Abstract
Epithelial-mesenchyme interactions during organogenesis are regulated by dynamic and reciprocal interactions between growth factors and extracellular matrix (ECM) components. Mouse embryonic submandibular gland (SMG) epithelium, isolated from its endogenous mesenchyme, undergoes branching morphogenesis when cultured ex vivo in a basement membrane extract in serum-free medium with growth factor stimulation. The resulting three-dimensional epithelial morphogenesis in the defined culture system makes this a useful model to analyze cell-cell and cell-matrix interactions, growth factor-mediated signaling and gene expression, proliferation, apoptosis, migration, lumen formation, and epithelial morphogenesis in a primary organ culture system. SMG epithelial culture is robust, reproducible, uses small amounts of reagents, and changes in gene expression are measured by real-time PCR using a limited amount of embryonic tissue. In this chapter, we describe a detailed protocol for isolating primary embryonic SMG epithelium and setting up an ECM and growth factor-dependent, serum-free assay of epithelial morphogenesis, with subsequent analysis of gene expression by real-time PCR.
Keywords: ECM, laminin, submandibular gland, FGF10, branching morphogenesis, FGFR signaling
1. Introduction
Branching morphogenesis of embryonic mouse submandibular glands (SMGs) is a complex process involving multiple cell types including epithelial, mesenchymal, endothelial and neuronal cells, and is influenced by multiple growth factors (recently reviewed in Patel et al., 2006; Tucker, 2007). However, a simplified organ culture system has been developed and refined over the years to specifically study SMG epithelial morphogenesis. This involves isolation of embryonic day 13 (E13) SMG epithelium, which undergoes growth factor-dependent branching morphogenesis when cultured in a basement membrane extract (Nogawa and Takahashi, 1991; Takahashi and Nogawa, 1991). Both EGF and FGF7 were originally described to promote SMG epithelial morphogenesis when cultured in serum-containing medium (Morita and Nogawa, 1999). Since SMG development is particularly sensitive to FGF signaling (reviewed in Patel et al., 2006), we have modified the culture conditions and use serum-free conditions to investigate the mechanisms by which FGF and ECM interactions regulate salivary gland development. We have focused on FGFR-dependent signaling and gene expression (Hoffman et al., 2002; Steinberg et al., 2005) and the role of laminin isoforms in the basement membrane during SMG morphogenesis (Rebustini et al., 2007). This assay can be used to investigate cell-matrix and cell-cell interactions, growth factor-mediated signaling and gene expression, proliferation, apoptosis, migration, and lumen formation during epithelial morphogenesis.
Branching morphogenesis of the intact SMG in culture involves duct elongation, epithelial bud expansion, clefting of the epithelial bud, and then repeated rounds of branching, with formation of new end buds and lateral branches of the main duct. The morphogenesis that occurs when the epithelium is cultured with individual growth factors does not replicate the intact gland, but discrete steps in the process are mimicked using either FGF10 or FGF7 in a laminin-111 matrix (Steinberg et al., 2005). FGF7 treatment results in end bud expansion, while FGF10 treatment produces duct elongation, making this culture system useful to investigate the role of individual growth factors, specific morphogenic events, and cell-ECM interactions during epithelial morphogenesis.
Isolated SMG epithelial culture has also been used to investigate cell migration and ECM dynamics during epithelial morphogenesis using adenovirus-GFP labeling of individual cells and fluorescent labeling of exogenous fibronectin in combination with a live-imaging confocal microscope to track both cell and matrix migration (Larsen et al., 2006). The isolation of epithelial rudiments, followed by their dissociation to a single cell suspension, was also used to investigate mechanisms of embryonic tissue assembly and subsequent functional differentiation (Wei et al., 2007). These observations are relevant to developing experimental approaches for functional organ regeneration.
Quantitative real-time PCR (qPCR) is a powerful tool for analyzing changes in gene expression using small amounts of embryonic tissue. We used SYBR-green qPCR to measure the expression levels of both the targeted gene as well as other changes in downstream gene expression in SMG organ cultures treated with antisense oligonucleotides (Hoffman et al., 2002; Steinberg et al., 2005) or siRNA (Sakai et al., 2003; Rebustini et al., 2007). Additionally, quantitative whole-mount immunofluorescence or Western blot analysis can be used to assess changes at the protein level (Rebustini et al., 2007).
