Abstract
Novel dimethyl-4,4′-dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-dicarboxylate (DDB) analogs were designed and synthesized to improve their chemosensitizing action on KBvin (vincristine resistant nasopharyngeal carcinoma) cells, a multi-drug resistant cell line over-expressing P-glycoprotein (P-gp). Structure-activity relationship analysis showed that aromatic and bulky aliphatic side chains at the 2,2′-positions effectively and significantly sensitized P-gp overexpressing multidrug resistant (MDR) cells to anticancer drugs, such as paclitaxel (TAX), vincristine (VCR), and doxorubicin (DOX). DDB derivatives 16 and 23 showed 5–10 times more effective reversal ability than verapamil (VRP) for TAX and VCR. Analog 6 also exhibited five times greater chemosensitizing effect against DOX than VRP. Importantly, no cytotoxicity was observed by the active DDB analogs against both non-MDR and MDR cells, suggesting that DDB analogs serve as the novel lead compounds for the development of chemosensitizers to overcome MDR phenotype. The mechanism of action studies demonstrated that effective inhibition of P-glycoprotein by DDB analogs dramatically elevated cellular concentration of anticancer drugs.
Introduction
Despite substantial biomedical research on cancer therapy, cancers still remain the leading cause of death. Among all factors resulting in the ultimate failure of cancer treatment, chemotherapy resistance is a significant player, and multidrug resistance (MDR), cross-resistance to different chemical drug classes, occurs in various cancer types. Cellular mechanisms of MDR include decreased uptake of chemotherapeutic agents, via expression of vacuolar ATPase (V-ATPase), or adaptation of cancer cells to the cytotoxic ability of chemotherapeutic agents, via down-regulation of topoisomerase II and over-expression of glutathione S-transferase-π.1–3 An emerging understanding of cancer resistance results from cancer stem cell-like features.4 However, over-expression of drug efflux transporters, such as P-glycoprotein (P-gp) and MDR-associated protein (MRP), is the primary cause leading to multidrug resistance.5 In order to surmount MDR, great efforts have been put into developing clinically usable chemosensitizing agents, categorized as either apoptosis modulators6, 7 or MDR modulators, also known as P-gp inhibitors.8 Verapamil (VRP) and cyclosporine A (CsA), two first-generation chemosensitizers, were precluded from clinical use due to significant toxicity, but are used in experiments as positive controls. Second- and third-generation chemosensitizers were developed subsequently; however, unsatisfactory toxicity and pharmacokinetic complications still impeded drug candidate development. Although several third-generation P-gp inhibitors, including tariquidar, are now in phase II cancer clinical trials,9 their clinical efficacies are not yet clear. Thus, the discovery of safe and effective MDR modulators is still attractive and greatly needed to overcome the MDR issue in the field of cancer chemotherapy.
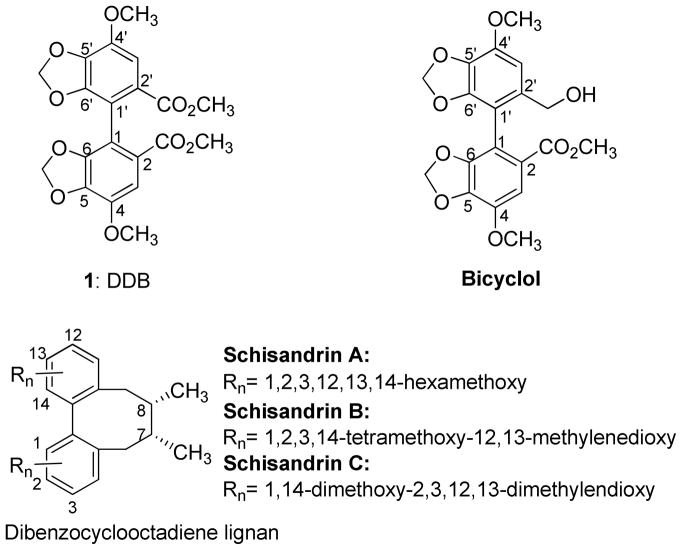
Schisandrin B (Figure 1), the most abundant dibenzocyclooctadiene lignan from Schisandra chinensis, was found to inhibit P-gp/MDR1 and MRP1/ABCC1.10,11 Structurally similar lignans, schisandrin A, schisandrol A, schisantherins A and B, also chemosensitized various anticancer drugs, including vincristine (VCR), daunorubicin, and etoposide, in human promyelocytic leukemia cell lines with over-expressed MRP1/ABCC1.12 Dimethyl-4,4′-dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-dicarboxylate (DDB, 1, Figure 1), which was discovered as a synthetic intermediate derivative of schisandrin C,13 shares the biphenyl partial structure of dibenzocyclooctadiene lignans. DDB (1) exhibited multidrug resistant reversal ability in MDR breast carcinoma MCF-7/Adr cells, KBv200, and intrinsic MDR human hepatocarcinoma Bel7402 cells via inhibition of P-gp and enhancement of apoptosis.14 However, a very high concentration (50 μM) was required for effective reversal action. Co-treatment of 1 with VCR using nude mice with KBv200 xenografts also enhanced antitumor activity at doses of 300 and 500 mg/kg.14 DDB (1) has been used to treat chronic viral hepatitis B patients in China for more than 20 years, as well as in Korea and Egypt for more than 10 years, without any significant adverse effects.15, 16 This important fact indicates that DDB analogs could be highly attractive MDR reversal agents with significant clinical potential due to their proven low toxicity. In addition, pharmacokinetic issues where chemosensitizers would interfere with the clearance of anticancer drugs often impede further development of an effective chemosensitizer. DDB was found not to alter the clearance of DOX by the evidence that plasma AUC0–24 of DOX alone and DOX plus DDB were similar in ICR mice bearing S180 sarcoma model.14 In 2006, Zhu et al reported that an asymmetric analog of DDB, bicyclol (Figure 1), also exhibited a chemosensitizing effect in two established MDR cancer cell lines, VinRKB and AdrRMCF-7.17, 18 Although DDB and bicyclol have high potentials as MDR reversal agents and various DDB analogs have been prepared, their MDR reversal abilities and structure activity relationship (SAR) correlations have been not investigated. To explore more potent non-toxic MDR reversal analogs with lower effective dosing and to study SAR, we designed and synthesized additional DDB analogs. Herein, we report the chemosensitizing effects of newly synthesized DDB analogs.
Figure 1.
Structures of DDB, Bicyclol and Dibenzocyclooctadiene Lignans
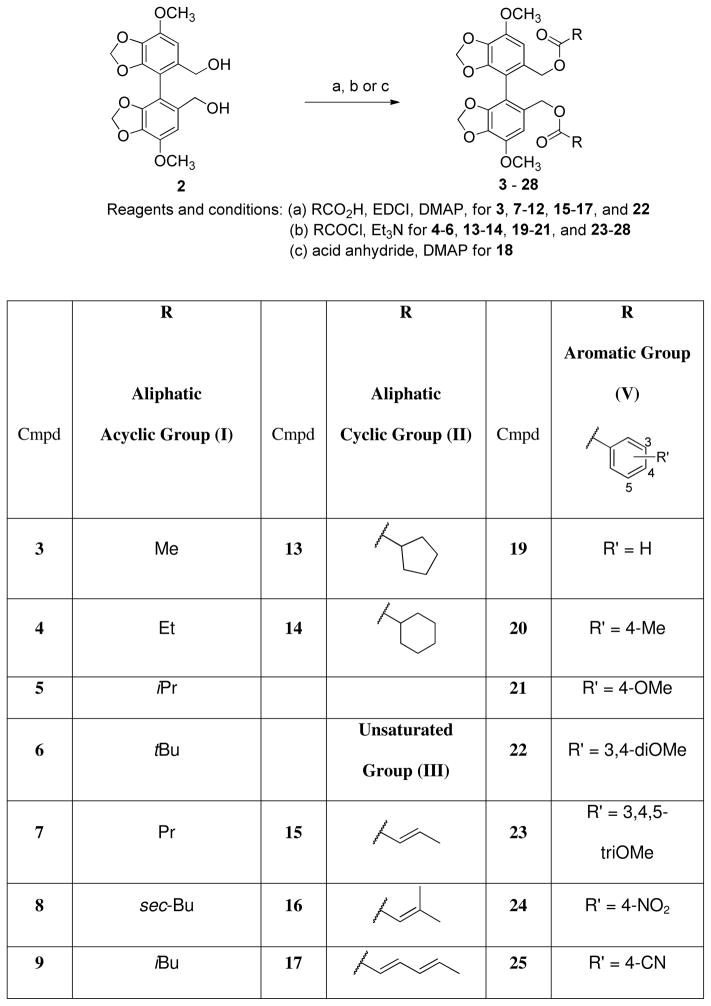
Design and Syntheses
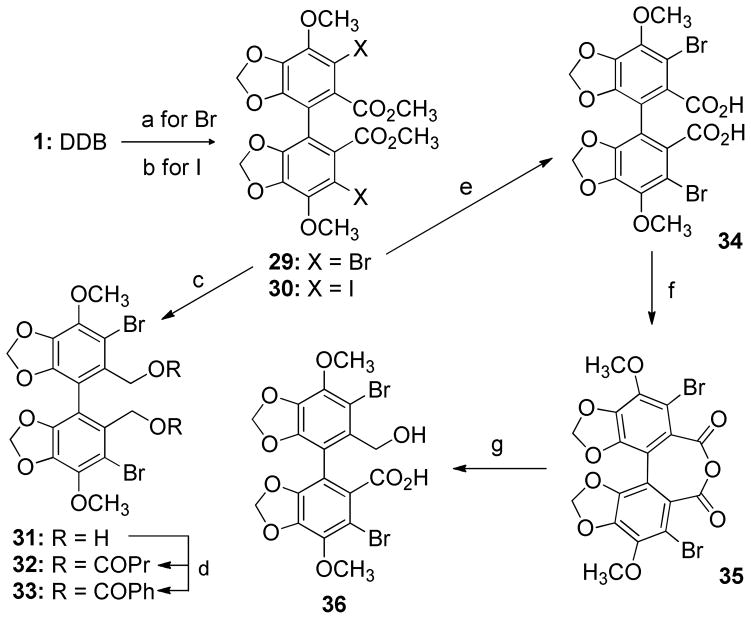
