To the Editor
Transglutaminase 1 (TG1)-deficient lamellar ichthyosis (LI) is associated with increased mortality in the neonatal period and has a dramatic impact on quality of life. No efficient treatment is available; current therapy only relieves some symptoms (Oji and Traupe, 2006). For the development of novel therapeutic approaches and to further investigate molecular mechanisms underlying the pathophysiology of LI, a stable, long-lived pre-clinical model is needed which fully recapitulates the human skin phenotype.
In vivo studies in human skin are limited by ethical and practical considerations. The Tgm1−/− mouse does not recapitulate the human skin phenotype. Moreover, the mice die within the first hours of life (Matsuki et al., 1998) due to impaired barrier function, underscoring the importance of TG1 for barrier formation (Candi et al., 2005). Only transplanted Tgm1−/− mouse skin resembled the skin seen in severe ichthyosis (Kuramoto et al., 2002). Choate and colleagues showed the feasibility of ex vivo and in vivo gene transfer for LI (Choate et al., 1996; Choate and Khavari, 1997), although their model system only allowed short-term human skin regeneration. Moreover, a model of rat skin with TG1-deficiency was described (O’Shaughnessy et al., 2010).
Using optimized tissue engineering and surgical conditions enabling stable human skin engraftment in athymic nude mice (Garcia et al. 2011) we have been able to develop a robust skin-humanized mouse model for TG1-deficient LI, involving persistent engraftment of bioengineered human skin, suitable for long-term, pre-clinical studies and for investigation of molecular mechanisms.
We analyzed two LI patients and identified the compound heterozygous TGM1 mutations c.377G>A (p.Arg126His) and c.876+2T>C (p.Glu253Valfs*2) (patient 1) and c.428G>A (p.Arg143His) and c.877-2A>G (p.Phe293Serfs*38 or p.Phe293Valfs*2) (patient 2), which were previously described (Farasat et al., 2009). Punch biopsies obtained from these patients were used to isolate keratinocytes and fibroblasts after obtaining written, informed consent of the probands and institutional approval in accordance with the Helsinki Guidelines. 4–6 weeks after grafting of bioengineered skin equivalents, regenerated human skin grafts became visible and persist in the recipient animals longer than 20 weeks indicating stable engraftment of epidermal stem cells (data not shown).
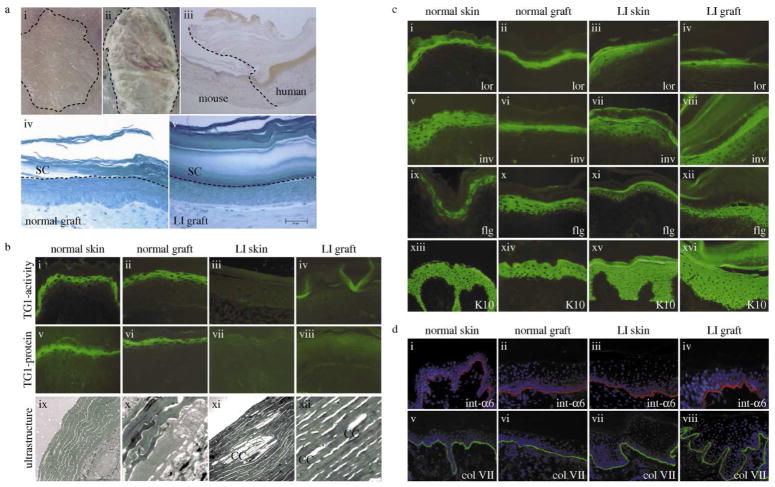
Macroscopically, epidermal hyperplasia and an increased scaly hyperkeratosis completely matched the human skin phenotype. Using a human specific antibody against involucrin we could confirm the human origin of the grafts and clearly delineate the border between mouse and human skin. Light microscopy displays a very thick and packed stratum corneum (SC) in the LI grafts (Fig. 1A).
Figure 1. Characterization of the skin-humanized mouse model for transglutaminase 1-deficient lamellar ichthyosis.
