Abstract
Parabiosis—conjoined surgery to provide a shared circulation between two mice—has been previously developed to study the hematopoietic system. This protocol describes the use of parabiosis for efficient transplantation of skin from a transgenic to a wild-type mouse. It can be used to study the role of stromal cells in a spontaneous model of distant cancer dissemination (metastasis). We have recently shown that primary tumor-derived stromal cells may facilitate metastasis by providing a provisional stroma at the secondary site. Studying the role of primary tumor–derived stroma cells requires methods for distinguishing and targeting stromal cells originating from the primary tumor versus their counterparts in the metastatic site. Parabiosis may also be used, taking advantage of the shared circulation between the parabiosed mice, to study tumor metastasis from one parabiont to another, or to investigate the role of circulating inflammatory cells or stem cells. Studying the role of stromal cells in metastasis using this model typically takes up to 11 weeks.
INTRODUCTION
Recent reports provide strong evidence for the contribution of nonmalignant stromal cells to the development of tumors1–5. Tumors actively recruit stromal cells and these cells facilitate tumor growth6–10. A metastatic cancer cell can only grow out to be an established metastasis when it successfully completes each of the sequential and interrelated steps of the metastatic cascade. Each of these steps can be rate limiting, and the failure to complete a step can abrogate the entire process11.
Host-derived nonmalignant stroma may increase the efficiency of this complex system12,13. Metastatic cells can reside in the lungs awaiting oncogenic activation14, or they can home to pre-existing ‘niches’ created by inflammation and immune cell or fibrocyte accumulation15–18. In addition, it has been shown that metastatic cells can proliferate intravascularly before extravasation into the lung tissue19. Cancer cell clumping and embolus formation in blood circulation may increase metastasis11,20–23. However, the clumps may also be fragments consisting of cancer cells and ‘passenger’ stromal cells carried over from the primary site.
The parabiosis model, in which two mice share the same circulation, may be used for a variety of studies of metastasis, such as tumor metastasis from one parabiont to another, or the role of circulating inflammatory cells or stem cells in the process11,24. However, previous parabiosis models were not ideal for the study of metastasis by large tumor–stromal cell clumps. Therefore, we have recently developed the transient parabiosis technique described in this protocol in which a large skin flap is transplanted. We have found that the host-derived cells increase cancer cell viability in circulation; they also serve as a provisional stroma in the secondary site and increase cancer’s metastatic efficiency13.
Overview of the technique
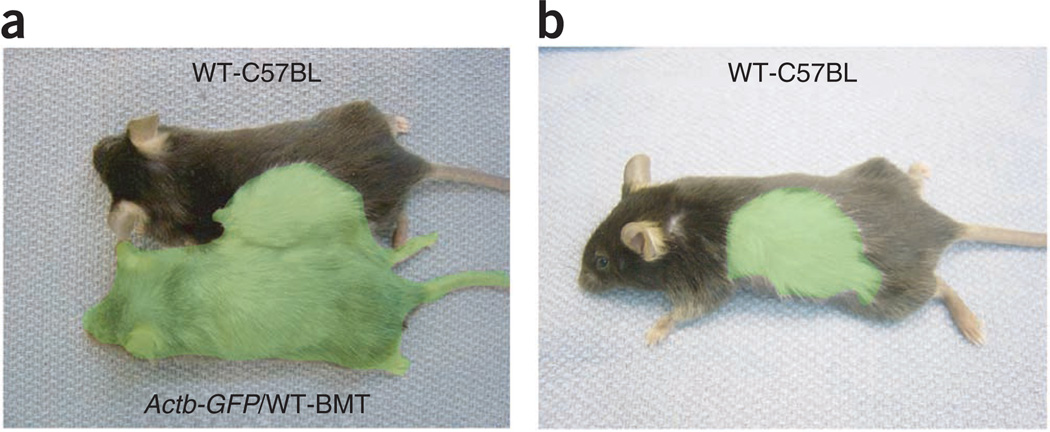
Previously, it has been shown that GFP and RFP (dsRed) could be used to color-code tumor and stroma in vivo25,26. This protocol describes an experiment for studying the involvement of stromal cells in a spontaneous metastasis formation model. The experimental design is depicted in Figure 1. In the parabiosis skin transplant model, we first generate a mouse chimera by reconstituting the bone marrow of GFP-expressing Actb-GFP/C57BL/6 mice with nonfluorescent (wild-type (WT)) bone marrow cells from C57BL/6 mice. This is necessary to exclude from the analysis GFP+ hematopoietic cells, which may be in the blood circulation during parabiosis. To this end, Actb-GFP/C57BL/6 mice are lethally irradiated using a dose of 12 Gy to the whole body in two fractions of 6 Gy 24 h apart. Then, the mice are rescued with a bone marrow transplant (BMT) from a nontransgenic (WT-C57BL/6) mouse. After 1 or 2 months, the reconstitution of the bone marrow is confirmed with flow cytometric analysis (i.e., chimerism of over 95%), using cells from non-GFP mice as controls. Next, the BMT-WT-ActbGFP and WT-C57BL/6 mice are parabiosed. The parabionts are conjoined for 3 weeks to establish a shared circulation and to ensure blood supply of the transplant from the recipient. Upon surgical separation, the large Actb-GFP skin transplant in WT mice is left to heal for 1–2 weeks before the implantation of red fluorescence-labeled tumor cells. The cancer cells in the transplanted Actb-GFP skin will generate primary tumors with GFP+ host-derived cells. Two to three weeks later, the GFP+ passenger stromal cells can be visualized in the lungs via multiphoton or confocal microscopy. Lung tissues from mice bearing non-GFP-labeled tumors are used as controls.
