Abstract
Precise, robust and scalable directed differentiation of pluripotent stem cells is an important goal with respect to disease modeling or future therapies. Using the AggreWell™400 system we have standardized the differentiation of human embryonic and induced pluripotent stem cells to a neuronal fate using defined conditions. This allows reproducibility in replicate experiments and facilitates the direct comparison of cell lines. Since the starting point for EB formation is a single cell suspension, this protocol is suitable for standard and novel methods of pluripotent stem cell culture. Moreover, an intermediate population of neural precursor cells, which are routinely >95% NCAMpos and Tra-1–60neg by FACS analysis, may be expanded and frozen prior to differentiation allowing a convenient starting point for downstream experiments.
Keywords: Pluripotent stem cells, differentiation, neural precursor, neurons
Introduction
Human pluripotent stem cells, both embryonic (hESCs) and induced (hiPSCs) hold great promise for the generation of cell types for disease-modeling, cell-based assays or indeed for future therapies. However, much of this is limited by the lack of effective standard protocols, which can generate differentiated cell types in sufficient numbers for such applications.
The efficient differentiation of human pluripotent stem cells to neurons has been the focus of much research (reviewed in Shwartz et al. [1]) with good progress being reported recently [2–4]. Many protocols, however, rely on the formation of embryoid bodies (EBs) or involve an EB-like step [2, 4–14] which, by its subjective nature, represents a great source of variability in any differentiation protocol [15, 16]. In the area of neuronal differentiation, efforts have been made to eliminate this step [3, 17–20] or to standardize it using mechanical dissection [21] or round-bottomed 96-well plates [22]. The AggreWell™400 system (Stem Cell Technologies) is a development of the latter concept whereby each well contains 1200 microwells of 400µm diameter. This allows 1200 EBs, of specific and uniform size up to 5000 cells per EB, to be generated from a single well thus simplifying harvest.
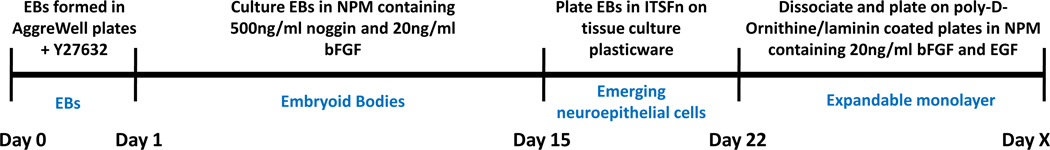
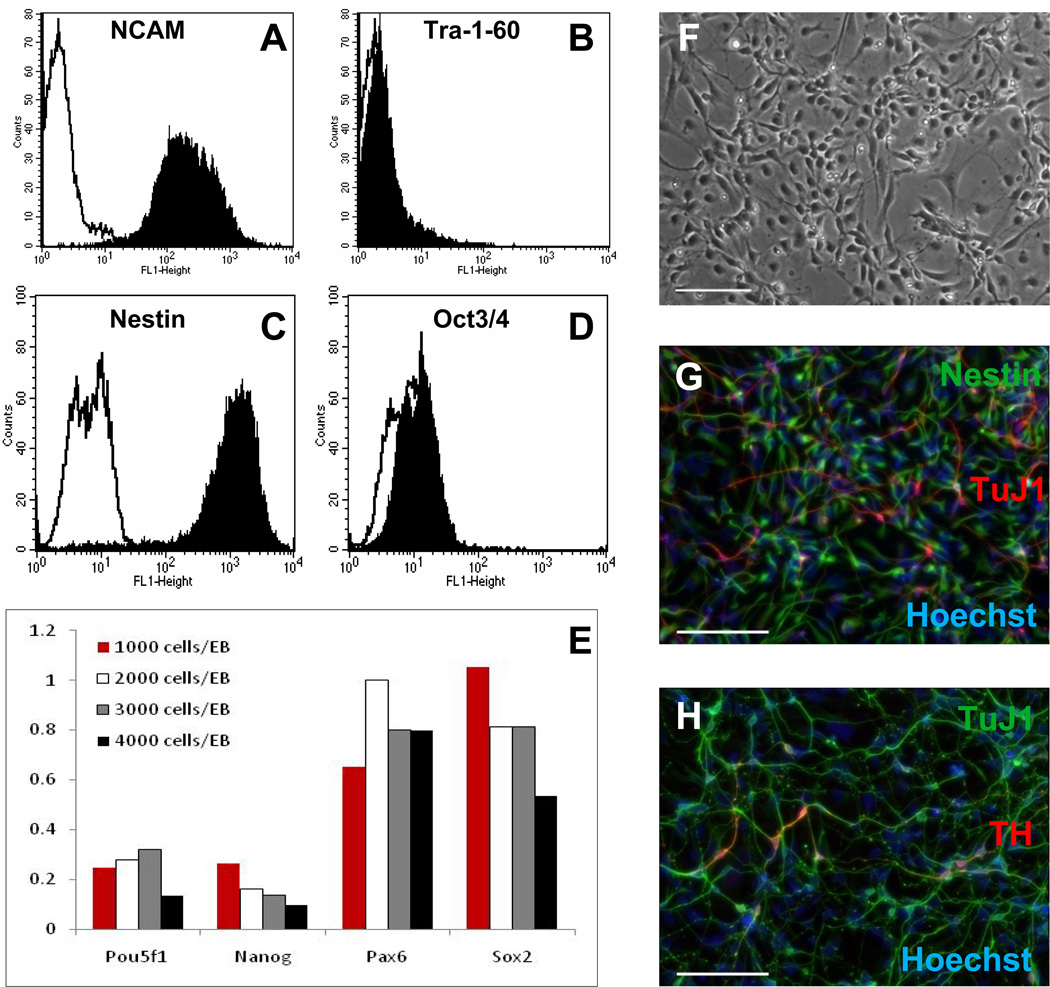
We have used AggreWell™400 plates to standardize the EB step in a modified version of the mouse ESC five-stage neuronal differentiation protocol of Lee et al. [23] (Fig. 1). This protocol results in a highly robust and scalable method for deriving neural precursor cells (NPCs) from hESCs or hiPSCs. Briefly, EBs are initially generated in hESC medium containing Y27632 (ROCK inhibitor) [22, 24] in an AggreWell™400 plate and