Abstract
Purpose/Objectives
Moderate Deep Inspiration Breath-hold (mDIBH), utilizing an Active breathing Control (ABC) device has been used in our clinic since 2002 to reduce cardiac dose for patients receiving left-sided breast irradiation. We report our routine use of the mDIBH technique in clinically localized breast cancer, treated to the intact breast, reconstructed breast, or chest wall.
Materials/Methods
Ninety-nine patients with left sided breast cancer were evaluated for ABC treatment, of which, 87 patients were treated with mDIBH. Plans for both the free-breathing (FB) and mDIBH CT scans were evaluated. Dose volume histograms (DVHs) were analyzed for the heart and ipsilateral lung, comparing results for mDIBH vs FB plans.
Results
Eighty-seven patients are included for analysis. Of those, 66% received adjuvant chemotherapy with cardiotoxic agents. The mean dose to the whole breast was 47.6 Gy. There was a statistically significant decrease in all DVH parameters evaluated, favoring the delivery of mDIBH over FB plans. mDIBH plans significantly reduced cardiac mean dose (4.23 Gy vs. 2.54 Gy; p<0.001), a relative reduction of 40%. As well, there were significant reductions in all other heart parameters evaluated (i.e volume of heart treated, V30, V25, V20, V15, V10, and V5). mDIBH also significantly reduced lung dose, including a reduction of the left lung mean dose (9.08 Gy vs. 7.86 Gy; p<0.001), a relative reduction of 13%, as well as significant reduction of all lung DVH parameters evaluated.
Conclusions
To date, this series represents the largest experience utilizing mDIBH to reduce cardiac irradiation during left-sided breast cancer treatment. Statistically significant reductions in all heart and lung DVH parameters were achieved with mDIBH over FB plans. mDIBH, for the treatment of left sided breast cancer, is a proven technique for reducing cardiac dose that may lead to reduced cardiotoxicity and can be routinely integrated into the clinic.
Keywords: Breast cancer, breath hold, radiation therapy, intensity modulated radiation therapy
Introduction
As breast conserving therapy has become the predominant therapeutic option for early-stage breast cancer, the majority of women with this diagnosis undergo some form of radiotherapy as a component of their treatment. Radiotherapy has been shown in a number of large, prospective, randomized trials to reduce the risk of breast cancer local recurrence (1, 2). As well, several studies have demonstrated the benefits of radiotherapy in the post-mastectomy setting. (3, 4) In addition, recent meta-analyses have also shown its benefits in terms of improving breast cancer survival (5) However, certain radiotherapy techniques may contribute to patient mortality from other causes. Namely, increased cardiac radiation exposure from the deep edge of the tangential beams, normally employed in the adjuvant therapy of left sided breast cancer has been shown to be associated with increased risks of heart disease and cardiac mortality (5).
Respiratory motion studies in the past have demonstrated that deep inspiration results in increased distance between the heart and left anterior chest wall. This has been shown in prior studies to reduce the volume of heart and, specifically, left ventricular myocardium receiving doses of radiation likely to be of biologic significance (6, 7). Additional studies evaluating myocardial perfusion utilizing SPECT (single photon emission computed tomography) imaging have correlated perfusion defects with regions of increased myocardial radiation exposure (8). While such studies have not yet been able to correlate such findings with clinical endpoints such as coronary artery disease, myocardial infarction, or cardiac mortality, such demonstrable changes, when combined with data from population studies and prospective trials with long-term follow-up, provide compelling reasons to pursue techniques to reduce myocardial exposure during left breast irradiation.
Prior data published from our institution have demonstrated the efficacy of Active Breathing Control (ABC) in terms of the reduction of irradiated myocardium as well as the practical feasibility of its implementation in the clinic (6, 9). We present in this report our updated experience with ABC in the adjuvant irradiation of early-stage, left-sided breast cancer patients.
Methods and Materials
Patient Selection
Of the ninety-nine patients evaluated to date, 87 patients had mDIBH plans that resulted in improvements in cardiac dose when compared with free-breathing plans and were included in this study. (WBH HIC 1997-082) All patients included had early localized breast cancer (AJCC stage I, II and III), left-sided breast cancer (forty percent of which required regional nodal irradiation). Initially, patient eligibility required that ≥2% of the contoured heart volume on standard, free-breathing (FB), planning computed tomography (CT) scan be receiving a dose of greater than 30 Gy (cardiac V30 ≥ 2%). Patients had to demonstrate a comprehension of the rationale of this treatment such that an optimal level of compliance could be attained. In the initial stages of this study, it was found that patients who had problems with claustrophobia had a more difficult time complying with the ABC treatment protocol, and consequently, severe claustrophobia became an exclusion criterion for this trial. After the enrollment of the first 5 patients on this study (all < age 55), no specific age criteria were implemented provided patients could demonstrate a minimum comfort level with the ABC device to allow for efficient daily treatment delivery. Ultimately, after the first 25 participants, patients were screened for possible ABC treatment at the treating physician’s discretion, taking in to account patient age, chemotherapy use, as well as Traztuzumab use.