Figure 1.
Design of the experiment. (a) Transient parabiosis between a GFP-transgenic mouse and a WT mouse to ensure vascularization of a large skin transplant onto the recipient mouse. (b) A large GFP+ skin transplant is created after separation of the parabionts. When tumors are grown in the transplant, GFP+ stromal cells will be recruited, which can be visualized during spontaneous metastases. Reproduced with permission from ref. 13. All animal procedures were performed according to the guidelines of public health service policy on humane care of laboratory animals and in accordance with an approved protocol by the Institutional Animal Care and Use Committee of MGH.
Comparison with other techniques
Several in vitro assays have been developed to study host–tumor cell interactions related to progression and invasion, including two complementary protocols from our group27–30.
As an alternative to growing primary tumors in previously transplanted Actb-GFP skin, one can use transplantation of tumor fragments that were originally grown in an Actb-GFP expressing mouse. In this tumor fragment graft model, tumor cells are first implanted subcutaneously in Actb-GFP–expressing mice and allowed to grow to a size of 6 mm in diameter. Then tumors are collected, fragmented into 1 × 1 mm pieces, and subsequently implanted in syngeneic WT (nontransgenic) recipient mice31. The advantage of this technique is the lack of circulating cell contamination in the model. The primary limitation of this technique is the discrepancy of doubling times between cancer and stromal cells, which results in rapid tumor growth with recruitment of mouse (nonfluorescent) stromal cells and depletion over time of the original GFP+ stromal cells from the transplanted fragment. This limits the ability to study the stromal cells during metastasis using GFP as a marker.
Selecting the appropriate experiment(s) for studying metastasizing stromal cells
We developed this spontaneous metastatic model to visualize passenger stromal cells that originate from the primary tumor in metastatic nodules. We have used this model—together with an isolated tumor perfusion model and a spontaneous metastases model where we can selectively deplete carcinoma-associated fibroblasts29,30—to unravel the role of passenger fibroblasts in tumor metastasis to the lungs13.
In order to select the appropriate technique for future studies, one should carefully consider which step of the metastatic process is of interest. Using the isolated tumor perfusion model, one could study tumor shedding and quantify the result of treatments or genetic manipulation in this specific step of metastasis30. The parabiosis skin transplantation model described here mimics the steps of spontaneous metastases from tumor shedding up to the outgrowth of micrometastases, taking advantage of the fluorescent labeling of the passenger stromal cells. Last, to facilitate depletion of the stromal compartment of the tumor microenvironment without affecting other organs, we developed a model of selective depletion of human carcinoma-associated fibroblasts29.
The key advantage of this protocol over other existing methods is that one gains the ability to image in vivo the passenger stromal cells during various steps of metastasis by using fluorescent tumor stroma in a nontransgenic mouse. One limitation is that many of the current examples are ectopic tumor metastasis models. Another limitation is that the main focus is on mesenchymal stromal cells (over immune cells, e.g., monocyte/macrophages, which are largely excluded from analysis in the BMT model).
List of applications of the parabiosis skin transplantation model for metastasis
The parabiosis model can be used to generate a large skin transplant from a syngeneic donor that can be genetically different from the recipient. Possible modifications could be knockdown or overexpression of certain genes, or gender mismatch. In this protocol, we focus on the role of stromal cells in metastases; however, other tumor stromal cells could potentially be studied using this protocol.
MATERIALS
 All reagents and equipment could be substituted with appropriate alternatives from other manufacturers.
All reagents and equipment could be substituted with appropriate alternatives from other manufacturers.