subsequently cultured in low attachment plates in medium containing B27 supplement minus Vitamin A (Neuronal Precursor Medium; NPM) with noggin and basic fibroblast growth factor (bFGF) [5]. After 2 weeks, the EBs are plated on standard tissue culture plasticware in a minimal medium containing insulin, transferrin, selenium and fibronectin (ITSFn) which has previously been shown to select for nestin-positive cells [25]. Neuroepithelial cells that have emerged from the EBs are collected after 7–8 days and replated on poly-D-ornithine/laminin-coated plates in medium supplemented with NPM, bFGF and epidermal growth factor (EGF). Neural precursor cells thus generated demonstrate appropriate cell morphology (Fig. 2F) and marker expression as determined by FACS analysis (Fig. 2A–D), RT-PCR (Fig. 2E) and immunostaining (Fig. 2G). The NPCs may be expanded in this growth medium through at least 10 passages (1000 fold increase) if maintained at high density. Cells may also be frozen at this stage to generate a bank of developmentally similar cells (Supplementary Fig. 1). This provides a convenient starting point for downstream analysis of neuronal differentiation pathways, amongst others, and facilitates comparison of multiple experiments.
Figure 1.
Timeline of steps involved in neural precursor cell generation from human pluripotent stem cells via EB formation.
Figure 2.
Analysis of neural precursor cells. A&B) FACS analysis of neural and pluripotent stem cell marker expression in SCU-i10-derived NPCs at p3 - A) IgM isotype control (white) and NCAM (black), B) IgM isotype control (white) and Tra-1–60 (black); C&D) FACS analysis of neural and pluripotent stem cell marker expression in H1-derived NPCs at p2 – C) IgG1 isotype control (white) and Nestin (black), D) IgG2b isotype control (white) and Oct3/4 (black). E) RT-PCR analysis of neural and pluripotent stem cell marker expression in HSF-6-derived NPCs at p0 from EBs of different sizes. Data were generated using a Sabiosciences PCR array and were analyzed and normalized using the company’s proprietary control gene panel and software. F) Phase image of SCU-i10 NPCs at p3, day1. Scale bar=100µm; G) Immunostaining of SCU-i10 NPCs p3, day 3 for neural precursors with anti-Nestin (green) and for neurons with anti-TuJ1 (red). Hoechst stain (blue). Scale bar=100µm; H) Immunostaining of differentiated SCU-i10 NPCs p2 for all neurons with anti-TuJ1 (green) and for dopaminergic neurons with anti-tyrosine hydroxylase (red). Hoechst stain (blue). Cells were plated in NPM with bFGF and EGF for 4 days, growth factors withdrawn for 3 days and further differentiated in Neurobasal medium containing B27, BDNF and GDNF for 4 days. Scale bar=100µm.