ABC Hardware
The ABC device has been described at length in previous publications but will be briefly reviewed (10). The same device was used during both the planning and treatment sessions and is the active breathing coordinator device commercially available through Elekta Oncology Systems. The device consists of a digital spirometer to record the patient’s real-time breathing trace and a balloon valve that is triggered to automatically inflate when the patient inspires to their pre-set mDIBH level. This allows for a predictable, consistent level of chest wall expansion with each mDIBH. A nose clip is used to prevent leakage and allow for accurate measurement of inspiratory volume. The trace is viewed on a laptop computer by radiation therapy staff outside the treatment room, and a prism glass positioned above the patient allows for patient viewing of their respiratory trace and anticipated duration of the breath hold. The details of several minor modifications made to the ABC system to facilitate RT delivery have been detailed in a prior publication (6).
Study Design
Following initial consultation at which time the patients’ candidacy for adjuvant radiotherapy were verified, all patients underwent an initial, free-breathing planning CT scan using Alpha-cradle immobilization. A prospective treatment plan was then generated incorporating medial and lateral non-divergent tangential beams to treat the entire left breast. A step-and-shoot IMRT approach that has been described at length previously in the literature was used to optimize dose homogeneity within the breast (11). For those patients requiring regional nodal irradiation, the third field encompassing the supraclavicular field was delivered, typically by a mono-isocentric technique. Cardiac volume was defined within the treatment planning system and a DVH analysis was performed.
Those who enrolled in this study underwent an initial training session. The patients were placed in their Alpha-cradle in the treatment position and connected to the ABC device. Their maximal inspiratory capacity was recorded according to the ABC spirometry software. A comfortable level of breath hold was then determined that would be used throughout the treatment course. This was generally set at approximately 75% maximal inspiratory capacity and was termed moderate deep inspiration breath hold (mDIBH). Once patients were able to demonstrate that they could comfortably maintain this level of breath hold for 20–25 seconds, they were deemed candidates for this treatment.
Patients then underwent repeat planning CT scan in mDIBH while connected to the ABC device. The planning process was then repeated using unopposed tangential beams to treat the entire left breast +/− the supraclavicular field via implementation of a step-and-shoot IMRT technique. Treatment plans were prepared with both the free-breathing and mDIBH scans by the same dosimetrist using identical guideline for delineation of target volumes and organs at risk to assure that target coverage was identical between the two plans. A verification simulation was performed with the patient connected to the ABC device to confirm the geometric parameters generated during the virtual CT simulation.
Treatment Delivery
Treatment was administered daily to a median whole breast total dose of 45 Gy, implementing ABC for each treatment. Patients were coached by radiation therapy staff on a daily basis, typically to perform two normal inspiratory/expiratory cycles prior to initiating mDIBH. The radiation therapist would initiate treatment once the patient achieved mDIBH as documented by the digital respiratory trace. Each medial and lateral non-divergent tangential beams were delivered over 2–3 breath holds, whilst the third beam addressing the supraclavicular field, if necessary, was delivered during an additional 1–2 breath holds. Treatments were typically supplemented via direct en-face electron boost to a dose of ≥ 60 Gy. ABC was not used during the boost portion of treatment.
Data Generation and Analysis
All planning and dosimetric evaluation was performed on the Pinnacle3 treatment planning system v. 8.0 (Phillips Healthcare, Andover, MI). The biopsy cavity, breast/chest wall, regional lymphatics (if applicable) and ipsilateral/contralateral lung, as well as heart were contoured on both mDIBH and free-breathing (FB) plans. Calculated dose-volume histograms were compared between both techniques for each of the aforementioned volumes. Paired samples t-tests were performed using SPSS ver. 17 (Chicago SPSS, Chicago, IL) to compare parameters between treatment techniques.