REAGENTS
PBS (1×; Cellgro, cat. no. 20-031-CV)
Buprenorphine hydrochloride (0.3 mg ml−1; MGH Pharmacy, cat. no. 716510)
 It is poisonous and may cause prolonged respiratory depression. Wear protective clothing to avoid contact or inhalation. Buprenorphine is a controlled substance and should be handled according to institutional guidelines.
It is poisonous and may cause prolonged respiratory depression. Wear protective clothing to avoid contact or inhalation. Buprenorphine is a controlled substance and should be handled according to institutional guidelines.Ethanol (70% (vol/vol); Pharmco, cat. no. 111000190)
Fatal-Plus (Vortech, cat. no. NDC 298-9373-68)
 Fatal-Plus is a poisonous agent; caution should be exercised to avoid contact of the drug with open wounds or accidental self-inflicted injections.
Fatal-Plus is a poisonous agent; caution should be exercised to avoid contact of the drug with open wounds or accidental self-inflicted injections.Fluorescent-labeled LLC1 cells (e.g., DsRed-labeled cells; ATTC, cat. no. CRL-1642)
Hank’s balanced salt solution (1×; Gibco, cat. no. 14170)
Heparin sodium (1,000 USP U ml−1; APP Pharmaceuticals, cat. no. 504011)
Ketamine (100 mg ml−1; Massachusetts General Hospital pharmacy) and Xylazine (10 mg ml−1; Webster, cat. no. 200204.00) mixture per kg body weight
Mice, Actb-GFP/C75BL/6, C57BL/6, 10 weeks of age
 All animal studies must be reviewed and approved by the institutional animal care and use committees and conform all relevant ethics regulations.
All animal studies must be reviewed and approved by the institutional animal care and use committees and conform all relevant ethics regulations.
 All experiments must be performed under sterile conditions. Researchers should wear sterile hats, gowns and gloves.
All experiments must be performed under sterile conditions. Researchers should wear sterile hats, gowns and gloves.
OCT compound (Allegiance, cat. no. M7148-4)
-
Paraformaldehyde (10% (wt/vol); Polyscience, cat. no. 4018)
 Hazardous when exposed to skin, inhaled or swallowed. Preparation of 4% (wt/vol) paraformaldehyde should be carried out in a chemical hood with appropriate clothing.
Hazardous when exposed to skin, inhaled or swallowed. Preparation of 4% (wt/vol) paraformaldehyde should be carried out in a chemical hood with appropriate clothing. dH2O
EQUIPMENT
5-0 Vicryl sutures (Ethicon, cat. no. X698G)
Applicator tips (Owens minor, cat. no. 5937-W0D1002)
Autoclip applier (plus 9-mm autoclips; Roboz Surgical Instruments, cat. no. RS-9260 + RS-9262)
Caliper (Roboz Surgical Instruments, cat. no. RS-6466)
Clipper (Webster, cat. no. 78997-010)
Cryomolds (Cardinal Health, cat. no. M7144-13)
Cryostat (Microm, cat. no. HM-560)
Fluorescence microscope (Cambridge Research and Instrumentation)
Forceps (Roboz Surgical Instruments, cat. nos. RS-5153, RS 5150, RS-5132)
Heating pad (Shore Line, cat. no. 712.0000.04)
Needle holder (14 cm; Roboz Surgical Instruments, cat. no. RS-6412)
Permanent marker (Staedtler, cat. no. 342-9)
Scissors (Roboz Surgical Instruments, cat. nos. RS-5840, RS-5883)
Syringe (1 ml with a 26-G needle for anesthesia, 16-G and 30-G needles for BMT; Fisher Scientific, cat. no. 14-823-2E)
Cotton swabs
Blade and blade holder
Cell strainer
REAGENT SETUP
Paraformaldehyde, 4% (wt/vol)
Add 180 ml of 1× PBS to 120 ml of 10% (wt/vol) formaldehyde.  This reagent has a short shelf life (< 1 week) so it must be freshly made on the day of use.
This reagent has a short shelf life (< 1 week) so it must be freshly made on the day of use.
Buprenorphine hydrochloride
Dissolve 1 ml of the 0.3 mg ml−1 stock solution in 30 ml of 0.9% (wt/vol) sodium chloride. Store at 20 °C for up to 3 months. PBS (1×) Add 100 ml of 10× PBS to 900 ml of dH2O. Store at 20 °C for up to 9 months.
Ethanol, 70% (vol/vol)
Mix 1.7 liters of dH2O and 1 gallon of 100% ethanol. Store at 20 °C in a closed container.
Heparin
Dilute 0.3 ml of heparin stock solution in 30 ml of 0.9% (wt/vol) sodium chloride.
