Abstract
Malaysian tualang honey possesses strong antioxidant and anti-inflammatory properties. Here, we evaluated the effect of tualang honey on early biomarkers of photocarcinogenesis employing PAM212 mouse keratinocyte cell line. Keratinocytes were treated with tualang honey (1.0%, v/v) before a single UVB (150 mJ/cm2) irradiation. We found that treatment of tualang honey inhibited UVB-induced DNA damage, and enhanced repair of UVB-mediated formation of cyclobutane pyrimidine dimers (CPDs) and 8-oxo-7, 8-dihydro-2′-deoxyguanosine (8-oxodG). Treatment of tualang honey inhibited UVB-induced nuclear translocation of NF-κB, and degradation of IκBα in murine keratinocyte cell line. Treatment of tualang honey also inhibited UVB-induced inflammatory cytokines and inducible nitric oxide synthase protein expression. Furthermore, treatment of tualang honey inhibited UVB-induced COX-2 expression and PGE2 production. Taken together, we provide evidence that treatment of tualang honey to keratinocytes affords substantial protection from the adverse effects of UVB radiation via modulation in early biomarkers of photocarcinogenesis and provide suggestion for its photochemopreventive potential.
Introduction
Ultraviolet (UV) B radiation (290-320 nm) induced DNA damage is one of the earliest molecular events in the development of skin cancers (1-3). UVB causes DNA damage, predominantly in the form of cyclobutane pyrimidine dimers (CPD) and 6-4 photoproducts (6-4 PP). Nucleotide excision repair (NER) removes DNA damage by two distinct pathways, transcription coupled repair (TCR) and global genome repair (GGR) of DNA (4). Reactive oxygen species, which are generated endogenously by cellular oxygen metabolism or exogenously by UV, are environmental mutagens, and produce various types of DNA damage. 8-oxo-dG is one type of oxidative DNA damage that can result in stable mutations. In mammalian cells, the Ogg1 gene encodes 8-oxo-dG-DNA glycosylase, a repair enzyme, which removes the oxidized base from DNA. Base excision repair (BER) is the most active process for correcting these DNA alterations that arise from the inherent instability of DNA. A growing body of evidence indicates that components of NER are also involved in repair of oxidative damage (5).
UVB irradiation evokes a signalling response through two different pathways: one dependent on and the other independent of DNA damage. On one hand, DNA damage activates the tumor suppressor p53, which induces cell-cycle arrest and the concurrent processes of DNA repair and apoptosis (6, 7). On the other hand, UVB irradiation activates the transcription factor NF-κB (8). Oxidative stress in epidermal cells plays a crucial role in the photodamage pathway, because it contributes to DNA damage (9-11), activation of MAP kinases (12, 13), apoptosis (14, 15), and secretion of inflammatory cytokines (16). UVB-induced ROS are responsible for skin inflammation, gene mutation and immunosuppression, photoageing and skin cancer (17, 18).
The therapeutic role of honey in the treatment of various ailments has been receiving considerable attention recently, and its therapeutic value has been partly attributed to its antioxidant properties (19, 20). Malaysian tualang honey (TH) is collected from the combs of Asian rock bees (Apis dorsata), which build their hives high up in the tualang tree (Koompassia excelsa). Tualang honey is used commonly as a medicinal product (21, 22) and as food in Malaysia. Recent data suggest that the elevated free-radical scavenging and antioxidant activity observed in tualang honey is due to the increased level of phenolic compounds (23). In addition to its antibacterial, anticarcinogenic, and anti-inflammatory properties, its antioxidant properties make it important for human nutrition and health.
The objective of this study was to investigate the effect of tualang honey on early biomarkers of UVB induced damage processes employing murine PAM212 keratinocyte cell line model.
Materials and Methods
Cell line and reagents
Murine epidermal keratinocyte cell line PAM212 was obtained from Lonza Walkersville Inc. (Walkersville, MD). Tualang Honey used in this study was supplied by Federal Agricultural Marketing Authority (FAMA), Malaysia. The primary antibodies for COX-2, iNOS, EP4, β-actin and the secondary antibodies, horseradish peroxidase–linked anti-mouse IgG and anti-rabbit IgG, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for p65 and IkBα were procured from Cell Signaling Technology Inc. (Danvers, MA). Anti-8-oxo-dG antibody was purchased from Millipore (Billerica, MA) and anti-CPD antibody was purchased from Kamiya biomedical company (Tukwila, WA). Secondary Alexafluor 488 antibody and Alexafluor 594 antibody was purchased from Invitrogen (Carlsbad, CA). Anti-EP2 antibody and PGE2 ELISA kit were purchased from Cayman chemical company (Ann Arbor, MI). ELISA kits for IL-1β, IL-6 and TNF-α were purchased from Invitrogen (Carlsbad, CA). Bay 11-7082, celecoxib and aminoguanidine hemisulfate were purchased from Sigma (St. Louis, MO). The DC protein assay kit was obtained from Bio-Rad Laboratories (Hercules, CA) and the enhanced chemiluminescence western blotting detection reagents were purchased from Amersham Pharmacia Biotech (Piscataway, NJ).
