Abstract
Background
We sought to investigate whether higher concentrations of resistin and lower concentrations of adiponectin relate to incident atrial fibrillation (AF) and whether this association is mediated by AF risk factors and inflammation. Resistin and adiponectin are adipokines that have been associated with multiple known risk factors for AF including diabetes, obesity, inflammation, and heart failure.
Methods
We studied the relations between circulating concentrations of both adipokines and incident AF in participants of the Framingham Offspring Study.
Results
Participants (n = 2,487) had a mean age of 61 ± 10 years, and 54% were women. During a mean follow-up of 7.6 ± 2.0 years, 206 (8.3%) individuals (96 women) developed incident AF. Plasma resistin concentration was significantly associated with incident AF (multivariable-adjusted hazard ratio [HR] 1.17 per SD [0.41 ng/mL] of natural logarithmically transformed resistin, 95% CI 1.02–1.34, P = .028). The resistin-AF association was attenuated after further adjustment for C-reactive protein (HR per SD increase resistin 1.14, 95% CI 0.99–1.31, P = .073). Adiponectin concentrations were not significantly associated with incident AF (multivariable-adjusted HR of 0.95 per SD [0.62 µg/mL] of logarithmically transformed adiponectin, 95% CI 0.81–1.10, P = .478).
Conclusion
In our community-based longitudinal study, higher mean concentrations of resistin were associated with incident AF, but the relation was attenuated by adjustment for C-reactive protein. We did not detect a statistically significant association between adiponectin and incident AF. Additional studies are needed to clarify the potential role of adipokines in AF and mechanisms linking adiposity to AF.
Both the incidence and prevalence of atrial fibrillation (AF) in the United States are substantial and will likely increase in the future.1 Despite efforts to prevent AF-related morbidity and mortality, AF remains associated with increased risk of heart failure, stroke, and death.2 Established risk factors for AF include advancing age, male sex, diabetes, hypertension, valvular disease, and heart failure.2–5 More recently, obesity,6,7 elevated concentrations of C-reactive protein (CRP),8,9 and prolongation of the PR interval10 also have proven to be predictors of AF.
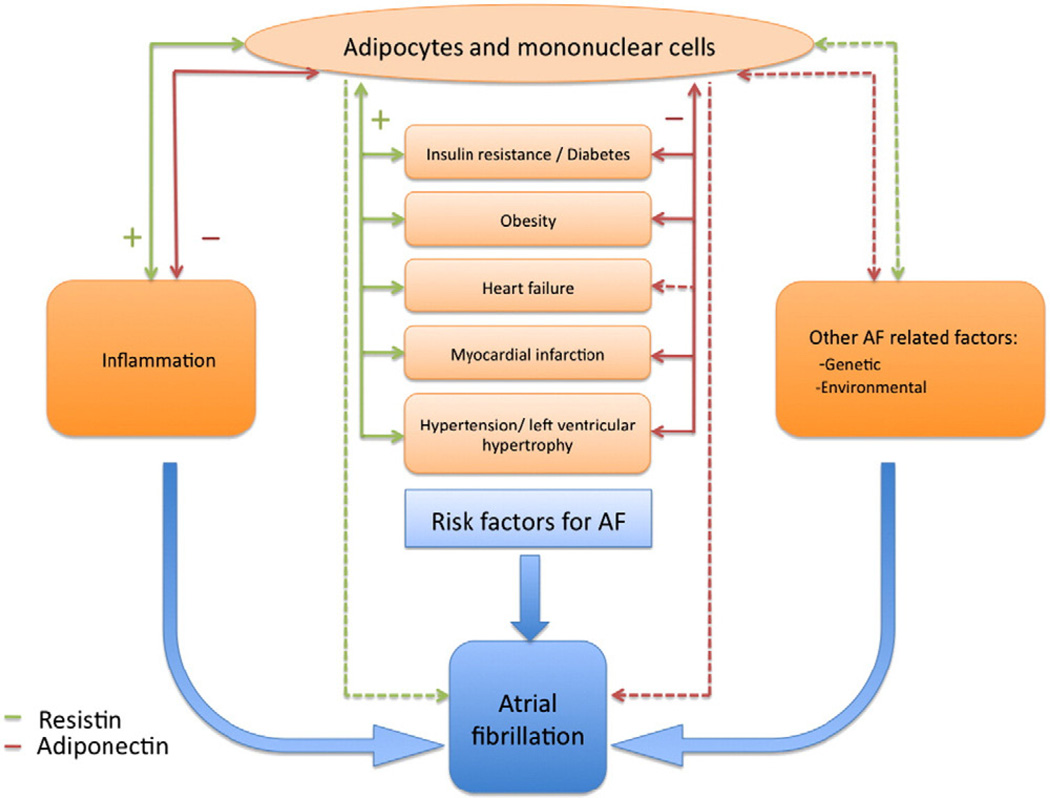
The exact pathophysiologic pathways linking adipokines and cardiovascular disease and its risk factors are not yet fully understood. Resistin has been associated with increased insulin resistance and has proinflammatory, prohypertrophic effects.11,12 Adiponectin has anti-inflammatory, atherogenic, and antihypertrophic consequences.13 Furthermore, clinical observations have shown that both resistin and adiponectin are associated with multiple known risk factors for AF, including inflammation, diabetes, obesity, myocardial infarction, and incident heart failure.14–21 A cross-sectional study reported that high concentrations of adiponectin were related to persistent AF.22 Thus, adipokines may be related to incident AF through several pathways, involving inflammation or through AF risk factors such as obesity and heart failure (Figure 1).