(A) Keratinocytes were seeded onto a fibroblast-populated fibrin-based matrix. Skin equivalents were grafted orthotopically onto the back of athymic nude mice. (i) normal human regenerated skin and (ii) regenerated LI skin (patient 1) 12 weeks after grafting; (iii) Peroxidase staining of involucrin (human specific) confirms the human skin phenotype; (iv) semithin sections, methylene blue staining, morphology of normal and (v) LI grafts showing a very thick and packed stratum corneum. Bar: D=E = 50μm. (B) TG1-activity (i–iv), TG1-protein (v–viii) and ultrastructure (ix–xii). Normal skin/grafts show the typical pericellular distribution of TG1-activity/protein in the stratum granulosum. Ultrastructurally no cholesterol clefts are visible. In contrast, LI skin and LI grafts lacked TG1-activity/protein but displayed cholesterol clefts as typical ultrastructural markers. (C) Characterisation of human and regenerated skin by immunostaining. TG1-substrates like loricrin (i–iv), involucrin (v–viii), and filaggrin (ix–xii) in LI samples show a more diffuse and slightly shifted staining pattern when compared to the normal samples. Keratin 10 (xiii–xvi) was expressed in suprabasal layers in all four samples. (D) The distributions of integrin-α6 (i–iv) and collagen VII (v–viii) in LI skin/grafts in comparison to normal skin/grafts are visualized by immunostaining. These components of the dermoepidermal junction show a comparable staining in all samples indicating a correct formation of the junction zone. Slides were counterstained with DAPI. CC, cholesterol clefts; LI, lamellar ichthyosis; TG1, transglutaminase 1.
TG1-activity and protein were absent in LI skin/grafts. Ultrastructural analysis revealed cholesterol clefts in the SC of the LI skin/grafts, which are important diagnostic markers typical for TG1-deficiency (Pigg et al., 1998). In contrast, skin/grafts derived from healthy individuals were completely normal (Fig. 1B).
The presence and spatial distribution of TG1-substrates, differentiation markers and dermoepidermal junction (DEJ) constituents were assessed by immunostaining. The comparison between normal graft/skin and LI graft/skin showed that normal grafts mirrored normal skin and, likewise, alterations of epidermal differentiation in LI grafts matched those found in LI skin. The diffuse and upwardly shifted distribution of loricrin and involucrin indicates insufficient cross-linking of these proteins into the CE. Filaggrin was expressed in the periphery of cells from the upper spinous layer in all samples. The early differentiation marker keratin 10, localized in viable suprabasal cell layers, remained unchanged (Fig. 1C). Integrin-α6 decorates the DEJ in a continuous, linear manner. Collagen VII was present along the DEJ, indicating its correct formation in the grafts (Fig. 1D).
To further explore changes in differentiation and to relate them to the previous results, we present to our knowledge previously unreported data on the analysis of the proteome of LI epidermis. Comparison between normal and LI skin/grafts provides valuable insights into molecular mechanisms involved in LI pathophysiology. Analysis of the skin-humanized mouse model gives very similar data to those observed in LI skin. Altogether 147 proteins were identified (supplementary table S1). We focused on TG1-substrates and some conspicuous proteins which may give insights into molecular differentiation mechanisms (Table 1).
Table 1.
Numbers of unique peptides of transglutaminase 1, cornified envelope components and desmosome/corneodesmosome components identified by MS/MS-analysis in 2 or 3 independent samples derived from normal human skin, normal grafts, human LI skin and LI grafts.
| protein | MW | number of unique peptides
|
|||
|---|---|---|---|---|---|
| normal skin | normal graft | LI skin | LI graft | ||
| transglutaminase 1 | 90 kDa | 17,10 | 10,11 | 0,0 | 0,41,0 |
|
| |||||
| filaggrin | 435 kDa | 74,110 | 76,84 | 10,13 | 13,12,16 |
| keratinocyte proline-rich protein | 64 kDa | 35,39 | 35,34 | 70,59 | 51,55,53 |
| involucrin | 68 kDa | 16,3 | 3,11 | 15,5 | 14,5,10 |
| loricrin | 26 kDa | 2,2 | 0,2 | 29,24 | 16,18,22 |
| late cornified envelope protein 1C | 12 kDa | 2,2 | 0,0 | 5,4 | 9,8,8 |
| small proline-rich protein 1B | 10 kDa | 0,0 | 0,0 | 2,2 | 2,2,3 |
| desmoplakin | 332 kDa | 77,28 | 40,36 | 146,128 | 135,136,137 |
| junctional plakoglobin | 82 kDa | 30,14 | 13,19 | 51,38 | 55,56,53 |
| desmoglein 1 | 114 kDa | 25,19 | 14,24 | 53,43 | 45,39,44 |
| desmocollin 1 | 100 kDa | 7,0 | 0,10 | 16,17 | 12,8,10 |
| corneodesmosin | 52 kDa | 0,0 | 0,0 | 3,4 | 2,4,3 |
four unique TG1-peptides (GSGVNAAGDGTIR; GTNPSAWVGSVEILLSYLR; YDTPFIFAEVNSDK; NPLPVTLTNVVFR) detected in only one sample are identical to mouse TG1-peptides
The numbers of unique peptides from identified proteins in the grafts were highly consistent with those found in human skin biopsies. No TG1-peptides could be detected in LI skin. The four TG1-peptides detected in one of three LI grafts (Table 1) were likely derived from surrounding mouse tissue since the sequences are identical in mouse and human.