For subsequent differentiation of the NPCs to neurons, cells are plated at low density in growth medium for 24 hours after which the bFGF and EGF are withdrawn. After a further 48 hours, the medium is again replaced with Neurobasal medium supplemented with B27, brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF). Under these conditions, the majority of cells exhibit positive immunostaining for the neuronal marker, β-tubulin III (TuJ1; Fig. 2H and Supplementary Fig. 2). It may be envisaged that optimization of growth factors and media may allow the selection of various neuronal subtypes. Since this protocol has been optimized for the generation of neurons using high concentrations of noggin [26], we find few cells positive for glial markers even after exposure to platelet derived growth factor (PDGF), ciliary neurotrophic factor (CNTF) and L-3,3’,5-Triiodothyronine (T3).
Materials and Equipment
hESC culture medium
400ml DMEM:F12 (Invitrogen #11330-032)
100ml Knockout Serum Replacer (Invitrogen #10828-028)
5ml Non essential amino acids 100x (Invitrogen #11140-050)
2.5ml L-Glutamine (1mM; Invitrogen #25030-081)
3.5µl β-mercaptoethanol (0.1mM; Sigma #7522)
- 4ng/ml Fibroblast growth factor-2 (bFGF, R&D Systems Inc. #233-FB)
- A stock solution of 10µg/ml is prepared in D-PBS containing 0.1% bovine serum albumin. Working aliquots are stored at −20°C and upon thaw should be stored at 4°C and used within 2 weeks.
Storage at 4°C, not to exceed 2 weeks
Neural Precursor Medium
48.5ml DMEM (Invitrogen #11965-044)
1ml B27 supplement minus Vitamin A (Invitrogen #12587-010)
0.5ml L-Glutamine (2mM; Invitrogen #25030-081)
Storage at 4°C, not to exceed 2 weeks
ITSFn Medium
49ml DMEM/F12 (Invitrogen #10565-018)
0.5ml Insulin-Transferrin-Selenium-A Supplement (100X) (Invitrogen #51300-044).
0.5ml L-glutamine (1mM; Invitrogen #25030-081)
5µg/ml human fibronectin (Roche Applied Sciences #11080938001)
Storage at 4°C, not to exceed 2 weeks
Reagents
D-PBS (Invitrogen #14190-250)
- Collagenase IV (Invitrogen #17104-019)
- A stock solution of 1.5mg/ml is prepared in DMEM:F12 (Invitrogen #11330-032), and frozen in working aliquots at –20°C. Aliquots are thawed immediately prior to use.
Accutase (Innovative Cell Technologies, Inc. #AT104)
- Y27632 (ROCK inhibitor - Selective inhibitor of Rho-associated protein kinase p160ROCK, EMD Biosciences #688000).
- A stock solution of 5mM is prepared by dissolving 0.5mg in 296µl of sterile distilled water. Working aliquots are stored at −20°C and upon thaw should be stored at 4°C and used within 2 weeks.
- Recombinant human Noggin (R&D Systems #6057-NG-100)
- A stock solution of 100µg/ml is prepared in D-PBS containing 0.1% bovine serum albumin. Working aliquots are stored at −20°C and upon thaw should be stored at 4°C and used within 2 weeks.
- Epidermal growth factor (EGF, R&D Systems #236-EG)
- A stock solution of 20µg/ml is prepared in D-PBS containing 0.1% bovine serum albumin. Working aliquots are stored at −20°C and upon thaw should be stored at 4°C and used within 2 weeks.
- Brain derived neurotrophic factor (BDNF, R&D Systems #248-BD)
- A stock solution of 10µg/ml is prepared in D-PBS containing 0.1% bovine serum albumin. Working aliquots are stored at −20°C and upon thaw should be stored at 4°C and used within 2 weeks.
