OVA-pulsed DCs that changes the character of the induced pulmonary immune response(s).
Figure 6. Eosinophil-dependent suppression of Th17 immune responses following transfer of OVA-pulsed myeloid DCs.
Bone marrow-derived and OVA-pulsed DCs (2–3 million cells) were transferred into the lungs of OVA-treated (OVA-sensitized/challenged) wild type or PHIL mice on day 24 of the acute OVA protocol described and assessed on day 29. Mice receiving DC via i.t. transfer are indicated by (DC) and transfer of vehicle alone is indicated by (Control). An additional cohort of OVA-treated PHIL mice also were adoptively transferred with 4 × 107 eosinophils i.p. on the same day (i.e., day 24) as the DC transfer (DC + Eos). Non-sensitized but OVA-challenged PHIL recipients adoptively transferred (i.t.) with DCs are indicated by NS-PHIL. LDLN-derived cells from each cohort of mice were incubated for 72 hours in the presence of OVA and culture supernatant levels of IL-17, IFN-γ, and IL-13 were assessed by ELISA. All data are derived from at least four independent experiments each with cohort sizes of 2–4 mice (Mean ± SEM). *p<0.05 (unpaired two-tailed student’s t-test).
DISCUSSION
Allergen exposure to the airways of sensitized mice results in a cascade of events that have been characterized as Th2-dominated immune responses leading to histopathologies and lung dysfunction pathognomonic of asthma. Although eosinophil accumulation in the lung has been considered a hallmark part of these Th2-dominated pulmonary immune responses (occurring in both patients (32) and animal models (33)), the definition of specific eosinophil-mediated mechanisms within the immune cascade associated with allergen provocation still has remained unclear. The seemingly incongruent observation that both dendritic cells (8, 10, 11) and eosinophils (5, 6) are necessary for Th2 pulmonary immune responses suggest previously unrecognized (and/or poorly understood) nodes exist whereby these two cells interact as part the development of pulmonary immune responses. The data presented in this current study now provide evidence for these nodes.
Our studies demonstrated that mice deficient in eosinophils were unable to promote the Th2 polarization of the lung microenvironment after allergen provocation due to a T cell activation/proliferation deficiency in LDLNs. This observation was linked, in part, to a failure of DCs to accumulate in the LDLN of OVA-treated PHIL mice, suggesting a previously underappreciated mechanism by which the steady-state presence of circulating/lymphatic eosinophils are necessary for pulmonary DC emigration to the LDLNs following allergen challenge. The mechanism of eosinophil-induced recruitment/accumulation of DCs is unknown, but may rely on release of eosinophil mediators to influence maturation/activation (29) and/or chemoattraction (29, 34). Significantly, adoptive transfer of eosinophils into PHIL mice only modulated the accumulation of the DC sub-type known to induce pulmonary inflammation (i.e., MHC IIhi myeloid DCs) and did not elicit the accumulation of antigen tolerance-inducing plasmacytoid DCs (35). In addition, adoptive cell transfer studies of MHC II-deficient eosinophils into PHIL recipient mice demonstrated that MHC II expression on eosinophils was not required for LDLN T cell proliferation in this allergen challenge model system. Thus, while eosinophils have the potential to act as APCs (14–16), it appears that the multitude of overlapping pathways offered by the various professional APCs available in the pulmonary compartment (in particular dendritic cells) are both necessary and sufficient providers of the APC activities needed for immune responses to allergen provocation.