Notably, the number of unique filaggrin peptides decreased markedly in both LI skin and grafts. Profilaggrin is proteolytically processed during keratinocyte differentiation and subsequently degraded into hydrophilic amino acids, their metabolites, and ions that contribute to moisture retention in the SC. Expression of filaggrin and its hydrolysis into these natural moisturizing factors (NMFs) are influenced by the SC microenvironment, including local pH, external humidity, and transepidermal water loss (TEWL) (O’Regan et al., 2008). The reduction of filaggrin detected by proteome analysis in LI skin/grafts may be attributed to an increased hydrolysis into NMFs to compensate for TEWL.
In contrast, the number of unique loricrin peptides increased in LI in comparison to normal skin/grafts. Loricrin becomes extensively cross-linked to numerous CE-components by different TGs (Candi et al., 2005). Insufficient intermolecular oligomerization by TG1 could result in an enhanced accessibility of trypsin cleavage sites during sample processing resulting in an increase of unique peptides. Likewise, a marked increase of unique peptides of keratinocyte proline-rich protein (KPRP) was found in LI skin/grafts. KPRP expression was markedly increased in psoriatic lesions suggesting that it could be extensively cross-linked by TG1 like small proline-rich proteins (SPRs) or some late cornified envelope proteins (LCEs) (Lee et al., 2005).
Interestingly, we did not observe changes in the number of unique peptides of involucrin, one of the first proteins to be cross-linked to initiate CE-assembly by forming a monomolecular layer adjacent to the cell membrane (Candi et al., 2005). We speculate that this location does not alter its accessibility to trypsin.
Desmoplakin, a structural cytoskeleton-constituent which participates in keratinocyte adhesion, and other desmosomal/corneodesmosomal proteins like desmoglein 1, desmocollin 1, junction plakoglobin and corneodesmosin display a marked increase in unique peptide numbers in LI skin/grafts. From a clinical perspective it is likely that increased expression of desmosomal adhesion molecules results in increased “stickiness” of corneocytes and thus explains why in LI the scales are large and plate-like, since normal, invisible desquamation did not occur. The rigid adhesion of cells is thought to be a compensatory effect to prevent TEWL (Steinert et al., 1998).
We conclude that the LI skin-humanized mouse model faithfully recapitulates the human disease phenotype and concomitant molecular changes and can be used as an excellent tool for testing of novel therapeutic approaches for this up-to-now untreatable genodermatosis.
MATERIALS AND METHODS
Patients
The study was approved by the Institutional Review Board of the University Hospital of Münster. All patients enrolled gave their written, informed consent. Biopsies were taken under local anesthesia. Patients were recruited in our specialized out patient clinic and had previously been characterized by clinical, immunohistologic, ultrastructural and biochemical means.
Animals
All animal studies have been approved by Centro de Investigaciones Energéticas Medioambientales y Tecnológicas’s Institutional Review Board and all experimental procedures were conducted according to European and Spanish laws and regulations.
Nude (nu/nu, NMRI background) mice were purchased from Elevage-Janvier (France) and housed in pathogen-free conditions at the Centro de Investigaciones Energéticas Medioambientales y Tecnológicas Laboratory Animals Facility (Spanish registration number 28079-21 A). Animals were housed in individually ventilated type II cages, with 25 air changes per hour and 10 KGy γ-irradiated soft wood pellets as bedding.
Genetic analysis
DNA was extracted from peripheral blood leukocytes using standard procedures. The translated exons 2–15 of TGM1 including the exon-intron boundaries were amplified by polymerase chain reaction (PCR) using intronic primers designed according to the genomic sequence of TGM1 (NC_000014.7). Reactions were performed with 0.2 μM of each primer, 0.1 mM dNTPs and 0.4 U Taq DNA polymerase in 30 cycles of 10 s at 94°C, 10 s at annealing temperature depending on the respective primer pair and 10 s at 72°C as described earlier (Hennies et al., 1998).