Based on previous literature, DDB had effects on inhibition of P-gp and activation of cellular caspase-3 leading to DOX-induced apoptosis of innate MDR cell line Bel7402 and acquired MDR MCF-7/Adr.14 Since DDB acts against multi-targets, which is a common feature of natural products or natural product- derived agents, ligand-based modification of DDB was applied. Bicyclol bears a primary alcohol at the C- 2′ position.17 Therefore, we selected the 2,2′-bis-hydroxymethyl biphenyl 2 as a base scaffold to design various esters 3–28 (Scheme 1) in order to define substituent effects at the C2 and C2′ positions. The ester groups (R in Scheme 1) were selected by considering size, hydrophobicity, and electron density. The diverse set included aliphatic acyclic (Group-I), cyclic (Group-II), unsaturated aliphatic groups (Group-III), polar groups (Group-IV), and aromatic groups (Group-V), which could be transformed into water-soluble salts, if necessary. Esterifications of 2 were performed by treatment with the related acid (RCO2H), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDCI) and 4- dimethylaminopyridine (DMAP) to produce 3, 7–12, 15–17, and 22, as well as by treatment with the related acid chloride (RCOCl) and Et3N to give 4–6, 13–14, 19–21, and 23–28 (Scheme 1). Halogenated DDB analogs with various functional groups were also designed to explore their effects (Scheme 2) since 3,3′-dibromo-DDB has showed good activity in anti-HIV research.19 3,33-Dibromo-DDB (29) was previously synthesized.19 Iodination of 1 was achieved by treatment with silver trifluoacetate and iodine to provide 30.20 The diester groups of 29 were reduced with diisobutylaluminium hydride (DIBAL) to afford bis-hydroxymethyl biphenyl 31 in good yield. The esters 32 and 33 were synthesized by esterification of 31 with butyric and benzoic acids, respectively, in the presence of EDCI and DMAP. Hydrolysis of dimethyl ester 29 under basic conditions resulted in dicarboxylic acid 34. Reflux of 34 in Ac2O gave anhydrous analog 35, followed by asymmetric cleavage of carbonyl anhydrous bridge with NaBH4 resulted in di-Br bicyclol analog 36.21 All synthesized analogs were prepared as the racemic mixtures.
Scheme 1. Syntheses of Diester Derivatives 3–28.
Scheme 2. Syntheses of Dihalogenated DDB Analogues.
Reagent and conditions: (a) Br2, CHCI3, 0°C, (b) CF3C02Ag, l2, (c) DIBAL, CH2CI2, (d) EDCI, DMAP, CH2CI2, CH3(CH2)2C02H for 32, and PhC02H for 33, (e) KOH, EtOH, reflux, (f) Ac20, reflux, (g) NaBH4, THF, MeOH
Results and Discussion
Evaluation of cytotoxicity and preliminary MDR reversal activity screening
All synthesized compounds were evaluated in a cytotoxic activity assay using four tumor cell lines, A549 (lung cancer), DU145 (prostate cancer), KB (epidermoid carcinoma of the nasopharynx) and a resistant sub-line, KBvin (over-expression of P-gp selected using increasing concentrations of vincristine). Verapamil, the first generation chemosensitizer precluded from clinical use due to toxicity, showed some cytotoxicity especially to KB (IC50 ~50 μM) and KBvin cells (IC50 ~ 80 μM). Although compounds 28 and 32 were slightly cytotoxic, most of the DDB-derived compounds did not exhibit significant cytotoxicity (IC50 > 100 μM), which implied low toxicity of these analogs (Suppl. Table S1).
For evaluating chemosensitizing activity, KB and KBvin cells were co-treated with test compounds at 10 μM and the anticancer drug paclitaxel (TAX) (Figure 2). As shown in Figure 2-B, DDB (1) and diol 2 did not exhibit MDR reversal effect at a concentration of 10 μM in KBvin cells. 3,3′-Dihalogenated DDB analogs with different 2,2′-functional groups did not show potent activity, except pentanoate 32 and benzoate 33, but 32 was slightly cytotoxic to KBvin cells, which may result from bromo substitution. While a 2,2′-methylester group [-CH2OC(O)R] appeared to be critical for reversal ability activity, 3,3′-halogenation reduced the potency (compare 33 with 19).
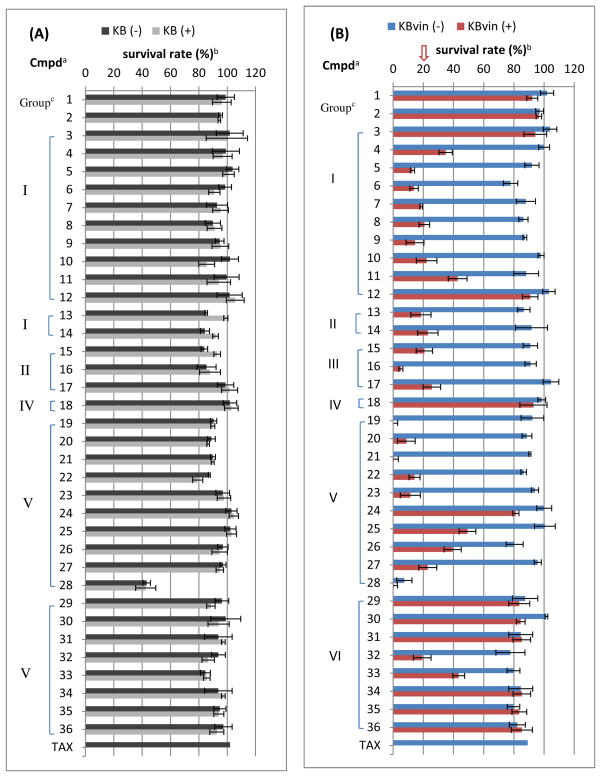
Figure 2. Screening of Reversal Abilities against KB (A) and KBvin (B).
Note: a Concentration of compounds: 10 μM, b Survival rate (%) was measured by SRB method using KB and KBvin cells in the presence (+) or absence (−) of paclitaxel (TAX). Compounds with survival rates below 20 % were considered very potent and moved to further experiments. cGroup (I) saturated acyclic alkyl; (II) cyclic alkyl ; (III) unsaturated; (IV) polar; (V): aromatic; (VI): halogenated.
Many of the analogs with aliphatic ester substituents (Group-I and -II in Figure 2) did show potent activity; however, acetate 3 (R = methyl) and dodecanoate 12 (R = undecanyl) were inactive, and 2-methylhexanoate 11 (R = 2-methybutyl) was only moderately active. The screening results suggested a rough SAR; at least a two carbon linear ester chain (R = ethyl) was necessary for chemosensitizing activity, but a long chain (R > approximately five carbons), led to reduced or no activity. Isobutyrate (R = isopropyl) 5 and pivalate (R = tert-butyl) 6 displayed the most significant survival rate (13–14%) among all saturated Group-I and-II compounds, and the unsaturated 3-methylbut-2-enoate (prenyl-like substituent) 16 in the Group-III compounds further increased the effect, showing a 5% survival rate. Among the Group-IV compounds with polar ester substituents, succinate 18 (R = CH2CH2COOH) was inactive, while compounds with phenyl or pyridinyl aromatic rings showed potent reversal ability, unless an electron-withdrawing group, such as nitro and cyano, was present on the aromatic ring. Especially, benzoate 19 and p-methoxybenzoate 21 exhibited less than 1% survival rate. From these findings, we speculated that decrease in electron density on the aromatic ring affected chemoreversal activity. Quinoline derivative 28 exhibited cytotoxicity against both KB and KBvin cells.
From these results, the Group 1 compounds (4–11), except for 3 and 12, the Group-II and -III (13–17), and the most active Group V compounds (19–23) were selected for further investigation. The remaining compounds (1–3, 12, 18, 24–27, 29–32, and 35–36), which generally had weaker reversal ability, as well as compounds as well as inherent cytotoxicity (28) were eliminated from further testing.
