Abstract
A series of novel 3-nitro-1H-1,2,4-triazole-(and in some cases 2-nitro-1H-imidazole)-based amides and sulfonamides were characterized for their in vitro anti-trypanosomal and antileishmanial activities as well as mammalian toxicity. Out of 36 compounds tested, 29 (mostly 3-nitro-1H-1,2,4-triazoles) displayed significant activity against T. cruzi intracellular amastigotes (IC50 ranging from 28 nM to 3.72 μM) without concomitant toxicity to L6 host cells (selectivity 66 to 2782). Twenty three of these active compounds were more potent (up to 58 fold) than the reference drug benznidazole, tested in parallel. In addition, 9 nitrotriazoles which were moderately active (0.5 μM ≤ IC50 < 6.0 μM) against T. b. rhodesiense trypomastigotes, were 5 to 31 fold more active against bloodstream-form T. b. brucei trypomastigotes engineered to overexpress NADH-dependent nitroreductase (TbNTR). Finally, 3 nitrotriazoles displayed a moderate activity against the axenic form of Leishmania donovani. Therefore, 3-nitro-1H-1,2,4-triazole-based amides and sulfonamides are potent anti-trypanosomal agents.
Keywords: nitrotriazoles, amides, sulfonamides, T. cruzi, Chagas disease, anti-trypanosomal agents
INTRODUCTION
The trypanosomatids protozoan parasites Trypanosoma cruzi (T. cruzi), Trypanosoma brucei (T. brucei), and various Leishmania species are the causative agents of Chagas disease, human African trypanosomiasis (HAT) and different forms of leishmaniasis, respectively. Over 20 million people are infected by T. cruzi, T. brucei and Leishmania, resulting in 100,000 of deaths per year.1 Chagas disease is transmitted by blood sucking triatomine insects and occurs mainly in Latin America. Despite the fact that in the past 20 years the number of incidences for both Chagas and HAT has significantly declined, primarily due to vector control initiatives,2 the number of cases in non-endemic regions such as the United States, Australia, Europe and Japan is on the rise.3 Reasons for this rise include population migration, drug usage and medical practices. With no immediate prospect for vaccines, chemotherapy is the only way to fight the parasite in the patient.
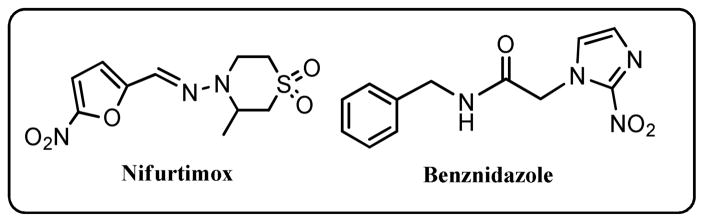
Currently, two nitroheterocycle prodrugs, nifurtimox (4-(5-nitrofurfurylidenamino)-3-methylthio-morpholine-1,1-dioxide) (Nfx) and benznidazole (N-benzyl-2(2-nitro-1H-imidazol-1-yl)acetamide) (Bnz) (Chart 1), are used to treat Chagas disease.4 However, their use is problematic as both can cause side effects, have limited efficacy, while some strains are refractory to treatment.5 In addition, the large quantities of medication required render it expensive, and the recommended long course of treatment is often not completed, resulting in the development of resistance. Therefore, the need for new, affordable and safer drugs to treat this disease is urgent.
Chart 1.
Most nitroheterocyclic compounds function as prodrugs and must undergo activation before mediating their cytotoxic effects. Initially it was proposed that the trypanocidal action of Nfx was due to its ability to induce oxidative stress through 1-electron reduction of its nitro-group and the subsequent formation of superoxide anions via a futile cycle.5–9 Several trypanosomal flavoproteins have been shown to mediate 1-electron reduction in vitro. However, more recent studies have shown that the above process does not occur to such a degree to cause toxicity to the parasites10 and that a type I nitroreductase (NTR)11 is responsible for Nfx and Bnz trypanocidal activity. This enzyme mediates a series of 2 electron reduction reactions resulting in the fragmentation of the heterocyclic ring and production of toxic metabolites.10,12 The fact that the activation of nitroheterocyclic prodrugs can be catalyzed by the type I NTR, which is absent from most eukaryotes, with trypanosomes being a major exception, have led to a renewed interest in the use of such compounds13–18 as antiparasitic agents.