Cell culture and preparation of tualang honey
Murine epidermal keratinocyte cell line PAM212 was cultured as monolayer in DMEM supplemented with 10% heat inactivated fetal bovine serum, 100 μg/ml penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA), and maintained in a humidified atmosphere of 95% air and 5% CO2 at 37°C. TH was initially dissolved in serum-free culture medium at a final concentration of 10% (v/v) and the mixture was filter-sterilized using 0.22 μm syringe filter unit (Millipore, USA). The honey mixture was freshly prepared before adding to cell cultures.
UVB irradiation
The source of UVB radiation was a band of four UVB lamps (Daavlin, UVA/UVB Research Irradiation Unit, Bryan, OH, USA) equipped with an electronic controller to regulate UV dosage at a fixed distance of 24 cm from the lamps to the surface of the cell culture plates. The majority of the resulting wavelengths were in the UVB (290–320 nm; about 80%) and UVA (about 20%) range and the peak emission was recorded at 314 nm.
Treatment of PAM212 keratinocytes
After determination of cellular viability by using MTT assay we have selected 1.0% dose of tualang honey for further study. To determine the effects of TH on UVB-induced markers of DNA damage or activation of signaling pathways, the sub-confluent (70–80%) cells were treated with 1.0% v/v tualang honey for 24h before UVB irradiation. In some sets of experiments, we also used pharmacological inhibitors like Bay 11-7082 (NF-kB inhibitor, 20 μM), Celecoxib (COX-2 inhibitor, 20 μM) and Aminoguanidine hemisulfate (iNOS inhibitor, 1.0 mM) in culture to demonstrate the essentiality and specificity of cellular changes mediated by honey. Thereafter, cells were washed three times with PBS to make them TH free and then cells in fresh 1.0 ml PBS were exposed to 150 mJ/cm2 of UVB. After UVB exposure, PBS was replaced with culture medium, and cells were again incubated. Cells were harvested at desired time points, either for preparation of lysates or CPD and 8-oxo-G staining.
MTT Assay for Cellular Viability
MTT assay is a colorimetric assay for measuring the activity of the cellular reductase enzymes in viable cells that reduce yellow 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma, St Louis, MO) substrate to purple formazan crystals. The effect of the tualang honey on cell viability was determined by MTT assay. Briefly, 1 × 104 cells/well in 1.0 ml of complete culture medium was plated in 96-well culture plates. After overnight incubation, the cells were treated with varying concentrations of tualang honey (0, 0.1, 0.2, 0.5, 1.0, 2.0, 3.0 and 5.0 %) dissolved in fresh culture medium supplemented with 2.0% fetal bovine serum and further incubated for at 24, 48 and 72 h at 37°C in a humidified chamber. At the end of the stipulated period, MTT (50 μl of 50 μg/ml stock) was added into each well and incubated for 3 h. The 96-well plate consisting of the cells was centrifuged at 280Xg for 5 min at 4°C. The MTT solution was removed from the wells by aspiration and the resulting formazan was then dissolved in 150 μl of DMSO and 200μl transferred into a 96 well-plate. The absorbance of the formazan in each well was recorded at 540 nm using a microplate reader (Bio-Rad; Hercules, CA, USA).
Immunocytochemical detection of CPD-positive and 8-oxo-dG-positive cells
UV-induced DNA damage in the form of CPD+ cells were detected using a protocol described previously with some modifications (24). Briefly, 1 × 104 cells were plated on round cover slips placed in a 10 mm cell culture dish (Nunc, Denmark). The cells were allowed to adhere on coverslips for overnight and the culture medium was replaced with fresh assay medium supplemented with 2.0% fetal bovine serum. The cells were treated with 1.0 % of tualang honey for 24 h before UVB irradiation. Thereafter, cells were washed three times with PBS and then cells of UVB and UVB+TH group were exposed to the 150 mJ/cm2 doses of UVB in 1.0 ml of PBS. After UVB exposure, PBS was replaced with culture medium, and cells were again incubated in dark for 30 min to make sure that CPD and 8-oxo-dG were generated in UVB irradiated cells. Thereafter, cells were fixed in 4.0% formaldehyde for 10 min and washed with PBS and subsequently permeabilized with 0.5% Triton-X 100 for 5 min on ice. DNA denaturation was performed by treating the cells with 2.0 M HCl at room temp for 20 min. after that cells were washed with PBS and incubated in blocking buffer, 10% anti-goat normal serum, at room temp for 1 h. Cells were then incubated with either CPD-specific or 8-oxo-G-specific mice monoclonal antibody for 1 h at room temperature and after washing the bound anti-CPD or 8-oxo-G antibody was detected by incubation with anti-mouse secondary IgG antibody Alexa Fluor 488 and Alexa Flour 594 respectively for 45 min. After washing with PBS, the cells were counterstained with DAPI. CPD+ and 8-oxo-G+ cells were counted under Olympus BX41 microscope at 5–6 different fields and the data were presented as the mean of the percentage of CPD+ and 8-oxo-dG+ cells±SD from at least three separate experiments.