The adoptive transfers of activated OVA-pulsed DCs into wild type vs. PHIL mice suggest a larger than previously suspected role for eosinophils in the Th2-associated pulmonary immune responses that occur following allergen challenge. In particular, these data suggested a reciprocal correlation exists between eosinophils and T cell immune responses such that in the absence of eosinophils, allergen-dependent T cell immunity is skewed and becomes a mixed Th2/Th17/Th1 collection of responses that now induces a pulmonary neutrophilia. This role for eosinophils in DC-mediated T cell polarization provides a parsimonious explanation for observations from several earlier studies investigating the activation and polarization of T cells in allergen-mediated pulmonary immune responses. For example, two recent studies (31, 36) demonstrated that in the absence of DCs (or the targeted knockout of MHC II expression on DCs), LDLN T cell proliferation occurs in mice but the Th2 polarization of these T cells following allergen challenge is dependent on another (i.e., non-DC) MHC II expressing cell(s). As a consequence, mice deficient in non-DC MHC II expressing cells develop a neutrophilic and IFNγ-dependent inflammation, although the status of Th17 was unknown in these models. An additional example of the proposed importance of one or more non-DC MHC II expressing cells on the skewing of antigen-induced immune responses is provided in studies depleting basophils with MAR-1 (FcєRIα) antibody in a papain inflammation model (37). Significantly, the data presented in this study showed (for reasons left unexplained) that this antibody also reduced eosinophil levels as well in these mice. In doing so, this leaves open the possibility that eosinophils and not basophils were the contributors skewing the immune responses in that study.
The mechanisms by which LDLN eosinophils suppress the production of Th17/Th1 cells are unknown, however, they appear to modulate DC-induced memory T cell polarization as they proliferate during their transition into effector T cells. That is, the presence of eosinophils in the immune environment where DC-mediated T cell activation/polarization is occurring provides (or in the case of PHIL mice fails to provide) additional signals to suppress the differentiation of Th17, and to some extend Th1, cells (38). Potential mechanisms by which eosinophils may modulate DC - T cell interactions abound. For example, Yang and colleagues demonstrated that human eosinophil granule proteins can stimulate DCs through a TLR2-depdendent pathway (29), which has been shown to be an important pathway to suppress Th17 responses (39) and induce Th2 pulmonary inflammation (40). Moreover, other studies have demonstrated that eosinophils are also a potentially significant source of the DC-modulating cytokine IL-25 that augments Th2 responses (41). Still other studies have shown that eosinophils express additional cytokines/enzymes with the potential ability(ies) to alter the relative balance of immune responses such as Th2 (IL-4) vs Th1 (IL-12) vs immune suppressive responses characterized by expression of TGF-β and IDO (19, 20). Finally, eosinophils may selectively activate or suppress T cells derived from a mixed pool of Th17/Th1/Th2 memory T cells that are plastic in phenotype (42).
The potential significance of eosinophil - DC - T cell interactions as part of cellular/molecular mechanism occurring in subsets of asthma patients offers unique and/or alternative explanations for confounding observations often associated with the care of these patients. Three examples are of particular interest: (i) Viral infections or post-viral complications represent significant events linked with asthma exacerbation leading to hospital visits (43). The assumption has been that viral-mediated lung pathology simply synergized with the difficulties already experienced by asthmatics leading to exacerbations events in this population (44). In addition, studies have demonstrated increased susceptibility to viral infections and extended/greater viral load, suggesting deficiencies in production of Th1 cytokine responses due to augmented Th2 cytokine production (45, 46). The hypothesis articulated here suggests that in asthmatic patients experiencing a viral infection, some DCs arriving in LDLNs encounter activated eosinophils which may differentially suppress Th17/Th1 events. This in turn would reduce targeted anti-viral immune responses, increasing the likelihood of infection and/or its severity (47). Moreover, the suppression of Th17/Th1 immune responses by activated eosinophils may also lead to the disproportional production of viral antigen-specific Th2 effector cells that upon recruitment to the lung enhance allergic respiratory inflammation in these patients (48). (ii) The paradigm of eosinophil - DC - T cell interactions suggests that inhaled corticosteroids (ICS) may attenuate local immune responses in the lung via a novel eosinophil-dependent pathway. Specifically, in addition to the ability of ICS to eliminate eosinophil activities through cytocidal affects (49), ICS treatment of patients would disrupt eosinophil-mediated positive regulatory loops promoting the production of Th2 effector cells in LDLNs and their subsequent recruitment to the lung. It is noteworthy that this same eosinophil-mediated disruption of immune regulatory loops may also provide an explanation for the observation that the most significant effect of anti-(IL-5) based treatment of asthma patients (i.e., Mepolizamab®(50, 51)) is not acute improvement of lung function with the resolution of symptoms but the reduction of exacerbation events over time. (iii) The proposed Th17 suppressive character of eosinophils may also provide a mechanism to explain the symptoms/pathologies experienced by patients with neutrophil-dominated severe asthma. In particular, corticosteroid administration that reduces pulmonary and LDLN associated eosinophils may also have the additional consequence of inducing/exacerbating severe and neutrophlic asthma (52) through elevated Th17 production in these patients (53).
