Abstract
Lightly touching normal skin near a site of itch can elicit itch sensation, a phenomenon known as alloknesis. To investigate the neural mechanisms of alloknesis, we have developed an animal model. Low-threshold mechanical stimulation of the skin normally does not elicit any response in naïve C57/BL6 mice. Following acute intradermal (id) injection of histamine in the rostral back, mechanical stimulation 7 mm from the injection site elicited discrete hindlimb scratch bouts directed toward the stimulus. This began at 10 min and peaked 20–40 min post-histamine, declining over the next hour. Histamine itself elicited bouts of scratching not associated with the mechanical stimulus, that ceased after 30 min. Histamine- and touch-evoked scratching was inhibited by the μ-opiate antagonist naltrexone. Touch-evoked scratching was observed following id 5-HT, a PAR-4 agonist and a MrgprC11 agonist BAM8-22, but not chloroquine or a PAR-2 agonist. The histamine H1 receptor antagonist terfenadine prevented scratching and alloknesis evoked by histamine, but not 5-HT, a PAR-4 agonist or a MrgprC11 agonist. In mice with experimental dry skin, there was a time-dependent increase in spontaneous and touch-evoked scratching. This animal model, which to our knowledge is previously unreported, appears to be useful to investigate neural mechanisms of itch and alloknesis.
INTRODUCTION
Chronic itch associated with dermatitis and systemic diseases considerably decreases the quality of life in patients (Ikoma et al., 2006). A potential mechanism underlying chronic itch is sensitization of itch-signaling pathways. In lesional skin of atopic dermatitis patients, pruritogens elicit stronger itch (hyperknesis) and noxious stimuli elicit itch instead of pain (Hosogi et al., 2006; Ikoma et al., 2004a; Ikoma et al., 2004b). Moreover, innocuous mechanical stimuli also frequently elicit itch sensation in patients suffering from chronic itch (Wahlgren et al., 1991). When delivered within a region of normal skin surrounding a site of experimental itch induced by intradermal injection of histamine, innocuous mechanical stimuli elicit itch (Heyer et al., 1997; Simone et al., 1991), a phenomenon called itchy skin or alloknesis (LaMotte, 1992). Alloknesis may reflect a central mechanism in which activation of low-threshold mechanoreceptors excites sensitized itch-signaling neurons in the spinal cord. However, this has not yet been tested due to the lack of an animal model for alloknesis.
Itch is defined as an unpleasant sensation that provokes the desire to scratch. The close relationship between itch and scratch has been exploited in rodent models in which hindlimb scratching is used to assess itch (Carstens and Kuraishi, 2004). Intradermal injection of histamine, 5-HT, agonists of protease-activated receptors PAR-2 and PAR-4, the MrgprA3 agonist chloroquine, or MrgprC11 agonist BAM8-22 elicits bouts of directed hindlimb scratching that are attenuated by μ-opioid antagonists but not by morphine (Akiyama et al., 2010a, c; Akiyama et al., 2009b; Akiyama et al., 2009c; Liu et al., 2009). These results are consistent with the antipruritic effects of μ-antagonists (Heyer et al., 1997) and the well-known ability of μ-agonists to induce or enhance itch, further validating the rodent itch models. In the present study, we investigated whether innocuous mechanical stimuli delivered to skin adjacent to a site of pruritogenic stimulation could elicit scratching.
We additionally wished to determine if innocuous mechanical stimuli elicit scratching in a model of chronic dry skin itch. Repeated treatment of murine skin with acetone/diethylether and water (AEW) induces dry skin characterized by increased transepidermal water loss, hyperplasia, and increased spontaneous scratching that was attenuated by μ-antagonists (Akiyama et al., 2010b, d). Moreover, there was a differential enhancement of scratching elicited by certain pruritogens (5-HT, PAR-2 agonist), accompanied by enhanced responses of primary sensory dorsal root ganglion (DRG) (Akiyama et al., 2010b) and second-order dorsal horn neurons (Akiyama et al., 2011). We hypothesized that the chronic dry skin itch is accompanied by alloknesis, such that innocuous mechanical stimuli delivered outside the perimeter of the dry skin area would elicit scratching behavior.