- Glial cell derived neurotrophic factor (GDNF, R&D Systems #212-GD)
- A stock solution of 20µg/ml is prepared in D-PBS containing 0.1% bovine serum albumin. Working aliquots are stored at −20°C and upon thaw should be stored at 4°C and used within 2 weeks.
Distilled water (Mediatech #25-055-CM)
- Poly-D-ornithine (Sigma #P3655)
- A stock solution of 10mg/ml is prepared in sterile distilled water. Working aliquots are stored at −20°C and upon thaw should be stored at 4°C and used within 2 weeks.
Mouse laminin (Sigma #L2020)
Neurobasal medium (Invitrogen #21103-049)
mTeSR1 medium (Stem Cell Technologies #05850)
ENStem-A™ Neuronal Differentiation Medium (Millipore #SCM017)
ENStem-A™ Neural Freezing Medium (Millipore #SCM011)
Equipment
Cell scrapers (Costar #3010)
AggreWell™400 plate (Stem Cell Technologies #27845)
Low attachment 6-well culture dishes (Corning #3471)
Tissue culture-treated 6-well plates
Benchtop low speed centrifuge
Microplate carrier for centrifuge
Tissue culture incubator −37°C/5% CO2
Methods
Embryoid Body (EB) Formation
If starting from colonies grown on feeders, wash cells once with D-PBS and add 1ml pre-warmed collagenase IV (1.5mg/ml) per well of a 6-well plate. If starting with cells on Matrigel go directly to step 6.
Incubate at 37°C for 20 mins at which time the plate should be tapped sharply. If the colonies do not detach at this time the plate should be incubated for a further 10–20mins and the process repeated. If the majority of colonies have not detached within 1 hour they may be removed using a cell scraper.
Remove colonies and transfer to a 15ml tube, wash each well with 1–2ml DMEM:F12 and add wash to the 15ml tube.
Allow to sediment for 3 minutes, aspirate down to the pellet, wash with 5ml DMEM:F12 without disrupting the colonies and allow to sediment again.
Wash with 5ml D-PBS and allow to sediment.
Add 1ml Accutase and incubate at 37°C for 10 mins, agitating by swirling the tube after 5 mins.
Add 5ml DMEM:F12 per 1ml Accutase used and pipet up and down gently.
Centrifuge at 200g for 5 mins.
Remove wash and resuspend in hESC culture medium to a density of 1.2×106 cells/ml in hESC medium containing 10µM Y27632 (ROCK inhibitor). This should generate approximately 1200 EBs of size 1000 cells per EB.
Prepare the AggreWell plate according to the manufacturer’s instructions using hESC culture medium containing 10µM Y27632 (ROCK inhibitor) and add 1ml of cell suspension per well.
Spin at 200g for 5 mins and incubate in the tissue culture incubator for 24 hours
To harvest, the EBs are dislodged from the AggreWell by forceful ejection of the culture medium present in the well using a 1ml pipetman and transferred to a 15ml tube using a 2ml pipette. Do not triturate.
A further 1.5–2ml of medium is added and step 12 repeated.
Repeat until the majority of EBs are removed – no more than 5 times total.
Allow the EBs to sediment for 5 mins and aspirate the supernatant.
Transfer EBs from one well of the AggreWell plate to one well of a low attachment 6-well culture dish in 3ml hESC medium per well and return to the incubator.
Generation of Neural Precursors
Twenty-four hours after harvest, transfer the EBs to a 15ml tube and allow to sediment for 5 mins.
Aspirate the medium and replace with NPM containing 500ng/ml noggin and 20ng/ml bFGF. Return EBs to the low-attachment well.
Change the medium by sedimentation every 2–3 days for 2 weeks.
Change the medium to ITSFn and transfer to standard tissue culture plasticware at a ratio of 1 well EBs to 1 well of a 6-well culture dish. EBs should attach and neuroepithelial-like cells should emerge.
Change the medium every other day for 7–8 days.
Wash the cells once with D-PBS, add 1ml of Accutase and incubate at 37°C for 10mins.
Remove all detached lumps and single cells to a 15ml tube and wash with NPM. Allow the biggest aggregates to sediment briefly (30secs), then remove the upper cell suspension to a new 15ml tube.