Figure 1.
Conceptual model of mechanisms and pathways relating resistin and adiponectin to incident AF: the potential mediatory role of known risk factors for AF and other factors. Solid lines indicate known association; dashed lines, potential association; green lines, resistin; red lines, adiponectin.
To our knowledge, adipokines have not been studied as potential predictors of incident AF. We hypothesized that higher concentrations of resistin and lower concentrations of adiponectin would be related to incident AF. In addition, we postulated that the association between adipokines and incident AF would be attenuated by adjustment for body mass index and CRP, which may constitute potential mediating mechanisms.
Methods
Participants
For the present analysis, we used a sample of the Framingham Heart Study Offspring cohort. The Offspring cohort was constituted in 1971 with the enrollment of 5,124 offspring (and their spouses) of the Original cohort.23 Between 1999 and 2001, 3,539 participants attended the seventh examination cycle. Of these participants, 150 had prevalent AF and were excluded from the present analysis. Because plasma adipokine measurements started partway through the seventh examination cycle, 722 attendees with missing adipokines data were excluded. Of the remaining 2,667 individuals, 180 additional people were excluded because of missing covariates (CRP concentrations, PR interval, body mass index, or valvular heart disease). The final sample comprised 2,487 attendees. Participants were monitored until the first AF event with a maximum follow-up duration of 10 years. The last follow-up date was September 9, 2009. The Framingham Heart Study protocol was approved by the Boston University Medical Center Institutional Review Board, and participants signed informed consent.
Clinical assessments
At each examination visit, a physician-administered medical history and physical examination and laboratory assessment were performed.24 Participants were diagnosed with AF if either atrial flutter or atrial fibrillation was present on an electrocardiogram obtained at a Framingham Heart Study clinic visit scheduled at 4 to 8 year intervals or on interim outside electrocardiograms. The Framingham Heart Study routinely ascertained electrocardiograms or Holter monitoring tests acquired during visits to outside clinicians or during hospitalizations. Atrial fibrillation electrocardiograms from any source were reviewed by 1 of 2 Framingham Heart Study cardiologists.
A clinically significant cardiac murmur was diagnosed if a systolic murmur that exceeded grade 3 of 6 or if any diastolic murmur was auscultated by a Heart Study clinic physician. Heart Study clinic physicians measured blood pressure as the average of 2 seated measurements. Ascertainment of antihypertensive treatment was by self-report. Individuals were diagnosed with heart failure based on standard major and minor clinical criteria.25 A committee of 3 Framingham Heart Study physicians adjudicated incident heart failure events.
Laboratory analyses
Blood samples were obtained at the Heart Study visit after an overnight fast and frozen at −80°C. Plasma CRP concentration was measured with Dade Behring BN100 nephelometer (Dade Behring, Deerfield, IL). The intraassay coefficient of variation was 3.2%. Plasma resistin and total adiponectin concentrations were measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). Intraassay coefficients of variation were 9.0% for resistin and 5.8% for adiponectin.