RESULTS
Alloknesis following id histamine
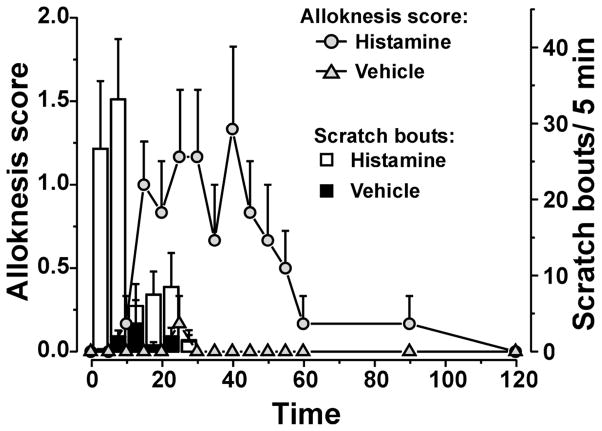
Histamine (271 nmol) elicited bouts of scratching that declined over a 30-min period (Fig. 1, open bars). These scratch bouts occurred independently of mechanical stimuli and presumably reflect ongoing itch from the histamine injection. Vehicle (saline) injection elicited very few scratch bouts (Fig. 1, filled bars). Prior to histamine, von Frey stimulation never elicited any scratch responses (Supplementary Video 1). Following id histamine, von Frey stimuli did not elicit scratching until 10 min-post injection, at which time the alloknesis score started to increase (Fig. 1,
 ). Supplementary Video 2 shows an example of a touch-evoked scratch bout. The alloknesis score reached a maximal level during the 15–45 min period post-histamine, and then gradually declined to zero over 2 hr. Following vehicle (saline) injection, von Frey stimuli never elicited any scratch bouts during this period (Fig. 1,
). Supplementary Video 2 shows an example of a touch-evoked scratch bout. The alloknesis score reached a maximal level during the 15–45 min period post-histamine, and then gradually declined to zero over 2 hr. Following vehicle (saline) injection, von Frey stimuli never elicited any scratch bouts during this period (Fig. 1,
 ).
).
Fig. 1.
Time course of scratching and alloknesis elicited by histamine. Bars: mean number of scratch bouts (right-hand y-axis) following histamine (□) or vehicle (■). Line graphs: mean alloknesis score (left-hand y-axis) following histamine (
 ) or vehicle (
) or vehicle (
 ). Error bars are S.E.M. (n = 6/ group). Following intradermal injection of histamine (271 nmol/10 μl; open bars and
). Error bars are S.E.M. (n = 6/ group). Following intradermal injection of histamine (271 nmol/10 μl; open bars and
 ) or saline (vehicle; filled bars and
) or saline (vehicle; filled bars and
 ) in the nape of the neck, the mouse was placed into the recording arena and videotaped for 30 min. At 5-min intervals, an innocuous von Frey filament (bending force 0.7 mN) was applied 3 times at separate sites oriented radially at a distance of 7 mm from the histamine injection site, and the presence or absence of a hindlimb scratch bout following each stimulus was noted (maximum alloknesis score = 3). The number of histamine- or vehicle-evoked hindlimb scratch bouts directed to the injection site (not associated with the von Frey stimulus) was counted after the experiment by reviewing the videotape.
) in the nape of the neck, the mouse was placed into the recording arena and videotaped for 30 min. At 5-min intervals, an innocuous von Frey filament (bending force 0.7 mN) was applied 3 times at separate sites oriented radially at a distance of 7 mm from the histamine injection site, and the presence or absence of a hindlimb scratch bout following each stimulus was noted (maximum alloknesis score = 3). The number of histamine- or vehicle-evoked hindlimb scratch bouts directed to the injection site (not associated with the von Frey stimulus) was counted after the experiment by reviewing the videotape.
It is noteworthy that the time courses of histamine-evoked scratch bouts and alloknesis differed, with the latter peaking after histamine-evoked scratching behavior had ceased. During the first 30 min post-histamine when scratching was observed, it is important to note that the von Frey stimulus was only applied at times when no histamine-related scratch bouts occurred. However, because the histamine-evoked scratch bouts could potentially interfere with von Frey-evoked scratch responses, in subsequent experiments we began alloknesis testing 30 min after injection of the pruritogen when the occurrence of histamine-evoked scratch bouts had ceased.