Preparation of cell lysates and western blotting
Either whole cell lysates or nuclear lysates were prepared from control, tualang honey, UVB, and UVB+tualang honey treated cells by using cytoplasmic and nuclear extraction kit of Active Motif (Carlsbad, CA). For western blot analysis 50-80μg protein was loaded in each well and resolved on 8, 10 and 12% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes. Membranes were incubated in blocking buffer for 2 h and then incubated with the primary antibodies in blocking buffer for 2 h at room temperature or overnight at 4°C. The membrane was then washed with TBS-T and incubated with secondary antibody conjugated with horseradish peroxidase. Protein bands were visualized using the enhanced chemiluminescence detection system (Amersham Life Science, Inc., Piscataway, NJ). To verify equal protein loading and transfer of proteins from gel to membrane, the blots were stripped and re-probed for β-actin or histone H3. The band density was analyzed using Image J software provided by NIH and the values were normalized to the β-actin band density.
PGE2 immunoassay for quantitation of prostaglandin E2
The analysis of PGE2 in cell homogenates was performed using the Cayman PGE2 Enzyme Immunoassay Kit (Cayman Chemicals) following the manufacturer's instructions. Homogenates were centrifuged and the supernatants were collected and analyzed for PGE2 concentration according to the manufacturer's instructions.
Measurement of inflammatory cytokines level
The levels of inflammatory cytokines were assayed in triplicate by using standard protocols of enzyme linked immunosorbent assay (ELISA) (Invitrogen, Carlsbad, CA). The plates were incubated with anti-IL-1β, IL-6 and TNFα antibody (25μg/ml; 30μl/well) at 4°C for overnight and then washed with PBS before adding the cell suspensions. Supernatants were harvested 24 h later after giving UVB radiation. The levels of cytokines were expressed as picograms per millilitre (pg/mL).
Statistical analysis
Statistical analysis was done using ANOVA followed by post hoc multiple comparison tests. The chemopreventive effect of tualang honey was considered significant if p < 0.05.
Results
Tualang honey does not inhibit cell growth of PAM212 murine keratinocytes
The treatment of PAM212 cells with 0.1-5.0% tualang honey did not result in significant cell death even after 72 h incubation (Fig. 1). However a maximum cell death of only about 30% was observed in 5.0% and 48 h TH treated cells. Whereas further incubation of cells for a longer period i.e.72 h results in a decrease in cell death. Since up to 1.0% concentration of tualang honey, more than 90% of the PAM212 cells survived, this is the reason to select this safer dose for the assessment of chemopreventive potential of tualang honey on PAM212 murine keratinocytes.
Figure 1.
Effect of tualang honey on the viability of murine PAM212 keratinocytes. Dose- and time-dependent effect of tualang honey on the proliferation of PAM212 keratinocytes was determined using MTT assay as described in Methods section. The values are represented as the percentage cell death. The data represents the mean ± SD of three independent experiments each conducted in triplicate.
Tualang honey reduces or repair DNA damage in UVB-exposed PAM212 keratinocytes
To determine whether tualang honey reduces or repair UVB-induced DNA damage in PAM212 keratinocytes, we evaluated the effect of tualang honey on UVB-induced DNA damage in the form of CPDs and 8-oxo-dG formation. For this purpose, cells were exposed to UVB (150 mJ/cm2) with or without the treatment with tualang honey. Cells were harvested either after 30 min or 24 h after UVB irradiation and subjected to the analysis of CPD-positive cells or 8-oxo-G positive cells following cytostaining using CPD-specific or 8-oxo-dG specific monoclonal antibody. CPD-positive cells were not detectable in both non-UVB-irradiated cells and tualang honey alone treated cells whereas in UVB-irradiated cells it was largely detected. When the cells were analyzed for CPDs (Fig. 2A) and 8-oxo-dG (Fig. 2B) immediately after UVB-exposure, there was a significant difference in the cells treated with or without tualang honey in terms of the number of CPD-positive cells (Fig. 2A) and 8-oxo-dG positive cells (Fig. 2B). When the cells were analyzed 24 h after UVB irradiation, the number and intensity of staining of CPD-positive cells (Fig. 2A) and 8-oxo-dG positive cells (Fig. 2B) was markedly decreased in tualang honey treated cells compared to the cells which were not treated with tualang honey but exposed to UVB, suggesting that tualang honey might accelerate the repair of UVB-induced CPDs and 8-oxo-dG in PAM212 keratinocytes.