The time and effort to isolate E13 SMGs, separate the epithelium from the mesenchyme, and perform primary organ culture, all of which are technique-sensitive, require an optimized protocol to obtain a maximal amount of information from a very small amount of tissue. E13 SMGs are less than 1 mm across and the isolated epithelia are only a few hundred microns across. The SMG epithelia are cultured in a 15 μl drop of basement membrane extract on top of a filter floating in a 200 μl culture well. The simplification and standardization of protocols with commercially available kits and SYBR-green qPCR analysis has made this a reproducible and routine procedure in the laboratory. In this chapter, we describe a detailed protocol to isolate SMG epithelial rudiments, perform ex vivo epithelial organ culture in a basement membrane extract, and analyze gene expression by qPCR.
2. Materials
2.1. Dissection of embryonic mouse SMGs and preparation of epithelial cultures using basement membrane extract (Matrigel) or purified laminin-111 as an extracellular matrix
-
1
Culture medium DMEM-F12/PS: DMEM-F12 (catalog # 11320, Invitrogen Corporation) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin (catalog # 4545, Invitrogen Corporation). The culture media is supplemented with 150 μg/ml vitamin C (various sources), and 50 μg/ml bovine transferrin (catalog # 11105-012, Invitrogen Corporation) (See Note 1).
-
2
Dispase I neutral protease (catalog number 11284908001, Roche Applied Sciences) stock solution. Prepare stock solution by diluting 5 mg of Dispase I neutral protease in 6.0 mL of H2O. Make aliquots of 133 μL each and store at −20 °C.
-
3
Protease-free BSA, 30% stock solution (catalog number A8577, SIGMA) (See Note 2)
-
4
Cultrex 3D Culture Matrix, either laminin-I at ~3.0 mg/mL (catalog number 3446-005-01, Trevigen) or Cultrex growth factor-reduced BME 13–16 mg/ml (catalog number 3431-005-01, Trevigen) (See Note 3).
-
5
Dissecting microscopes with a transmitted light base, such as the Zeiss Stemi SV-6.
-
6
An inverted microscope with a 5x objective and a digital camera attached for photographing the glands, such as a Zeiss Axiovert 25 microscope with a Fuji FinePix SLR camera. We find that using a digital SLR camera is quicker and easier for multiple users than using a computer-controlled digital camera.
-
7
Whatman Nuclepore Track-etch 13 mm filters with 0.1 μm pore size (catalog number 110405, Whatman VWR).
-
8
50-mm glass-bottom microwell dishes (catalog number p50G-1.5-14F, MatTek)
-
9
Tissue culture dishes: 150 X 25 mm (catalog number 353025, Falcon) and 35 X 10 mm (catalog number 430165, Corning).
-
10
Micropipettes, Rnase-free barrier tips and 1.5 mL Eppendorf tubes (various sources).
-
11
Dissection instruments: fine forceps No. 5 (catalog number OF-224-027.0003, Dumont), surgical scissors (many sources), dressing forceps 5” (catalog number REF V96-6, McConnell Group) and sterilized carbon steel surgical blades (Miltex, PA).
-
15
Ethanol 70% solution. Prepare prior to use by adding 30.0 mL of MilliQ H2O to 70.0 mL of absolute ethanol.
-
16
Pyrex 3-depression spot plates (catalog number 7223-34 Corning).
2.2. Analysis of gene expression in epithelial cultures by real-time PCR
Micro RNAqueous reagent kit (catalog number AM1931, Ambion)
DEPC-H2O (multiple sources).
Spectrophotometer Nanodrop ND-1000 (Nanodrop Technologies).
Heating blocks (Eppendorf Thermomixer R).
I-Script cDNA synthesis kit (catalog # 170-8891, BioRad) or TaqMan reverse transcription reagents (catalog # N808-0234, Applied Biosystems)
iQ SYBR-Green PCR master mix (catalog number 170-8880, BioRad)
Oligonucleotides for PCR are designed using Beacon Design Software (Premier Biosoft International). Importantly, the design parameters should be consistent; we typically design primers with a 3 prime bias, 18–30 bp long, with amplicons at 75–150 bp, with Tm of 65 +/− 5 °C. All primers must be tested for amplification efficiency and that they amplify a single product, usually by melt-curve analysis.