Naltrexone attenuates histamine-evoked alloknesis and spontaneously-occurring scratch bouts
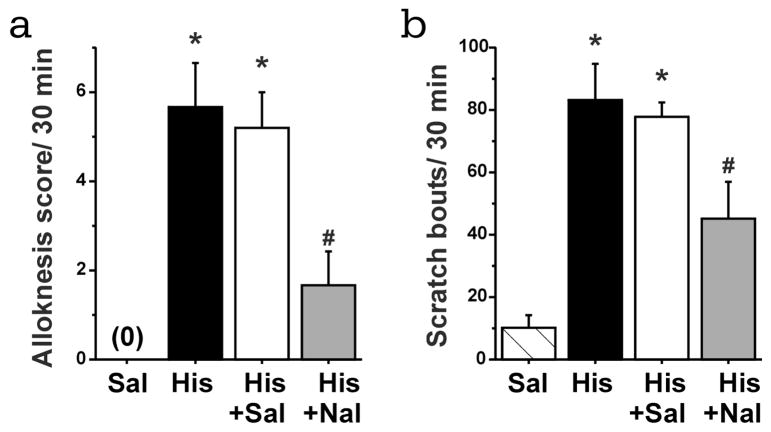
The mean histamine-evoked alloknesis score over the period from 30–60 min following id histamine, and mean number of histamine-evoked scratch bouts, are shown in Fig. 2A and B, respectively (black bars). Pretreatment with naltrexone resulted in a significant reduction in the mean alloknesis score (Fig. 2A, gray bar) and number of spontaneously-occurring scratch bouts (Fig. 2B, gray bar). Pretreatment with systemic administration of saline (vehicle) did not affect the alloknesis score or number of spontaneous scratch bouts elicited by id histamine (Fig. 2A, B, white bars). Figure 2A shows the average of the sum of the alloknesis score over 30–60 min followed by histamine injection. The id injection of saline resulted in an alloknesis score of 0 (Fig. 2A) and evoked a minimal number of scratch bouts (Fig. 2B, striped bar).
Fig. 2.
Effects of naltrexone on alloknesis and scratch evoked by histamine. A) Bar graph of the mean sum of alloknesis score over 30 to 60 min after histamine injection. The striped and black bars show, respectively, the sum of the alloknesis score evoked by saline and histamine (271 nmol/10 μl). The white and grey bars show the sum of score for alloknesis evoked by histamine when preceded by subcutaneous saline or by naltrexone (1 mg/kg), respectively. B) Same as in A for mean scratch bouts/ 30 min. Error bars are S.E.M. * p < 0.05, significant difference from saline group (n = 6/group). # p < 0.05, significant difference from histamine + saline group (n = 6/group).
Scratching and alloknesis elicited by other pruritogens
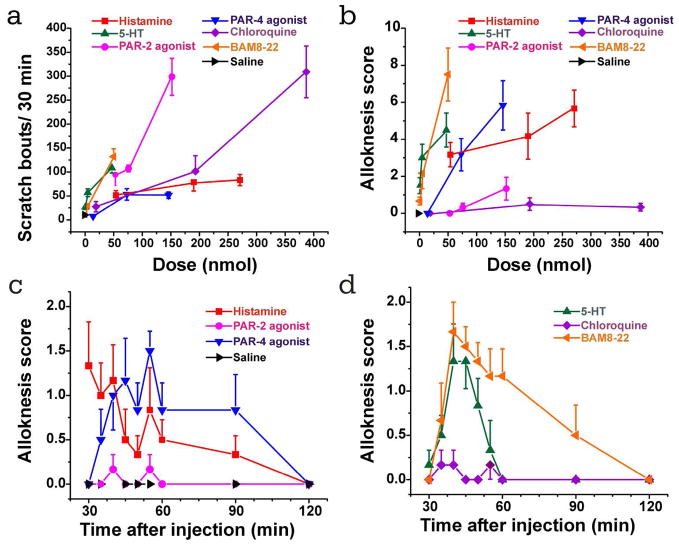
We additionally assessed scratching and alloknesis following id injection of a variety of pruritogens. Figs. 3A and B show dose-response curves of scratch bouts and alloknesis scores, respectively, elicited by various pruritogens. Histamine, 5-HT, the PAR-4 agonist and BAM8-22 each elicited dose-dependent alloknesis while the PAR-2 agonist and chloroquine elicited weak or no alloknesis. The time courses of alloknesis elicited by these agents at the intermediate or high dose are shown in Figs. 3C and D. The PAR-4 agonist, 5-HT and BAM8-22 elicited alloknesis that peaked around 40–45 min post- injection and declined by 60–120 min. Saline did not elicit any alloknesis and minimal scratching.