Results
Patients and Treatments
Eighty-seven patients, median age of 49 years (range 29–70), are included for analysis (Table 1). The median tumor size was 1.8 cm and the majority of patients had ≤T2 invasive tumors of the upper outer quadrant. As well, patients were nearly evenly split with respect to having involvement of 1 or more lymph nodes and those who had a negative sentinel lymph node biopsy. Table 2 demonstrates the treatment characteristics of the patients, with approximately 2/3’s of which received radiation therapy as a part of breast conserving therapy (BCT). Further, 66% received adjuvant chemotherapy with cardiotoxic agents, 45% with Anthracycline-based regimens and 9% with Trastuzamab.
Table 1.
Patient Disease Characteristics
| Patient Characteristics n=87 | |
|---|---|
| Median Age (yrs) | 49 (29–70) |
| Tumor Size (cm) | 1.8 (0.07–10.5) |
| T stage | |
| Tis | 15% |
| T1 | 39% |
| T2 | 32% |
| T3 | 12% |
| T4 | 2% |
| Tumor Location | |
| UOQ | 57% |
| UIQ | 20% |
| LOQ | 11% |
| LIQ | 7% |
| Central | 5% |
| Histology | |
| DCIS | 16% |
| IDC | 79% |
| ILC | 5% |
| Lymph Node status | |
| Negative | 51% |
| Positive | 49% |
Table 2.
Treatment Characteristics
| Treatment Characteristics | |
|---|---|
| Lumpectomy | 66% |
| Mastectomy | 34% |
| Radiation Dose (cGy) | |
| Median Breast/Chestwall | 4500 |
| Range | (1080–5400) |
| Median Boost | 1600 |
| Range | (600–1600) |
| Radiation Treatment Fields | |
| Simple Tangents | 48 (55%) |
| Lumpectomy+S CL/III | 9 (10%) |
| Chestwall+SCL/III | 13 (15%) |
| Reconstructed+SCL/III | 13 (15%) |
| Reconstructed Tangents | 4 (5%) |
| Systemic Treatment | |
| Chemotherapy | 56 (64%) |
| Adriamycin | 36 (64%) |
| Herceptin | 8 (14%) |
| Adriamycin + Herceptin | 3 (5%) |
| Hormone Therapy | 28 (32%) |
Cardiac Doses
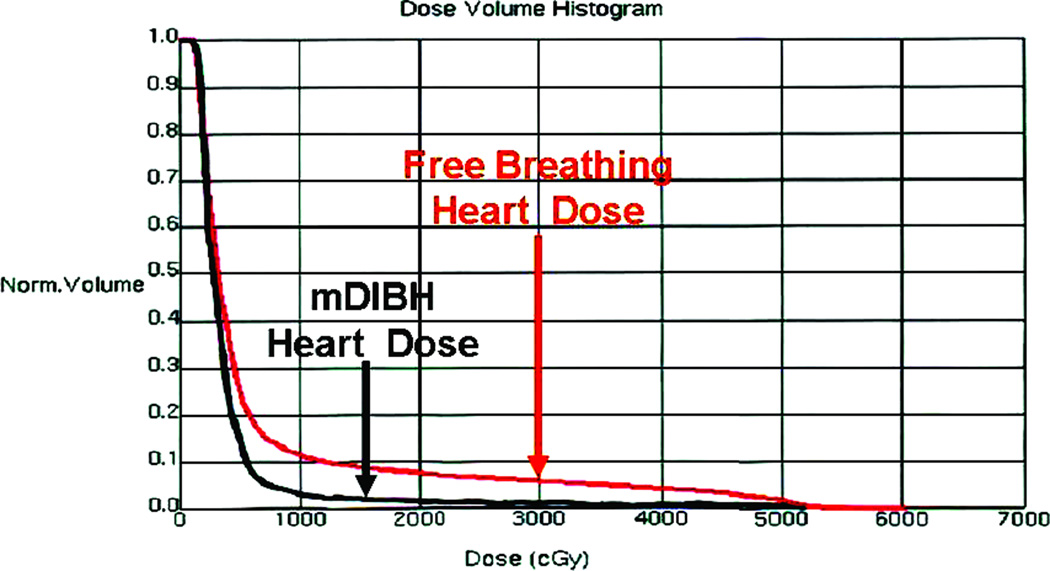
As a result of moderate deep inspiration breath-hold, the left-sided breast and the heart were separated during radiation treatment, excluding substantial heart volumes from the high-dose area. (Figure 1). As demonstrated by the Dose Volume Histogram (Figure 2), there is significantly less dose delivered to the heart with the mDIBH plan compare to the free-breathing plan. The delivered plan via mDIBH results in significant decrease in the heart volume treated: 0.79 mL vs. 2.3 mL (p<0.0001) as well as mean heart dose: 2.5 Gy vs. 4.2 Gy (p<0.0001) as shown in Table 3. All volumetric parameters evaluated: V30, V25, V20, V15, V10 and V5 were also significantly decreased as compared to the free-breathing plan. As well, for 34% of the patients, the V25 was 0% for the delivered mDIBH plan.
