Psoriatic skin contains more nerve fibers (Naukkarinen et al., 1989), has increased levels of sensory nerve-derived calcitonin gene related peptide (CGRP) and substance P (SP) (Chan et al., 1997; Jiang et al., 1998), and psoriasis disease severity can be exacerbated by stress (Griffiths and Richards, 2001) possibly by increasing cutaneous nerve numbers and SP and CGRP expression (Joachim et al., 2007; Remrod et al., 2007). The clinical observation that psoriasis undergoes remission following loss of innervation, nerve function or nervous system injury (Dewing, 1971; Farber et al., 1990; Joseph et al., 2005; Perlman, 1972; Stratigos et al., 1998) further supports a role for the nervous system in psoriasis pathogenesis; however, the neural-mediated mechanisms underlying disease resolution in these cases have not been explored. Recent work by our group identified increases in cutaneous nerve fibers and nerve-derived SP and CGRP in the KC-Tie2 murine model of psoriasiform dermatitis, providing an experimental paradigm to explore neural contributions to psoriasis pathogenesis. Surgical elimination of the cutaneous nerves in KC-Tie2 mouse dorsal skin resulted in a 30% improvement in acanthosis, a 40% decrease in CD11c+ dendritic cells (DCs) and a 30% decrease in CD4+ T cells. These outcomes were SP and CGRP dependent; as reconstitution of SP and CGRP in denervated KC-Tie2 skin prevented improvement in the phenotype and inhibition of SP and CGRP in innervated KC-Tie2 skin recapitulated the findings elicited by experimental denervation in a sensory neuropeptide specific manner (Ostrowski et al., 2011). These findings provide insight into potential mechanisms underlying clinical reports of disease improvement following nervous system injury and identify key roles for nerve derived SP and CGRP in sustaining chronic psoriasiform skin inflammation.
Botulinum neurotoxins of various serotypes act by inhibiting the exocytosis of neurotransmitters from nerve endings. Botulinum neurotoxin A (BoNT-A, known commonly by the tradenames BOTOX®, Dysport® and Xeomin®) does so by cleaving the SNAP25 protein, and is best known for its capacity to inhibit acetylcholine release in neuromuscular junctions and at the neurovascular interface and therefore is often used to treat glabellar lines, hemifacial spasm, cervical dystonia, blepharospasm, hyperhidrosis and Raynaud's disease. However BoNT-A also inhibits nerve-derived release of CGRP and SP and can be used for treating pain syndromes and potentially neurogenic inflammation (Carmichael et al., 2010; Meng et al., 2007). This is particularly significant and provides mechanistic insight into the recent unpublished observation of psoriatic plaque remission in a patient injected with BoNT-A for the treatment of upper limb spasticity related to stroke (Dr. Jim Andrews, personal communication) and the subjective clinical observation of disease improvement in inverse psoriasis following BoNT-A administration (Zanchi et al., 2008).
This study sought to investigate whether one intradermal injection of BoNT-A into KC-Tie2 mouse skin would provide similar levels of improvement in skin disease as surgical denervation and/or chemical inhibition of SP and CGRP. Adult KC-Tie2 mouse dorsal skin was intradermally injected with BoNT-A (Dysport®; 9units/kg/100ul) and saline (100ul volume) in anatomically separate locations, the regions were marked and images were taken to ensure that the same locations were harvested for analyses. Two (n=5) or six weeks (n=6) later, mice were sacrificed, and BoNT-A and saline injected skin was harvested and processed for histological and immunostaining analyses as described previously (Ostrowski et al., 2011). All animal protocols were approved by the Case Western Reserve University institutional animal care and use committee.
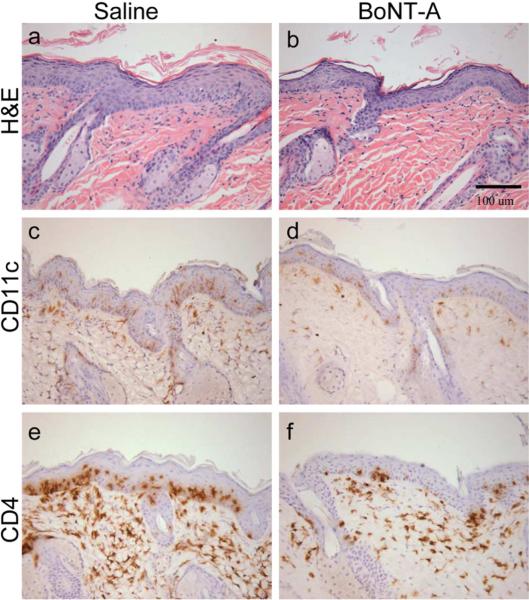
Dorsal skin injected once with BoNT-A showed significant improvement in acanthosis compared to saline injected skin at 2 weeks (~17% decrease; P=0.031) and at 6 weeks (~25% decrease; P=0.011; Figure 1a–b; Figure 2). Dermal DC infiltration was also significantly reduced, evidenced by a significant decrease in the number of dermal CD11c+ DCs in BoNT-A injected skin compared to saline injected skin (Figure 1c–d; Figure 2) at 2 weeks (29% decrease; P=0.002) and at 6 weeks (38% decrease; P<0.0001). CD4+ T cell numbers also decreased significantly in BoNT-A injected skin compared to saline injected skin (Figure 1e–f; Figure 2) at 2 weeks (24% decrease; P=0.017) and at 6 weeks (34% decrease; P<0.002). The number of F4/80+ macrophages and CD8+ T cells did not differ between BoNT-A and saline injected skin, nor were any changes observed for dermal angiogenesis (blood vessel number or size) at either of the time points examined (not shown).