Chemoreversal ability of DDB analogs with TAX, VCR, and doxorubicin (DOX)
A quantitative evaluation of the reversal ability of DDB analogs 4–11, 13–17, and 19–23 was performed using MDR KBvin cells with various concentrations of TAX, VCR, and DOX, which are clinically used and known as significant P-gp substrates, partly accounting for their resistance (Table 1). The IC50 value of anticancer drugs in the presence of test compounds at 10 μM concentration was calculated and fold reversal was determined by dividing the IC50 of anticancer drug alone by the IC50 of anticancer drugs plus DDB analog. For chemoreversal ability against TAX resistance, compounds 9, 15, 16, 19, and 23 were 3–10 times more potent than the positive control VRP. Especially, 16, with a prenyl-like ester substituent, and trimethoxybenzoate 23 effectively reversed the sensitivity of TAX in KBvin cells by 326- and 222-fold, respectively. Most of the tested analogs, including VRP, showed greater reversal against VCR resistance compared with TAX resistance. The reversal effects of compounds 6, 10, 13–16, 22, and 23 against VCR were significantly better than that of VRP. Especially, 16 and 23 showed 560-fold reversal effect, which was 5.1 times greater than that of VRP. The following SAR correlations were proposed based on the chemosensitizing effects against TAX and VCR. In compounds with aliphatic esters, unsaturated Group III compounds 15 and 16 were generally more potent than saturated group I and II compounds. Compounds 15 and 16 displayed greater chemoreversal ability than 7 and 9, which contain structurally related saturated groups. In the case of compounds with aromatic esters (Group V), an electron donating group, such as methyl and methoxy, at the para-position reduced the reversal ability, while additional methoxy groups at the meta-position enhanced the ability. The following rank order of potency was seen: 3,4,5-tri-OMe (23) > 3,4-di-OMe (22) > H (19) > 4-Me (20) ≈ 4-OMe (21). Different SAR correlations between TAX and VCR were found in the saturated aliphatic group. Cyclic aliphatic side chains (Group II, 13–14) were more effective than non-cyclic aliphatic side chains (Group I, 4–11) in the case of VCR resistance, while this difference was not present for TAX resistance. Within the tested aliphatic acyclic Group I compounds (4–11), 2-methylbutyrate (R = iso-butyl) 9 was most potent for TAX, while pivalate (R = tert-butyl) 6 and petanoate (R = n-butyl) 10 were most potent for VCR. In addition, 10 exhibited greater activity than the related unsaturated analog 17 against VCR resistance. All of the tested compounds were less effective at reversing DOX resistance than against TAX and VCR. This phenomenon might be correlated with the difference of efflux pump on the cell membrane with that on the nuclear membrane, because both TAX and VCR act by binding tubulin, while DOX interacts with DNA by intercalation.22 However, compounds 6, 8, 11–14, and 20–23 still showed greater reversal effects than VRP for DOX chemosensitivity in MDR cells. Especially, pivalate 6, with a bulky and short aliphatic ester chain, reversed the activity most effectively, exhibiting medium more sensitivity than VRP. SAR against DOX was slightly different from that against VCR. Compounds 6, 8, 11, 13, and 14 with a branched substituent at the ester α-position showed significant MDR reversal activity. Benzoates with an electron donating group, such as para-methyl (20) and –methoxy (21), had little effect. Although an additional methoxy group at the meta-position increased the ability, no difference was found between trimethoxy 23 and dimethoxy 22. The effects of substituent on phenyl ring resulted in the following order of potency: 3,4-di-OMe (22) ≈3,4,5-tri-OMe (23) > H (19) ≈4-Me (20) ≈4-OMe (21). In conclusion, analogs 16 and 23 showed significant TAX and VCR cytotoxic reversal ability, and analog 6 exhibited the most potent DOX cytotoxic reversal ability.
Table 1.
Reversal Effects with Paclitaxel (TAX), Vincristin (VCR), and Doxorubicin (DOX) in KBvin
| Group | Cmpda | IC50 of TAX (nM)b | Foldc | IC50 of VCR (nM) | Fold | IC50 of DOX (nM) | Fold |
|---|---|---|---|---|---|---|---|
| TAX | 976.8 | - | 2520.7 | - | 1942.3 | - | |
| I | 4 | 82.93 | 12 | 80.96 | 31 | 507.5 | 4 |
| 5 | 46.52 | 21 | 41.42 | 61 | 284.0 | 7 | |
| 6 | 26.15 | 37 | 7.22 | 349 | 42.6 | 46 | |
| 7 | 30.50 | 32 | 50.81 | 50 | 510.9 | 4 | |
| 8 | 37.06 | 26 | 35.92 | 70 | 93.9 | 21 | |
| 9 | 8.72 | 112 | 31.75 | 79 | 339.7 | 6 | |
| 10 | 40.74 | 24 | 15.65 | 161 | 221.5 | 9 | |
| 11 | 39.11 | 25 | 47.31 | 53 | 61.9 | 31 | |
| II | 13 | 48.65 | 20 | 8.14 | 310 | 86.3 | 23 |
| 14 | 39.89 | 24 | 10.55 | 239 | 65.8 | 30 | |
| III | 15 | 9.55 | 102 | 10.98 | 230 | 547.8 | 4 |
| 16 | 2.99 | 326 | 4.50 | 560 | 291.5 | 7 | |
| 17 | 47.45 | 21 | 46.53 | 54 | 573.9 | 3 | |
| V | 19 | 7.65 | 167 | 20.21 | 101 | 157.7 | 9 |
| 20 | 46.82 | 27 | 39.61 | 51 | 130.8 | 11 | |
| 21 | 47.23 | 27 | 40.53 | 50 | 131.4 | 11 | |
| 22 | 14.25 | 89 | 10.10 | 202 | 68.80 | 21 | |
| 23 | 4.41 | 222 | 4.45 | 566 | 84.2 | 23 | |
| VRP | 31.87 | 31 | 23.04 | 109 | 219 | 9 |
Concentration of compound: 10 μM,
SD was shown in Supporting Information,
The reversal fold values were calculated as: Reversal fold = IC50 (anticancer drug alone) / IC50 (anticancer drug + test compound).
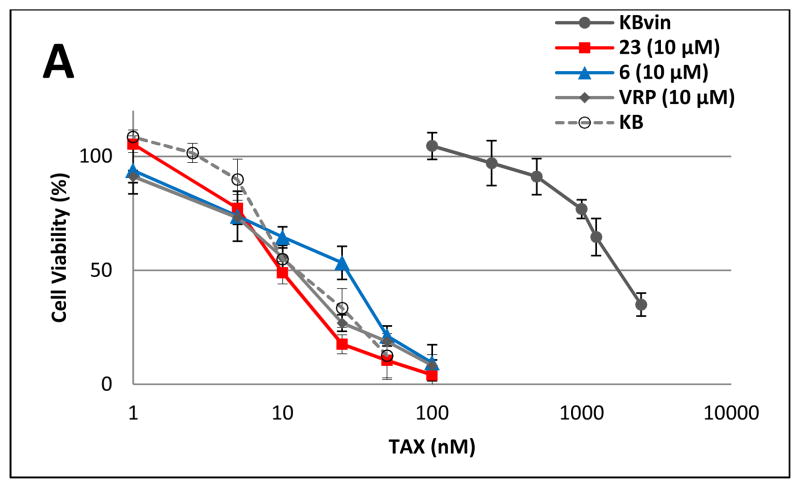
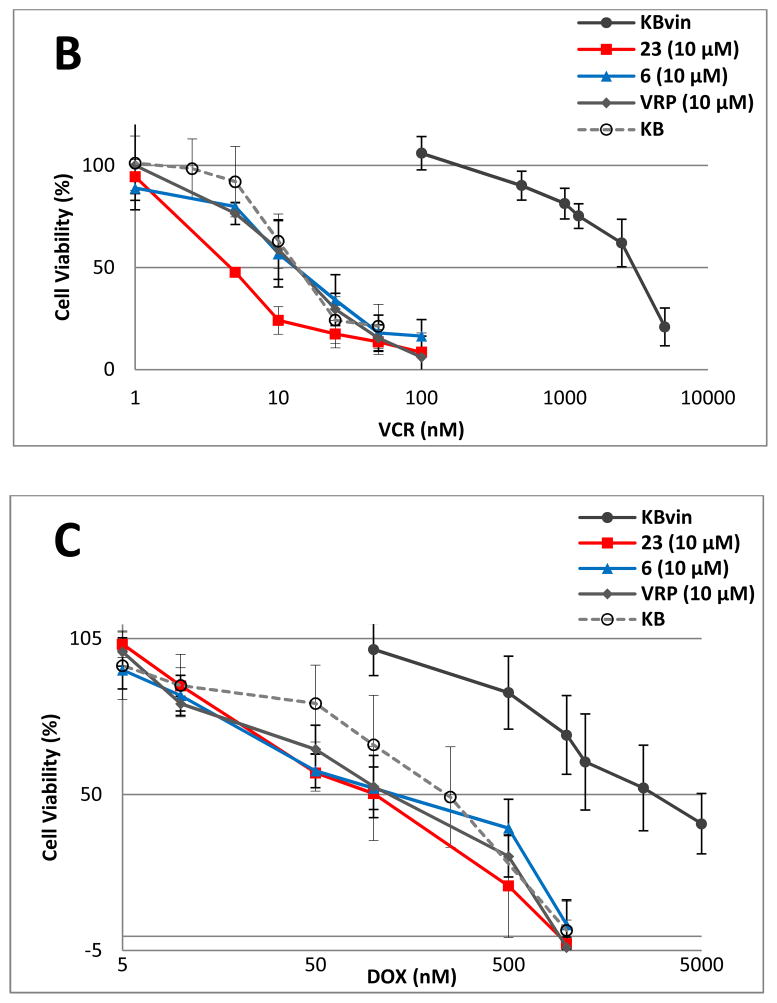
Based on the above results, compounds 6 and 23 were selected for further evaluation of chemosensitizing efficacy. The dose-response proliferation inhibitory effects of TAX, VCR, and DOX at 10 μM were analyzed against KB and KBvin cells (Figure 3). In the absence of DDB compound or VRP, KBvin cells were resistant to all three anticancer drugs, resulting in IC50 values over 1000 nM. When 10 μM of compound 6 or 23 or VRP was added, the sensitivity of KBvin cells to each anticancer drug was dramatically increased to at least the same levels of KB cells. The chemosensitizing efficacy of 6 or 23 was either similar or better than VRP. KBvin became even more sensitive to VCR than KB, when KBvin cells were co-treated with 10 μM 23 and VCR. These results demonstrated that 10 μM of 6 and 23 effectively chemosensitized MDR cells.