Primary Cultures of normal and transglutaminase 1-deficient human Keratinocytes and Fibroblasts
Primary keratinocytes and fibroblasts were obtained by enzymatic digestion from 3–5 mm punch biopsies according to a standard protocol described previously (Rheinwald and Green, 1995) with minor changes. Briefly, biopsies were incubated over night at 4°C in 0.5 mg/ml protease X (Sigma, Munich, Germany). Epidermal sheets were peeled off the dermis and incubated in 0.25% trypsin/0.02% EDTA (PAA, Pasching, Austria) for 15 min at 37°C in order to achieve single-cell suspension. Trypsin activity was stopped with FCS containing medium. The suspension was centrifuged at 1000×g for 5 min and cells were resuspended in serum-free culture medium (KSFM) supplemented with 10 ng/ml epidermal growth factor (EGF), 50 mg/ml bovine pituitary extract (BPE) (all from Invitrogen, Darmstadt, Germany), 2 mM glutamine and 100 U/ml penicillin, and 100 mg/ml streptomycin (PAA, Pasching, Austria).
To obtain fibroblasts, the dermis was incubated for 2–4 h in 0.5 mg/ml collagenase IA (Sigma, Taufkirchen, Germany). After centrifugation cells were resuspended in DMEM supplemented with 10% FCS (Invitrogen, Darmstadt, Germany), 2 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin. Medium was renewed every 2–3 days and cells were usually passaged at 80% confluence.
For bioengineered skin preparation primary keratinocytes were cultured on a feeder layer of lethally irradiated (X-ray; 50 Gy) 3T3-J2 cells as described previously (Bernerd et al., 2001).
Bioengineered Skin Preparation and Grafting to Immunodeficient Mice
As the dermal scaffold of the bioengineered skin a fibrin-matrix populated with live fibroblasts was prepared following a procedure previously described (Del Rio et al., 2002; Llames et al., 2004). In brief, a fibrinogen solution (cryoprecipitate of human blood donors) was added to DMEM supplemented with 10% fetal calf serum (ratio 1:4) containing 5× 105 dermal fibroblasts and 500 IU of bovine aprotinin (Trasylol, Bayer, West Haven, CT, USA). Immediately afterwards, 0.025 mM CaCl2 with 11 IU of bovine thrombin (Sigma, St. Louis, MO, USA) was added. Finally, the mixture was poured in 6 well culture plates and allowed to solidify at 37°C. Keratinocytes (1–5× 105 cells per well) were seeded on the fibrin-matrix to form the epidermal layer. When confluent, bioengineered skins were grafted onto immunodeficient mice under sterile conditions using 6 week old female nude mice.
In brief, full thickness 35 mm diameter circular wounds were created on the dorsum of the mice and bioengineered equivalents were placed orthotopically on the wound. The removed mouse skin was devitalized by three cycles of freezing and thawing and used as a biological bandage, fixed with sutures to protect and hold the skin substitute in place during the take process. Dead mouse skin was sloughed off, generally within 4–6 weeks after grafting, and regenerated human skin became visible.
Ultrastructural analysis and Histology of engrafted human skin
All specimens were fixed for at least 2 h at room temperature (RT) in 3% glutaraldehyde, cut into pieces of approximately 1 mm3, post-fixed 1% osmium tetroxide, rinsed in water, dehydrated through graded ethanol solutions, transferred in propylene oxide, and embedded in epoxy resin (glycidether 100). Semithin and ultrathin sections were cut with an ultramicrotome (Reichert Ultracut E, Depew, NY, USA.). Semithin sections were stained with methylene blue. Ultrathin sections were treated with uranyl acetate and lead citrate, and examined with an electron microscope (Philips EM 400, FEI Eindhoven, The Netherlands).
For immunoperoxidase staining, formalin-fixed paraffin sections (4–6 μm) were prepared using standard protocols. Sections were incubated overnight at 4°C with a human-specific primary antibody against involucrin (Sigma, Taufkirchen, Germany) to visualize the human origin of the regenerated skin.
In situ monitoring of transglutaminase-activity
In situ monitoring of transglutaminase activity on cryosections was performed as described elsewhere (Oji et al., 2006) using biotinyl cadaverin (Molecular Probes, Leiden, The Netherlands) as a substrate.
Immunofluorescent staining
Unfixed or acetone fixed cryosections were incubated for 45 min at RT with the following primary antibodies: transglutaminase 1 (Zedira, Darmstadt, Germany), loricrin, keratin 10 (both from Convance, Emeryville, CA, USA), involucrin, collagen type VII (both from Sigma, Taufkirchen, Germany), filaggrin (Novocastra Laboratories Ltd, Newcastle, UK), integrin α6 (Chemicon, Hofheim, Germany).