Figure 1. Botulinum neurotoxin A (BoNT-A) improves skin disease severity in KC-Tie2 mice.
Representative images of H&E (a–b), CD11c (c–d) and CD4 (e–f) immunostained back skin of KC-Tie2 animals 6 weeks following a single intradermal injection of either saline (a, c, e) or BoNT-A (b, d, f; Dysport®, 9units/kg). Scale bar = 100 μM.
Figure 2. Acanthosis, CD11c+ DC and CD4+ T cell numbers decrease following BoNT-A injection.
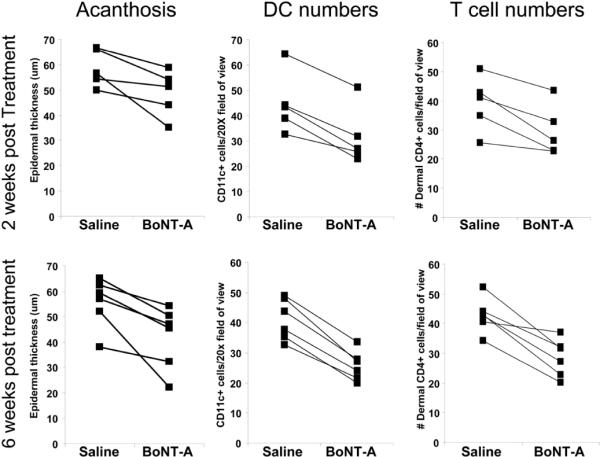
Acanthosis (epidermal thickness; in μm), CD11c+ DC and CD4+ T cell numbers are presented for saline and BoNT-A injected skin of individual animals at 2 (n=5) and 6 weeks n=6). Acanthosis improves in BoNT-A compared to saline injected skin at 2 weeks (~17% decrease, P=0.031) and at 6 weeks (~25% decrease; P=0.011). Dermal CD11c+ DC numbers decrease in BoNT-A compared to saline injected skin at 2 weeks (29% decrease; P=0.002) and at 6 weeks (38% decrease; P<0.0001). CD4+ T cell numbers decrease significantly in BoNT-A compared to saline injected skin at 2 weeks (24% decrease; P=0.017) and at 6 weeks (34% decrease; P<0.002). All data was analysed with a paired Student's T-test.
These findings demonstrate a significant improvement in psoriasiform skin inflammation and epidermal hyperplasia following one intradermal injection of BoNT-A, and identify decreases in infiltrating CD4+ T cells and CD11c+ DCs that occur concomitant with the improvement in acanthosis. These findings are similar to those we previously reported, and the percent improvement for each outcome was comparable to that observed following both surgical elimination of the cutaneous nerves and following 4 weeks of CGRP and SP inhibition (Ostrowski et al., 2011) and suggest that BoNT-A might serve as a useful psoriasis therapeutic. However, the use of BoNT-A as monotherapy for patients with high PASI scores (>50) is not a practical approach. Nonetheless, in some psoriasis patients, recalcitrant plaques remain, even following treatment with strong systemic and biological therapies such as TNFα inhibitors. The persistence of some plaques may be explained by the local microenvironment within the tissue combined with signals yet unaccounted for that have strong proinflammatory effects, such as nerve-derived SP and CGRP. Our previous results (Ostrowski et al., 2011) identified these neuropeptides as necessary to sustain and exacerbate tissue hyperproliferation and suggest that the presence of nerves and their derived proteins play critical roles in the cascade of events that sustain inflammation and acanthosis. The ability to interfere with cutaneous nerve signaling presents an opportunity to address recalcitrant plaque psoriasis, thus BoNT-A may serve as a supplemental therapeutic approach to otherwise successful topical or biologic regimens. The current data validates this idea and demonstrates that a single intradermal injection of BoNT-A, a known inhibitor of CGRP/SP release, leads to significant improvement in disease severity as early as two weeks post treatment and provides new insight into the cellular mechanisms underlying disease improvement following BoNT-A administration in psoriasis patients.