Fig. 3.
Scratching and alloknesis elicited by different pruritogens. A. Dose-response curve for scratch bouts (assessed over 30 min) elicited by id injection of pruritogens indicated in the legend. Error bars: SEM (n=6/group). B. Dose-response curve for alloknesis score elicited by the same pruritogens. C: time course of alloknesis for histamine (271 nmol/10 μl), PAR-2 agonist SLIGRL-NH2 (76 nmol/10 μl), PAR-4 agonist AYPGKF-NH2 (146 nmol/10 μl), and saline vehicle. D: time course of alloknesis for 5-HT (47 nmol/10 μl), chloroquine (193 nmol/10 μl), and BAM8-22 (50 nmol/10 μl).
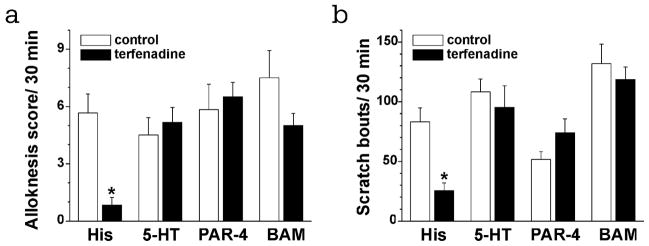
Histamine-dependent and -independent types of itch accompanied by alloknesis. Pretreatment with the H1 histamine receptor antagonist terfenadine almost completely abolished histamine-evoked alloknesis (Fig. 4A) and scratching (Fig. 4B). In contrast, pretreatment with terfenadine failed to reduce the mean alloknesis score (Fig. 4A) or number of pruritogen-evoked scratch bouts (Fig. 4B) following id injection of 5-HT, the PAR-4 agonist, or BAM8-22.
Fig. 4.
Effects of terfenadine on alloknesis and scratching evoked by four pruritogens. A. Bar graph of the mean alloknesis score during the time period 30–60 min after injection of the following: histamine (271 nmol/10 μl), 5-HT (47 nmol/10 μl), PAR-4 agonist AYPGKF-NH2 (146 nmol/10 μl), BAM8-22 (50 nmol/10 μl). Graph shows the alloknesis scores evoked by each pruritogen without (open bars) or following pretreatment with terfenadine (filled bars, 30 mg/kg), respectively. B. As in A for mean scratch bouts/ 30 min. Error bars are S.E.M. * p < 0.05, significant difference from control group (n = 6/group).
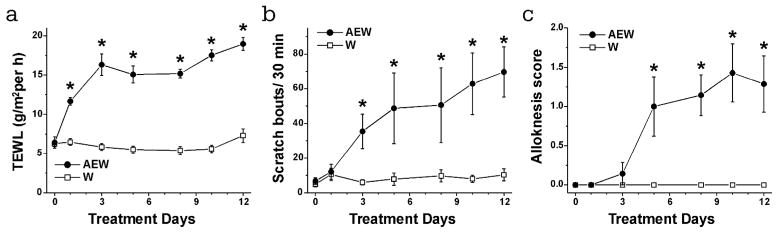
Alloknesis in AEW-treated mice
TEWL increased significantly on the first day of AEW treatment and reached a plateau by day 3 (Fig. 5A, ●). Spontaneous scratching behavior increased significantly by treatment day 3 (Fig. 5B, ●) and continued to gradually rise. Alloknesis elicited by weak mechanical stimulation at the perimeter of the AEW treatment area increased significantly by day 4 and appeared to reach a plateau (Fig. 5C, ●). There were no significant changes in any of these measures in the control W treatment group (Fig. 5A–C, □).