Figure 3. Reversal of chemosensitivity of KBvin by 6 or 23.
Chemoresistant KBvin cells were incubated with various concentrations of anticancer drugs TAX (A), VCR (B), or DOX (C) in the presence of test compounds, as indicated, for 72 h to evaluate the effect on chemosensitization. KB cells (○) were sensitive to anticancer drugs, while KBvin (●) were resistant in the absence of test compounds. Chemosensitization of KBvin cells was observed when the cells were co-treated with 10 μM of 6 (▲), 23 (■), or VRP (◆).
Dose-response effect of compounds 6 and 23 on sensitization of KBvin to TAX
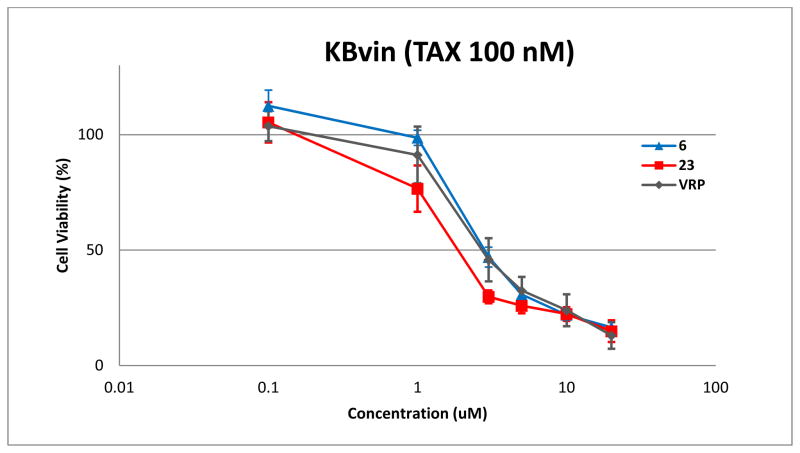
To evaluate the reversal activity of 6 and 23 in a dose-response manner, KBvin cells were cultured with non-toxic concentration of TAX (100 nM) in the presence of various concentrations of compounds (Figure 4). As we expected, compounds 6 and 23 exhibited reversal activity in a dose dependent manner. Although the median effective concentration (EC50) value of 6 (2.81 μM) was similar to that of VRP (2.71 μM), compound 23 (1.87 μM) exhibited a lower EC50 than VRP (P values are given in suppl. Table S3). These results demonstrate that 23 could be more effective than VRP in chemosensitizing the MDR cells to TAX.
Figure 4. Dose response effect of compounds 6 and 23 on sensitization of KBvin to TAX.
Multidrug resistant KBvin cells were treated with various concentrations of compounds 6 or 23 in the presence of 100 nM TAX, an absolutely non-toxic concentration for KBvin. Data are expressed as mean ± SE of three independent experiments. Calculated median effective concentration (EC50) of compounds 6, 23, or VRP was 2.81 μM, 1.87 μM or 2.71 μM, respectively.
The effect of DDB analogs on P-gp function in KBvin cells
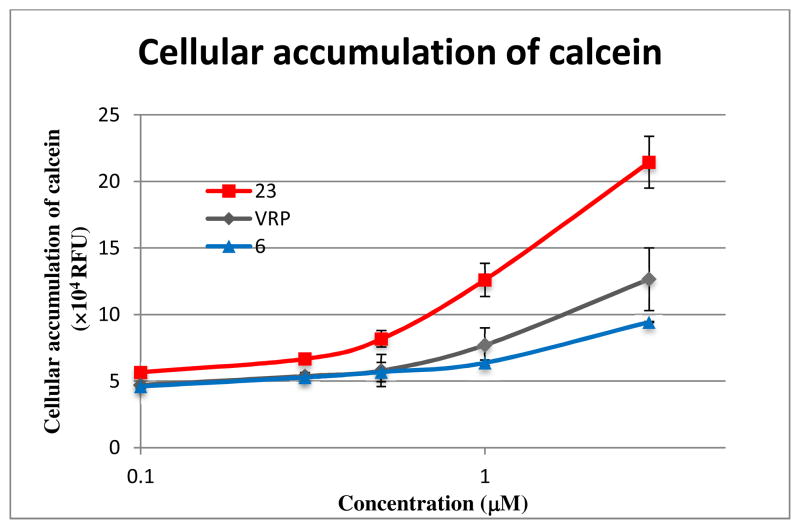
To confirm our hypothesis that DDB analogs inhibit efflux activity of P-gp resulting in elevated concentration of anticancer drugs in MDR cells, the effect of compounds 6 and 23 on P-gp function in KBvin cells using calcein-AM as a fluorogenic P-gp substrate was investigated (Figure 5, suppl. Table S4). Dose-dependent intracellular accumulation of calcein was observed in the presence of compounds. Although 6 was slightly less potent than VRP, 23 was up to two-fold more potent than VRP, especially at concentrations around the EC50 value (1.87 μM) of 23. Therefore, these results clearly indicated that DDB analogs, especially 23, are effective P-gp inhibitors.
Figure 5. Effect of compounds on P-gp function in KBvin cells.
KBvin cells were pre-treated with compounds followed by addition of calcein-AM. The cellular accumulation of calcein is represented by the relative fluorescent unit (×104 RFU). Cellular accumulation of calcein demonstrates inhibition of efflux activity of P-gp. Data with mean ± SD of three independent experiments are shown in suppl. Table S2.
To demonstrate the effective efflux inhibition of anticancer drugs, direct measurement of cellular accumulation of DOX in KBvin cells was studied as the intensity of intrinsic fluorescence of DOX (Figure 6). KBvin cells were pre-treated with compounds followed by addition of DOX. Intracellular accumulation of DOX was measured as the fluorescence intensity and standardized as fold ratio. All DDB analogs induced DOX accumulation in KBvin cells at 1.2- to 2.4-fold. The cellular accumulation of DOX by DDB analogs was consistent with sensitization of KBvin cells to DOX (Table 1). Thus, these data further support that DDB-derived chemosensitizers function as P-gp inhibitors resulting in cellular accumulation of anticancer drugs.
Figure 6. Recovered DOX Accumulation in DDB Analog-treated Drug Resistant KBvin Cells.
KBvin cells were incubated in DOX medium (final conc. 10 μM) for 3 h in the presence of 10 μM DDB analogs, and then cellular accumulation of DOX was measured as intrinsic fluorescence intensity of DOX. Fluorescence intensity of DOX was expressed as the ratio of effect of compound to negative control (Dox). Intracellular accumulation of DOX was clearly observed in the presence of DDB-derived compounds. Data represent as mean ± SD, n= 3. P-gp inhibitor VRP (10 μM) or cyclosporine (CsA) (5 μM) was used as a positive control.
Further screening studies demonstrated that DDB analogs sensitized NIH3T3-MDR cells (murine fibroblast NIH3T3 cells with overexpressing human P-gp protein) to TAX and VCR (unpublished data). These results also support our conclusion that DDB analogs interfere with drug efflux function of P-gp.
Hydrophobicity evaluation of active DDB analoges
P-gp contains a large drug-binding pocket with a volume of around 6000Å.23 The pocket includes predominantly hydrophobic and aromatic residues in its upper half and more polar and charged residues in its lower half. Thus, because of similarity, hydrophobic substrates would bind to the hydrophobic residues, and aromatic substrates would overlap with the π-orbitals of aromatic residues in the binding pocket. Further evaluation of the chemical structures of various known P-gp inhibitors identified common features, including high hydrophobicity, two or more aromatic rings, a methoxy group on the aromatic ring (hydrogen bond acceptor), and one or two protonatable nitrogens.24, 25 Because the upper half of the presumptive drug-binding pocket is composed of mainly hydrophobic and aromatic residues,23 it is possible that hydrophobicity of DDB analogs could influence their MDR reversal activity. Table 2 shows the clog P values of synthesized compounds. Although a few exceptions were present, the chemosensitizing effects of compounds were moderately correlated with their clog P values. Active compounds had clog P values of 4–8; those with clog P lower than 4 tended to lose chemosensitizing activity. The clog P values of 6, 16, and 23, which were significantly active as described above, were between 4.8 and 6.2, which is close to that of VRP. This fact implied that hydrophobicity is an important parameter in P-gp inhibition.