We have recently reported19 that 3-nitro-1H-1,2,4-triazole-based aromatic and aliphatic amines demonstrate excellent in vitro activity against intracellular T. cruzi amastigotes and in some cases activity against T. b. rhodesiense and T. b. brucei parasites. We have also shown that 3-nitrotriazole-based amines are activated by type I nitroreductase and that blood stream form T. b. brucei parasites overexpressing NTR are hypersensitive to these compounds. Moreover, these compounds were significantly less toxic in host cells compared to parasites, and up to 34 fold more potent than the reference compound benznidazole.19 Interestingly, the 3-nitrotriazole-based amines that were evaluated in the Ames test, were found negative for mutagenicity, in contrast to their 2-nitroimidazole analogs (unpublished data). Treatment of T. cruzi-infected mice with one aromatic amine, NTLA-1,19,20 given at just 2 mg/kg/day × 50 days, resulted in a rapid and persistent drop in peripheral parasite levels and in a fraction of cures.21 Importantly, there was an absolute correlation between treatment efficacy as determined parasitologically and the increase in the fraction of T. cruzi-specific CD8+ T cells with a T central memory phenotype in the peripheral blood of treated mice.21 Several other 3-nitrotriazole-based amines are currently being investigated in vivo for antichagasic activity. Encouraged by these results, we have expanded our investigation to the classes of 3-nitro-1H-1,2,4-triazole-based amides and sulfonamides. Here we describe the synthesis and in vitro evaluation of such compounds as anti-trypanosomal agents.
CHEMISTRY
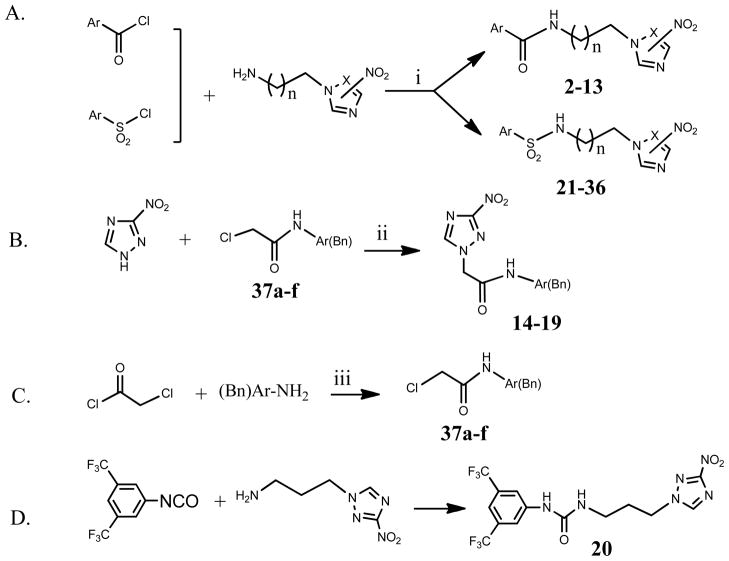
The structure of all compounds is depicted on Table 1. Their synthesis is straightforward and based on well-established chemistry, outlined in Scheme 1. Compound 1 has been described before.22 Amides 2–13 and sulfonamides 21–36 were synthesized at room temperature by nucleophilic substitution of the appropriate arylcarbonyl/arylsulfonyl chloride by the appropriate nitrotriazole/nitroimidazole alkyl amine23 in the presence of triethyl amine (Scheme 1A). For compounds 3, 5, 22, 26, 30 and 32 the hydrochloride salt of 2-(3-nitro-1H-1,2,4-triazole)ethylamine was used because the free amine was too water soluble to be extracted by an organic solvent after its synthesis. Amides 14–19 were synthesized as depicted in Scheme 1B, according to the literature24. First, 3-nitro-1,2,4-triazole was converted to its potassium salt by treating with KOH in acetonitrile under mild heating and then this mixture was added to a solution of the appropriate α-chloroacetamide 37a–f in acetonitrile for a nucleophilic substitution, which occurred under refluxing conditions (8 h). The 2-chloro-N-arylacetamides 37a–f were synthesized through nucleophilic acyl substitution of an appropriate arylamine with 2-chloroacetyl chloride in dry dichloromethane24 (Scheme 1C). The yields of the final compounds in Table 1 were in general good to very good with the exception of some compounds (14, 19, 22, 26, 29, 30–32) with yields < 50%. However, the yields are higher if they are calculated on the basis of recovered starting material, since on many occasions unreacted chloride was isolated from the reaction mixture. Finally, the urea 20 was formed by addition of 3-(3-nitro-1H-1,2,4-triazolyl)propylamine to 3,5-bis(trifluoromethyl)phenyl isocyanate.