 Freshly prepare for each experiment.
Freshly prepare for each experiment.
PROCEDURE
Bone marrow transplantation
-
1|
Calculate the necessary number of mice for the experiment. Lethally irradiate the mice using 12 Gy in two fractions. Start on day 1 with a dose of 6 Gy to the whole body of Actb-GFP/C57BL/6 mice.
 During parabiosis, the donor and recipient share a common circulation. To prevent GFP+ circulating hematopoietic cells from the donor from confounding the results after separation of the parabionts, the donor must receive a WT BMT at least 4 weeks before conjoining.
During parabiosis, the donor and recipient share a common circulation. To prevent GFP+ circulating hematopoietic cells from the donor from confounding the results after separation of the parabionts, the donor must receive a WT BMT at least 4 weeks before conjoining.
-
2|
On the next day, repeat the whole-body irradiation as in Step 1.

-
3|
On the next day (i.e., 1 day after second fraction of radiation), collect bone marrow from the femurs of WT-C57BL/6. Euthanize mice by injecting 0.1 ml of Fatal-Plus intra-abdominally. Dissect skin and muscles from the femurs (hind limbs) of the mouse, and cut off the joints using a blade and blade holder. Wash the bone marrow out of each bone by flushing them with 3 ml of 4% (vol/vol) heparin/PBS solution using a 30-G needle, and collect the marrow in 15-ml tubes kept on ice. Centrifuge the bone marrow cells for 3 min at 500g at 4 °C, and then remove the supernatant. Resuspend the bone marrow cell pellet obtained from one mouse (two femurs) in 600 µl of PBS by using a 16-G needle and filtering through a 70-µm cell strainer. Infuse 150 µl of bone marrow into the tail vein of the recipient Actb-GFP/C57BL/6 mouse of at least 8 weeks of age (i.e., the bone marrow cells from one mouse could be used to transplant three lethally irradiated mice).
 Do not crush the bone when cutting off the ends, as it makes bone marrow flushing difficult.
Do not crush the bone when cutting off the ends, as it makes bone marrow flushing difficult. Keep bone marrow cells on ice at all times before transplantation.
Keep bone marrow cells on ice at all times before transplantation. -
4|
Wait 4 weeks for the bone marrow to engraft before proceeding to the next steps, while keeping mice in regular cages under sterile conditions.

Parabiosis surgery
-
5|
Anesthetize both mice using 0.4 ml of ketamine/xylazine and a 30-G syringe.
 To make sure they wake up at the same time, it is important to anesthetize mice at the same time with the same amount of anesthetic.
To make sure they wake up at the same time, it is important to anesthetize mice at the same time with the same amount of anesthetic. -
6|
Use a clipper to shave the entire right flank of the donor mouse and left flank of the recipient and clean their skin using 70% (vol/vol) ethanol.
-
7|
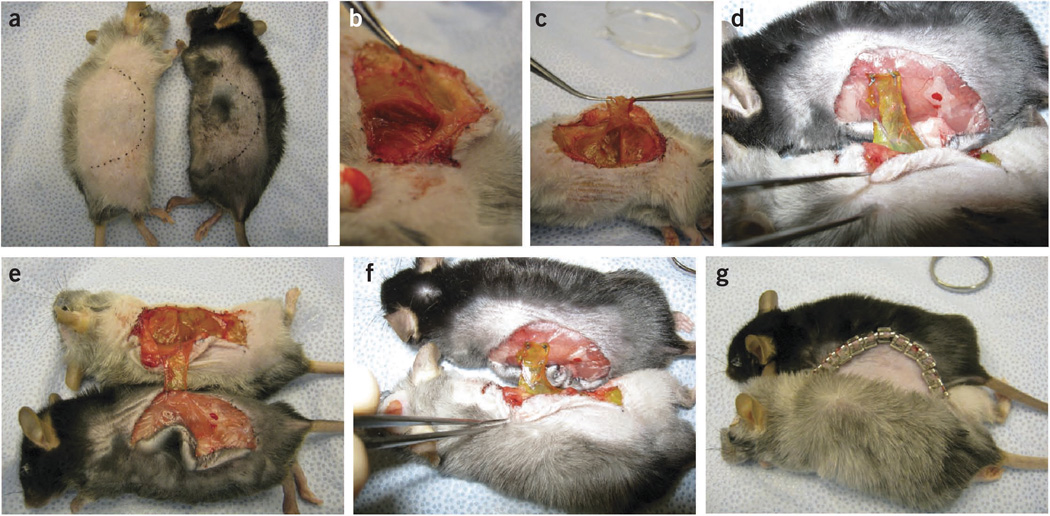
Mark the incision line on the skin of the mouse using permanent marker. This should be half an ellipse starting from behind the ear to a point 1 cm anterior-lateral to the tail. For the Actb-GFP/C57BL/6 skin donor, this line should be on the right flank of the animal going from the ear to the ventral side. For the WT recipient, the incision line should be on the left flank going from behind the ear toward the back of the mouse and the tail (Fig. 2a).