Spin the cell suspension at 200g for 5 mins.
Resuspend in NPM containing 20ng/ml each of bFGF and EGF and plate on pre-coated poly-D-ornithine/laminin plates at 1.5–2×106 cells per well of a 6-well dish. This is considered p0 for neural precursors.
Cells may be passaged 1:2 or 1:3 (first passage only) with Accutase for expansion or 1:12 for differentiation.
Cells may be frozen using ENStem-A™ Neural Freezing Medium. We recommend the addition of 10µM Y27632 (ROCK inhibitor) upon thawing.
Poly-D-ornithine/Laminin Coated Plates
Add 2ml of filter-sterilized 20µg/ml poly-D-ornithine in distilled water per well of a tissue culture-treated 6-well plate.
Incubate overnight at 37°C.
Wash once with distilled water and add 2ml of 10µg/ml laminin diluted in DPBS.
Incubate overnight at 37°C.
To use, aspirate laminin immediately prior to plating cells. Do not wash.
For culture on glass, the concentrations of poly-D-ornithine and laminin should be adjusted to 50µg/ml and 20µg/ml respectively.
Precoated plates may be stored at −20°C after incubation is complete – do not remove the laminin.
Differentiation of Neural Precursor Cells to Neurons
Passage the neural precursor cells 1:12 onto poly-D-Ornithine/laminin coated plates in NPM containing 20ng/ml each of bFGF and EGF.
After 24 hours, exchange the medium for NPM without growth factors.
After a further 2 days, exchange the medium for Neurobasal medium supplemented with 2mM glutamine, 1× B27 supplement, 10ng/ml BDNF and 2ng/ml GDNF.
Exchange medium every two to three days for up to 10 days or longer if cultures remain healthy.
Notes
This protocol describes a robust, reproducible method for the generation of embryoid bodies using the AggreWell™400 system. Neural precursors may also be generated from colony-derived EBs, which does not require the use of the AggreWell™400 plate and associated reagents. Detached colonies may be cultured directly in NPM containing noggin and bFGF (Generation of Neural Precursors step 2.). Similarly, single cell suspensions may be aggregated without the use of the AggreWell™400 plate using 24-well low attachment dishes or in 96-well plates as described by Eiraku et al [22]. In 24-well plates, 1.5×106 cells are plated per well in hESC medium containing 10µM Y27632 (ROCK inhibitor) and incubated overnight at which time EBs from all wells may be combined for step 2 of Generation of Neural Precursors. Under these conditions, however, the uniformity and reproducibility of the AggreWell™ is lost. It should be noted that high quality cultures with a low proportion of spontaneous differentiation are essential for success with any approach.
For differentiation, cells may also be cultured in mTeSR1 medium (Stem Cell Technologies) or in Neuronal Differentiation Medium (NDM: Millipore) (Supplementary Fig. 2). Cells differentiated in mTeSR1 appear to maintain some proliferative capacity and serial passaging may enrich for neuronal precursors. After initial differentiation in mTeSR1, medium may be exchanged for other differentiation media such as NDM or Neurobasal medium containing B27, BDNF and GDNF. Cells at this stage may also be differentiated on 1.25% Matrigel (BD Biosciences) instead of poly-D-Ornithine/laminin. Precoated poly-D-Ornithine/laminin plates from BD may also be used but, in our hands, do not perform as well as self-coated plates.
This protocol has been used successfully for the differentiation of 6 hESC lines (H1/WA01, H7/WA07, H9/WA09, H14/WA14, HSF-6/UC06, SA01) and 3 iPSC lines derived in-house. Figure 2 shows representative data from H1 and HSF-6 hESCs and an iPSC line (SCU-i10) which was derived from bone marrow stromal cells [27] by lentiviral transduction of the four Yamanaka reprogramming factors [28, 29] using StemCCA (Millipore). SCU-i10 expresses pluripotent stem cell markers, SSEA-4, Tra-1–60 and Tra-1–81, is negative for SSEA-1 (Supplementary Fig. 3A) and had a normal karyotype at p40 (Supplementary Fig. 3B). Using published methods [30, 31], this line was able to differentiate not only along the ectodermal lineage, as we show here by neuronal differentiation, but also toward endoderm, as exhibited by HNF4A and albumin immunostaining (Supplementary Fig. 3C), and mesoderm, demonstrated by spontaneously beating patches of cells (Supplementary Fig. 4D and Supplementary Movie 1).