Figure 2.
Inhibitory effect of tualang honey on UVB-induced formation of cyclobutane primidine dimers and 8-oxo-7, 8-dihydro-2′-deoxyguanosine in PAM212 keratinocytes. PAM212 were exposed to UVB (150 mJ/cm2) and treated with tualang honey as described in the Methods section. Immunocytochemical staining for cyclobutane pyrimidine dimers (CPDs) (A), and 8-oxodG (C) was performed using appropriate antibodies. Representative pictures are shown. The number of CPD positive cells (B) and 8-oxodG positive cells (D) after immunostaining were counted in five different areas of the sections under a microscope. The numbers of CPD and 8-oxodG positive cells are represented as percent of CPD and 8-oxo-dG positive cells, respectively. The data represent the mean ± SD of cells in triplicate (***=P < 0.001 and *= P < 0.05 vs control; ###=P < 0.001 and ##=P < 0.01 vs UVB). The representative micrographs are shown from three independent experiments. Bar=50uM.
Treatment of tualang honey inhibits UVB-induced activation of NF-κB in PAM212 keratinocytes
Nuclear factor kappa B is present in the cytosol as a heterodimer usually consisting of its p50 and p65 subunits bound to its inhibitory proteins IκB. One of the critical events in NF-κB activation is its dissociation from inhibitory protein IκB (25). We determined whether treatment of tualang honey inhibits UVB-mediated activation of NF-κB. UVB irradiation resulted in degradation of IκBα protein in the cytosol. Our data clearly demonstrated that treatment of tualang markedly inhibited UVB-mediated degradation of IκBα (Fig. 3). We then investigated whether treatment of tualang honey inhibited UVB-induced activation and nuclear translocation of NF-κB/p65 in PAM212 keratinocytes. Employing Western blot analysis, we found that UVB exposure resulted in the activation and nuclear translocation of NF-κB/p65, which was markedly inhibited by tualang honey (Fig. 3) and this is in accordance with well known inhibitory potential NF-kB inhibitor Bay 11-7082. Histone H3 was used as a loading control for NF-κB.
Figure 3.
Inhibitory effect of tualang honey on UVB-induced nuclear translocation of p65 and degradation of IκBα in PAM212 keratinocytes. Cells were treated with either tualang honey or Bay 11-7082 (20 μM) and exposed to UVB (150 mJ/cm2) as described in the Methods section. Cytosolic and nuclear protein lysates were prepared and Western blot analysis was performed to determine the protein expression level of p65 and IkB-α. Equal loading was confirmed by stripping the Western blot and re-probing it for β-actin or histone H3. The representative blots are shown from three independent experiments (***=P < 0.001, **= P < 0.01 and *= P < 0.05 vs control; ##=P < 0.01 vs UVB).
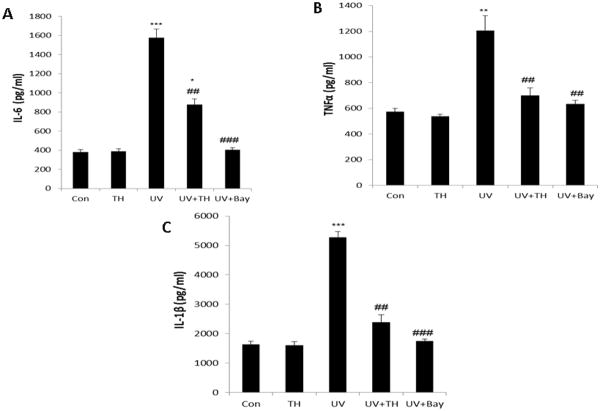
Tualang honey treatment inhibits UVB-induced activation of inflammatory cytokines
Keratinocytes are the major target of UVR and play a central role in the inflammatory and immune modulatory changes observed after UV exposure, at least partly via the UV-induced release of cytokines (IL-1, IL-6, IL-8, IL-10, GM-CSF, TNF-α) (26). Our data shows that tualang honey treatment of PAM212 keratinocytes resulted in decrease in proinflammatory cytokines IL-1β, IL-6, and TNF-α by ELISA (Fig. 4) which could be due to the inhibition of NF-kB that resembles with well known NF-kB inhibitor Bay 11-7082. Inhibition of UVB-induced inflammatory cytokines signifies the anti-inflammatory potential of tualang honey.