Statistical analyses
In our primary analysis, we tested the hypotheses that higher mean resistin concentrations and lower mean adiponectin concentrations, considered separately, were related to incident AF using Cox proportional hazards regression analysis. Because both resistin and adiponectin measurements were positively skewed, the values were natural logarithmically transformed (loge). We used one SD of the loge continuous adipokine to quantify the effect size. Cox proportional hazards models were estimated in the following sequence: adjusted for age and sex; adjusted for age, sex, and body mass index; adjusted for the previously published Framingham Heart Study AF risk score26; and adjusted for the AF risk score and CRP (mg/L). The AF risk score includes the following variables: age, sex, body mass index, systolic blood pressure, treatment of hypertension, PR interval, clinically significant cardiac murmur, and heart failure. We used the composite AF risk score to ensure parsimony, given the modest number of incident AF events during follow-up.
As an exploratory analysis, we additionally adjusted for interim heart failure. In secondary analyses, we explored the dose-response relations of resistin, adiponectin, and incident AF in the multivariable-adjusted model using splines.27 All analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC), and a 2-tailed P < .05 was considered statistically significant.
The authors are solely responsible for the design and conduct of the study, all study analyses, the drafting and editing of the manuscript, and its final contents. The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute (contract NO1-HC-25195).
Results
Study sample characteristics
The characteristics of the 2,487 attendees (mean age 61 ± 10 years, 54% women) included in the analysis are reported in Table I. Median plasma resistin concentration was 12.7 ng/mL (25th percentile 10.0 ng/mL, 75th percentile 16.4 ng/mL) and median plasma adiponectin concentration was 8.6 µg/mL (25th percentile 5.5µg/mL, 75th percentile 13.2µg/mL). The SDs of the loge resistin and adiponectin were 0.41 ng/mL and 0.62 µg /mL, respectively.
Table I.
Characteristics of study participants, expressed as total population
| Variable | Study sample (n = 2487) |
|---|---|
| Age (y) | 61 ± 10 |
| Women | 1348 (54%) |
| Body mass index (kg/m2) | 28.1 ± 5.3 |
| Systolic blood pressure (mm Hg) | 127 ± 19 |
| Antihypertensive therapy | 821 (33%) |
| PR interval duration (ms) | 168 ± 27 |
| Heart murmur* | 57 (2%) |
| Heart failure | 13 (0.5%) |
| CRP (mg/L)† | 2.2 (1.0–5.2) |
| Adiponectin (µg/mL)† | 8.6 (5.5–13.2) |
| Loge adiponectin | 2.1 ± 0.6 |
| Resistin (ng/mL)† | 12.7 (10.0–16.4) |
| Loge resistin | 2.6 ± 0.4 |
Data presented as mean ± SD, n (%), or in the case of untransformed biomarkers† median (25th and 75th percentile).
Heart murmur: any systolic murmur that exceeded grade 3 of 6, or any diastolic murmur.
Adipokines and AF risk
Incident AF developed in 206 individuals (8.3%, 96 women) during a mean follow-up of 7.6 ± 2.0 years. Age was modestly correlated to resistin (r = 0.11, P < .0001) and adiponectin (r = 0.15, P < .0001) concentrations. Adiponectin was not statistically significantly related to incident AF; the hazard ratio (HR) was approximately 1 in all models. With the multivariable-adjusted model for the AF risk score and CRP, the per SD increase for incident AF was 0.95, 95% CI 0.82 to 1.11, P = .55 (Table II). Plasma resistin was related to incident AF in a Cox proportional hazards regression model adjusted for the AF risk score (HR per SD increase 1.17, 95% CI 1.02–1.34, P = .03) (Table II). C-reactive protein was statistically significantly correlated with resistin (r = 0.15, P < .0001). When we added CRP (HR of CRP per SD increase 1.16, 95% CI 1.05–1.29, P = .005) to the multivariable model, the association of resistin with incident AF was attenuated (HR per SD increase 1.14, 95% CI 0.99–1.31, P = .07) (Table II).
Table II.