Fig. 5.
Time-dependent changes in scratch bouts, TEWL and alloknesis score in dry skin mice. The dry skin was developed by treating with AEW twice a day for 12 days. A) TEWL was mesured on the day 0, 1, 3, 5, 8, 10 and 12. Black circle and white square show, respectively, AEW-treated and W-treated groups. B) As in A for Spontaneous scratching. C) As in A for Alloknesis score. Error bars are S.E.M. * p < 0.05, significant difference from day 0.
DISCUSSION
Innocuous mechanical stimuli that normally do not elicit a behavioral response in C57/BL6 mice elicited scratch bouts when delivered following id application of the pruritogens histamine, 5-HT, the PAR-4 agonist and BAM8-22. Similarly, following local application of histamine, 5-HT, or BAM8-22 in humans, innocuous mechanical stimuli elicit itch, a phenomenon called alloknesis (Heyer et al., 1997; Simone et al., 1991; Weisshaar et al., 2004; Sikand et al., 2011). The incidence of mechanical stimulus-evoked scratching peaked at 10–40 min following histamine injection and declined after that. Post-histamine mechanically-evoked scratching in mice was significantly attenuated by the μ–opioid antagonist naltrexone, consistent with the ability of another antagonist, naloxone, to reduce alloknesis in humans (Heyer et al., 1997) and supporting the view that touch-evoked scratching represents a novel animal model of alloknesis. Innocuous mechanical stimulation also elicited scratching when delivered at the edge of a region of AEW treatment in mice, suggesting the presence of alloknesis in this animal model of chronic dry skin itch.
It has been recently reported that Mrgprs present in subsets of small-diameter sensory neurons play an important role in itch. MrgprA3 and MrgprC11 are activated by chloroquine and BAM8-22, respectively (Liu et al., 2009). The subpopulation of BAM8-22-responsive DRG neurons is part of the subpopulation of chloroquine-responsive DRG neurons. Chloroquine failed to elicit alloknesis, while BAM8-22 elicited alloknesis (Fig. 3), suggesting that these agonists activate separate itch signaling pathway. In support of this, BAM8-22-elicited signaling requires PLC, whearas chloroquine-elicited signaling requires Gβγ (Wilson et al., 2011).
The PAR-2 agonist SLIGRL-NH2 failed to elicit alloknesis (Fig. 3). It was recently reported that SLIGRL-NH2 activates PAR-2 as well as MrgprC11 (Liu et al., 2011). SLIGRL-NH2 differentially elicits hyperalgesia via PAR-2 rather than MrgprC11, whereas it elicits scratching via MrgprC11 rather than PAR-2. Consistent with this, we observed that the MrgprC11 agonist BAM8-22 elicited scratching with accompanying alloknesis (Fig. 3). We speculate that the inability of the PAR-2 agonist SLIGRL-NH2 to elicit alloknesis is due to its hyperalgesic action mediated via PAR-2. Consistent with this idea, it was previously reported that histamine failed to elicit alloknesis when injected into an area of capsaicin-evoked allodynia (Brull et al., 1999).
Recent studies indicate that there are separate mechanisms and pathways for histamine-dependent and other types of itch (Davidson and Giesler, 2010; Steinhoff et al., 2003) In particular, id insertion of cowhage spicules, which contain proteases (mucunain), induce a largely histamine-independent type of itch (Johanek et al., 2007). Current evidence also suggests that histamine and cowhage may excite largely separate populations of primary afferents, namely mechanically-insensitive C-afferents and polymodal nociceptors, respectively (Johanek et al., 2008; Namer et al., 2008; Schmelz et al., 1997). Moreover, histamine and cowhage excite separate populations of primate spinothalamic tract neurons (Davidson et al., 2007). These findings support the notion of separate, histamine-dependent and histamine-independent itch pathways. We were interested to determine if histamine-dependent and -independent types of itch are accompanied by alloknesis. 5-HT, the PAR-4 agonist, and BAM8-22 each elicited scratching and alloknesis. The alloknesis evoked by these agents was not affected by pretreatment with the H1 histamine receptor terfenadine. In contrast, histamine-evoked scratching and alloknesis were significantly attenuated by the H1 antagonist (Fig. 4). This result is consistent with human psychophysical data showing that n H1 histamine receptor antagonist failed to reduce the BAM8-22-elicited itch sensations and allokensis (Sikand et al., 2011). Our data indicate that 5-HT, the PAR-4 agonist, and BAM8-22 elicit itch and alloknesis in a histamine-independent manner.