Acknowledgments
The authors apologize to colleagues whose work could not be cited because of the space constraints. NLW is supported by grants from the National Institutes of Health (P30AR39750 and P50AR05508), National Psoriasis Foundation and the Murdough Family Center for Psoriasis.
Footnotes
Conflict of Interest. Dr. Gilbert is a consultant for Allergan, Merz and for Michelson Diagnostics Ltd.
Literature Cited
- Carmichael NM, Dostrovsky JO, Charlton MP. Peptide-mediated transdermal delivery of botulinum neurotoxin type A reduces neurogenic inflammation in the skin. Pain. 2010;149:316–324. doi: 10.1016/j.pain.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Chan J, Smoller BR, Raychauduri SP, Jiang WY, Farber EM. Intraepidermal nerve fiber expression of calcitonin gene-related peptide, vasoactive intestinal peptide and substance P in psoriasis. Arch Dermatol Res. 1997;289:611–616. doi: 10.1007/s004030050249. [DOI] [PubMed] [Google Scholar]
- Dewing SB. Remission of psoriasis associated with cutaneous nerve section. Arch Dermatol. 1971;104:220–221. [PubMed] [Google Scholar]
- Farber EM, Lanigan SW, Boer J. The role of cutaneous sensory nerves in the maintenance of psoriasis. Int J Dermatol. 1990;29:418–420. doi: 10.1111/j.1365-4362.1990.tb03825.x. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Richards HL. Psychological influences in psoriasis. Clin Exp Dermatol. 2001;26:338–342. doi: 10.1046/j.1365-2230.2001.00834.x. [DOI] [PubMed] [Google Scholar]
- Jiang W-Y, Raychaudhuri SP, Farber EM. Double-labeled immunofluorescence study of cutaneous nerves in psoriasis. International Journal of Dermatology. 1998;37:572–574. doi: 10.1046/j.1365-4362.1998.00533.x. [DOI] [PubMed] [Google Scholar]
- Joachim RA, Kuhlmei A, Dinh QT, Handjiski B, Fischer T, Peters EM, et al. Neuronal plasticity of the “brain-skin connection”: stress-triggered up-regulation of neuropeptides in dorsal root ganglia and skin via nerve growth factor-dependent pathways. J Mol Med. 2007;85:1369–1378. doi: 10.1007/s00109-007-0236-8. [DOI] [PubMed] [Google Scholar]
- Joseph T, Kurian J, Warwick DJ, Friedmann PS. Unilateral remission of psoriasis following traumatic nerve palsy. Br J Dermatol. 2005;152:185–186. doi: 10.1111/j.1365-2133.2005.06330.x. [DOI] [PubMed] [Google Scholar]
- Meng J, Wang J, Lawrence G, Dolly JO. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci. 2007;120:2864–2874. doi: 10.1242/jcs.012211. [DOI] [PubMed] [Google Scholar]
- Naukkarinen A, Nickoloff BJ, Farber EM. Quantification of cutaneous sensory nerves and their substance P content in psoriasis. J Invest Dermatol. 1989;92:126–129. doi: 10.1111/1523-1747.ep13071340. [DOI] [PubMed] [Google Scholar]
- Ostrowski SM, Belkadi A, Loyd CM, Diaconu D, Ward NL. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J Invest Dermatol. 2011;131:1530–1538. doi: 10.1038/jid.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman HH. Remissiona of psoriasis vulgaris from the use of nerve-blocking agents. Arch Dermatol. 1972;105:128–129. doi: 10.1001/archderm.1972.01620040088028. [DOI] [PubMed] [Google Scholar]
- Remrod C, Lonne-Rahm S, Nordlind K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch Dermatol Res. 2007;299:85–91. doi: 10.1007/s00403-007-0745-x. [DOI] [PubMed] [Google Scholar]
- Stratigos AJ, Katoulis AK, Stavrianeas NG. Spontaneous clearing of psoriasis after stroke. J Am Acad Dermatol. 1998;38:768–770. doi: 10.1016/s0190-9622(98)70210-5. [DOI] [PubMed] [Google Scholar]
- Zanchi M, Favot F, Bizzarini M, Piai M, Donini M, Sedona P. Botulinum toxin type-A for the treatment of inverse psoriasis. J Eur Acad Dermatol Venereol. 2008;22:431–436. doi: 10.1111/j.1468-3083.2007.02457.x. [DOI] [PubMed] [Google Scholar]