Figure 1.
CT Simulation Comparing Free Breathing (A) with mDIBH (B) as well as beam’s eye view of breast with heart contoured in red for Free Breathing Plan (C) and mDIBH (D) plan.
Figure 2.
Dose Volume Histogram comparing heart dose with the mDIBH plan and the Free Breathing Plan
Table 3.
Improvement in Heart Dose Parameters with ABC Treatments
| Parameter | Free Breathing |
mDIBH ABC |
Relative Reduction |
p-value |
|---|---|---|---|---|
| Heart Vol Treated | 2.3 mL | 0.79 mL | 66% | <0.0001 |
| Mean Heart Dose | 4.2 Gy | 2.5 Gy | 40% | <0.0001 |
| Dose/Vol Parameters | ||||
| Heart V30 | 3.2% | 0.39% | 88% | <0.0001 |
| Heart V25 | 3.8% | 0.52% | 86% | <0.0001 |
| Heart V20 | 4.3% | 0.69% | 84% | <0.0001 |
| Heart V15 | 5.1% | 0.95% | 81% | <0.0001 |
| Heart V10 | 6.4% | 1.5% | 77% | <0.0001 |
| Heart V5 | 12% | 5.1% | 58% | <0.0001 |
For 34% of patients, the heart V25 was 0% for the delivered mDIBH plan.
Lung Doses
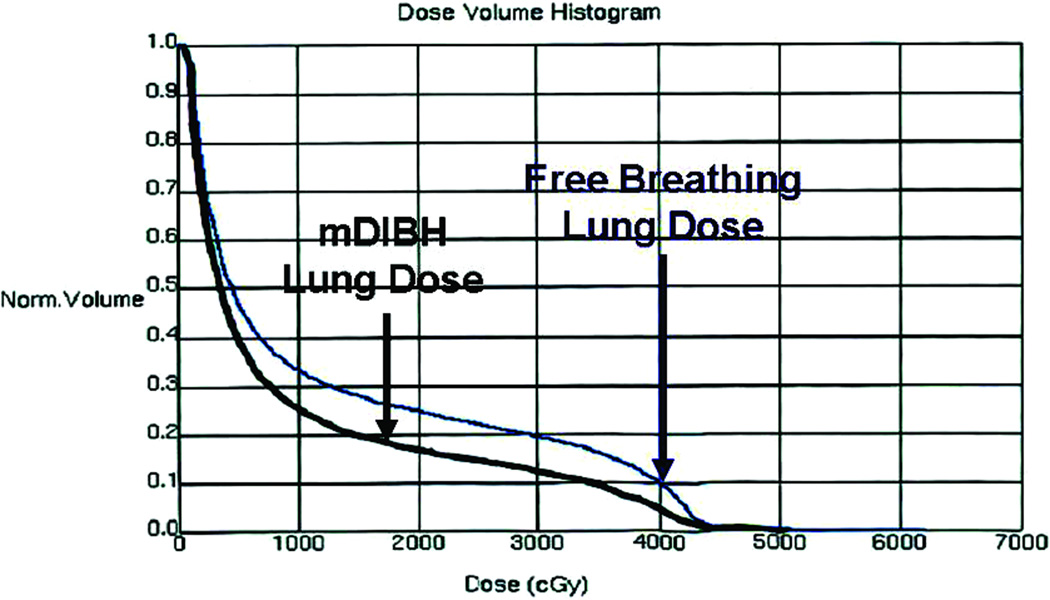
In addition to cardiac dose reductions, mDIBH also significantly reduced lung dose, as demonstrated by the dose volume histogram for the mDIBH plan versus that of the free-breathing plan (Figure 3). As displayed in Table 4, the mDIDH technique decreased the ipsilateral mean lung dose from 9.1 Gy to 7.9 Gy (p< 0.001) as well at the volume of lung treated, from 8.6 mL to 7.8 mL (p< 0.001). There were also statistically significant decreases in the Left Lung V20, V15, V10 and V5 volumes when evaluating the delivered mDIBH plan versus the free-breathing plan.
Figure 3.
Dose Volume Histogram comparing lung dose with the mDIBH plan and the Free Breathing Plan
Table 4.