96-well unskirted PCR plate (catalog number MLP-9601, BioRad).
Optical tape microseal B film (catalog number MSB-1001, BioRad).
Real-time PCR machine such as MyiQ PCR Thermocycler (BioRad).
Eppendorf repeater plus automatic pipette.
3. Methods
3.1. Dissection of embryonic mouse SMGs and preparation of epithelial cultures using laminin-111 basement membrane substrate
3.1.1. Prepare in advance solutions and materials necessary to start and terminate SMG dissections
Prepare culture medium prior to use (See 2.1.1 Materials).
Sterilize all dissection instruments by rinsing with 70% ethanol.
Prepare 10% BSA working solution by diluting BSA stock solution in DMEM-F12/PS and keeping it on ice.
In a Corning 3 well glass dish, fill 2 wells with 700 μL of 10% BSA and one well with DMEM-F12/PS.
Prepare Dispase I working solution by adding 367 μL of DMEM-F12/PS to 133 μL of Dispase stock (keep on ice prior to use).
Prepare culture dishes: pipette 200 μL of culture medium into a 50 mm glass- bottom microwell dish and float a Whatman Nuclepore Track-Etch membranes on top of the media. Pipette 15 μL of ECM (either BME or laminin-111) in the center of the filter, and spread it out over the center of the filter surface with the pipette tip, being careful not to form bubbles. Generally we spread 15 μl of laminin into a 5 mm diameter drop (See Note 4). The culture medium must be supplemented with recombinant growth factors: FGF7 (200 ng/mL), FGF10 (1000 ng/mL), or HB-EGF (20 ng/mL) (See Note 5). The dish is covered at room temperature until the dissections are completed, by which time (more than 30 mins) the laminin-1 or BME has polymerized. A diagram of the culture dish is shown in figure 1-D.
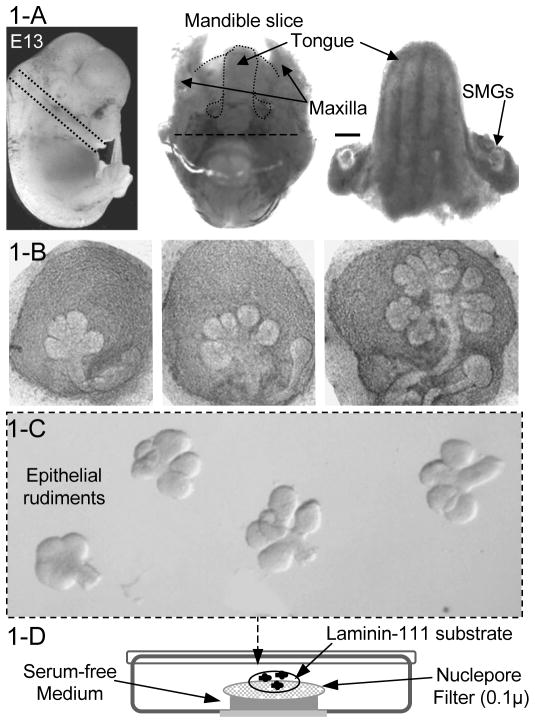
Figure 1.
Dissection of embryonic mouse SMGs and epithelial tissue and ex vivo organ culture system. Figure 1-A: After harvesting embryos at 13 days of development (left panel) the glands are dissected as follow: the head of the embryo (left panel) is removed at the neck and ~1 mm slice of the inferior mandible is removed (middle panel), the posterior half of the slice with the brain stem is removed (bar), and the tongue is separated from the mandible with the SMGs attached (right panel). The two SMGs, positioned at the base of the tongue, are finally separated with fine forceps. Scale bar = 1 mm. Figure 1-B shows the variation of SMGs morphologies at E13 depending on the time of mating. Earlier glands, shown in the left and middle panels, are suitable for epithelial-mesenchyme dissections; glands at late E13 have undergone a lot of branching and the epithelium is difficult to separate intact from the mesenchyme. After dissection, SMGs are placed in media and the epithelial rudiments separated from the mesenchyme as shown in Figure 1-C. The diagram in Figure 1-D shows the culture set up; epithelial rudiments are plated in a drop of laminin-111 substrate on top of a filter, floating on 200μl of medium.