The duration of alloknesis in mice appeared to be longer compared to histamine-evoked alloknesis in humans that peaks approximately 12 min post-injection and declines within about 40 minutes (Simone et al., 1991). In the latter study it was shown that the development of alloknesis was delayed by skin cooling (Simone et al., 1991). In our experiments, the mice were freely able to scratch at and around the injection site, whereas in the human studies the magnitude of itch sensation was rated in the absence of scratching. We suggest that scratching may have delayed the build up of alloknesis in our studies to result in a longer time course.
One potential mechanism underlying alloknesis is the sensitization of itch pathways to touch. We previously showed that a subpopulation of neurons in the superficial doral horn responds to id injection of pruritogens over a time course consistent with scratching behavior (Akiyama et al., 2009a; Akiyama et al., 2009b), representing candidate itch-signaling neurons. We are currently investigating if id injection of histamine or other pruritogens enhances low-threshold mechanically-evoked respnses of pruritogen-responsive dorsal horn neurons in mice. We hypothesize that acute and chronic itch conditions might sensitize either peripheral mechanosensitive afferents, and/or spinal itch-signaling neurons, to increase touch-evoked activity in the itch pathway.
Alloknesis was also observed in our present experimental model of chronic dry skin pruritus. The onset of alloknesis was delayed relative to the onset of spontaneous scratching. The increase in spontaneous scratching may reflect sensitization of itch-signlaing pathways. We recently reported that in mice receiving similar hindpaw AEW treatment, lumbar superficial dorsal horn neurons recorded ipsilateral to the dry-skin treatment exhibited significantly heightened spontaneous activity and pruritogen-evoked responses compared to neurons ipsilateral to the control (water-treated) or untreated hindpaw (Akiyama et al., 2011). However, we did not observe enhancement of low-threshold mechanically-evoked responses of neurons ipsilateral to the hindpaw AEW treatment (Akiyama et al., 2011). In the present study, alloknesis was only elicited by stimulation at the border of the dry skin-treated area, but not within the treatment area. In the electrophysiological study, neuronal receptive fields were within the hindpaw dry skin area where mechanical stimulation would not be expected to elicit enhanced responses.
In conclusion, we have developed a murine model of alloknesis that to our knowledge has not been previously reported. Innocuous mechanical stimulation of skin adjacent to a site of acute itch, or chronic dry skin treatment on the rostral back, elicited scratching behavior in both a histamine-dependent and –independent manner. This model should prove useful in future studies that investigate the mechanisms and pathways underlying itch.
MATERIALS AND METHODS
Experiments were conducted using adult C57BL/6 mice (Harlan, Oxnard CA) (19–32 g body weight) under a protocol approved by the UC Davis Animal Care and Use Committee.
Behavior
The fur on the nape of the neck was shaved and mice were habituated to a Plexiglas recording arena with a transparent cover one week prior to testing. On the test day, the animal received an intradermal (id) microinjection of 10 μl of one of the following: vehicle (isotonic saline), histamine (54, 190, 271 nmol; Sigma-Aldrich, St. Louis MO), PAR-2 agonist SLIGRL-NH2 (53, 76, 152 nmol; Quality Controlled Biochemicals, Hopkinton, MA), PAR-4 agonist AYPGKF-NH2 (14, 73, 146 nmol; QCB), 5-HT (0.47, 4.7, 47 nmol; Alfa Aesar, Ward Hill, MA), chloroquine (19, 193, 387 nmol; Sigma) or BAM8-22 (0.5, 5, 50 nmol; Genemed Synthesis Inc., San Antonio, TX). Briefly, microinjections were made id in the nape of the neck using a 30-g needle attached to a Hamilton microsyringe by PE-50 tubing. Immediately following the id microinjection, mice were placed into the arena and videotaped from above. In the first experiment, the animal was tested for alloknesis at 5-min intervals, starting 5 min post-injection. In the remainder of the experiments, investigators left the room for 30 min and then returned to test for alloknesis, starting 30 min post-injection.