Improvement in Lung Dose Parameters with ABC Treatments
| Parameter | Free Breathing |
mDIBH ABC |
Relative Reduction |
p-value |
|---|---|---|---|---|
| Left Lung Vol Treated | 8.6 mL | 7.8 mL | 9.6% | <0.0001 |
| Mean Left Lung Dose | 9.1 Gy | 7.9 Gy | 13% | <0.0001 |
| Dose/Vol Parameters | ||||
| Left Lung V20 | 17% | 14% | 16% | <0.0001 |
| Left Lung V15 | 19% | 16% | 13% | <0.0001 |
| Left Lung V10 | 22% | 20% | 10% | <0.0001 |
| Left Lung V5 | 32% | 30% | 5% | <0.0001 |
Discussion
Several population based mortality studies have demonstrated an increase in cardiac mortality in the setting of patients irradiated for left-sided breast cancer (12–16). A recent analyses of 42,000 women treated on 78 randomized trials using RT for breast cancer has demonstrated small but significant reductions in breast cancer mortality within the first ten years after treatment, primarily due to excess mortality due to heart disease (rate ratio, 1.27; SE, 0.07; 2p=0.0001) and lung cancer (rate ratio, 1.78; SE, 0.22; 2p=0.0004)(5). These small improvements in survival appear to diminish with time due to the late effects of RT on adjacent normal tissues. It has long been speculated that one of the primary factors responsible for the detrimental effect of RT on survival was cardiac toxicity related to RT exposure. Adding concern is that most breast cancer patients are now also receiving cardiotoxic systemic therapy. As increased use of post-mastectomy RT can be expected, given that three recent studies (3, 4) demonstrated improved survival with RT for all patients with positive lymph nodes, it is vital that new RT techniques be developed which can provide improved sparing of the heart.
Confounding the data is the fact that cardiac, and for that matter, pulmonary toxicities attributed to irradiation are late effects of radiation therapy and the true clinical presentation may lag decades behind the radiation treatments (17). Clearly, the improvements in techniques have paralleled decreases in late complications such as cardiac morbidity. Risk from death from ischemic heart disease associated with radiation for breast cancer has substantially decreased over time. (15)
However, the improvements in radiotherapy techniques have been paralleled by the integration of known cardiotoxic agents in the management of breast cancer. For instance, Anthracyclines are associated with dose dependent acute cardiotoxic side effects including: pericarditis, myocardistis, left ventricular dysfunction, and arrhythmias (18). Delayed effects of Anthracyclines include congestive heart failure and systolic dysfunction (19). There are reports that the use of concurrent doxorubicin-based chemotherapy and radiotherapy do indeed increase the risk of long-term cardiac sequelae (20, 21)
Trastuzumab, a humanized monoclonal antibody to HER2/ErbB2, has been successfully integrated into the management of all stages of breast cancer in patients who over-express the product of the Her2/neu gene. Although the mechanism of its effects on the heart are not well understood, the use of Trastuzumab is several clinical trials has been associated with and increased incidence of congestive heart failure (22–25) Further the concurrent use of Trastuzumab during radiotherapy has resulted in significant decreased in left ventricular ejection fraction (26) The use of concurrent Trastuzumab and Anthracylines, a common combination used in breast cancer, is also associated with increased cardiovascular morbidity than the use of Trastuzumab alone or in combination with other agents such as Paclitaxel (27)
As the clinical implications of left-sided breast irradiation are long delayed, with respect to the treatment interval, there has been a movement towards cardiac testing to evaluate the effects of radiation on the heart to look for effects that predate the realization of the potential cardiogenic phenotype created as a result of cardiac radiation exposure. In one such study patients receiving left sided adjuvant radiation for breast cancer had detectable single-photon emission computed tomography (SPECT) scan changes evident 3 to 6 years post-radiation therapy (8). In another study, Correa et al. demonstrated that, at a follow-up at 12 years, there was a statistically significant higher prevalence of stress test abnormalities found among left (27 of 46; 59%) versus right-side irradiated patients and, further, there was a skewing of cardiac abnormal cardiac catherizations along the distribution of the left anterior descending artery (28) Such studies uniformly recommend that, “every effort should be made to minimize incidental irradiation of the heart while maintaining adequate coverage of target volumes (8).