3.1.2. Harvest embryos at 13 days of development, dissect out SMGs and place them in 200 μL of DMEM-F12/PS
Prior to removing the embryo sacs the fur of the pregnant mouse must be liberally sprayed with 70% ethanol to decrease potential contamination of the cultures.
Place the embryo sacs into a 100 mm plastic dish containing DMEM-F12/PS, open each sac to remove the embryos, and then place them in another dish with fresh medium.
Remove the head of the embryo at the neck and make a 1 mm dorsal-ventral section of the mandible containing the tongue as shown in Figure 1-A. The ventral half of the section, containing the brain stem may be removed with a scalpel blade (see figure 1-A). The section is placed tongue-side down in ~ 50 μl media under a dissecting microscope. Separate the SMGs from the mandible slice using fine forceps and place them in a glass-bottom microwell dish containing 200 μL DMEM-F12/PS. The SMGs should have 3–5 buds at this stage (See Note 6).
3.1.3. Separate SMG epithelial rudiment from the mesenchyme and culture them in different extracellular matrices
Carefully aspirate DMEM-F12/PS using a pipette, and incubate SMGs in 200 μL of Dispase working solution for 20 minutes at 37 °C (See Note 7).
Carefully remove Dispase working solution with a pipette, add 10% BSA to the glands to wash the dispase away. Repeat this washing step twice, remove the SMGs with a pipette and transfer them to the first well the 3-well depression dish (See Section 3.1.1 and Note 8).
Mechanically separate the epithelium from the mesenchyme using fine forceps. Hold the mesenchyme with one pair of forceps and carefully peel off the mesenchyme from the epithelial tissue with the other. Transfer the epithelial rudiments using a P20 pipette to the second BSA-containing well in the dish (never grasp the rudiments with forceps). After separating 10–15 rudiments, wash them by pipetting up and down a few times to remove residual mesenchymal cells, and transfer them to the third well containing DMEM-F12/PS where the BSA is washed away by pipetting up and down again. Figure 1-B shows the intact SMGs before dispase treatment, and Figure 1-C shows the epithelium after dissecting from the mesenchyme.
Aspirate the epithelial rudiments (3–5 at a time) using a P2 pipette in a minimal volume (0.5–2 μl) of DMEM-F12/PS, and transfer them to the laminin-1 matrix where they can be separated in the matrix with the tip of fine forceps (See Note 9).
Incubate the epithelial rudiments at 37° C in 5% CO2. Monitor the epithelial morphology and make lysates for RNA isolation at different times: 0, 6, 18, 24, 30 and 48 hours (See Note 10). FGF7 and FGF10 induce different morphologies by 48 hours as shown in Figure 2.
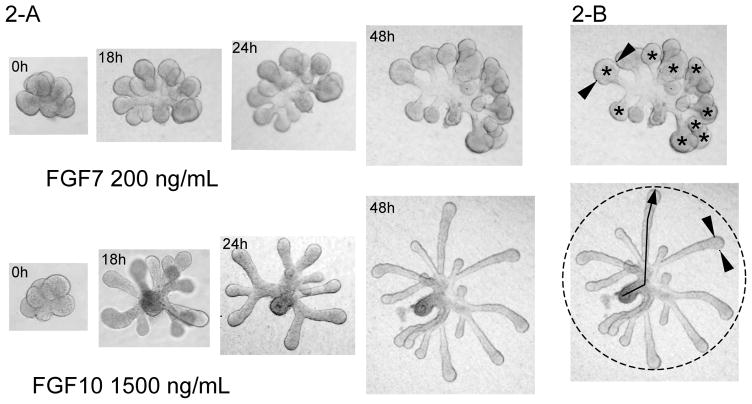
Figure 2.