 It is important that both incision lines are of the same length and shape.
It is important that both incision lines are of the same length and shape.
-
8|
Make a skin incision along the incision line in the donor using forceps and scissors. Dissect skin and fat tissue from underlying muscles.
 Do not cut the right brachial artery, as this will cause massive bleeding.
Do not cut the right brachial artery, as this will cause massive bleeding. -
9|
Use forceps to lift the muscle from the thoracic ribs and dissect a muscle layer with small scissors and cotton swabs. Cut the intercostal muscles. Work from the ventral to the dorsal side and from anterior to posterior (Fig. 2b).

-
10|
After dissecting the muscle from the thoracic wall, continue separating toward the tail. Use cotton swabs to separate the muscle from the peritoneum and use scissors to cut the ventral edges of the muscle layer. Create a muscle flap of about 2–3 cm in size that folds to the back of the mouse (Fig. 2c).
 The muscle layer and peritoneum join in the ventral midline. To avoid damaging the peritoneum, dissect up to 3 mm from the ventral line.
The muscle layer and peritoneum join in the ventral midline. To avoid damaging the peritoneum, dissect up to 3 mm from the ventral line. -
11|
Move to the recipient mouse and make a skin incision along the incision line. Dissect the skin from the underlying muscle.

-
12|
Position the mice next to each other with their heads facing down in the same direction. Use forceps, a needle holder and sutures to position the donor’s muscle flap on the intact muscle of the recipient mouse. Make two mattress stitches on the lateral edges of the far end of the muscle flap (Fig. 2d).

-
13|
Position the pair of mice on their backs and secure the muscle transplant with two more stitches as proximal to the donor as possible (Fig. 2e). This will create a fixed overlay of 1 cm2 of muscle tissue to ensure development of a shared circulation (Fig. 2f).
-
14|
Close the skin by positioning the donor skin onto the back of the recipient mouse with wound clips (Fig. 2g).
When you are finished, turn the pair and close the ventral skin. Use 5.0 Vicryl sutures to close the corners in the ear and tail areas. The mice should look similar to those in Figure 1a.
-
15|
Administer 0.2 ml of buprenorphine and house every pair of mice individually in a special cage with easy water and food access.
-
16|
Remove half of the wound clips (alternating, i.e., every other clip) after 1 week.
-
17|
Remove the remaining wound clips after 2 weeks.
Figure 2.
GFP+ skin transplantation through parabiosis surgery under general anesthesia. (a–g) The fur is shaved and the size of the transplant marked on the skin (a); a muscle flap is dissected from the thoracic cage and the peritoneum using a cotton swab (b); a 1–2 cm muscle flap (c,d) of the muscle flap is attached onto the non-GFP mouse with two sutures, and the pair of mice is turned onto their backs to apply two more sutures ~1 cm apart from the first set (e); a 1 cm2 of GFP+ muscle is positioned on the non-transgenic mouse to ensure the development of a shared circulation (f); and the skin is closed with wound clips to finalize the parabiosis surgery (g). All animal procedures were performed according to the guidelines of public health service policy on humane care of laboratory animals and in accordance with an approved protocol by the Institutional Animal Care and Use Committee of MGH.
Separation of parabionts (3 weeks after initial conjoinment)
-
18|
Three weeks after initial conjoinment, anesthetize the mice with ketamine/xylazine.
-
19|
Cut the donor skin and subcutaneous tissues so that a large skin transplant (2–3 cm) is created from the Actb-GFP/C57BL/6 donor mouse to be included in the skin of the WT mouse (see Fig. 1b for an example of the final appearance). Close the wound edges with wound clips. After separation, euthanize the donor mouse by cervical dislocation or by injecting 0.1 ml of Fatal-Plus intraperitoneally with a 30-G needle. House the transplanted mouse under pathogen-free conditions and allow the wound to heal (~1 week).

Tumor inoculation and resection
-
20|
Anesthetize mice and inject 5 × 105 (fluorescence-labeled) metastatic cells suspended in 100 µl of Hank’s buffered salt solution in the middle of the skin transplant.
-
21|
Monitor the tumor size daily by caliper measurement. When the tumor diameter reaches 10 mm, resect the primary tumor and close the wound with wound clips.