Table 1.
In vitro biological and physical properties of 3-nitrotriazole-based amides/sulfonamides.
| Comp. | T.b.rhod.a | SI | T. cruzib | SI | L.don. ax.c | SId | L6e | IC50 Bnz/IC50 Comp | logP | PSA | Chemical Structure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | IC50(μM) | IC50 (μM) | (Å2) | |||||||
| Melars. | 0.012 | Reference | |||||||||
| Bnz | 1.562 | Reference | |||||||||
| Miltef. | 0.382 | Reference | |||||||||
| Podoph. | 0.022 | Reference | |||||||||
| 1 | 21.374 | 6.053 | 29 | 13.77 | 176.6 | 0.3 | 2.52 | 92.74 |  |
||
| 2 | 46.648 | 3.715 | 74 | 36.03 | 274 | 0.4 | 3.07 | 102 | 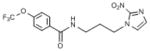 |
||
| 3 | 3.161 | 0.438 | >625 | 33.44 | >273.6 | 3.6 | 2.09 | 105.6 | |||
| 4 | 0.501 | 208 | 0.176 | 591 | 7.93 | 104.3 | 8.9 | 2.66 | 105.6 | ||
| 5 | 1.986 | >131 | 0.73 | >357 | 29.25 | >260.9 | 2.1 | 2.64 | 114.9 | ||
| 6 | 1.391 | >193 | 0.113 | >2381 | 12.98 | >268 | 13.8 | 3.22 | 114.9 | ||
| 7 | 3.761 | 0.353 | >826 | 37.32 | >292 | 4.4 | 2.15 | 105.6 | |||
| 8 | 16.4 | 11.4 | 0.642 | 290.7 | 12.34 | 186.6 | 2.4 | 3.03 | 105.6 |  |
|
| 9 | 3.546 | 3.459 | >84 | 96.51 | >291 | 0.5 | 1.32 | 118.5 | |||
| 10 | 3.22 | 0.138 | 1579 | 19.94 | 217.98 | 11.3 | 1.81 | 118.5 | |||
| 11 | 4 | 0.132 | 691 | 13.41 | 91.5 | 11.8 | 2.33 | 118.5 | |||
| 12 | 34.862 | 0.807 | >379 | 62.69 | >306 | 1.9 | 0.98 | 131.4 | |||
| 13 | 0.587 | 199 | 0.043 | 2782 | 8.37 | 117 | 36.3 | 2.92 | 105.6 |  |
|
| 14 | 9.96 | 3.383 | 102 | 28.12 | 344.83 | 0.5 | 0.95 | 105.6 | |||
| 15 | 6.474 | 0.970 | 133 | 10.39 | 128.88 | 1.6 | 1.83 | 105.6 |  |
||
| 16 | 3.404 | 0.307 | 468 | 51.37 | 143.77 | 5.1 | 2.17 | 96.84 | |||
| 17 | 11.51 | 14.8 | 1.799 | 94.4 | 5.91 | 28.7 | 169.8 | 0.9 | 2.58 | 118.5 |  |
| 18 | 48.45 | 3.4 | 6.588 | 24.8 | 5.82 | 28 | 163.1 | 0.2 | 2.67 | 118.5 | |
| 19 | 34.42 | <1 | 7.876 | 3.3 | 4.68 | 5.6 | 26.3 | 0.2 | 1.88 | 131.66 |  |
| 20 | 6.03 | 4 | 0.734 | 33 | 11.43 | 24.18 | 2.1 | 3.08 | 117.66 |  |
|
| 21 | 27.51 | 1.659 | 106 | 15.55 | 175.99 | 0.9 | 2.27 | 109.81 | |||
| 22 | 2.79 | 0.803 | 248.5 | 32.38 | 199.5 | 1.9 | 1.84 | 122.70 | |||
| 23 | 0.504 | 467 | 0.359 | 656 | 13.09 | 235.33 | 4.4 | 1.9 | 122.70 | ||
| 24 | 0.354 | 240 | 0.71 | 120 | 7.79 | 84.91 | 2.2 | 2.42 | 122.70 | ||
| 25 | 10.313 | 0.644 | 178 | 46.09 | 114.77 | 2.4 | 2.78 | 122.70 | |||
| 26 | 36.7 | 3 | 1.677 | 66.2 | 33.26 | 111.1 | 0.9 | 2.72 | 122.70 | 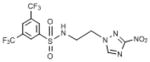 |
|
| 27 | 11.3 | 9.6 | 0.322 | 337.6 | 20.74 | 108.7 | 4.9 | 2.78 | 122.70 |  |
|
| 28 | 2.54 | 121 | 0.412 | >746.8 | 38.15 | >8.1 | >307.7 | 3.8 | 1.54 | 122.70 |  |
| 29 | 0.477 | 234.9 | 0.203 | 551.7 | 7.8 | 14.4 | 112 | 7.7 | 2.97 | 131.93 | |