Cox proportional hazards models relating loge resistin and adiponectin to incident AF
| Adjustment factors |
Loge resistin | Loge adiponectin | ||
|---|---|---|---|---|
| HR* | P | HR* | P | |
| No adjustment | 1.33 (1.17–1.52) | <.001 | 0.95 (0.83–1.09) | .439 |
| Age and sex | 1.19 (1.04–1.37) | .011 | 0.91 (0.78–1.06) | .205 |
| Age, sex, BMI | 1.15 (1.00–1.32) | .050 | 0.97 (0.83–1.14) | .719 |
| AF risk score | 1.17 (1.02–1.34) | .028 | 0.95 (0.81–1.10) | .478 |
| AF risk score and CRP | 1.14 (0.99–1.31) | .073 | 0.95 (0.82–1.11) | .547 |
| AF risk score and interim heart failure | 1.16 (1.01–1.33) | .034 | 0.95 (0.81–1.11) | .486 |
AF, Atrial fibrillation; BMI, body mass index.
AF risk score (age, sex, body mass index, systolic blood pressure, treatment of hypertension, PR interval, clinically significant cardiac murmur, and heart failure).26
Hazard ratios are relative to 1 SD (0.41 ng/mL for resistin and 0.62 µg/mL for adiponectin) increase in loge resistin or adiponectin, respectively, and 95% CI.
Secondary analyses
In an exploratory analysis, we additionally adjusted for interim heart failure because AF and heart failure are strongly interrelated and resistin has been related to incident heart failure.16 Adjusting for incident heart failure did not change the observed relation between resistin and incident AF (Table II). Spline modeling of loge multivariable-adjusted resistin and adiponectin revealed a linear dose-response relation for resistin and a fairly flat dose-response relation for adiponectin. Thus, we found no evidence of any threshold effects (online Appendix Supplementary Figure 1).
Statistical power
Adjusting for the AF risk score alone, the 95% CI for loge adiponectin included a protective relation (lower bound 0.81) and a small deleterious relation (upper bound 1.10). To determine whether we had adequate statistical power to detect a meaningful association between adiponectin and incident AF, we performed a post hoc power analysis. With 206 incident AF events, we had 80% power at an α of .05 to detect an HR of 0.70 for incident AF per 1 SD decrement in loge adiponectin.
Discussion
In our community-based sample, we observed that higher plasma concentrations of resistin were related to incident AF during up to 10 years of follow-up, after adjusting for traditional AF risk factors. Numerous prior clinical and preclinical studies have demonstrated a relation between inflammation, largely as assessed by concentrations of CRP and AF.28 Resistin, produced by mononuclear cells both within and outside adipose tissue, contributes both to insulin resistance and is related to inflammatory triggers. For example, proinflammatory mediators such as tumor necrosis factor α, interleukin 1β, interleukin 6, or lipopolysaccharide can strongly increase the expression of resistin in peripheral blood mononuclear cells in humans.11 The relation between inflammation and resistin has served as the basis for several studies investigating the potential mechanistic links between resistin and cardiovascular diseases.14,15,29
There are several potential explanations for the relation between resistin and AF. Resistin may be related to incident AF directly or indirectly (Figure 1). Studies in mice demonstrated that resistin promotes insulin resistance and diabetes30 but also has effects on cardiac myocytes leading to hypertrophy12 and decreased cardiac contractility.12 Furthermore, direct vascular effects of resistin promoting endothelial cell activation and smooth muscle cell proliferation have been reported.31,32 From clinical studies, it is known that resistin is related to multiple risk factors for AF, including obesity, diabetes, hypertension, coronary artery disease, and heart failure.14,15,20,21,29,33 However, after adjusting for standard AF risk factors, including age, sex, body mass index, hypertension, and prevalent and interim heart failure, the relation between resistin and AF remained present, suggesting that these AF risk factors are not the (only) mediators involved (Figure 1). Another potential mediatory pathway involved in the relation between resistin and AF is inflammation (Figure 1). Resistin is associated with increased concentrations of inflammatory markers, such as CRP, interleukin 6, and tumor necrosis factor α.11 Concentrations of these inflammatory markers are associated with increased risk of AF, in multiple experimental and clinical studies.28 We, therefore, adjusted for CRP in our analysis and observed attenuation of the association between resistin and AF, suggesting that the association between resistin and AF may be, in part, mediated by inflammation. The precise role of resistin in inflammatory pathways in humans is not completely understood; thus, it is also possible that inflammation may be an initiator rather than a consequence of increased concentrations of resistin (ie, resistin represents a confounder of the relation between inflammation and AF). In addition, we cannot exclude residual confounding or chance as possible explanations of our findings.