Figure 4.
Treatment of tualang honey reduces UVB-induced secretion of pro-inflammatory cytokines by PAM212 keratinocytes. Cells were treated with either tualang honey or Bay 11-7082 (20 μM) and exposed to UVB (150 mJ/cm2) as described in the Methods section. Cell supernatant was collected for estimation of cytokines IL-1β, IL-6, and TNF-α by ELISA. The representative data are shown from three independent experiments (***=P < 0.001, **= P < 0.01 and *= P < 0.05 vs control; ##=P < 0.01vs UVB).
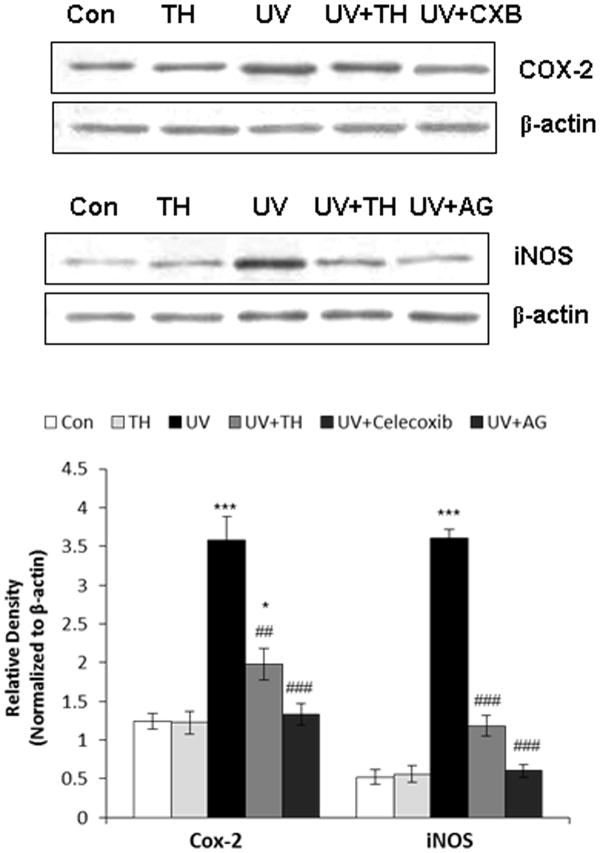
Tualang honey inhibits UVB-induced iNOS and COX-2 protein expression in PAM212 keratinocytes
Exposure to UVB radiation induces the IKKβ activity, phosphorylates extracellular signal-regulated kinase (ERK) and p38 MAP kinase, activates NF-κB and elevates the expression iNOS in mouse skin. Pharmacological inhibition of UVB-induced IKKβ activity results in the inhibition of ERK and p38 MAP kinase phosphorylation, downregulation of NF-κB activation, and suppression of iNOS expression in hairless mouse skin (27). In order to determine whether tualang honey could downregulate UVB-induced iNOS production, whole cell extracts were analysed by Western blotting for iNOS. Our data shows that tualang honey treatment of PAM212 keratinocytes resulted in decreased expression of inflammatory marker iNOS (Fig. 5) that was confirmed by using a well-known iNOS inhibitor Aminoguanidine hemisulfate. Studies have demonstrated that the expression of proinflammatory enzyme COX-2 is induced by UVB exposure (28). Therefore, we determined the effect of tualang honey on UVB-induced epidermal COX-2 protein expression. Western blot analysis revealed that UVB exposure to PAM212 keratinocytes resulted in a marked increase in COX-2 protein expression compared with control group. Whereas prior treatment of tualang honey significantly decrease the COX-2 protein level (Fig. 4). Celecoxib (COX-2 inhibitor) was used to confirm the inhibitory potential of taulang honey against COX-2.
Figure 5.
Inhibitory effect of tualang honey on UVB-induced cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) protein expression in PAM212 keratinocytes. Cells were treated with either tualang honey or with celecoxib (CXB, 20 μM) and aminoguanidine (AG, 1.0 mM) and exposed to UVB (150 mJ/cm2) as described in the Methods section. Western blot analysis was performed to determine the protein expression using COX-2 and iNOS specific antibodies. Equal loading was confirmed by stripping the Western blot and reprobing it for β-actin. The representative blots are shown from three independent experiments (***=P < 0.001, **= P < 0.01 and *= P < 0.05 vs control; ###=P < 0.001 and ##=P < 0.01 vs UVB).