Alloknesis was assessed as follows. At 5-min intervals, the mouse received 3 separate innocuous mechanical stimuli delivered using a von Frey filament (bending force: 0.7 mN) at separate, randomly-selected sites oriented radially 7 mm away from the injection site. The 0.7 mN von Frey filament was selected because it never elicited scratch bouts in naïve mice, and was the minimum strength to elicit scratch bouts when delivered to skin surrounding the site of histamine injection or dry skin treatment. The presence or absence of a positive response, i.e., a hindlimb scratch bout directed to the site of mechanical stimulation, was noted for each stimulus before the next one was given. The alloknesis score was the total number of positive responses elicited by the three stimuli, i.e., 0, 1, 2 or 3. The sequence was repeated at 5-min intervals out to 60 min post-injection, and again at 90 and 120 min post-injection time points. In many experiments, an overall alloknesis score per 30 min was calculated as the sum of individual alloknesis scores at each 5-min inter over the 30 min period beginning 30 min post-injection.
In one set of experiments we tested the effect of the μ-opiate antagonist naltrexone on scratching and alloknesis. Naltrexone (1 mg/kg sc, Dupont; Garden, NY) or saline was administered 10 min prior to the id microinjection of histamine as described in our recent study (Akiyama et al., 2009c).
In another set of experiments we tested the effect of the H1 histamine receptor antagonist terfenadine on scratching and alloknesis. Terfenadine (30 mg/kg ip, Sigma) was administered 30 min prior to the id microinjection of histamine, 5-HT, the PAR-4 agonist or BAM8-22.
Videotapes were played back and the number of scratch bouts counted at 5-min intervals over the 30-min recording period by two independent observers blinded as to the treatment. A scratch bout is defined as one or more rapid back-and-forth motion of the hindpaw directed to the site of injection, and ending with licking or biting of the toes and/or placement of the hindpaw on the floor. Between-group comparisons of counts of scratch bouts or alloknesis scores were made by unpaired t-test with p<0.05 considered to be significant.
Dry skin model
To induce chronic dry skin on the nape of the neck, we followed a previously-reported procedure (Akiyama et al., 2010b; Miyamoto et al., 2002; Nojima et al., 2003). Briefly, a mixture of acetone and diethylether (1:1) was applied to a circumscribed area (approx. 15 × 15 mm spanning the midline) at the nape of the neck for 15 s, followed immediately by distilled water for 30 sec, twice-daily for a maximum of 12 days (designated as AEW treatment). Control mice were treated in the same manner with application of water only for 45 sec (designated as W treatment). The animals’ toenails were clipped; mice were free to direct hindlimb scratch movements to the treatment area such that the skin was rubbed by the toes but not scratched. Sixteen to 20 h after the second treatment on the 1st, 3rd, 5th, 8th, 10th and 12th treatment day, spontaneous behavior was recorded for 30 min after which alloknesis testing was conducted in a manner similar to that noted above. In pilot experiments we determined that application of von Frey stimuli within the AEW treatment area was ineffective, whereas application at or outside the border of the AEW treatment area elicited an approximately equivalent incidence of positive scratch responses. For the data shown in Fig. 4, von Frey stimuli were applied on the border of the AEW (or W) treatment area at 3 randomly selected sites. Trans-epidermal water loss (TEWL) was measured by applying a Vapo Meter® (Delfin Technologies Ltd., Kuopio, Finland) to the skin for 10 s.
Acknowledgments
Supported by a Collaborative Research Grant from the International Association for the Study of Pain (to AI and EC), and by grants DE021183 and AR057194 from the National Institutes of Health (to EC). TA received a postdoctoral fellowship from the Japan Society for the Promotion of Science.
ABBREVIATIONS
- 5-HT
5-hydroxytryptamine (serotonin)
- AEW
acetone-ether-water
- id
intradermal
- Nal
naltrexone
- PAR
protease-activated receptor
- TEWL
trans-epidermal water loss
- W
water
Footnotes
CONFLICT OF INTEREST: none.