Skin samples for Proteomic Analysis
For proteomic analysis, epidermis was isolated using the heating method described previously (Baumberger et al., 1942) with minimal modifications. In brief, biopsies were incubated for exactly 2.5 min in sterile H2O at 55°C and transferred into ice cold PBS for exactly 3 min. Epidermis was peeled off the dermis and was freezed in liquid nitrogen. Duplicate samples from normal control skin, LI skin and normal grafts were from different individuals, the triplicate samples from LI grafts were independently cultured from the same individual (patient 1).
Proteomic sample preparation
Samples were extracted 3–5 times in 2% SDS in 0.1 M sodium phosphate buffer (pH 7.8) for 5 min in a boiling water bath and recovered by centrifugation. Samples were resuspended in 0.4 ml 2% SDS in phosphate buffer containing 25 mM dithioerythritol (DTE) and gently stirred for 45 min. After addition of iodoacetamide to a final concentration of 50 mM, stirring was continued for 45 min in the dark. Proteins were precipitated by addition of 1 ml of ethanol, rinsed with 70% ethanol, then fresh 0.1 M ammonium bicarbonate and resuspended in 0.4 ml of the bicarbonate adjusted to 10% in acetonitrile. The proteins were digested for 3 days at RT by addition of methylated trypsin (Worthington Biochemical Corporation, Lakewood, NJ, USA) (Rice et al., 1977) to ≈1% by weight at daily intervals.
Mass Spectrometry
Digested peptides were analyzed using LC-MS/MS on an LTQ with Michrom Paradigm LC and CTC Pal autosampler. Peptides were directly loaded onto an Agilent ZORBAX 300SB C18 reverse-phase trap cartridge which, after loading, was switched in-line with a Michrom Magic C18 AQ 200 μm × 150 mm nano-LC column connected to a Thermo-Finnigan LTQ iontrap mass spectrometer through a Michrom Advance Plug and Play nanospray source. The nano-LC was used with a binary solvent gradient; buffer A was composed of 0.1% formic acid and buffer B composed of 100% acetonitrile. The 120 min gradient consisted of the steps 2–35% buffer B in 85 min, 35–80% buffer B in 23 min, hold for 1 min, 80–2% buffer B in 1 min, then hold for 10 min, at a flow rate of 2 μl/min for the maximum separation of tryptic peptides. MS and MS/MS spectra were acquired using a top 10 method, where the top 10 ions in the MS scan were subjected to automated low energy CID. An MS survey scan was obtained for the m/z range 375–1400, and MS/MS spectra were acquired using the three most intense ions from the survey scan. An isolation mass window of 2 Da was used for the precursor ion selection, and normalized collision energy of 35% was used for the fragmentation. A 2 min duration was used for the dynamic exclusion.
Protein Identification
Tandem mass spectra were extracted with Xcalibur version 2.0.7. All MS/MS samples were analyzed using X! Tandem (www.thegpm.org; version TORNADO (2010.01.01.4)). X! Tandem was set up to search the IPI human database (version 3.83, 91,000 entries) assuming the digestion enzyme trypsin. X! Tandem was searched with a fragment ion mass tolerance of 0.40 Da and a parent ion tolerance of 1.8 Da. Iodoacetamide derivative of cysteine was specified in X! Tandem as a fixed modification. Deamidation of asparagine and glutamine, oxidation of methionine and tryptophan, sulfone of methionine and acetylation of the N-terminus were specified in X! Tandem as variable modifications. Scaffold (version Scaffold_3_00_08, Proteome Software, Inc., Portland, OR, USA) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95% probability as specified by the Peptide Prophet algorithm (Keller et al., 2002). Protein identifications were accepted if they could be established at greater than 99% probability and contained at least 2 identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al., 2003) Proteins that contained similar peptides and could not be distinguished by MS/MS analysis alone were grouped for parsimony. Numbers of unique peptides, which permit semi-quantitative comparisons of parallel samples (Ishihama et al., 2005), were tabulated as a basis for selecting prominent proteins for further analysis.