SMG epithelial rudiments cultured with FGF7 or FGF10. Figure 2-A shows epithelial rudiments cultured for 48 h and the corresponding bud expansion or duct elongation promoted by FGF7 or FGF10, respectively. Figure 2-B illustrates how to quantitate morphological events during SMG epithelial morphogenesis. The upper figure shows how the width is measured (indicated by arrowheads) and the number (*) of end buds is counted; the bottom figure shows that the diameter (dotted circle) and the length of the ducts (arrow) can be measured.
3.2. Analysis of gene expression in epithelial cultures by real-time PCR
3.2.1. Prepare total RNA using a micro-RNAqueous kit for PCR
Aspirate the epithelial rudiments with a P20 pipette and place them in a 1.7 mL Eppendorf tube containing 100 μL of lysis solution from the RNAqueous kit, and vortex the tube for a few seconds (See Note 11).
Purify the RNA following the manufacturer’s instructions (See Note 12) and elute the spin-columns with 10 μL of elution buffer previously heated to 75 °C.
Treat all RNA samples with DNase for 45 min at 37 °C using the reagents in the RNAqueous kit (See Note 13), followed by a 2 min incubation with 3.0 μL of DNase inactivation reagent. Collect the DNase-free RNA samples in a new tube and assess the quality and quantity using 1μl of RNA sample and a Nanodrop spectrophotometer. The expected quantity of RNA/E13 epithelial rudiments is ~40.0 ng and after 48 hours of FGF7- or FGF10-induced culture, this increases to ~60.0–80.0 ng RNA/rudiment, respectively.
3.2.2. Prepare cDNA reactions using either the i-Script cDNA synthesis or TaqMan RT kits prior to use for quantification of gene expression using real-time PCR
Use 100–1,000ng of total RNA for cDNA synthesis according to the manufacturer’s specifications. Briefly, dilute the RNA aliquots using DEPC-H2O to complete a volume of 15 μL, and then add 1.0 μL of Reverse Transcriptase and 4.0 μL of the appropriate RT-buffer, followed by incubations at room temperature for 5 min, 42 °C for 30 min, and inactivation at 85 °C for 5 min. Adjust the volume of each cDNA aliquot using DEPC-H2O in order to obtain cDNA solutions at 1.0 ng/μL (See Note 14).
Design PCR primers using Beacon Design Software (See Note 15), and once having the primers dilute them in DEPC H2O in order to make a stock solution of 20 μM each forward and reverse primer.
Perform PCR reactions in 96-well plates using an Eppendorf Repeater Pipettor to add 10.0 μL of cDNA (total of 0.5–1.0 ng) and 15 μL of PCR master mix reaction to a final reaction volume of 25 μL. The PCR mix contains 0.5 μL of oligonucleotides (forward and reverse, each one at final concentration 2.5 μM), 12.5 μL of SYBR-Green PCR master mix and 2.0 μL of DEPC-H2O. There are 3 technical replicates/sample.
Typically the PCR cycle program is 10 min at 94 °C, followed by 40 cycles at 62 °C for 45 sec and 94 °C for 15 sec (See Note 16).
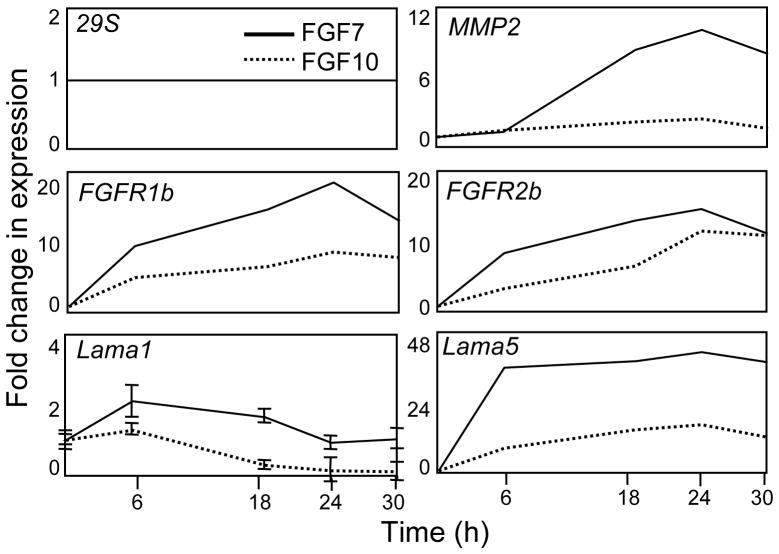
Fold change in gene expression is calculated using the deltaCt-deltaCt method, comparing threshold cycle numbers (Cts) (See Note 17). Briefly, normalize the gene expression of each cDNA to a housekeeping gene (such as GAPDH or 29S) and then to the control levels of expression for each gene analyzed. Transform the profiles of gene expression to a fold change and plot the data in a graph in a time-course dependent manner, as visualized in Figure 3.