Table 2.
cLogP Values of Synthesized DDB-derived Compounds
| Cmpd | cLog Pa | Cmpd | cLog P |
|---|---|---|---|
| 1 | 4.27 | 19 | 6.93 |
| 2 | 1.26 | 20 | 7.93 |
| 3 | 2.98 | 21 | 7.28 |
| 4 | 4.03 | 22 | 6.69 |
| 5 | 4.65 | 23 | 5.93 |
| 6 | 5.45 | 24 | 6.42 |
| 7 | 5.09 | 25 | 5.80 |
| 8 | 5.71 | 26 | 7.31 |
| 9 | 5.89 | 27 | 6.11 |
| 10 | 6.15 | 28 | 6.21 |
| 11 | 6.77 | 29 | 4.68 |
| 12 | N/A | 30 | 5.02 |
| 13 | 5.92 | 31 | 2.60 |
| 14 | 7.04 | 32 | 7.49 |
| 15 | 5.24 | 33 | 8.27 |
| 16 | 6.04 | 34 | 1.96 |
| 17 | 6.51 | 35 | 5.34 |
| 18 | 2.40 | 36 | 2.28 |
| VRP | 4.58 |
cLogP was calculated by ChemDraw Ultra Version 12.0
Conclusions
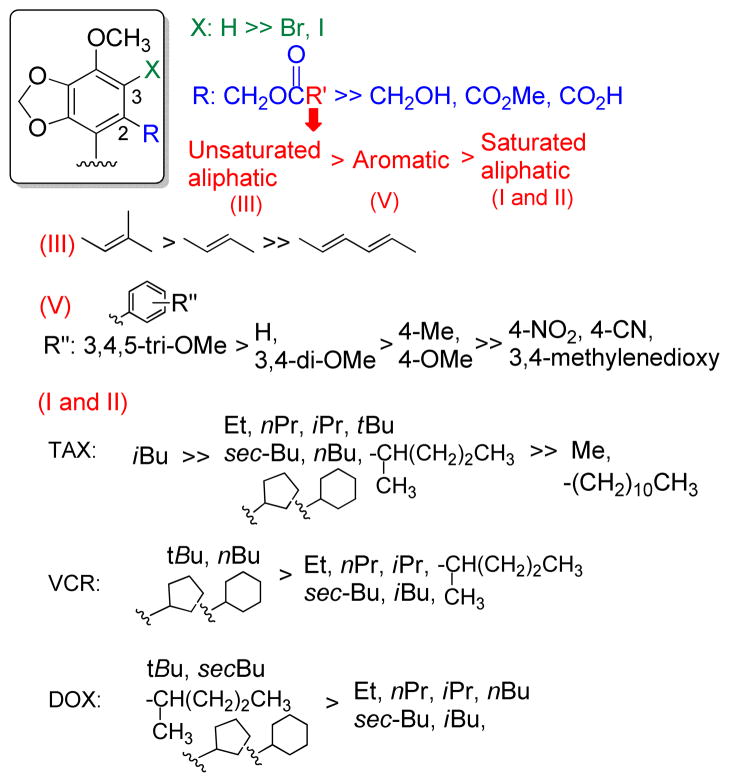
Multidrug resistance is still a serious barrier to successful cancer chemotherapy. Among the possible reasons for multidrug resistance, reducing intracellular concentration of anticancer drug caused by the drug efflux pumps, such as P-gp, is a major cause of chemoresistance. Effective P-gp modulators are still unavailable for clinical use, due partly to the toxicity of the currently available compounds. We selected DDB, a clinically used hepatoprotective compound, as a lead, and 33 new DDB analogs were newly designed and synthesized. All synthesized analogs were evaluated for MDR chemosensitizing effects on clinically used anticancer drugs, such as TAX, VCR, and DOX. We succeeded to improve chemoreversal action by introducing a 2,2′-methylester group to DDB, and SAR studies were summarized in Figure 7. Insertion of halogen onto the 3-position reduced the ability. In the 2-position of the ester side chain, bulky groups, such as pivalate, 2-methylbutanoate, cyclic aliphatic, and trimethoxyphenyl, tended to enhance the reversal ability of DDB analogs. Among all tested compounds, DDB derivatives 16 and 23 were 5–10 times more effective than VRP for TAX and VCR reversal ability. Analog 6 also showed five-fold greater chemosensitizing effect against DOX than VRP. Importantly, active DDB analogs displayed no cytotoxicity against tumor cells established from different tissues, suggesting that our novel DDB analogs are significant lead compounds for further clinical development to overcome the MDR phenotype. Intracellular accumulation studies using Calcein-AM and DOX in KBvin cells clearly demonstrated that DDB analogs interfere with P-gp, drug efflux pump.
Figure 7.
Study of Structure-Activity Relationship
To conclude, newly synthesized DDB analogs were identified as P-gp inhibitors with new scaffold for non-toxic chemosensitizer drug development.
Experimental section
General
1H NMR (400 MHz) spectra were measured on a Varian Inova spectrometer with TMS as the internal standard. Mass spectra were measured on a Shimazu LCMS-IT-TOF. All reactions were monitored by thin-layer chromatography (TLC) on aluminum sheets (silica gel 60 F254 plate, 20 × 20, Merck). Melting points were recorded on a Fisher Johns melting apparatus without correction. Medium-pressure column chromatography was used in Biotage Flash and Isco companion systems with silica 40 μM columns from Grace Inc. All final compounds are >95 % pure based on HPLC. Anhydrous solvents were purchased from commercial suppliers.
General synthetic procedure for compounds 3, 7–12, 15–17, and 22
To a solution of 2 in CH2Cl2, an appropriate carboxylic acid (5 equiv mol), N-(3-dimethylaminopropyl)- N′-ethylcarbodiimide hydrochloride (5 equiv mol) and 4-(dimethylamino)pyridine (1 equiv mol) were added and stirred overnight. The reaction mixture was then applied directly to preparative TLC (hexane-EtOAc) without work-up.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) diacetate (3)
Yield 85%; colorless oil; 1H NMR (CDCl3) δ 6.69 (s, 2H), 5.97 (s, 2H), 5.95 (s, 2H), 4.85 (s, 4H), 3.95 (s, 6H), 1.99 (s, 6H); HRMS calcd for C22H22NaO10 (M+Na)+ 469.1111, found 469.1116.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) dibutyrate (7)
Yield 97%; colorless oil; 1H NMR (CDCl3) δ 6.68 (s, 1H), 5.96 (d, J = 1.4 Hz, 2H), 5.95 (d, J = 1.4 Hz, 2H) 4.86 (s, 4H), 3.93 (s, 6H), 2.22 (t, J = 7.2, 4H), 1.59 (sex, J = 7.2 Hz, 4H), 0.90 (t, J = 7.2 Hz, 6H); HRMS calcd for C26H30NaO10 (M+ Na)+ 525.1737, found 525.1759.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(2-methylbutanoate) (8)
Yield 83%; colorless oil ; 1H NMR (CDCl3) δ 6.68 (s, 2H), 5.97 (s, 2H), 5.95 (s, 2H), 4.91–4.82 (m, 4H), 3.93 (s, 6H), 2.32 (sex, J = 6 Hz, 2H), 1.67–1.56(m, 2H), 1.48–1.36 (m, 2H), 1.09 (d, J = 7.2 Hz, 3H), 1.07 (d, J = 7.2 Hz, 3H), 0.84 (dd, J = 7.4, 14.6 Hz, 6H); HRMS calcd for C28H34NaO10 (M + Na)+ 553.2050, found 553.2063.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(3-methylbutanoate) (9)
Yield 91%; colorless oil; 1H NMR (CDCl3) δ 6.68 (s, 2H), 5.96 (s, 2H), 5.95 (s, 2H), 4.86 (s, 4H), 3.93 (s, 6H), 2.12 (d, J = 6.4 Hz, 4H), 2.03 (m, 2H), 0.90 (dd, J = 2.8, 6.6 Hz, 12H); HRMS calcd for C28H34NaO10 (M + H)+ 531.2225, found.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) dipentanoate (10)
Yield 99%; colorless oil; 1H NMR (CDCl3) δ 6.68 (s, 2H), 5.96 (d, J = 1.5 Hz, 2H), 5.94 (d, J = 1.5 Hz, 2H), 4.85 (s, 4H), 3.93 (s, 6H), 2.23 (t, J = 7.6 Hz, 4H), 1.54 (pent, J = 7.6 Hz, 4H), 1.29 (sex, J = 7.6 Hz, 4H), 0.88 (t, J = 7.6 Hz, 6H); HRMS calcd for C28H34NaO10 (M + Na)+ 553.2050, found 553.2067.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(2-methylpentanoate) (11)
Yield 85%; colorless oil; 1H NMR (CDCl3) δ 6.68 (s, 2H), 5.97 (s, 2H), 5.95 (s, 2H) 4.91–4.81 (m, 4H), 3.93 (s, 6H), 2.37 (dd, J = 7.2, 14 Hz, 2H), 1.64–1.13 (m, 8H), 1.09 (d, J = 7 Hz, 3H), 1.07 (d, J = 7 Hz, 3H), 0.86 (t, J = 6.8 Hz, 6H); HRMS calcd for C30H38NaO10 (M + Na)+ 609.2676, found 609.2688.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) didodecanoate (12)
Yield 88%; colorless oil; 1H NMR (CDCl3, 400 MHz) δ 6.68 (s, 2H), 5.96 (d, J = 1.5 Hz, 2H), 5.94 (d, J = 1.5 Hz, 2H), 4.85 (s, 4H), 3.93 (s, 6H), 2.22 (t, J= Hz, 4H), 1.57–1.19 (m), 0.87 (t, J = 6.8 Hz, 6H); HRMS calcd for C42H62NaO10 (M + Na)+ 749.4247, found 749.4225.