Since the initial state of pluripotent cells required for EB formation is a single-cell suspension, the protocol may be used with cells grown under various culture conditions. Apart from standard feeder-dependent colony-based culture, cells grown under feeder-free conditions using standard substrates (reviewed by Mallon et al [32]) or novel alternative substrates such as Synthemax (Corning) and StemAdhere (Stem Cell Technologies) may also be used. On these alternative substrates, colony size and fragments recovered may be relatively small and otherwise not suitable for EB formation.
Supplementary Material
Supplementary Figure 1 – Thawed neural precursor cells. A&B) H1-derived NPCs p10, 0.5 well frozen and thawed in the presence of 10µM Y27632 - A) 4 hours post thaw; B) 24 hours post thaw; C&D) SCU-i10-derived NPCs p5, 1 well frozen and thawed in the presence of 10µM Y27632 - C) 4 hours post thaw; D) 24 hours post thaw. Scale bars=100µm.
Supplementary Figure 2 – Immunostaining of SCU-i10 NPCs differentiated for 11 days with antibodies to MAP2 (top row; red) and TuJ1 (second row; green). Merged images of both immunostains with Hoechst nuclear stain (third row; blue) are shown in the bottom row. Scale bars=100µm. Three differentiation media were compared - standard Neurobasal medium containing BDNF and GDNF (left column), mTeSR1 (center column) and NDM (right column).
Supplementary Figure 3 - Characterization of the SCU-i10 human iPSC line. A) FACS analysis shows SCU-i10s are positive for SSEA-4, Tra-1–60 and Tra-1–81 and negative for SSEA-1; B) Karyotype is normal at p40 (performed by Cell Line Genetics, Madison, WI); C) Immunostaining of cells differentiated to endodermal lineage with antibodies to hepatocyte markers HNF4A (red) and albumin (green) with Hoechst nuclear stain (blue). Scale bar=100µm; D) Image captured from Supplementary Movie 1 which shows spontaneously beating area of differentiated cells.
Acknowledgements
We gratefully acknowledge Dr. Sergei Kuznetsov and Dr. Pamela Robey of the National Institute for Dental and Craniofacial Research for providing the bone marrow stromal cells from which the iPSC line was derived. We would also like to thank Dr. Ron McKay and Dr. Josh Chenoweth of the Lieber Institute for Brain Development for helpful discussions. This research was supported by the Intramural Research Program of the NIH, NINDS.
Footnotes
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Schwartz PH, Brick DJ, Stover AE, Loring JF, Muller FJ. Differentiation of neural lineage cells from human pluripotent stem cells. Methods. 2008;45(2):142–158. doi: 10.1016/j.ymeth.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falk A, Koch P, Kesavan J, et al. Capture of neuroepithelial-like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PloS One. 2012;7(1):e29597. doi: 10.1371/journal.pone.0029597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemati S, Hatami M, Kiani S, et al. Long-term self-renewable feeder-free human induced pluripotent stem cell-derived neural progenitors. Stem Cells Dev. 2011;20(3):503–514. doi: 10.1089/scd.2010.0143. [DOI] [PubMed] [Google Scholar]
- 4.Nistor G, Siegenthaler MM, Poirier SN, et al. Derivation of high purity neuronal progenitors from human embryonic stem cells. PloS One. 2011;6(6):e20692. doi: 10.1371/journal.pone.0020692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MA, Itsykson P, Reubinoff BE. Neural differentiation of human ES cells. Chapter 23, Unit 23 27. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb2307s36. [DOI] [PubMed] [Google Scholar]
- 6.Iacovitti L, Donaldson AE, Marshall CE, Suon S, Yang M. A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: Studies in vitro and in vivo. Brain Res. 2007;1127(1):19–25. doi: 10.1016/j.brainres.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch P, Opitz T, Steinbeck JA, Ladewig J, Brustle O. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci USA. 2009;106(9):3225–3230. doi: 10.1073/pnas.0808387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar M, Bagchi B, Gupta SK, Meena AS, Gressens P, Mani S. Neurospheres derived from human embryoid bodies treated with retinoic Acid show an increase in nestin and ngn2 expression that correlates with the proportion of tyrosine hydroxylase-positive cells. Stem Cells Dev. 2007;16(4):667–681. doi: 10.1089/scd.2006.0115. [DOI] [PubMed] [Google Scholar]
- 9.Lim UM, Sidhu KS, Tuch BE. Derivation of Motor Neurons from three Clonal Human Embryonic Stem Cell Lines. Curr Neurovasc Res. 2006;3(4):281–288. doi: 10.2174/156720206778792902. [DOI] [PubMed] [Google Scholar]