| 30 | 8.39 | >38.3 | 6.463 | >48 | 112.86 | >321.5 | 0.2 | 0.73 | 122.70 |  |
|
| 31 | 6.49 | >47.4 | 2.237 | >137.6 | 79.38 | >307.7 | 0.7 | 0.79 | 122.70 | ||
| 32 | 35.88 | >9.3 | 83.39 | 4 | >332.2 | >332.2 | 0.0 | −0.25 | 140.52 |  |
|
| 33 | 21.9 | >14.5 | 20.57 | >15 | 223.17 | >317.5 | 0.1 | −0.19 | 140.52 |  |
|
| 34 | 1.99 | 122 | 0.438 | 556 | 33 | 7.4 | 243.5 | 3.6 | 1.74 | 122.70 | |
| 35 | 1.049 | 0.028 | 1764 | 7.54 | 50 | 55.8 | 2.67 | 122.70 |  |
||
| 36 | 6.519 | 0.4 | 519 | 32.87 | 208 | 3.9 | 1.18 | 135.59 | |||
| active | moderately active | active but cytotoxic, low specificity | |||||||||
T.b. rhodesiense, strain STIB 900 trypomastigotes;
T. cruzi, strain Tulahuen C4 amastigotes;
L. donovani axenic, strain MHOM-ET-67/L82 amastigotes;
SI is the ratio: IC50 in L6 cells/IC50 in each parasite.
Cytotoxicity in L6 cells. Reference drugs: Melarsoprol (Melars), Benznidazole (Bnz), Miltefosine (Miltef), Podophylotoxin (Podoph). The IC50 value of each reference is the mean from 36 measurements in parallel with each compound (SD was 0.001, 0.011 and 0.005 for Melars, Bnz and Miltef, respectively). PSA: polar surface area; All physical properties were predicted by using the Marvin Calculator (www.chemaxon.com).
Scheme 1.
i) Et3N (2 eq), CH2Cl2, RT, 12 h; n = 1–3; X= C, 2-NO2; X= N, 3-NO2; when n =1, the HCl salt was used instead of the free amine with 4 eq of Et3N.
ii) KOH, CH3CN, mild heating. iii) Et3N, CH2Cl2.
RESULTS AND DISCUSSION
Anti-Trypanosomal activity of nitrotriazole/nitroimidazole-based amides and sulfonamides
The in vitro growth inhibitory properties of all compounds against bloodstream form T. b. rhodesiense trypomastigotes, T. cruzi amastigotes (in infected L6 myoblasts), axenically cultured L. donovani amastigotes and rat skeletal myoblasts (L6 cells) were evaluated by using standard drug screens.25 From resultant dose response curves, IC50 values in μM were determined (Table 1). The criteria used for activity take into account the complex life cycles of the parasites and the fact that T. cruzi and L. donovani are, in contrast to T.b. rhodesiense, intracellular parasites. These criteria were established by the TDR’s “compound screeners network”, published in a review26 and are as follows: For T. b. rhodesiense, compounds that gave an IC50 < 0.5 μM, were designated as ‘active’, while those yielding an IC50 = 0.5–6.0 μM or an IC50 > 6.0 μM were designated ‘moderately active’ and ‘inactive’, respectively. For T. cruzi, IC50 < 4.0 μM, ‘active’; IC50 = 4.0–60 μM, ‘moderately active’; IC50 > 60 μM, ‘inactive’. For L. donovani, IC50 < 1 μM, ‘active’; IC50 = 1.0–6.0 μM, ‘moderately active’; IC50 > 6.0 μM, ‘inactive’.