The inhibitory effect of tualang honey on COX-2 is associated with inhibition of UVB-induced PGE2 production in PAM212 keratinocytes
As the COX-2 metabolite, PGE2, has been implicated in COX-2-mediated effects; we determined the levels of PGE2 in the tualang honey-treated cells. Our results revealed that treatment with tualang honey for 24 h resulted in significant inhibition of PGE2 production in PAM212 cells (Fig. 6A), suggesting that tualang honey-induced reduction in PGE2 production is associated with an inhibitory effect of the tualang honey on COX-2 in these cells which was further confirmed by using celecoxib.
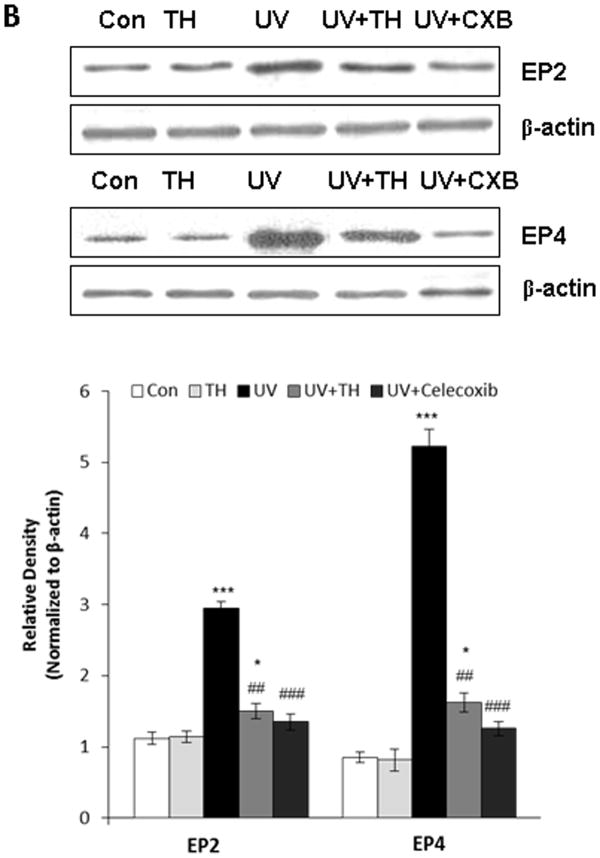
Figure 6.
Treatment of PAM212 keratinocytes with tualang honey decreases UVB-induced expression level of PGE2 and PGE2 receptors, EP2 and EP4. (A). Treatment of PAM212 keratinocytes with tualang honey decreases UVB-induced expression level of PGE2 as measured by ELISA. (B) Treatment of PAM212 keratinocytes with tualang honey also decreases UVB-induced expression level of PGE2 receptors EP2 and EP4. Cells were treated with either tualang honey or celecoxib (CXB, 20 μM) and exposed to UVB (150 mJ/cm2) as described in the Methods section. Western blot analysis was performed to determine the levels of EP2 and EP4 receptors using EP2- and EP4-specific antibodies. Equal loading was confirmed by stripping the Western blot and reprobing it for β-actin. The representative blots are shown from three independent experiments (***=P < 0.001 and *= P < 0.05 vs control; ##=P < 0.01 vs UVB).
Tualang honey decreases the levels of UVB-induced PGE2 receptors in PAM212 keratinocytes
As it has been shown that PGE2 manifests its biological activity via four known G-protein-couples receptors (i.e. EP1–EP4) (29), we determined the effect of tualang honey on the UVB induced levels of PGE2 receptors in PAM212 keratinocytes. Western blot analysis revealed that treatment of PAM212 keratinocytes with tualang honey for 24 h resulted in a significant attenuation in the levels of EP2 and EP4 (Fig. 6B).
Discussion
The UVB component of solar UV radiation is believed to be the major cause of the variety of cutaneous disorders including skin cancers (1-3, 30, 31). Antioxidants from natural plant products consumed by the human population have gained considerable attention as photoprotective and photochemopreventive agents against skin cancers (32). Antioxidants, which are abundant in natural honey, are free-radical scavengers that either reduce the formation of or neutralize free radicals. It has been reported that the elevated free-radical scavenging and antioxidant activity observed in tualang honey is due to the increased level of phenolic compounds. In another study, 27 compounds were indentified after methanol extraction of tualang honey, some of which had anti-proliferative properties on keloid fibroblasts. Tualang honey that was extracted using methanol contained lipid including fatty acid esters. Phenolic compounds were not detected in methanolic extraction of tualang honey. Different methods need to be used to determine the phenolic content in honey for example using ethyl acetate extract and analyzing with thin layer chromatography (33). We believe that the medicinal properties of tualang honey are not based on single compound but a combination of many different components. More studies need to be done to identify the active compounds in tualang honey.