- 10.Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, Mattson MP. Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells. BMC Dev Biol. 2008;8:90. doi: 10.1186/1471-213X-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuldiner M, Eiges R, Eden A, et al. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001;913(2):201–205. doi: 10.1016/s0006-8993(01)02776-7. [DOI] [PubMed] [Google Scholar]
- 12.Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003;4:27. doi: 10.1186/1471-2202-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swistowski A, Peng J, Liu Q, et al. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28(10):1893–1904. doi: 10.1002/stem.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 15.Bauwens CL, Peerani R, Niebruegge S, et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26(9):2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- 16.Sachlos E, Auguste DT. Embryoid body morphology influences diffusive transport of inductive biochemicals: a strategy for stem cell differentiation. Biomaterials. 2008;29(34):4471–4480. doi: 10.1016/j.biomaterials.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Axell MZ, Zlateva S, Curtis M. A method for rapid derivation and propagation of neural progenitors from human embryonic stem cells. J of Neurosci Meth. 2009;184(2):275–284. doi: 10.1016/j.jneumeth.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27(3):275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erceg S, Lainez S, Ronaghi M, et al. Differentiation of human embryonic stem cells to regional specific neural precursors in chemically defined medium conditions. PloS One. 2008;3(5):e2122. doi: 10.1371/journal.pone.0002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23(9):1234–1241. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- 21.Joannides AJ, Fiore-Heriche C, Battersby AA, et al. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells. 2007;25(3):731–737. doi: 10.1634/stemcells.2006-0562. [DOI] [PubMed] [Google Scholar]
- 22.Eiraku M, Watanabe K, Matsuo-Takasaki M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3(5):519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18(6):675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25(6):681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 25.Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996;59(1):89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 26.Itsykson P, Ilouz N, Turetsky T, et al. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005;30(1):24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Bianco P, Kuznetsov SA, Riminucci M, Gehron Robey P. Postnatal skeletal stem cells. Meth Enzym. 2006;419:117–148. doi: 10.1016/S0076-6879(06)19006-0. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 30.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93(1):32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 31.Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51(1):297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallon BS, Park KY, Chen KG, Hamilton RS, McKay RD. Toward xeno-free culture of human embryonic stem cells. Int J Biochem Cell Biol. 2006;38(7):1063–1075. doi: 10.1016/j.biocel.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 – Thawed neural precursor cells. A&B) H1-derived NPCs p10, 0.5 well frozen and thawed in the presence of 10µM Y27632 - A) 4 hours post thaw; B) 24 hours post thaw; C&D) SCU-i10-derived NPCs p5, 1 well frozen and thawed in the presence of 10µM Y27632 - C) 4 hours post thaw; D) 24 hours post thaw. Scale bars=100µm.
Supplementary Figure 2 – Immunostaining of SCU-i10 NPCs differentiated for 11 days with antibodies to MAP2 (top row; red) and TuJ1 (second row; green). Merged images of both immunostains with Hoechst nuclear stain (third row; blue) are shown in the bottom row. Scale bars=100µm. Three differentiation media were compared - standard Neurobasal medium containing BDNF and GDNF (left column), mTeSR1 (center column) and NDM (right column).
Supplementary Figure 3 - Characterization of the SCU-i10 human iPSC line. A) FACS analysis shows SCU-i10s are positive for SSEA-4, Tra-1–60 and Tra-1–81 and negative for SSEA-1; B) Karyotype is normal at p40 (performed by Cell Line Genetics, Madison, WI); C) Immunostaining of cells differentiated to endodermal lineage with antibodies to hepatocyte markers HNF4A (red) and albumin (green) with Hoechst nuclear stain (blue). Scale bar=100µm; D) Image captured from Supplementary Movie 1 which shows spontaneously beating area of differentiated cells.