In contrast to resistin, adiponectin is produced by adipose tissue and has anti-inflammatory, antiatherogenic, and antihypertrophic effects, via a multiplicity of cell-signaling mechanisms.13 Adiponectin expression and subsequent release from adipocytes are stimulated by activation of peroxisome proliferator-activated receptor γ, a key transcriptional factor involved in adipocyte differentiation.13 Adiponectin is inversely related to cardiovascular risk factors such as hypertension34 and CRP.17 It has been observed that increased plasma adiponectin levels are associated with a lower risk of myocardial infarction.19
We did not find any evidence of a relation of adiponectin with incident AF in our cohort. Our findings are in contrast to one prior report in which high concentrations of adiponectin were related to persistent AF, and not to paroxysmal AF, in a cross-sectional hospital-based cohort.22 The discrepancy may have resulted from a difference in the form of adiponectin measured. In the current study, we measured concentrations of total adiponectin rather than high-molecular-weight adiponectin. There are conflicting data whether total adiponectin or high-molecular-weight adiponectin is the more biologically active form. We did measure on average lower levels of adiponectin in our community-based sample than in the cross-sectional analysis of adiponectin and AF.22 Our contrasting results also may be caused by differences in the study samples. We studied a community-based sample with incident AF rather than a hospital-based sample with prevalent AF. Thus, we may have a smaller proportion of individuals with other comorbidities that may result in lower concentrations of adiponectin.
Strengths and limitations
The community-based setting, the longitudinal design, the standardized measures of clinical variables, and standardized adjudication of incident AF in the Framingham Heart Study represent important strengths. However, our study has limitations that merit consideration. To avoid overfitting of the regression model, we used the linear risk predictor of the AF risk model26 as representative of conventional risk factors into 1 variable, rather than testing each conventional AF risk factors individually in the model. We cannot exclude the possibility of residual confounding. We acknowledge that other covariates associated with AF, rather than resistin and the conventional AF risk factors, could account for the increased risk of incident AF. We also note that, with an observational study design, we cannot establish a causal relation between resistin and AF and we cannot distinguish whether CRP is a confounder or an intermediate mechanism linking resistin to AF. We cannot exclude power limitations studying the relation of adiponectin and incident AF, although our post hoc power calculations determined that we had sufficient power to detect moderately large-sized associations. We observed a small estimated effect size (HR 1.17 per SD of loge resistin) of the relation between resistin and incident AF, suggesting that it is unlikely that resistin will serve as a clinically useful marker for risk prediction or reclassification of incident AF. Furthermore, our sample consisted of predominantly middle-aged or elderly ambulatory individuals of European ancestry; so our results may not be generalizable to other races/ethnicities, younger individuals, or samples with a higher burden of acute or serious illnesses.
Future directions
Our findings are consistent with the available literature that adipokines may play a role in the pathophysiology of a variety of cardiovascular diseases. In addition, the relation between resistin and AF provides additional support for the role of inflammation in AF initiation. Insulin-sensitizing agents such as thiazolidinediones and metformin have known favorable effects on adipokines. 35 In an experimental heart failure model, pioglitazone has been reported to attenuate atrial remodeling and AF promotion to the same extent as candesartan, an angiotensin-receptor blocker.36 One of the proposed mechanisms by which thiazolidinediones may suppress AF is by depressing resistin secretion and stimulating adiponectin secretion from adipocytes and thereby inhibiting inflammatory, oxidative, and hypertrophic signaling pathways involved in atrial remodeling.30,35,37 Future studies are needed to elucidate the exact relation between adipokines and AF. In addition, if our findings are replicated, future research is required to evaluate whether therapies that modulate adipokines concentrations and reduce adiposity decrease AF risk.
Implications
In our community-based longitudinal study, higher concentrations of resistin were related to incident AF; the relation was attenuated upon adjustment for CRP. We were unable to detect a statistically significant association between adiponectin and incident AF. Future studies are warranted to clarify the role of adipokines in AF.
Supplementary Material
Acknowledgments
The Framingham Heart Study is supported by National Heart, Lung, and Blood Institute; Framingham Heart Study (NHLBI/NIH contract N01-HC-25195); and the Boston University School of Medicine. Dr Rienstra is supported by a grant from the Netherlands Organization for Scientific Research (Rubicon grant 825.09.020). This work was supported by grants from the National Institutes of Health to Drs Benjamin and Ellinor (1R01HL092577); Dr Benjamin (1RC1HL101056, 1R01HL102214, R01AG028321; and support via 6R01-NS17950) and Dr Ellinor (5R21DA027021, 5RO1HL104156, 1K24HL105780); and Dr Vasan (R01-DK-080739). Dr Magnani is supported by American Heart Association Award 09FTF2190028. This work was partially supported by the Evans Center for Interdisciplinary Biomedical Research ARC on “Atrial Fibrillation” at Boston University (http://www.bumc.bu.edu/evanscenteribr/).