We suggest that the respective activities and importance of pulmonary and LDLN eosinophils may be greater than previously suspected. In particular, the roles of eosinophils as unique mediators of local immune responses provide new insights as to the larger contributory role of this leukocyte in the complex inflammatory cascades leading to disease pathology. We would also suggest that these roles are likely to extend beyond asthma and allergic diseases and may provide explanations for the involvement of eosinophils in other inflammatory diseases that have been linked with these cells, including helminth infection, gastrointestinal diseases, cancer, and organ transplant rejection (54).
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank the invaluable contribution of numerous individuals not listed as authors, including Dr. Sergei Ochkur, Ralph Pero, Alfred Doyle, and Dr. Michael McGarry. Moreover, we also wish to acknowledge the tireless efforts of the Mayo Clinic Arizona medical graphic artist Marv Ruona and Joseph Esposito of Research Library Services. In addition, we wish to express our gratitude to the Lee Laboratories administrative staff (Linda Mardel and Charlie Kern), without whom we could not function as an integrated group.
Footnotes
The work presented was supported by the Mayo Foundation and grants from the National Institutes of Health to J.J. Lee (HL065228 and K26-RR019709), N.A. Lee (HL058723), and E.A. Jacobsen (HL08514). Additional support was provided by grants from the American Heart Association to J.J. Lee (0855703G) and N.A. Lee (0555639Z).
The authors have no conflicting financial interests.
REFERENCES
- 1.Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126:1081–1091. doi: 10.1016/j.jaci.2010.06.025. quiz 1092-1083. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, Francois-Bernard M. Eosinophilic inflammation in asthma [see comments] New England Journal of Medicine. 1990;323:1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 3.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4+ Th2 lymphocyte function. J Immunol. 2001;167:3146–3155. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 4.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, Rothenberg ME, Matthaei KI, Young IG, Foster PS. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ. Allergic Pulmonary Inflammation in Mice is Dependent on Eosinophil-induced Recruitment of Effector T Cells. Journal of Experimental Medicine. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, August A. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J. Exp. Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dua B, Watson RM, Gauvreau GM, O'Byrne PM. Myeloid and plasmacytoid dendritic cells in induced sputum after allergen inhalation in subjects with asthma. J Allergy Clin Immunol. 2010;126:133–139. doi: 10.1016/j.jaci.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 10.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model [see comments] J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. Journal of Clinical Investigation. 2000;105:945–953. doi: 10.1172/JCI8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun. 2006;74:3232–3238. doi: 10.1128/IAI.02067-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Pozo V, De Andres B, Martin E, Cardaba B, Fernandez JC, Gallardo S, Tramon P, Leyva-Cobian F, Palomino P, Lahoz C. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. European Journal of Immunology. 1992;22:1919–1925. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- 17.Duez C, Dakhama A, Tomkinson A, Marquillies P, Balhorn A, Tonnel A-B, Bratton DL, Gelfand EW. Migration and accumulation of eosinophils toward regional lymph nodes after airway allergen challenge. Journal of Allergy and Clinical Immunology. 2004;114:820–825. doi: 10.1016/j.jaci.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang H-B, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. Journal of Immunology. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moqbel R. Eosinophil-derived cytokines in allergic inflammation and asthma. [Review] [41 refs] Annals of the New York Academy of Sciences. 1996;796:209–217. doi: 10.1111/j.1749-6632.1996.tb32583.x. [DOI] [PubMed] [Google Scholar]
- 21.Turley SJ, Fletcher AL, Elpek KG. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat Rev Immunol. 2010;10:813–825. doi: 10.1038/nri2886. [DOI] [PubMed] [Google Scholar]