Supplementary Material
Acknowledgments
This work was supported by the Bundesministerium für Bildung und Forschung as part of the Network for rare diseases NIRK [grant numbers: 01GM0901 and 01GM0902]; the Foundation for Ichthyosis and Related Skin Types (F.I.R.S.T.); the National Institutes of Health (NIH) [grant number: P42 ES004699]; and the Selbsthilfe Ichthyose e.V. FL was supported in part by the Institudo the Salud Carlos III (ISCIII) [Grant number: PI081054], MDR was supported by the Ministerio de Ciencia y Innovación (MICINN) [Grant number: SAF2010-16976]. HCH was further supported by the Deutsche Forschungsgemeinschaft (DFG) [Grant number: HE3119/5-1] and Köln Fortune [Grant number: 79/2011].
We are grateful to all the patients and other probands who participated in the study. The excellent technical assistance of Blanca Duarte, Androniki Kolovou, Marc Nätebus, Anette Peffekoven, and the Proteomics Core Facility (Brett Phinney, Rich Eigenheer), University of California, Davis is gratefully acknowledged. Special thanks to Mrs. Brigitte Willis.
ABBREVIATIONS
- ARCI
autosomal recessive congenital ichthyosis
- TG1
transglutaminase 1
- LI
lamellar ichthyosis
- CE
cornified envelope
- DEJ
dermoepidermal junction
- TEWL
transepidermal water loss
- NMF
natural moisturizing factor
- KPRP
keratinocyte proline-rich protein
- SPR
small proline-rich protein
- LCE
late cornified envelope protein
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
References
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6(4):328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Choate KA, Khavari PA. Direct cutaneous gene delivery in a human genetic skin disease. Hum Gene Ther. 1997;8(14):1659–65. doi: 10.1089/hum.1997.8.14-1659. [DOI] [PubMed] [Google Scholar]
- Choate KA, Medalie DA, Morgan JR, et al. Corrective gene transfer in the human skin disorder lamellar ichthyosis. Nat Med. 1996;2(11):1263–7. doi: 10.1038/nm1196-1263. [DOI] [PubMed] [Google Scholar]
- Farasat S, Wei MH, Herman M, et al. Novel transglutaminase-1 mutations and genotype-phenotype investigations of 104 patients with autosomal recessive congenital ichthyosis in the USA. J Med Genet. 2009;46(2):103–11. doi: 10.1136/jmg.2008.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García M, Larcher F, Hickerson RP, et al. Development of skin-humanized models of Pachyonychia Congenita. J Invest Dermatol. 2011;131:1053–60. doi: 10.1038/jid.2010.353. [DOI] [PubMed] [Google Scholar]
- Kuramoto N, Takizawa T, Takizawa T, et al. Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase 1. J Clin Invest. 2002;109(2):243–50. doi: 10.1172/JCI13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Jang S, Lee JS, et al. Molecular cloning and expression of human keratinocyte proline-rich protein (hKPRP), an epidermal marker isolated from calcium-induced differentiating keratinocytes. J Invest Dermatol. 2005;125(5):995–1000. doi: 10.1111/j.0022-202X.2005.23887.x. [DOI] [PubMed] [Google Scholar]
- Matsuki M, Yamashita F, Ishida-Yamamoto A, et al. Defective stratum corneum and early neonatal death in mice lacking the gene for transglutaminase 1 (keratinocyte transglutaminase) Proc Natl Acad Sci. 1998;95(3):1044–9. doi: 10.1073/pnas.95.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oji V, Traupe H. Ichthyoses: differential diagnosis and molecular genetics. Eur J Dermatol. 2006;16(4):349–59. [PubMed] [Google Scholar]
- O’Shaughnessy RF, Choudhary I, Harper JI. Interleukin-1 alpha blockade prevents hyperkeratosis in an in vitro model of lamellar ichthyosis. Hum Mol Genet. 2010;19:2594–605. doi: 10.1093/hmg/ddq145. [DOI] [PubMed] [Google Scholar]
- O’Regan GM, Sandilands A, McLean WH, et al. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;124:R2–6. doi: 10.1016/j.jaci.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Pigg M, Gedde-Dahl T, Jr, Cox D, et al. Strong founder effect for a transglutaminase 1 gene mutation in lamellar ichthyosis and congenital ichthyosiform erythroderma from Norway. Eur J Hum Genet. 1998;6:589–96. doi: 10.1038/sj.ejhg.5200224. [DOI] [PubMed] [Google Scholar]
- Steinert PM, Candi E, Kartasova T, et al. Small proline-rich proteins are cross-bridging proteins in the cornified cell envelopes of stratified squamous epithelia. J Struct Biol. 1998;122(1–2):76–85. doi: 10.1006/jsbi.1998.3957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.