Figure 3.
Time-course of gene expression during SMG epithelial culture. Total RNA is collected at the times indicated to make cDNA and perform real-time PCR to assess FGF7- (solid line) or FGF10-induced (dashed line) gene expression. The expression of each gene of interest is compared to a housekeeping gene 29S, which does not change with FGF7 or FGF10 treatment, and then the corresponding values of fold change in gene expression at different times (6, 18, 24 and 30 h) are compared to the respective fold change in gene expression in a control group (time 0 h). For more details, see Methods 3.2.2.
Footnotes
For each experiment, add 10.0 μL of single-use aliquots from frozen stock solutions of vitamin C (75 mg/mL in H2O) and transferrin (25 mg/mL in H2O) to 5.0 mL of DMEM/F12 (supplemented with penicillin and streptomycin).
It is important to use high-grade protease-free BSA, as lower grades will result in loss of epithelial viability.
Laminin-111 and growth factor-reduced BME are liquid at 4° C and form a 3D gel at 37° C, therefore they must be thawed on ice and kept cold. Laminin-111 forms a 3D gel at a minimal concentration of ~1 mg/mL, but if it is too dilute it will not gel. The BME can be diluted to at least 1:3–1:5 (usually in culture medium). Dilution of the laminin-111 and BME stock with ice-cold media should be done depending on the actual protein concentration of the batch. We generally use 15 μL ECM for epithelial cultures.
Other experimental setups are possible, particularly for live-cell imaging, to avoid imaging through the filters. BME can be added directly on the glass surface of a MatTek dish, the epithelial rudiments are placed on the top, and a Nuclepore filter is used to cover the system before adding culture medium (Larsen et al., 2006). For this protocol, laminin-111 at 1 mg/mL does not work as the gel is too soft and the rudiments will not grow properly.
Growth factors can be used in a range of concentrations that vary according to their biological activity. It is recommended to perform a dose-response of each growth factor used to optimize the final working concentration for specific experiments. For example, FGF7 is used in a range of 20–200 ng/mL, and FGF10 is 200–1500 ng/mL. Alternatively, combinations of different growth factors can be used in specific experiments. Without growth factors, the epithelium degenerates in serum-free ex vivo organ cultures.
At the E13 stage of development, SMGs have condensed mesenchyme tissue and visible sublingual glands developing in the same mesenchyme tissue. SMGs at E13 show a range of morphologies starting from a gland with 3–5 buds and a single duct, following the second and third rounds of cleft formation with 6–10 epithelial buds, and finally having multiple buds and lateral secondary ducts (See Figure 2-B).
Notice that Dispase I incubation is previously optimized. With experience, 20–30 SMGs can be treated each time; longer times may disrupt the epithelial tissue, and shorter times, lower temperatures, or too many glands treated at the same time may compromise the efficiency of dissection.
Multiple washings in BSA working solution inactivate Dispase I. Typically, 2 washes are used in order to eliminate neutral proteases. The epithelium should not be stored in the serum-free media in the third well since this decreases viability. They should only be washed free of BSA just before they are plated into the laminin.
Usually, about 6–10 epithelial rudiments are plated in the same culture dish on top of ECM substrate. Given the variations on the thickness in the center or on the border of the laminin-111 gel, the rudiments may grow more or less three-dimensionally, respectively.