On the basis of these criteria, all but compound 32 were active or moderately active against T. cruzi, 16 compounds (47 %) were active or moderately active against T. b. rhodesiense, and only 3 compounds (~ 8%) were moderately active against L. donovani parasites (Table 1). However, for a compound to be considered for further in vivo investigation, the growth inhibitory effect against the mammalian cell line L6 has to be evaluated from which a measure of a compound’s cytotoxicity can be deduced. Thus, the selectivity index (SI), namely the ratio of IC50 against L6 cells to IC50 against each parasite, is also an important parameter and both IC50 and SI values are used to rank compounds.26 This SI must be ≥ 100 for T.b. rhodesiense, ≥ 50 for T. cruzi and ≥ 20 for L. donovani axenic amastigotes.
On the basis of the above, only 9 compounds (4–6, 13, 23, 24, 28, 29 and 34) were moderately active/active and selective against T. b. rhodesiense, whereas 30 compounds (83%), namely 1–17, 21–31 and 34–36 were active (with the exception of 30 which was moderately active) and selective against T. cruzi (Table 1). Compounds 17 and 18, which were moderately active against L. donovani have also an acceptable selectivity. Therefore, as in the case of 3-nitrotriazole-based amines,19 the majority of these 3-nitrotriazole-based amides/sulfonamides act as antichagasic agents.
Evaluation of SARs: Analysis of the nitroheterocyclic ring
On the basis of our previous experience that the 2-nitroimidazole-based aromatic and aliphatic amines tend to be significantly less potent as anti-trypanosomal agents and more toxic to the host cells than their 3-nitrotriazole analogs19, we focused more on the synthesis and evaluation of 3-nitrotriazole-based amides/sulfonamides. Therefore, only two 2-nitroimidazole-based amides (1 and 2) and one sulfonamide (21) were included. Because of the very limited number of such compounds, no solid conclusions can be obtained regarding the effect of the nitroheterocyclic ring on the anti-trypanosomal activity of these classes. However, it is apparent that all of these compounds were inactive against T. b. rhodesiense, and in general, they were less potent anti-chagasic agents than their closely related 3-nitrotriazoles or benznidazole (compare 1 with 3, 4 and 7; 2 with 5 and 6; 21 with 23) (Table 1).
Analysis of amides in which the 3-nitrotriazole ring is linked through the amino group
Comparing the antichagasic activity of the N-(3-nitrotriazolyl-alkyl)benzamides 3–8, it is observed that activity increases with the length of the linker between the 3-nitrotriazole ring and amido group (Table 1, compare 3 with 4 and 7; 5 with 6). The same rule applies for the activity against T. b. rhodesiense as well. Replacing the trifluoromethyl group in 3 with the trifluoromethoxy group, resulted in decreased activity and selectivity against T. cruzi in 5; however the opposite effect was observed in the case of compounds 4 and 6. Interestingly, the more lipophilic 6, was slightly less toxic to L6 cells compared to the less lipophilic 5 and, because of its increased potency against T. cruzi, resulted in a very high selectivity of > 2381. It is also worthy mentioning that the trifluoromethoxy group increased lipophilicity to the same degree as two methylene groups (Table 1). However, this increased lipophilicity was not always translated to increased antichagasic or anti-HAT activity and the length of the linker played a more important role. The addition of an extra trifluoromethyl group in the phenyl ring of 8 resulted also in decreased antichagasic activity and selectivity, as well as in inactivity against T.b. rhodesiense (Table 1). Exchanging the phenyl group with a pyridino- in 9, significantly decreased the activity and selectivity against T. cruzi but did not have any dramatic effect on the moderate activity against T. b. rhodesiense (compare 7 with 9).
Quinoline-2-carboxamides 10 and 11 demonstrated exceptional in vitro activity against T. cruzi and very good selectivity. The additional methylene in the linker of 11 naturally increased lipophilicity of this compound and led to a decreased selectivity (Table 1). Going from the quinoline-2-carboxamide 10 to the quinoxaline analog 12, we observe a decrease in the antichagasic activity and selectivity, and complete inactivity against T. b. rhodesiense (Table 1). A significant drop in logP value compared to 10 (Table 1) may be related to this inactivity. Finally, the 4-phenylbenzamide 13 was the most potent derivative against T. cruzi, with an IC50 of 43 nM (36 times more potent than benznidazole) and selectivity of 2782, the highest selectivity observed in all compounds. Compound 13 was also moderately active against T. b. rhodesiense (Table 1).