- 22.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 23.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–1344. [PubMed] [Google Scholar]
- 24.van Rijt LS, Vos N, Hijdra D, de Vries VC, Hoogsteden HC, Lambrecht BN. Airway eosinophils accumulate in the mediastinal lymph nodes but lack antigen-presenting potential for naive T cells. J Immunol. 2003;171:3372–3378. doi: 10.4049/jimmunol.171.7.3372. [DOI] [PubMed] [Google Scholar]
- 25.Lutz MB, Rossner S. Factors influencing the generation of murine dendritic cells from bone marrow: the special role of fetal calf serum. Immunobiology. 2007;212:855–862. doi: 10.1016/j.imbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol. 2006;176:3578–3584. doi: 10.4049/jimmunol.176.6.3578. [DOI] [PubMed] [Google Scholar]
- 27.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat Immunol. 2005;6:895–890. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 28.Davoine F, Cao M, Wu Y, Ajamian F, Ilarraza R, Kokaji AI, Moqbel R, Adamko DJ. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol. 2008;122:69–77. e61–e62. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermaelen K, Pauwels R. Accelerated airway dendritic cell maturation, trafficking, and elimination in a mouse model of asthma. Am J Respir Cell Mol Biol. 2003;29:405–409. doi: 10.1165/rcmb.2003-0008OC. [DOI] [PubMed] [Google Scholar]
- 31.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garlisi CG, Falcone A, Hey JA, Paster TM, Fernandez X, Rizzo CA, Minnicozzi M, Jones H, Billah MM, Egan RW, Umland SP. Airway eosinophils, T cells, Th2-type cytokine mRNA, and hyperreactivity in response to aerosol challenge of allergic mice with previously established pulmonary inflammation. American Journal of Respiratory Cell & Molecular Biology. 1997;17:642–651. doi: 10.1165/ajrcmb.17.5.2866. [DOI] [PubMed] [Google Scholar]
- 33.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 34.Rose CE, Jr, Lannigan JA, Kim P, Lee JJ, Fu SM, Sung SS. Murine lung eosinophil activation and chemokine production in allergic airway inflammation. Cell Mol Immunol. 2010;7:361–334. doi: 10.1038/cmi.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MAM, Hoogsteden HC, Lambrecht BN. Essential Role of Lung Plasmacytoid Dendritic Cells in Preventing Asthmatic Reactions to Harmless Inhaled Antigen. J. Exp. Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu N, Laufer T, Homer RJ, Cohn L. Cutting edge: Limiting MHC class II expression to dendritic cells alters the ability to develop Th2- dependent allergic airway inflammation. J Immunol. 2009;183:1523–1527. doi: 10.4049/jimmunol.0901349. [DOI] [PubMed] [Google Scholar]
- 37.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loures FV, Pina A, Felonato M, Calich VL. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J Immunol. 2009;183:1279–1290. doi: 10.4049/jimmunol.0801599. [DOI] [PubMed] [Google Scholar]
- 40.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 41.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lees JR, Farber DL. Generation, persistence and plasticity of CD4 T-cell memories. Immunology. 2010;130:463–470. doi: 10.1111/j.1365-2567.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 44.Green RM, Custovic A, Sanderson G, Hunter J, Johnston SL, Woodcock A. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. B. M. J. 2002;324:763. doi: 10.1136/bmj.324.7340.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, Jeffery PK, Stanciu LA, Johnston SL. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105:13562–13567. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol. 2002;168:2944–2952. doi: 10.4049/jimmunol.168.6.2944. [DOI] [PubMed] [Google Scholar]
- 48.Alwan WH, Kozlowska WJ, Openshaw PJ. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Druilhe A, Letuve S, Pretolani M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis. 2003;8:481–495. doi: 10.1023/a:1025590308147. [DOI] [PubMed] [Google Scholar]
- 50.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, Marshall RP, Bradding P, Green RH, Wardlaw AJ, Pavord ID. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O'Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 52.Mann BS, Chung KF. Blood neutrophil activation markers in severe asthma: lack of inhibition by prednisolone therapy. Respir Res. 2006;7:59. doi: 10.1186/1465-9921-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 54.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in Health and Disease: The LIAR Hypothesis. Clinical and Experimental Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.