(2E,2’E)-(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(but-2-enoate) (15)
Yield 8%; colorless oil; 1H NMR (CDCl3) δ 6.96–6.87 (m), 6.7 (s, 2H), 5.94 (s, 4H), 5.78 (d, J= 15.6 Hz, 2H), 4.94 (d, J = 12.4 Hz, 2H), 4.85 (d, J = 12.4 Hz, 2H), 3.93 (s, 6H), 1.85 (d, J = 6.8 Hz, 6H); HRMS calcd for C26H26NaO10 (M + Na)+ 521.1424, found 521.1449.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(3-methylbut-2-enoate) (16)
Yield 51%; colorless oil; 1H NMR (CDCl3) δ 6.70 (s, 2H), 5.94 (d, J = 1.5 Hz, 2H), 5.93 (d, J = 1.5 Hz, 2H), 5.62 (s, 2H), 4.95 (d, J = 12.5 Hz, 2H), 4.82 (d, J = 12.5 Hz, 2H), 3.93 (s, 6H), 2.12 (s, 6H), 1.86 (s, 6H); HRMS calcd for C28H30NaO10 (M + Na)+ 549.1737, found 549.1761.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(hexa-2,4-dienoate) (17)
Yield 55%; colorless oil; 1H NMR (CDCl3) δ 7.19 (d, J = 9.8 Hz, 1H), 7.15 (d, J = 9.8 Hz, 1H), 6.70 (s, 2H), 6.18–6.08 (m, 4H), 5.93 (d, J = 1.5 Hz, 4H), 5.71 (s, 1H), 5.68 (s, 1H), 4.96 (d, J = 12.3 Hz, 4H), 4.87 (d, J = 12.3 Hz, 4H), 3.93 (s, 6H), 1.83 (d, J= 5.2 Hz, 6H); HRMS calcd for C30H30NaO10 (M + Na)+ 573.1737, found 573.1746.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(3,4-dimethoxybenzoate) (22)
Yield 90%; colorless oil; 1H NMR (CDCl3) δ 7.55 (dd, J = 2, 8.4 Hz, 2H), 7.43 (d, J = 2 Hz, 2H), 6.78 (d, J = 8.4 Hz, 2H), 6.76 (s, 2H), 5.91 (s, 2H), 5.81 (s, 2H), 5.14 (d, J = 12.4 Hz, 2H), 5.04 (d, J = 12.4 Hz, 2H), 3.93 (s, 6H), 3.89 (s, 6H), 3.88 (s, 6H); HRMS calcd for C36H34NaO14 (M + Na)+ 713.1846, found 713.1839.
General procedure for compound 4–6, 13–14, 19–21, and 23–28
To a flask with anhydrous dichloromethane, compound 2 and triethylamine (5–10 equiv mol) were added first and then the appropriate acyl chloride (2.2 equiv mol) was added at 0 °C under nitrogen. The reaction was warmed up to room temperature gradually and stirred for 1–3 h. After the reaction was completed, water and saturated sodium carbonate solution were added and the reaction mixture was extracted with dichloromethane, dried over sodium sulfate, and concentrated. Further purification was done by Combiflash (hexane-EtOAc gradient).
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) dipropionate (4)
Yield 99%; colorless oil; 1H NMR (CDCl3) δ 6.68 (s, 2H), 5.95 (d, J = 6.5 Hz, 4H), 4.85 (s, 4H), 3.93 (s, 6H), 2.26 (q, J = 7.2 Hz, 4H), 1.08 (t, J = 7.6 Hz, 6H); HRMS calcd for C24H27O10 (M + H)+ 497.1424, found 497.1432.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(2-methylpropanoate) (5)
Yield 83%; colorless oil; 1H NMR (CDCl3) δ 6.68 (s, 2H), 5.96 (dd, J = 9.2 Hz, 4H), 4.88 (d, J = 12.6 Hz, 2H), 4.84 (d, J = 12.6 Hz, 2H), 3.93 (s, 6H), 2.49 (sept, J = 7.2 Hz, 2H), 1.11 (t, J = 7.2 Hz, 12H); HRMS calcd for C26H30NaO10 (M + Na)+ 525.1737, found 525.1754.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(2,2-dimethylpropanoate) (6)
Yield 82%; colorless oil; 1H NMR (CDCl3) δ 6.68 (s, 2H), 5.96 (d, J = 13.8 Hz, 4H), 4.91 (d, J = 12.8 Hz, 2H), 4.82 (d, J = 12.8 Hz, 2H), 3.93 (s, 6H), 1.15 (s, 18H); HRMS calcd for C28H34NaO10 (M + Na)+ 553.2050, found 553.2054.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) dicyclopentanecarboxylate (13)
Yield 99 %; colorless oil; 1H NMR (CDCl3) δ 6.68 (s, 2H), 5.95 (d, J = 7.9 Hz, 4H), 4.88 (d, J = 12.6 Hz, 2H ), 4.84 (d, J = 12.6 Hz, 2H), 3.93 (s, 6H), 2.71–2.65 (m, 2H), 1.88– 1.52 (m, 16H); HRMS calcd for C30H34NaO10 (M + Na)+ 577.2050, found 577.2052.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) dicyclohexanecarboxylate (14)
Yield 99%; colorless oil; 1H NMR (CDCl3) δ 6.67 (s, 2H), 5.95 (d, J = 7.6 Hz, 4H), 4.87 (d, J = 12.6 Hz, 2H), 4.83 (d, J= 12.6 Hz, 2H), 3.93 (s, 6H), 2.27–2.20 (m, 2H), 1.95– 1.13 (m, 20H); HRMS calcd for C32H38NaO10 (M + Na)+ 605.2363, found 605.2387.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) dibenzoate (19)
Yield 59%; colorless amorphous; 1H NMR (CDCl3) δ 7.94–7.92 (m, 4H), 7.52–7.47 (m, 2H), 7.38–7.34 (m, 4H), 6.76 (s, 2H), 5.90 (s, 2H), 5.79 (s, 2H), 5.15 (d, J = 12.3 Hz, 2H), 5.06 (d, J = 12.3 Hz, 2H), 3.93 (s, 6H); HRMS calcd for C32H26NaO10 (M + Na)+ 593.1424, found 593.1452.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(4-methylbenzoate) (20)
Yield 34%; colorless amorphous; 1H NMR (CDCl3) δ 7.81 (d, J = 8.0 Hz, 4H), 7.15 (d, J = 8.0 Hz, 4H), 6.75 (s, 2H), 5.91 (s, 2H), 5.81 (s, 2H), 5.12 (d, J = 12.5 Hz, 2H), 5.04 (d, J = 12.5 Hz, 2H), 3.92 (s, 6H), 2.35 (s, 6H) ; HRMS calcd for C34H30NaO10 (M + Na)+ 621.1737, found 621.1761.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(4-methoxybenzoate) (21)
Yield 30%; colorless amorphous; 1H NMR (CDCl3) δ 7.90–7.86 (m, 4H), 6.85– 6.81 (m, 4H), 6.75 (s, 2H), 5.91 (s, 2H), 5.82 (s, 2H), 5.12 (d, J = 12.3 Hz, 2H), 5.03 (d, J = 12.3 Hz, 2H), 3.93 (s, 6H), 3.80 (s, 6H); HRMS calcd for C34H30NaO12 (M + Na)+ 653.1635, found 653.1615.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(2,3,4-trimethoxybenzoate) (23)
Yield 98%; colorless amorphous; 1H NMR (CDCl3) δ 7.18 (s, 4H), 6.73 (s, 2H), 5.90 (s, 2H), 5.77 (s, 2H), 5.16 (d, J = 12.3 Hz, 2H), 5.04 (d, J = 12.3 Hz, 2H), 3.92 (s, 6H), 3.87 (s, 6H), 3.86 (s, 12H); HRMS calcd for C38H39O16 (M + H)+ 773.2058, found 773.2050.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(4-nitrobenzoate) (24)
Yield 52%; colorless amorphous; 1H NMR (CDCl3) δ 8.22–8.19 (m, 4H), 8.09–8.06 (m, 4H), 6.75 (s, 2H), 5.92 (s, 2H), 5.85 (s, 2H), 5.18 (d, J = 12.4 Hz, 2H), 5.12 (d, J = 12.4 Hz, 2H), 3.94 (s, 6H); HRMS calcd for C32H25N2O14 (M + H)+ 683.1125, found 683.1119.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(4-cyanobenzoate) (25)
Yield 38%; colorless amorphous; 1H NMR (CDCl3) δ 8.02–7.99 (m, 4H), 7.69–7.66 (m, 4H), 6.73 (s, 2H), 5.91 (s, 2H), 5.81 (s, 2H), 5.16 (d, J = 12.4 Hz, 2H), 5.08 (d, J = 12.4 Hz, 2H), 3.94 (s, 6H); HRMS calcd for C34H24N2NaO10 (M + Na)+ 643.1329, found 643.1314.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(benzo[d][1,3]dioxole-5-carboxylate) (26)
Yield 85%; colorless amorphous; 1H NMR (CDCl3) δ 7.53 (m, 2H), 7.34 (s, 2H), 6.77 (s, 2H), 6.75 (s, 2H), 5.99 (s, 4H), 5.93 (s,2H), 5.88 (s, 2H), 5.10 (d, J = 12.4 Hz, 2H), 5.02 (d, J = 12.4 Hz, 2H), 3.93 (s, 6H); HRMS calcd for C34H26NaO14 (M + Na)+ 681.1220, found 681.1188.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis[6-(trifluoromethyl)nicotinate] (27)
Yield 61%; colorless oil; 1H NMR (CDCl3) δ 9.15 (m, 2H), 8.38 (m, 2H), 7.71 (m, 2H), 6.74 (s, 2H), 5.93 (dd, J= 1.6, 8.8 Hz, 4H), 5.21 (d, J= 7.6 Hz, 4H), 3.93 (s, 3H); HRMS calcd for C32H22F6N2NaO10 (M + Na)+ 731.1076, found 731.1076.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) bis(quinoline-2-carboxylate) (28)
Yield 97%; orange needles; mp: 129–130 °C; 1H NMR (CDCl3) δ 8.25 (m, 2H), 8.16 (m, 2H), 7.98 (m, 2H), 7.77–7.70 (m, 4H), 7.59–7.55 (m, 2H), 6.86 (s, 2H), 5.88 (dd, J= 1.6, 19.6 Hz, 4H), 5.32 (d, J= 2.4 Hz, 4H), 3.93 (s, 6H); HRMS calcd for C38H29N2O10 (M + H)+ 673.1822, found 673.1798.