All the 3-nitrotriazole-based amides in which the nitrotriazole ring was linked through the amino group (3–13), with the exception of 9, were 1.9–36 fold more potent than benznidazole against T. cruzi amastigotes (Table 1).
Analysis of amides in which the 3-nitrotriazole ring is linked through the carbonyl group
A small number of amides (14–19) in which the 3-nitrotriazole ring is linked through the carbonyl group were also synthesized for comparison with benznidazole. Compound 14 was 0.5 fold less potent against T. cruzi amastigotes than its 2-nitroimidazole-bearing analog, benznidazole (Table 1), perhaps due to its decreased lipophilicity (logP 0.95 versus 1.32 for benznidazole). Indeed, the more lipophilic amides 15 and 16 were also more potent antichagasic agents than benznidazole (Table 1).
Interestingly, despite their relatively high lipophilicity, the benzothiazole acetamides 17 and 18 and the benzoxazole acetamide 19 were less potent against T. cruzi amastigotes compared to benznidazole. Similarly, all three compounds were inactive against T. b. rhodesiense (Table 1). However, compounds 17–19 demonstrated a moderate anti-leishmanial activity and could be considered as initial scaffolds for further investigation for such drugs.
To further expand the class of amides, we have evaluated one urea (20). Although urea 20 was similarly active against T. cruzi with the analogous amide 8, it was significantly more toxic, resulting in an unacceptable selectivity of 33 (Table 1). Lipophilicity alone could not account for the toxicity of 20, since both 8 and 20 have similar logP values (Table 1).
Analysis of N-(3-nitrotriazole-alkyl) arene-sulfonamides
Evaluating sulfonamides 21–36, it is observed that all but the methyl-imidazole sulfonamides 32 and 33, were potent antichagasic agents. Looking at Table 1, it is apparent that compounds 32 and 33 were the only ones with negative logP values and PSA > 140 Å, indicative of poor penetration through cell membranes.
The 2-nitroimidazole-based sulfonamide 21 was a more potent antichagasic agent than the analogous amides 1 and 2, but still slightly less active than the reference drug benznidazole (Table 1). These results imply that perhaps further evaluation of 2-nitroimidazole-based sulfonamides as antichagasic agents is worthwhile. However, as was mentioned previously, both 2-nitroimidazole-based amides and sulfonamides were not effective anti-HAT agents compared to their 3-nitrotriazole-based analogs.
As in the case of N-(3-nitrotriazolyl-alkyl)benzamides, activity of N-(3-nitrotriazolyl-alkyl) benzene sulfonamides 22–24 against T. b. rhodesiense, proportionally increases with the length of the linker between the 3-nitrotriazole ring and sulfamido group (Table 1). The same rule, however, does not apply here for activity against T. cruzi, although it is clear that two methylene-linker corresponds to the lowest activity (Table 1).
In general, sulfonamides were slightly less potent antichagasic agents compared to their analogous amides (compare 22 with 3; 24 with 4; 29 with 6). However, sulfonamides 27 and 35 were more potent than amides 8 and 13, respectively, against T. cruzi (Table 1). A second trifluoromethyl group on the phenyl ring (25, 26 and 27) resulted in inactivity against T. b. rhodesiense, independently of its position on the ring (25, 26, 27), with the linker length (26) being the most determinant parameter. However, the effect of the second trifluoromethyl group on the antichagasic activity of sulfonamides was not clear (Table 1). Replacing the trifluoromethyl group in 24 with a trifluoromethoxy group in 29 increased the activity and selectivity against T. cruzi but slightly reduced the activity and selectivity against T. b. rhodesiense. Membrane permeability issues, due to a greater PSA value in 29, may be the reason for this slight reduction in anti-HAT activity (Table 1).