More recently however, there has been a randomized phase III trial data reported in a study designed to assess changes in cardiac perfusion, as measured by SPECT, in women randomized to left chest wall or breast irradiation with or without ABC. The findings demonstrate, quite unexpectedly, that there was significant worsening of aprical cardiac perfusion at 6 months post-radiation in patients receiving ABC treatments compared to those treated with free-breathing techniques (29). Two plausible explanations for these phenomena include the fact that the cardiac vasculature is far more sensitive to lower doses of radiation or that mDIBH induces a conformational change in the heart, uncovering sensitive aprical segments. (R. Zellers, personnel communication) None the less, long term follow up on this cohort is necessary to help resolve this issue.
Despite significant improvements in the technical aspects of radiation delivery in recent years, it remains technically ‘challenging’ to comprehensively irradiate the entire cancer breast/chest wall and/or regional lymphatics (IM nodes) for many left-sided breast patients, without either compromise target volume coverage or on the heart avoidance. A recent CT planning study of optimized contemporary RT techniques, aiming to irradiate the chest wall and the regional nodes, showed that none of these methods was able to attain this goal in a satisfactory manner. However, it did appear that the use of partially wide tangent fields did result in the lowest heart V30 (30). Further, decreases in heart dose have been achieved through the use of 3D conformal and best yet by intensity modulated approaches (31)
An effective older approach to reduce cardiac radiation exposure is simply to alter the patient treatment position. Canney et al (32) and Hurkmans et al (33) both reported a reduction in the irradiated volumes of both the heart and lung with the use of a simple forearm support instead of the conventional "L-bar" support. At our institution, an alpha cradle immobilization system is used which more closely produces an effect that resembles that of the forearm support than the "L-bar" support. However, our experience with tangential treatment suggests that, for some patients, portions of the heart will still remain in the field during free breathing that cannot be avoided solely by setup modification.
As mentioned above, methods of IMRT have also been exploited in planning studies to improve sparing of the heart. Unfortunately, these IMRT plans are generally complex, requiring the use of additional gantry angles that can potentially increase the dose to the contralateral breast (34–36) and other normal tissues (some not typically included in treatment plans). These complex treatments can also require lengthy delivery times and attention must be given to ensure that the patient setup remains stable. In addition, the effects of setup error and breathing motion on the dosimetry of these IMRT deliveries have not been studied for determining the appropriate treatment margin.
We also describe that the mDIBH technique significantly decreases the dose to the ipsilateral lung. This seems counter-intuitive, as there would be more lung within the tangential beam when the heart moves out of the treatment field during mDIBH. However, it has been previously demonstrated that the use of deep inspiration breath hold technique in breast cancer patients can reduce the MLD by 15% and the Lung V20 by 17%, when compare to free breathing techniques (37). This compares with reductions seen in the current study. It is believed that Deep Inspiration Breath Hold Technique reduces ipsilateral lung dose by inflation, so that less tissue remains in the irradiated region and that modern planning systems can sufficiently account for such complex computations (37).
In this paper, we present our routine integration of mDIBH with an ABC device in the treatment of patients with early stage, left-sided breast cancer. As well, we have combined our ABC procedure with our “step and shoot” IMRT method designed primarily to improve homogeneity in the target volume (11). We currently aim to treat all of our patients less than 60 years old with left sided lesions with mDIBH, of which greater than 95% can tolerate the breath hold well enough to complete the ABC treatments. We have demonstrated that our technique has significantly decreased the volume of heart treated, mean heart dose, as well as all other dosimetric parameters evaluated. A large majority of our patients also received potentially cardiotoxic chemotherapy. Further, the ABC plans allowed for reductions in ipsilateral lung dose and volume. The treatments are well tolerated by all the patients who complete the practice sessions during the planning process. The purpose of our study was to present the dosimetry and feasibility of the routine integration for ABC techniques for patients receiving adjuvant radiation for left sided breast cancer, and not a study to demonstrate the decreased cardiac morbidity associated with the adoption of such techniques. However, this cohort will undoubted need to be followed to assess cardiac toxicities compared the historic controls.
In our present study, we specifically have not presented data pertaining to Normal Tissue Complication Probability (NTCP). First, the true NTCP for the small volumes of heart irradiated is uncertain (38). Second, some preliminary anatomic data (39) suggest that volumes of heart irradiated with ‘standard techniques’ may involve more critical structures such as the coronary vessels. Third, most NTCP models use pericarditis as an endpoint and do not consider the effects of cardio-toxic drugs such as the anthracyclines and trastuzamab. As a result, NTCP may underestimate the true risk of long-term cardiac disease for breast patients treated with combined radio- and chemo- therapy.