UVB causes considerable amount to DNA damage which includes oxidative stress. This study was designed to assess the photochemopreventive effect of tualang honey after single UVB irradiation to the mouse keratinocyte cell line PAM212. Our data clearly demonstrate that treatment of tualang honey affords significant inhibition against UVB-induced inflammation and DNA damage. UVB radiation to mammalian skin is known to alter cellular function via oxidation of macromolecules, DNA damage, generation of ROS and alterations in signaling pathways (9-11).
Cyclooxygenase-2 has been implicated in UVB-induced skin inflammation and photocarcinogenesis, and its overexpression has been shown to enhance cell proliferation, induce angiogenesis, regulate antiapoptotic cellular defenses and augment immunological response through production of PGE2 (32). A considerable body of evidence suggests that inhibition of COX-2 expression or activity is important for not only alleviating inflammation, but also for prevention of cancer. Our results demonstrate the inhibitory effect of treatment of tualang honey against UVB-mediated induction of epidermal COX-2 protein expression. These results indicate that tualang honey targets COX-2 expression in exerting its photoprotective effects.
UVB is a potent carcinogen known to damage DNA directly or through the generation of ROS. Both CPDs and 8-oxo-dG are formed in epidermal DNA after UVB irradiation and are considered as important biomarkers of DNA damage. Our data clearly demonstrated that treatment of tualang honey to PAM212 cells resulted in marked reduction in the number of CPDs and 8-oxo-dG positive cells and these may be due to enhanced DNA repair. Activated NF-κB is a crucial factor for the immunoinflammatory responses and is also implicated in tumorigenesis (34). Therefore, NF-κB has emerged as one of the most promising molecular targets in the prevention of cancer. NF-κB is sequestered in the cytoplasm as a heterotrimer consisting of p50, p65 and IκBα subunits. Upon phosphorylation and subsequent proteolytic degradation of IκBα, NF-κB activates and translocates to the nucleus where it binds to DNA and activates the target genes by binding to the DNA regulatory element (35). In the present study, we have demonstrated that treatment of tualang honey inhibited UVB-induced NF-κB and IKKα activation.
Our data suggests that treatment of tualang honey to PAM212 keratinocytes affords substantial protection from the adverse effects of UVB radiation via modulation in early biomarkers of photocarcinogenesis and provide suggestion for its photochemopreventive potential.
Acknowledgments
This work was supported by Pilot ' Feasibility Study to NY from NIH funded UAB Skin Disease Research Center grant P30AR050948.
Footnotes
This paper is part of the Special Issue in commemoration of 70th birthday of Dr. David R. Bickers.
References
- 1.Kovach BT, Sams HH, Stasko T. Systemic strategies for chemoprevention of skin cancers in transplant recipients. Clin Transplant. 2005;19:726–34. doi: 10.1111/j.1399-0012.2005.00412.x. [DOI] [PubMed] [Google Scholar]
- 2.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;4:1133–4. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 3.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–6. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 4.Berneburg M, Krutmann J. Photoimmunology, DNA repair and photocarcinogenesis. J Photochem Photobiol B. 2000;54:87–93. doi: 10.1016/s1011-1344(00)00024-5. [DOI] [PubMed] [Google Scholar]
- 5.D'Errico M, Lemma T, Calcagnile A, Proietti De Santis L, Dogliotti E. Cell type and DNA damage specific response of human skin cells to environmental agents. Mutat Res. 2007;3:37–47. doi: 10.1016/j.mrfmmm.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Campbell C, Quinn AG, Angus B, Farr PM, Rees JL. Wavelength specific patterns of p53 induction in human skin following exposure to UV radiation. Cancer Res. 1993;15:2697–9. [PubMed] [Google Scholar]
- 7.Latonen L, Laiho M. Cellular UV damage responses--functions of tumor suppressor p53. Biochim Biophys Acta. 2005;25:71–89. doi: 10.1016/j.bbcan.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;10:1442–5. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 9.Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705–20. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 10.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;1:107–20. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6:561–70. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- 12.Peus D, Vasa RA, Beyerle A, Meves A, Krautmacher C, Pittelkow MR. UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J Invest Dermatol. 1999;112:751–6. doi: 10.1046/j.1523-1747.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- 13.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFkappaB and TNFalpha signal transduction pathways. Arch Dermatol Res. 2010;302:5–17. doi: 10.1007/s00403-009-0994-y. [DOI] [PubMed] [Google Scholar]