4,4′-[(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene)]bis(oxy)bis(4-oxobutanoic acid) (18)
To a solution of 2 (26 mg, 0.072 mmol) in anhydrous THF (2.0 mL), succinic anhydride (9 mg, 0.090 mmol) and DMAP (5 mg, 0.040 mmol) was added. The mixture was refluxed overnight. After cooling to rt, the whole was acidified with 1N HCl aq. and partitioned with EtOAc. The organic phase was concentrated. The residue was purified by preparative TLC (CH2Cl2:MeOH:TFA =95:5:0.25). Yield 99%; colorless oil; 1H NMR (CDCl3) δ 6.67 (s, 2H), 5.96 (d, J = 16.8 Hz, 4H), 4.94 (d, J = 12.6 Hz, 2H), 4.89 (d, J = 12.6 Hz, 2H), 3.93 (s, 6H), 2.59–2.54 (m, 8H); HRMS calcd for C26H26NaO14 (M + Na)+ 585.1220, found 585.1228.
Dimethyl 3,3′-diiodo-4,4′-dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-dicarboxylate (30)
Silver trifluoroactate (89 mg, 0.4 mmol) was added to a solution containing 1 (42.1 mg, 0.1 mmol) and CHCl3(1 mL). Then iodine (102.2 mg, 0.4 mmol) was poured into the solution, and the reaction mixture was stirred overnight at room temperature. Further isolation was done by preparative TLC (hexane-EtOAc: 7:3). Mono-and di-iodo products were found with this condition. Yield 7 %; colorless amorphous; 1HNMR (CDCl3) δ 6.01 (d, J = 1.6 Hz, 2H), 5.99 (d, J = 1.2 Hz, 2H), 4.05 (s, 6H), 3.68 (s, 6H); HRMS calcd for C20H16I2NaO10 (M + Na)+ 692.8731, found 692.8704.
3,3′-Dibromo-4,4′-dimethoxy-5,6,5′,6′-bis(methylenedioxy)biphenyl-2,2′-dimethanol (31)
To a stirred solution containing 1 (95.2 mg, 0.165 mmol) and 1mL anhydrous CH2Cl2 under nitrogen at around −20 °C (ice with brine), Diisobutylaluminum hydride (DIBAL) (0.83 mL, 0.83 mmol) was added dropwise. After one h, another portion of DIBAL (0.4 mL, 0.4 mmol was added and stirring continued until starting material disappeared. The reaction was quenched with MeOH (3 mL), 10% Rochelle salt solution (3 mL) was added, and the mixture stirred for 30 min. Water was added to the mixture, which was then extracted with EtOAc three times, dried over sodium sulfate, and concentrated. The compound has low solubility in various solvents (EtOAc, CH2Cl2, MeOH, acetone). A small portion was taken for further purification for bioassay. The remaining portion was used in the next reaction without further purification. Colorless amorphous solid; 1H NMR (CDCl3) δ 5.96 (s, 4H), 4.67 (d, J = 12.4 Hz, 2H), 4.26 (d, J = 12 Hz, 2H), 4.09 (s, 6H), 3.19 (bs, 2H); HRMS calcd for C18H16Br2NaO8 (M + Na)+ 542.9089, found 542.9091.
(3,3′-Dibromo-4,4′-dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) dibutyrate (32)
Compound 31 (16.8 mg, 0.033 mmol), butyric acid (0.02 mL, 0.21 mmol), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (31.5 mg, 0.16 mmol), 4- (dimethylamino)pyridine (4.1 mg, 0.033 mmol) were mixed together in CH2Cl2 overnight. The reaction mixture was subjected to preparative TLC to give the desired compound, which was recrystallized from CH2Cl2 -hexane. Yield 86 %; colorless prisms; mp: 109–110 °C; 1H NMR (CDCl3) δ 5.94 (d, J = 1.2 Hz, 2H), 5.92 (d, J = 1.6 Hz, 2H), 4.97 (d, J = 1.2 Hz, 2H), 4.89 (d, J = 1.2 Hz, 2H), 4.06 (s, 6H), 2.18 (t, J = 7.6 Hz, 6H), 1.57 (sex, J = 7.6 Hz, 4H), 0.88 (t, J = 7.2 Hz, 6H); HRMS calcd for C28H32Br2NaO10 (M + Na)+ 682.9926, found 682.9916.
(4,4′-Dimethoxy-5,6,5′,6′-dimethylenedioxybiphenyl-2,2′-diyl)bis(methylene) dibenzoate (33)
The same procedure as for compound 32, but benzoic acid was used instead of butyric acid. Yield 74 %; colorless prisms; mp: 127–128 °C; 1H NMR (CDCl3) δ 7.92 (d, J = 7.6 Hz, 4H), 7.46–7.52 (m, 2H), 7.36 (t, J = 7.6 Hz, 4H), 5.88 (s, 2H), 5.70 (s, 2H), 5.31 (d, J = 11.6 Hz, 2H), 5.12 (d, J = 11.6 Hz, 2H), 4.07 (s, 3H); HRMS calcd for C32H24Br2NaO10 (M + Na)+ 750.9613, found 750.9614.
3,3′-Dibromo-4,4′-dimethoxy-5,6,5′,6′-bis(methylenedioxy)biphenyl-2-hydroxymethyl-2′-carboxylic acid (36)
To a stirred solution containing anhydrous THF (5 mL) and 35 (69.4 mg, 0.13 mmol) under nitrogen, sodium borohydride (15 mg, 0.39 mmol), followed by MeOH (0.1 mL), was added at room temperature. After the reaction was completed, 2 N HCl solution was added to acidify the solution to pH= 2. The solution was extracted with CH2Cl2 (with less than 10 % MeOH), and the organic layer was dried over sodium sulfate. Flash chromatography (CH2Cl2—MeOH) was used to purify the desired compound. Yield 88 %; amorphous solid; 1H NMR (DMSO, 400 MHz) δ 13.2 (bs), 6.04 (d, J = 34 Hz, 2H), 5.97 (d, J = 40 Hz, 2H), 4.55 (bs), 4.28 (dd, J = 11.2, 25.8 Hz, 4H), 3.99 (s, 6H), 3.95 (s, 6H); HRMS calcd for C18H14Br2NaO9 (M + Na)+ 582.9029, found 582.9038.
Cell culture
A549 (lung carcinoma), DU-145 (prostate cancer), K562 (chronic myelogenous leukemia) and KB (epidermoid carcinoma) cell lines were obtained from Lineberger Comprehensive Cancer Center (UNC-CH). KBvin (vincristine-resistant KB subline) was generously provided by Professor Y.-C. Cheng (Yale University, CT). Cells were cultured in RPMI 1640 medium supplemented with 25 mM HEPES, 2 mM L-glutamine (Mediatech), 10 % heat inactivated fetal bovine serum (Hyclone), 100 IU penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Mediatech). KBvin cells were maintained in the culture medium containing 100 nM VCR. Cells were maintained at 37 °C in a humidifier with 5 % CO2 atmosphere.
Cytotoxicity analysis (SRB assay)
Cytotoxicity was determined by the sulforhodamine B (SRB) colorimetric assay. Cells (3–5 × 103 cells/well) were seeded in 96-well plates filled with culture medium containing various concentrations of samples for 72 h. At the end of the exposure period, the supernatant was removed and cells were washed with 100 μL fresh culture medium. The proliferated cells were fixed with 50% trichloroacetic acid for 30 min, and stained with 0.04% SRB (Sigma Chemical Co.) for 30 min. Protein-bound SRB dye was dissolved from stained cells in 10 mM Tris base, and absorbance was measured at 515 nm on Microplate Reader ELx800 (Bio-Tek instruments, Winooski, VT) with a Gen5 software.
MDR reversal activity
For screening of chemosensitizing ability of test compounds, MDR and parental chemosensitive cells were incubated with test compound in the presence of 100 nM TAX, which did not affect the cell growth. Multidrug resistant KBvin and parental KB cells were seeded at 5–7 × 103 cells/well into 96-well plates and incubated with 10 μM test compound with 100 nM TAX for 72 h, and cell density was determined by a SRB assay. To assess the reversal activity of MDR by candidate compounds, it was evaluated by comparing IC50 values of anticancer drugs (vincristine, paclitaxel, and doxorubicin) in the absence or presence of 10 μM of test compound. IC50 values were calculated by log–linear interpolation of data points. Verapamil, as a known P-gp inhibitor/modulator, was used as a positive control in all experiments. The reversal fold values, as potency parameter of test compounds, were calculated as: Reversal fold = IC50 (anticancer drug alone) / IC50 (anticancer drug + test compound). All experiments were performed at least three times.