Tissue collect and processing
-
22|
After 3 weeks, or once metastases have formed, euthanize the mice by injecting 0.1 ml of Fatal-Plus intraabdominally and collect the lungs or other target organs for metastases if you are using a different cell line (e.g., liver).
-
23|
Fix the lungs in 4% (wt/vol) paraformaldehyde for 6 h, wash them with PBS, and then dehydrate them in 30% (wt/vol) sucrose overnight.
 Do not overfix the tissue as this may cause difficulties for immunohistochemical analyses.
Do not overfix the tissue as this may cause difficulties for immunohistochemical analyses. -
24|
Fill appropriate-sized molds with OCT, embed collected tissues and store at − 80 °C.
 Collected tissues can be sectioned and stained at any time. OCT-embedded blocks can be stored at − 80 °C for over a year.
Collected tissues can be sectioned and stained at any time. OCT-embedded blocks can be stored at − 80 °C for over a year. -
25|
Use cryostat to cut 20 µm tissue sections. Use standard antibody and counterstaining protocols to further analyze the tissue using a fluorescent microscope.
![]()
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1 | Death of the mouse seconds after infusion of the bone marrow | Pulmonary embolism | Repeat the procedure after carefully filtering the bone marrow cell suspension. Inject slowly (over 1–2 min) |
| 2 | Mouse is not fully anesthetized | Dose is insufficient | Different strains of mice may have disparate sensitivities to a particular anesthesia. The dose prescribed in the protocol generally provides a safe and appropriate response; however, this dose may not be sufficient in all situations |
| 4 | Death of the mouse days/weeks after infusion of the bone marrow | Failure of the bone marrow to engraft because of poor viability or a small number of cells | Keep the bone marrow on ice during the time it is outside an animal. Be sure to infuse a proper amount of cells (agitate syringe before injection) |
| 7 | Damage to the peritoneum | Incomplete separation of the muscle layers before cutting the muscle flap | Use 5.0 vicryl and the mattress suturing technique to close the hole in the peritoneum |
| 9 | Difficulties suturing through the muscle flap | Sutures are covered with blood | Use 70% (vol/vol) ethanol and a sponge to clean the suture before proceeding |
| 11 | Skin is attached to underlying tissues | Tissue dries easily during prolonged surgery | Apply saline to the dried tissue using a cotton swab |
| 12 | Skin does not heal | The outside part of the skin was rolled inside when wound clips were applied | Ensure that only the inside parts of the skin are touching each other before applying wound clips |
| 19 | No formation of metastases | Prolonged in vitro culture could change the cell phenotype. Fluorescent labeling of tumor cells might affect their phenotype | Repeat the experiment using original cell stock or another fluorophore (that might be less toxic) |
Steps 1 and 2, irradiation of Actb-GFP/C57BL/6 mice: 1 h
Step 3, bone marrow transplantation: 1.5 h
Step 4, bone marrow engraftment: 4 weeks
Steps 5–17, parabiosis surgery: 1 h
Step 18, separation surgery and skin graft: 15 min
Step 19, skin graft wound healing: 1 week
Step 20, tumor implantation in skin transplant: 10 min
Step 21, tumor growth: 2–3 weeks
Step 22, lung tissue collect and evaluation of metastatic burden: 45 min
Step 23, fixation and dehydration of lung tissue: 2 d
Step 23, embedding lung tissue in OCT: 15 min
Step 24, immunohistochemical analysis: depending on the protocol used, usually 1–2 d.