Conclusion
Reductions in the percentage of the heart receiving 30 Gy can be achieved in patients with left-sided breast cancer using an ABC device to immobilize lung volume at mDIBH. As well, reduction in lung dose parameters can also be achieved. The technique is simple, well tolerated by patients and can be implemented for routine use in a busy clinic. The routine integration of this experience suggests that mDIBH, utilizing an ABC device may provide one of the most promising methods of improving the efficacy of EBRT, for patients with left-sided breast cancer. Long term follow-up is needed to determine the clinical outcomes associated with decreased dose to the heart.
Acknowledgements
This work was funded in part by an Educational Grant from Elekta Oncology Systems, the Alfred Berkowitz Foundation, the William Beaumont Hospital Foundation and the National Cancer Institute (NCI) grant 1R01CA76182.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as an oral presentation at the 2009 ASTRO Annual Meeting, Chicago, IL
Conflict of Interest Notification:
None of the authors have any pertinent disclosures to declare.
References
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen HM, Overgaard M, Grau C, et al. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24:2268–2275. doi: 10.1200/JCO.2005.02.8738. [DOI] [PubMed] [Google Scholar]
- 4.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 5.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 6.Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 7.Krauss DJ, Kestin LL, Raff G, et al. MRI-based volumetric assessment of cardiac anatomy and dose reduction via active breathing control during irradiation for left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2005;61:1243–1250. doi: 10.1016/j.ijrobp.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Prosnitz RG, Hubbs JL, Evans ES, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007;110:1840–1850. doi: 10.1002/cncr.22965. [DOI] [PubMed] [Google Scholar]
- 9.Remouchamps VM, Letts N, Vicini FA, et al. Initial clinical experience with moderate deep-inspiration breath hold using an active breathing control device in the treatment of patients with left-sided breast cancer using external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:704–715. doi: 10.1016/s0360-3016(03)00010-5. [DOI] [PubMed] [Google Scholar]
- 10.Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 1999;44:911–919. doi: 10.1016/s0360-3016(99)00056-5. [DOI] [PubMed] [Google Scholar]
- 11.Kestin LL, Sharpe MB, Frazier RC, et al. Intensity modulation to improve dose uniformity with tangential breast radiotherapy: initial clinical experience. Int J Radiat Oncol Biol Phys. 2000;48:1559–1568. doi: 10.1016/s0360-3016(00)01396-1. [DOI] [PubMed] [Google Scholar]
- 12.Rutqvist LE, Johansson H. Mortality by laterality of the primary tumour among 55,000 breast cancer patients from the Swedish Cancer Registry. Br J Cancer. 1990;61:866–868. doi: 10.1038/bjc.1990.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paszat LF, Mackillop WJ, Groome PA, et al. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol. 1998;16:2625–2631. doi: 10.1200/JCO.1998.16.8.2625. [DOI] [PubMed] [Google Scholar]
- 14.Paszat LF, Mackillop WJ, Groome PA, et al. Mortality from myocardial infarction following postlumpectomy radiotherapy for breast cancer: a population-based study in Ontario, Canada. Int J Radiat Oncol Biol Phys. 1999;43:755–762. doi: 10.1016/s0360-3016(98)00412-x. [DOI] [PubMed] [Google Scholar]
- 15.Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97:419–424. doi: 10.1093/jnci/dji067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roychoudhuri R, Robinson D, Putcha V, et al. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer. 2007;7:9. doi: 10.1186/1471-2407-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muren LP, Maurstad G, Hafslund R, et al. Cardiac and pulmonary doses and complication probabilities in standard and conformal tangential irradiation in conservative management of breast cancer. Radiother Oncol. 2002;62:173–183. doi: 10.1016/s0167-8140(01)00468-6. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg JS, Cohen AJ, Wasserman AG, et al. Acute arrhythmogenicity of doxorubicin administration. Cancer. 1987;60:1213–1218. doi: 10.1002/1097-0142(19870915)60:6<1213::aid-cncr2820600609>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: a prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol. 2002;13:699–709. doi: 10.1093/annonc/mdf132. [DOI] [PubMed] [Google Scholar]