- 14.Mori E, Takahashi A, Kitagawa K, Kakei S, Tsujinaka D, Unno M, Nishikawa S, Ohnishi K, Hatoko M, Murata N, Watanabe M, Furusawa Y, Ohnishi T. Time course and spacial distribution of UV effects on human skin in organ culture. J Radiat Res Tokyo. 2008;49:269–77. doi: 10.1269/jrr.07106. [DOI] [PubMed] [Google Scholar]
- 15.Ryu HC, Kim C, Kim JY, Chung JH, Kim JH. UVB Radiation Induces Apoptosis in Keratinocytes by Activating a Pathway Linked to “BLT2-Reactive Oxygen Species”. J Invest Dermatol. 2010;130:1095–1106. doi: 10.1038/jid.2009.436. [DOI] [PubMed] [Google Scholar]
- 16.Averbeck M, Beilharz S, Bauer M, Gebhardt C, Hartmann A, Hochleitner K, Kauer F, Voith U, Simon JC, Termeer C. In situ profiling and quantification of cytokines released during ultraviolet B-induced inflammation by combining dermal microdialysis and protein microarrays. Exp Dermatol. 2006;15:447–54. doi: 10.1111/j.0906-6705.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 17.Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–4. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Von Thaler AK, Kamenisch Y, Berneburg M. The role of ultraviolet radiation in melanomagenesis. Exp Dermatol. 2010;19:81–8. doi: 10.1111/j.1600-0625.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- 19.Aljadi AM, Kamaruddin MY. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chemistry. 2004;85:513–518. [Google Scholar]
- 20.Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chemistry. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- 21.Ainul Hafiza AH, Yusof N, Maimon A. Potential of Malaysian local honey as an antibacterial agent. Sains Malaysiana. 2005;34:17–20. [Google Scholar]
- 22.Ghazali FC. Morphological characterization study of Malaysian honey - A VPSEM, EDX randomised attempt. Ann Microscopy. 2009;9:93–102. [Google Scholar]
- 23.Kassim M, Achoui M, Mustafa MR, Mohd MA, Yusoff KM. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. J Nutr Res. 2010;30:650–9. doi: 10.1016/j.nutres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell DL, Volkmer B, Breitbart EW, Byrom M, Lowery MG, Greinert R. Identification of a non-dividing subpopulation of mouse and human epidermal cells exhibiting high levels of persistent ultraviolet photodamage. J Invest Dermatol. 2001;117:590–5. doi: 10.1046/j.0022-202x.2001.01442.x. [DOI] [PubMed] [Google Scholar]
- 25.Adhami VM, Afaq F, Ahmad N. Suppression of ultraviolet B exposure-mediated activation of NF-kappaB in normal human keratinocytes by resveratrol. Neoplasia. 2003;5:74–82. doi: 10.1016/s1476-5586(03)80019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pupe A, Moison R, De Haes P, van Henegouwen GB, Rhodes L, Degreef H, Garmyn M. Eicosapentaenoic acid, a n-3 polyunsaturated fatty acid differentially modulates TNF-alpha, IL-1alpha, IL-6 and PGE2 expression in UVB-irradiated human keratinocytes. J Invest Dermatol. 2002 Apr;118(4):692–8. doi: 10.1046/j.1523-1747.2002.01615.x. [DOI] [PubMed] [Google Scholar]
- 27.Chang EJ, Kundu JK, Liu L, Shin JW, Surh YJ. Ultraviolet B radiation activates NF-κB and induces iNOS expression in HR-1 hairless mouse skin: role of IκB kinase-β. Mol Carcinog. 2011;50:310–7. doi: 10.1002/mc.20646. [DOI] [PubMed] [Google Scholar]
- 28.Van Dross RT, Hong X, Essengue S, Fischer SM, Pelling JC. Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol Carcinog. 2007 Apr;46(4):303–14. doi: 10.1002/mc.20281. [DOI] [PubMed] [Google Scholar]
- 29.Herschman HR. Regulation of prostaglandin synthase-1 and prostaglandin synthase-2. Cancer Metastasis Rev. 1994;13:241–256. doi: 10.1007/BF00666095. [DOI] [PubMed] [Google Scholar]
- 30.De Gruijl FR, Rebel H. Early events in UV carcinogenesis—DNA damage, target cells and mutant p53 foci. Photochem Photobiol. 2008;84:382–387. doi: 10.1111/j.1751-1097.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 31.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 32.Afaq F, Adhami VM, Mukhtar H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat Res. 2005;571:153–173. doi: 10.1016/j.mrfmmm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Syazana MSN, Halim AS, Gan SH, Shamsuddin S. Antiproliferative effect of methanolic extraction of tualang honey on human keloid fibroblasts. BMC Complement Altern Med. 2011;11:82–89. doi: 10.1186/1472-6882-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang YM, Lo CP, Chen YP, Wang SY, Yang NS, Kuo YH, Shyur LF. Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br J Pharmacol. 2005;146:352–363. doi: 10.1038/sj.bjp.0706343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karin M. The IkappaB kinase—A bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]