ANTICIPATED RESULTS
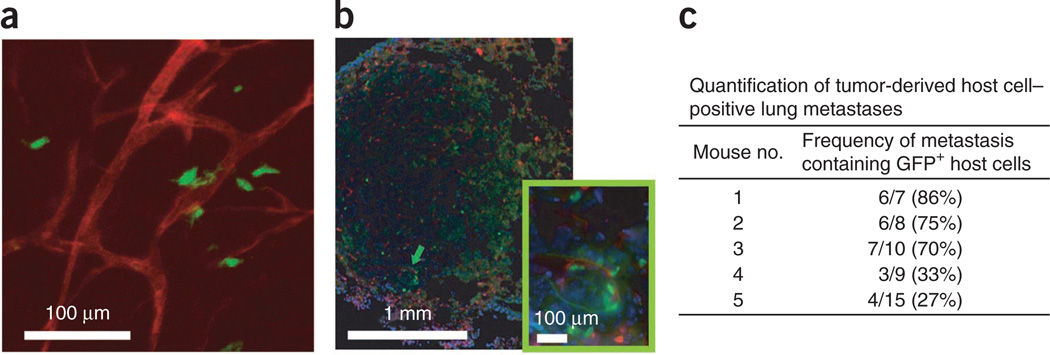
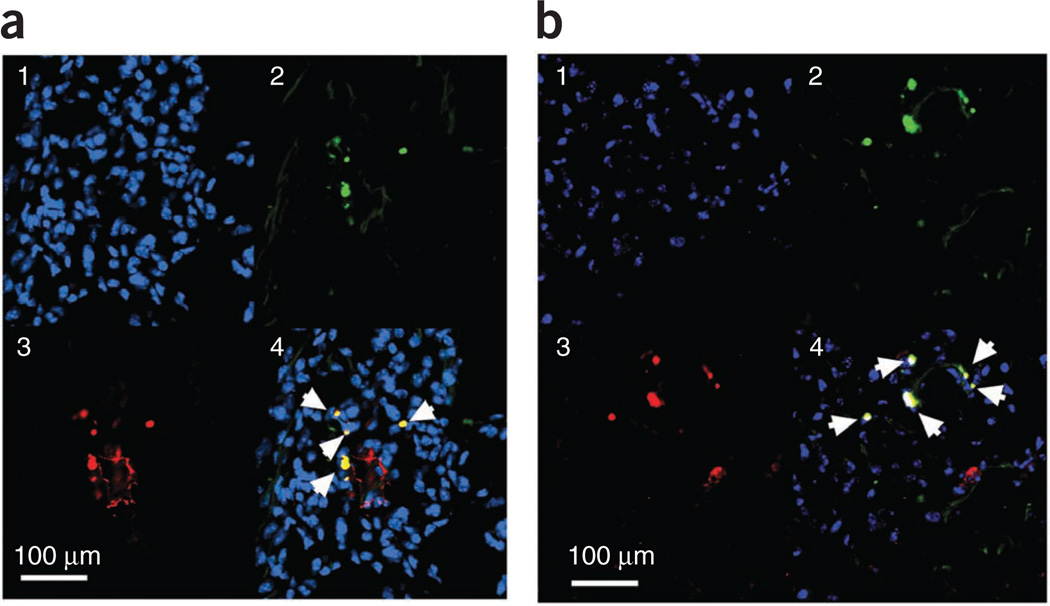
We used this parabiosis model to quantitatively evaluate the participation of primary site–derived host cells. LLC1 cells were implanted subcutaneously in the GFP+ skin transplant, allowed to grow up to a diameter of 10 mm, and then primary tumors were excised to allow metastases to grow. GFP+ host-derived cells were detectable in lung metastases in all five animals in which the primary tumor contained GFP+ host cells (Fig. 3). The frequency of the involvement of host-derived cells from primary tumors in distant metastases varied between 27% and 86% of the metastatic nodules. The frequency of GFP+ host cells in metastases correlated with the density of GFP+ cells in the primary tumor (data not shown). Next, we collected the lungs containing LLC1 metastases with GFP+ passenger stromal cells. Immunohistochemical analysis of skin-derived, GFP+ stromal cells in the lungs revealed that the majority (~75%) of these cells are of mesenchymal origin13 (Fig. 4).
Figure 3.
Quantification of tumor-derived GFP+ stromal cells in lung metastases. Large skin flaps were transferred from Actb-GFP/C57BL mice (after C57BL/6 bone marrow transplantation) to C57BL/6 mice through parabiosis. GFP+ host cells were detected in the lung metastases of subcutaneously grown LLC-1 tumors (implanted in the transplanted skin area). (a,b) Multiphoton (a) and confocal microscopy (b) detection of GFP+ cells in the lung metastatic foci. Inset, magnified image of the area indicated by the green arrow. (c) Quantification of the frequency of GFP+ cells in metastatic nodules in the skin transplant model. Images are 315 µm (a), 1.72 mm (b) and 420 µm (inset in b) across. Blood vessels are enhanced by rhodamine-labeled infusion (shown in red) in a; DAPI nuclear counterstaining is shown in blue in b. Reproduced with permission from ref. 13. All animal procedures were performed according to the guidelines of public health service policy on humane care of laboratory animals and in accordance with an approved protocol by the Institutional Animal Care and Use Committee of MGH.
Figure 4.
Passenger stromal cells in spontaneous metastasis. (a,b) GFP+ stromal cells that have metastasized spontaneously from the skin transplant to the lungs. Immunohistochemical markers have been used to show colocalization of these stromal cells with α-SMA (a) and FSP-1 (b) expression. (1) In blue, DAPI nuclear staining; (2) in green, GFP + stromal cells, (3) in red, expression of α-SMA (in a) and FSP-1 (in b), and (4) overlay of the three colors; arrows indicate colocalization of GFP and α-SMA or FSP-1 expression. Reproduced with permission from ref. 13. All animal procedures were performed according to the guidelines of public health service policy on humane care of laboratory animals and in accordance with an approved protocol by the Institutional Animal Care and Use Committee of MGH.