REFERENCES CITED
- Akiyama T, Carstens MI, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role in itch. J Neurophysiol. 2009a;102:2176–83. doi: 10.1152/jn.00463.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Differential itch- and pain-related behavioral responses and micro-opoid modulation in mice. Acta Derm Venereol. 2010a;90:575–81. doi: 10.2340/00015555-0962. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010b;151:378–83. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol. 2010c;104:2442–50. doi: 10.1152/jn.00563.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Carstens MI, Carstens E. Spontaneous itch in the absence of hyperalgesia in a mouse hindpaw dry skin model. Neurosci Lett. 2010d;484:62–5. doi: 10.1016/j.neulet.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. J Neurophysiol. 2011 doi: 10.1152/jn.01124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Carstens MI, Carstens E. Activation of Superficial Dorsal Horn Neurons in the Mouse by a PAR-2 Agonist and 5-HT: Potential Role in Itch. Journal of Neuroscience. 2009b;29:6691–9. doi: 10.1523/JNEUROSCI.6103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. Scratching Behavior and Fos Expression in Superficial Dorsal Horn Elicited by Protease-Activated Receptor Agonists and Other Itch Mediators in Mice. Journal of Pharmacology and Experimental Therapeutics. 2009c;329:945–51. doi: 10.1124/jpet.109.152256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford R. Experiments relating to the itch sensation, its peripheral mechanism, and central pathways. Clin Sci. 1938;3:377–86. [Google Scholar]
- Brull SJ, Atanassoff PG, Silverman DG, Zhang J, Lamotte RH. Attenuation of experimental pruritus and mechanically evoked dysesthesiae in an area of cutaneous allodynia. Somatosens Mot Res. 1999;16:299–303. doi: 10.1080/08990229970366. [DOI] [PubMed] [Google Scholar]
- Carstens E, Kuraishi Y. Animal models of itch: scratching away at the problem. In: Yosipovitch G, Greaves MW, Fleischer AB, McGlone F, editors. Itch: Basic Mechanisms and Therapy. Monticello, NY: Marcel Dekker; 2004. pp. 35–50. [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–8. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–14. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer G, Dotzer M, Diepgen TL, Handwerker HO. Opiate and H1 antagonist effects on histamine induced pruritus and alloknesis. Pain. 1997;73:239–43. doi: 10.1016/S0304-3959(97)00098-5. [DOI] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126:16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus - Central sensitization for itch. Neurology. 2004a;62:212–7. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Handwerker H, Miyachi Y, Schmelz M. Neuronal sensitization for itch in patients with chronic pruritus. Experimental Dermatology. 2004b;13:589. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–47. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–69. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte RH. Subpopulations of nocifensor neurons contributing to pain and allodynia, itch and alloknesis. Am Pain Soc J. 1992;1:115–26. [Google Scholar]
- LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol. 2009;101:1430–43. doi: 10.1152/jn.91268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–65. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. 2011;4:ra45. doi: 10.1126/scisignal.2001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. 2002;88:285–92. doi: 10.1254/jjp.88.285. [DOI] [PubMed] [Google Scholar]
- Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–9. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Carstens MI, Carstens E. c-fos expression in superficial dorsal horn of cervical spinal cord associated with spontaneous scratching in rats with dry skin. Neurosci Lett. 2003;347:62–4. doi: 10.1016/s0304-3940(03)00609-8. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Dong X, Lamotte RH. BAM8–22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci. 2011;31:7563–7. doi: 10.1523/JNEUROSCI.1192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Alreja M, LaMotte RH. Psychophysical studies of the itch sensation and itchy skin (“alloknesis”) produced by intracutaneous injection of histamine. Somatosens Mot Res. 1991;8:271–9. doi: 10.3109/08990229109144750. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–80. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren CF, Hagermark O, Bergstrom R. Patients’ perception of itch induced by histamine, compound 48/80 and wool fibres in atopic dermatitis. Acta Derm Venereol. 1991;71:488–94. [PubMed] [Google Scholar]
- Weisshaar E, Dunker N, Rohl FW, Gollnick H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp Dermatol. 2004;13:298–304. doi: 10.1111/j.0906-6705.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]