Additionally, the distinct morphologies promoted by FGF7 and FGF10 can be measured using image analysis software such as NIH Image (http://rsb.info.nih.gov/nih-image) or MetaMorph 7 (Molecular Devices, www.molecular.devices.com). Take Photographs of the epithelial rudiments after times of FGF7 or FGF10 treatments to evaluate the morphology by counting the number of epithelial end buds, measuring the length of epithelial ducts, the radius of epithelial rudiment, or the width of end bud, as shown in Figure 2-B.
For RNA preparations, all materials such as pipettes, tips and Eppendorf tubes must be Rnase-free following appropriate decontamination protocols. Wear gloves during all proceedings.
Alternatively, lysates for RNA extraction can be stored at −80 °C from 6–12 months prior to RNA extractions.
The time of DNase treatment of RNA samples varies according to specific experiments. Usually 45 min is sufficient to eliminate SMG DNA contamination using 5–10 epithelial rudiments. Longer DNase treatments using a small amount of tissue may compromise the quality and the final RNA concentration.
The final concentration of cDNA is calculated by approximation based on the initial RNA amount used for RT reactions. cDNAs should be stored as concentrated stock solutions and, once diluted to 0.5–1.0 ng/10μl, should be used as soon as possible with minimal freeze/thaws.
Design the PCR oligonucleotides using the SYBR-Green Design Option in the Beacon Design software. For templates, use sequences of mRNA of interest from NCBI (www.ncbi.nlm.nih.gov), and perform primers search limiting the sizes of the forward and reverse oligonucleotides to between 18–30 base pairs, and the PCR amplification product to between 75–150 base pairs. Preferentially, oligonucleotides are designed at the 3’ end of the corresponding mRNA of interest. The annealing temperature is typically 65° C +/− 5 °C. Finally, compare oligonucleotides to a data bank of known genes using the BLAST option in the software in order to determine the specificity of the gene of interest.
Before using primers to calculate the fold change in gene expression, melt-curve analysis and the efficiency of the oligonucleotides for PCR amplification must be determined. First, perform an additional heating cycle to generate melt-curves after running the PCR amplification cycles. Melt-curve analysis of the amplification products must show a single PCR product corresponding to each pair of oligonucleotides tested. Then perform PCR using a serial dilution of a standard cDNA sample to verify the efficiency of product amplification by linear regression analysis. Oligonucleotides that fail in either melt-curve analysis or amplification efficiency must not be used to calculate fold change in gene expression and should redesigned.
Fold change in gene expression reflects an indirect comparison based on Threshold Cycle (Ct) numbers obtained after PCR amplifications. The relative abundance of each cDNA is inversely proportional to Ct numbers, and usually housekeeping genes show low Ct values compared to regulatory genes such as transcription factors, for example.
References
- Hoffman MP, Kidder BL, Steinberg ZL, Lakhani S, Ho S, Kleinman HK, Larsen M. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development. 2002;129:5767–78. doi: 10.1242/dev.00172. [DOI] [PubMed] [Google Scholar]
- Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J Cell Sci. 2006;119:3376–84. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- Morita K, Nogawa H. EGF-dependent lobule formation and FGF7-dependent stalk elongation in branching morphogenesis of mouse salivary epithelium in vitro. Dev Dyn. 1999;215:148–54. doi: 10.1002/(SICI)1097-0177(199906)215:2<148::AID-DVDY7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Nogawa H, Takahashi Y. Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development. 1991;112:855–61. doi: 10.1242/dev.112.3.855. [DOI] [PubMed] [Google Scholar]
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–64. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, Hoffman MP. Laminin α5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through β1 integrin signaling. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.04.031. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–81. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, Larsen M, Hoffman MP. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–34. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Nogawa H. Branching morphogenesis of mouse salivary epithelium in basement membrane-like substratum separated from mesenchyme by the membrane filter. Development. 1991;111:327–35. doi: 10.1242/dev.111.2.327. [DOI] [PubMed] [Google Scholar]
- Tucker AS. Salivary gland development. Semin Cell Dev Biol. 2007 doi: 10.1016/j.semcdb.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Wei C, Larsen M, Hoffman MP, Yamada KM. Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 2007;13:721–35. doi: 10.1089/ten.2006.0123. [DOI] [PubMed] [Google Scholar]