Footnotes
Disclosures
None.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 4.Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 5.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 6.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 7.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish diet, cancer, and health study. Am J Med. 2005;118:489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 9.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 10.Cheng S, Keyes MJ, Larson MG, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301:2571–2577. doi: 10.1001/jama.2009.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filkova M, Haluzik M, Gay S, et al. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin Immunol. 2009;133:157–170. doi: 10.1016/j.clim.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Kim M, Oh JK, Sakata S, et al. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45:270–280. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmazyn M, Purdham DM, Rajapurohitam V, et al. Signalling mechanisms underlying the metabolic and other effects of adipokines on the heart. Cardiovasc Res. 2008;79:279–286. doi: 10.1093/cvr/cvn115. [DOI] [PubMed] [Google Scholar]
- 14.Azuma K, Katsukawa F, Oguchi S, et al. Correlation between serum resistin level and adiposity in obese individuals. Obes Res. 2003;11:997–1001. doi: 10.1038/oby.2003.137. [DOI] [PubMed] [Google Scholar]
- 15.Frankel DS, Vasan RS, D'Agostino RB, Sr, et al. Resistin, adiponectin, and risk of heart failure the Framingham Offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay RS, Funahashi T, Hanson RL, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 19.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 20.Weikert C, Westphal S, Berger K, et al. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab. 2008;93:2647–2653. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 21.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Serum resistin concentrations and risk of new onset heart failure in older persons: the health, aging, and body composition (Health ABC) study. Arterioscler Thromb Vasc Biol. 2009;29:1144–1149. doi: 10.1161/ATVBAHA.109.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimano M, Shibata R, Tsuji Y, et al. Circulating adiponectin levels in patients with atrial fibrillation. Circ J. 2008;72:1120–1124. doi: 10.1253/circj.72.1120. [DOI] [PubMed] [Google Scholar]
- 23.Feinleib M, Kannel WB, Garrison RJ, et al. The Framingham Offspring study. Design and preliminary data. Prev Med. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 24.Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families. The Framingham Offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 25.Ho KK, Anderson KM, Kannel WB, et al. Survival after the onset of congestive heart failure in Framingham Heart study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 26.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hastie T, Tibshirani R. Generalized additive models for medical research. Stat Methods Med Res. 1995;4:187–196. doi: 10.1177/096228029500400302. [DOI] [PubMed] [Google Scholar]
- 28.Issac TT, Dokainish H, Lakkis NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. 2007;50:2021–2028. doi: 10.1016/j.jacc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 29.Reilly MP, Lehrke M, Wolfe ML, et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 30.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 31.Verma S, Li SH, Wang CH, et al. Resistin promotes endothelial cell activation: further evidence of adipokine-endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 32.Calabro P, Samudio I, Willerson JT, et al. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–3340. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]
- 33.Takata Y, Osawa H, Kurata M, et al. Hyperresistinemia is associated with coexistence of hypertension and type 2 diabetes. Hypertension. 2008;51:534–539. doi: 10.1161/HYPERTENSIONAHA.107.103077. [DOI] [PubMed] [Google Scholar]
- 34.Iwashima Y, Katsuya T, Ishikawa K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 35.Bajaj M, Suraamornkul S, Hardies LJ, et al. Plasma resistin concentration, hepatic fat content, and hepatic and peripheral insulin resistance in pioglitazone-treated type II diabetic patients. Int J Obes Relat Metab Disord. 2004;28:783–789. doi: 10.1038/sj.ijo.0802625. [DOI] [PubMed] [Google Scholar]
- 36.Shimano M, Tsuji Y, Inden Y, et al. Pioglitazone, a peroxisome proliferator-activated receptor-gamma activator, attenuates atrial fibrosis and atrial fibrillation promotion in rabbits with congestive heart failure. Heart Rhythm. 2008;5:451–459. doi: 10.1016/j.hrthm.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 37.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.