- 20.Zambetti M, Moliterni A, Materazzo C, et al. Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J Clin Oncol. 2001;19:37–43. doi: 10.1200/JCO.2001.19.1.37. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro CL, Hardenbergh PH, Gelman R, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol. 1998;16:3493–3501. doi: 10.1200/JCO.1998.16.11.3493. [DOI] [PubMed] [Google Scholar]
- 22.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 23.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 24.Rastogi P, Jeong J, Geyer CE, et al. Five year update of cardiac dysfunction on NSABP B-31, a randomized trial of sequential doxorubicin/cyclophosphamide (AC)→paclitaxel (T) vs. AC→T with trastuzumab(H) ASCO Proc. 2007;Vol 25 [Google Scholar]
- 25.Perez EA, Romond EH, Suman VJ, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer. Proc ASCO. 2007;Vol 25 [Google Scholar]
- 26.Belkacemi Y, Gligorov J, Ozsahin M, et al. Concurrent trastuzumab with adjuvant radiotherapy in HER2-positive breast cancer patients: acute toxicity analyses from the French multicentric study. Ann Oncol. 2008;19:1110–1116. doi: 10.1093/annonc/mdn029. [DOI] [PubMed] [Google Scholar]
- 27.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 28.Correa CR, Litt HI, Hwang WT, et al. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol. 2007;25:3031–3037. doi: 10.1200/JCO.2006.08.6595. [DOI] [PubMed] [Google Scholar]
- 29.Zellars R, Valenzuela PB, Tryggestad E, et al. Active Breathing Control (ABC) May Worsen Cardiac Toxicity in Women Receiving Radiation (XRT) for Left-sided Breast Cancer: Preliminary Results of a Randomized-controlled Phase III Trial. Int J Radiat Oncol Biol Phys. 2010;78:S50. [Google Scholar]
- 30.Pierce LJ, Butler JB, Martel MK, et al. Postmastectomy radiotherapy of the chest wall: dosimetric comparison of common techniques. Int J Radiat Oncol Biol Phys. 2002;52:1220–1230. doi: 10.1016/s0360-3016(01)02760-2. [DOI] [PubMed] [Google Scholar]
- 31.Hurkmans CW, Cho BC, Damen E, et al. Reduction of cardiac and lung complication probabilities after breast irradiation using conformal radiotherapy with or without intensity modulation. Radiother Oncol. 2002;62:163–171. doi: 10.1016/s0167-8140(01)00473-x. [DOI] [PubMed] [Google Scholar]
- 32.Canney PA, Deehan C, Glegg M, et al. Reducing cardiac dose in post-operative irradiation of breast cancer patients: the relative importance of patient positioning and CT scan planning. Br J Radiol. 1999;72:986–993. doi: 10.1259/bjr.72.862.10673950. [DOI] [PubMed] [Google Scholar]
- 33.Hurkmans CW, Borger JH, v Giersbergen A, et al. Implementation of a forearm support to reduce the amount of irradiated lung and heart in radiation therapy of the breast. Radiother Oncol. 2001;61:193–196. doi: 10.1016/s0167-8140(01)00406-6. [DOI] [PubMed] [Google Scholar]
- 34.Krueger EA, Fraass BA, McShan DL, et al. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:1023–1037. doi: 10.1016/s0360-3016(03)00183-4. [DOI] [PubMed] [Google Scholar]
- 35.Ma CM, Ding M, Li JS, et al. A comparative dosimetric study on tangential photon beams, intensity-modulated radiation therapy (IMRT) and modulated electron radiotherapy (MERT) for breast cancer treatment. Phys Med Biol. 2003;48:909–924. doi: 10.1088/0031-9155/48/7/308. [DOI] [PubMed] [Google Scholar]
- 36.Popescu CC, Olivotto I, Patenaude V, et al. Inverse-planned, dynamic, multi-beam, intensity-modulated radiation therapy (IMRT): a promising technique when target volume is the left breast and internal mammary lymph nodes. Med Dosim. 2006;31:283–291. doi: 10.1016/j.meddos.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Zurl B, Stranzl H, Winkler P, et al. Quantitative assessment of irradiated lung volume and lung mass in breast cancer patients treated with tangential fields in combination with deep inspiration breath hold (DIBH) Strahlenther Onkol. 2010;186:157–162. doi: 10.1007/s00066-010-2064-y. [DOI] [PubMed] [Google Scholar]
- 38.Gagliardi G, Lax I, Rutqvist LE. Partial irradiation of the heart. Semin Radiat Oncol. 2001;11:224–233. doi: 10.1053/srao.2001.23483. [DOI] [PubMed] [Google Scholar]
- 39.Krueger EA, Schipper MJ, Koelling T, et al. Cardiac chamber and coronary artery doses associated with postmastectomy radiotherapy techniques to the chest wall and regional nodes. Int J Radiat Oncol Biol Phys. 2004;60:1195–1203. doi: 10.1016/j.ijrobp.2004.04.026. [DOI] [PubMed] [Google Scholar]