Measurement of Intracellular Accumulation of Calcein or Doxorubicin
KBvin cells (5 × 103 cell/well) were seeded in 96-well plates and pretreated with samples for 1 h before Calcein-AM or doxorubicin was added to make final concentrations of 1 μM Calcein-AM and 10 μM doxorubicin. After 10 min for Calcein-AM or 3 h for DOX, the medium was removed by aspiration, and the cells were washed with ice-cold PBS followed by lysing with PBS containing 1% sodium dodecyl sulfate (SDS). The mean fluorescence intensity of Calcein or DOX was measured at Ex: 494 nm/Em: 517 nm or Ex: 488 nm/Em: 580 nm, respectively, by a fluorescence microplate reader (Plate Chameleon Multilabel Detection Platform, Hidex Oy, Turku, Finland) with MikroWin software. VRP or CsA was used as a positive control of P-gp modulator. All data for intracellular accumulation of DOX were calculated as the fluorescence intensity, and were represented as fold changes by treatment with compound compare with vehicle (DMSO) treatment.
Supplementary Material
Acknowledgments
This study was supported by grant CA-17625-32 from the National Cancer Institute, NIH, awarded to K. H. Lee. Support in part was also due to the Cancer Research Center of Excellence (CRC) (DOH-100-TD-C-111-005).
Abbreviations used
- DDB
dimethyl-4,4′-dimethoxy-5,6,5′,6′-dimethylene dioxybiphenyl-2,2′-dicarboxylate
- MDR
multi-drug resistance
- P-gp
P-glycoprotein
- MRP
multidrug resistance-associate protein
- TAX
paclitaxel
- VCR
vincristine
- DOX
doxorubicin
- VRP
verapamil
- EDCI
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- DMAP
4-dimethylaminopyridine
- DIBAL
diisobutylaluminium hydride
Footnotes
Supporting Information Available: Cytotoxicity of DDB analogs (Table S1); IC50 ± standard deviation (SD) for reversal effects with paclitaxel (TAX), vincristine (VCR), and doxorubicin (DOX) in KBvin (Table S2); Standardized P values of compounds 6, 23, and VRP (Table S3); Effect of compounds 6 and 23 on P-gp function in KBvin cells (Table S4). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F, Diz PG, Rey JM, Garcia-Garcia A. Multidrug resistance in oral squamous cell carcinoma: The role of vacuolar ATPases. Cancer Lett. 2010;295:135–143. doi: 10.1016/j.canlet.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Zhou T, Shi Q, Bastow KF, Lee KH. Antitumor agents 286. Design, synthesis, and structure-activity relationships of 3′R,4′R-disubstituted-2′,2′-dimethyldihydropyrano[2,3-f]chromone (DSP) analogues as potent chemosensitizers to overcome multidrug resistance. J Med Chem. 2010;53:8700–8708. doi: 10.1021/jm101249z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fodale V, Pierobon M, Liotta L, Petricoin E. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J. 2011;17:89–95. doi: 10.1097/PPO.0b013e318212dd3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres-Romero D, Munoz-Martinez F, Jimenez IA, Castanys S, Gamarro F, Bazzocchi IL. Novel dihydro-[small beta]-agarofuran sesquiterpenes as potent modulators of human P-glycoprotein dependent multidrug resistance. Org Biomol Chem. 2009;7:5166–5172. doi: 10.1039/b915678j. [DOI] [PubMed] [Google Scholar]
- 6.Das SG, Doshi JM, Tian D, Addo SN, Srinivasan B, Hermanson DL, Xing C. Structure-activity relationship and molecular mechanisms of ethyl 2-amino-4-(2-ethoxy-2-oxoethyl)-6-phenyl-4h-chromene-3-carboxylate (sha 14–1) and its analogues. J Med Chem. 2009;52:5937–5949. doi: 10.1021/jm9005059. [DOI] [PubMed] [Google Scholar]
- 7.Mor G, Montagna MK, Alvero AB. Modulation of apoptosis to reverse chemoresistance. Methods Mol Biol. 2008;414:1–12. doi: 10.1007/978-1-59745-339-4_1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang PY, Wong IL, Yan CS, Zhang XY, Jiang T, Chow LM, Wan SB. Design and syntheses of permethyl ningalin B analogues: potent multidrug resistance (MDR) reversal agents of cancer cells. J Med Chem. 2010;53:5108–5120. doi: 10.1021/jm100035c. [DOI] [PubMed] [Google Scholar]
- 9.Robey RW, Shukla S, Finley EM, Oldham RK, Barnett D, Ambudkar SV, Fojo T, Bates SE. Inhibition of P-glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1®. Biochem Pharmacol. 2008;75:1302–1312. doi: 10.1016/j.bcp.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun M, Xu X, Lu Q, Pan Q, Hu X. Schisandrin B: A dual inhibitor of P-glycoprotein and multidrug resistance-associated protein 1. Cancer Lett. 2007;246:300–307. doi: 10.1016/j.canlet.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Qiangrong P, Wang T, Lu Q, Hu X. Schisandrin B--A novel inhibitor of P-glycoprotein. Biochem Biophys Res Commun. 2005;335:406–411. doi: 10.1016/j.bbrc.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Pan Q, Sun M, Lu Q, Hu X. Dibenzocyclooctadiene lignans -- A class of novel inhibitors of multidrug resistance-associated protein 1. Life Sci. 2007;80:741–748. doi: 10.1016/j.lfs.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Liu GT. Chemopreventive effect of dimethyl dicarboxylate biphenyl on malignant transformation of WB-F344 rat liver epithelial cells. Acta Pharmacol Sin. 2005;26:1339–1344. doi: 10.1111/j.1745-7254.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 14.Jin J, Sun H, Wei H, Liu G. The anti-hepatitis drug DDB chemosensitizes multidrug resistant cancer cells in vitro and in vivo by inhibiting P-gp and enhancing apoptosis. Invest New Drugs. 2007;25:95–105. doi: 10.1007/s10637-006-9001-z. [DOI] [PubMed] [Google Scholar]
- 15.Yu HQ, Yang XY, Zhang YX, Shi JZ. Biphenyl-dimethyl dicarboxylate in treating and preventing hepatitis due to drug poisoning. Chin Med J (Engl) 1987;100:122–123. [PubMed] [Google Scholar]
- 16.Huber R, Hockenjos B, Blum HE. DDB treatment of patients with chronic hepatitis. Hepatology. 2004;39:1732–1733. doi: 10.1002/hep.20247. [DOI] [PubMed] [Google Scholar]
- 17.Zhu B, Liu GT, Zhao YM, Wu RS, Strada SJ. Chemosensitizing multiple drug resistance of human carcinoma by Bicyclol involves attenuated p-glycoprotein, GST-P and Bcl-2. Cancer Biol Ther. 2006;5:536–543. doi: 10.4161/cbt.5.5.2655. [DOI] [PubMed] [Google Scholar]
- 18.Zhu B, Liu GT, Wu RS, Strada SJ. Chemoprevention of bicyclol against hepatic preneoplastic lesions. Cancer Biol Ther. 2006;5:1665–1673. doi: 10.4161/cbt.5.12.3377. [DOI] [PubMed] [Google Scholar]
- 19.Xie L, Xie JX, Kashiwada Y, Cosentino LM, Liu SH, Pai RB, Cheng YC, Lee KH. Anti-AIDS (acquired immune deficiency syndrome) agents. 17. New brominated hexahydroxybiphenyl derivatives as potent anti-HIV agents. J Med Chem. 1995;38:3003–3008. doi: 10.1021/jm00016a002. [DOI] [PubMed] [Google Scholar]
- 20.Molander GA, George KM, Monovich LG. Total synthesis of (+)-isoschizandrin utilizing a samarium(II) iodide-promoted 8-endo ketyl-olefin cyclization. J Org Chem. 2003;68:9533–9540. doi: 10.1021/jo0347361. [DOI] [PubMed] [Google Scholar]
- 21.Soai K, Yokoyama S, Mochida K. Reduction of symmetric and mixed anhydrides of carboxylic acids by sodium borohydride with dropwise addition of methanol. Synthesis. 1987:647–648. [Google Scholar]
- 22.Calcabrini A, Meschini S, Stringaro A, Cianfriglia M, Arancia G, Molinari A. Detection of P-glycoprotein in the nuclear envelope of multidrug resistant cells. Histochem J. 2000;32:599–606. doi: 10.1023/a:1026732405381. [DOI] [PubMed] [Google Scholar]
- 23.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pajeva IK, Wiese M. Pharmacophore model of drugs involved in P-glycoprotein multidrug resistance: explanation of structural variety (hypothesis) J Med Chem. 2002;45:5671–5686. doi: 10.1021/jm020941h. [DOI] [PubMed] [Google Scholar]
- 25.Pajeva IK, Globisch C, Wiese M. Combined pharmacophore modeling, docking, and 3D QSAR studies of ABCB1 and ABCC1 transporter inhibitors. ChemMedChem. 2009;4:1883–1896. doi: 10.1002/cmdc.200900282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.