Acknowledgments
This work is supported by US National Cancer Institute grants P01-CA80124, R01-CA115767, R01-CA85140, R01-CA126642 and T32-CA73479 (R.K.J.), R01-CA96915 (D.F.), R21-CA139168 and R01-CA159258 (D.G.D.) and Federal Share Proton Beam Program grants (R.K.J., D.F. and D.G.D.); Department of Defense Innovator Award W81XWH-10-1-0016 (R.K.J.) and Predoctoral Fellowship W81XWH-06-1-0781 (A.M.M.J.D.); American Cancer Society grant RSG-11-073-01TBG (D.G.D.); and Stichting Michael Van Vloten Fonds and the Stichting Jo Kolk (A.M.M.J.D.). We acknowledge the outstanding technical assistance of J. Kahn, S. Roberge and P. Huang with animal models.
Footnotes
AUTHOR CONTRIBUTIONS D.G.D., D.F. and R.K.J. designed the studies; A.M.M.J.D. and M.K. performed the experiments; D.G.D., D.F., A.M.M.J.D., M.K. and R.K.J. analyzed the data; and A.M.M.J.D., D.G.D. and D.F. wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
References
- 1.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–1260. [PubMed] [Google Scholar]
- 2.Bhowmick NA, et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N. Engl. J. Med. 1999;340:430–436. doi: 10.1056/NEJM199902113400604. [DOI] [PubMed] [Google Scholar]
- 5.Elenbaas B, et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Tlsty TD. Stromal cells can contribute oncogenic signals. Semin. Cancer Biol. 2001;11:97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- 10.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 12.Bouvet M, et al. In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res. 2006;66:11293–11297. doi: 10.1158/0008-5472.CAN-06-2662. [DOI] [PubMed] [Google Scholar]
- 13.Duda DG, et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl. Acad. Sci. USA. 2010;107:21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podsypanina K, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiratsuka S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Deventer HW, et al. C-C chemokine receptor 5 on pulmonary fibrocytes facilitates migration and promotes metastasis via matrix metalloproteinase 9. Am. J. Pathol. 2008;173:253–264. doi: 10.2353/ajpath.2008.070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Mehdi AB, et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 20.Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–894. [PubMed] [Google Scholar]
- 21.Chertow BS, Fidler WJ, Fariss BL. Graves’ disease and Hashimoto’s thyroiditis in monozygous twins. Acta Endocrinol (Copenh) 1973;72:18–24. doi: 10.1530/acta.0.0720018. [DOI] [PubMed] [Google Scholar]
- 22.Ruiter DJ, van Krieken JH, van Muijen GN, de Waal RM. Tumour metastasis: is tissue an issue? Lancet Oncol. 2001;2:109–112. doi: 10.1016/S1470-2045(00)00229-1. [DOI] [PubMed] [Google Scholar]
- 23.Sahai E. Illuminating the metastatic process. Nat. Rev. Cancer. 2007;7:737–749. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 24.Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur. J. Cancer. 1973;9:223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- 25.Fukumura D, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 26.Yang M, et al. Dual-color fluorescence imaging distinguishes tumor cells from induced host angiogenic vessels and stromal cells. Proc. Natl. Acad. Sci. USA. 2003;100:14259–14262. doi: 10.1073/pnas.2436101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albini A, Benelli R. The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation. Nat. Protoc. 2007;2:504–511. doi: 10.1038/nprot.2006.466. [DOI] [PubMed] [Google Scholar]
- 28.Bayless KJ, Kwak HI, Su SC. Investigating endothelial invasion and sprouting behavior in three-dimensional collagen matrices. Nat. Protoc. 2009;4:1888–1898. doi: 10.1038/nprot.2009.221. [DOI] [PubMed] [Google Scholar]
- 29.Duyverman AMMJ, Steller EJ, Fukumura D, Jain RK, Duda DG. Studying carcinoma-associated fibroblast involvement in cancer metastasis in mice. Nat. Protoc. 2012;7:756–762. doi: 10.1038/nprot.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duyverman AMMJ, et al. An isolated tumor perfusion model in mice. Nat. Protoc. 2012;7:749–755. doi: 10.1038/nprot.2012.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duda DG, et al. Differential transplantability of tumor-associated stromal cells. Cancer Res. 2004;63:5920–5924. doi: 10.1158/0008-5472.CAN-04-1268. [DOI] [PubMed] [Google Scholar]