Replacing the trifluoromethyl group in 23 with a methyl group in 28 resulted in slightly decreased activity and slightly increased selectivity against T. cruzi, perhaps due to a slight decrease in lipophilicity (Table 1). However, this slight decrease in lipophilicity of 28 had a more dramatic decrease in both activity and selectivity against of T. b. rhodesiense (Table 1). Exchanging the tolyl group in 28 with a benzyl group in 31 further decreased the logP value and resulted in lower activity and selectivity against both T. cruzi and T. b. rhodesiense (Table 1). Finally, shortening the linker of 31 by one methylene group in 30 significantly decreased the activity against T. cruzi and T. b. rhodesiense and resulted in unacceptable selectivity (Table1). Interestingly, both benzyl sulfonamides 30 and 31 were less potent antichagasic agents than benznidazole.
As in the case of 4-phenylbenzamide 13, the 4-phenylbenzene sulfonamide 35 was the most potent antichagasic compound in the series of sulfonamides, with an IC50 of 28 nM (~56 times more potent than benznidazole) and selectivity of 1764. Sulfonamide 35 was more potent against T. cruzi than the analogous amide 13, but less active than 13 against T. b. rhodesiense. In addition, increased toxicity of 35 to L6 host cells, independently of lipophilicity, resulted in decreased selectivity as compared to 13 (Table 1).
Replacing the phenyl ring with a chloro-thiophene in 34, slightly decreased the potency against T. cruzi and had a more significant impact in selectivity due to an increase in toxicity (compare 28 with 34). However, the activity against T. b. rhodesiensie and selectivity of 34 was similar to that of 28 (Table 1). Replacing the benzene ring with an 8-quinoline in 36, did not affect the antichagasic potency but resulted in increased toxicity and decreased selectivity as compared to 28. The decreased anti-HAT activity of 36 compared to 28, may be related to a decreased lipophilicity and an increased PSA value (Table 1).
The involvement of type I nitroreductase in the activation of 3-nitrotriazole-based amides/sulfonamides
Nitroheterocyclic prodrugs must undergo enzyme-mediated activation within the pathogen to have cytotoxic effects, a reaction catalyzed by nitroreductases. Both Nfx and Bnz are activated by the NADH-dependent, oxygen insensitive, mitochondrially localized, bacterial-like, type I NTR, and down-regulation of this enzyme resulted in resistance to these compounds.10–12
Several compounds from all sub-categories in Table 1 have been evaluated for anti-HAT activity against bloodstream form T. b. brucei (Table 2). With few exceptions (3, 13, 20, 35), most compounds demonstrated a greater IC50 value or were inactive against T. b. brucei compared to T. b. rhodesiense (Table 2). Compounds with an IC50 ≤ 5 μM against T. b. brucei were tested in a parasite line engineered to overexpress tetracycline-inducible TbNTR, in order to examine the involvement of this enzyme in their activation (Table 2).
Table 2.
The effect of type I nitroreductase (TbNTR) expression on the activity of selected compounds against bloodstream form T. b. brucei parasites
| ID No | T.b. rhod.a | T.b. bruceib | TbNTRc | TbNTRc | Ratio |
|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | −tet | +tet | −tet/+tet | |
| 3 | 3.16 | 1.3 ± 0.4 | 1.26 ± 0.27 | 0.09 ± 0.02 | 14 |
| 4 | 0.50 | 0.9 ± 0.1 | 1.30 ± 0.28 | 0.13 ± 0.01 | 10 |
| 6 | 1.39 | 3.6 ± 0.7 | 1.05 ± 0.05 | 0.10 ± 0.00 | 11 |
| 7 | 3.76 | > 10 | ndd | nd | nd |
| 8 | 16.4 | >10 | nd | nd | nd |
| 9 | 3.55 | 7.9 ± 0.2 | nd | nd | nd |
| 10 | 3.22 | > 10 | nd | nd | nd |
| 11 | 4.00 | > 10 | nd | nd | nd |
| 12 | 34.86 | > 10 | nd | nd | nd |
| 13 | 0.59 | 0.3 ± 0.0 | 0.28 ± 0.02 | 0.05 ± 0.01 | 6 |
| 14 | 9.96 | > 10 | nd | nd | nd |
| 15 | 6.47 | 8.5 ± 0.2 | nd | nd | nd |
| 16 | 3.40 | > 10 | nd | nd | nd |
| 20 | 6.03 | 1.0 ± 0.0 | 0.81 + 0.07 | 0.18 + 0.02 | 5 |
| 22 | 2.79 | > 10 | nd | nd | nd |
| 23 | 0.50 | 3.4 ± 0.6 | 7.83 ± 0.50 | 0.25 ± 0.01 | 31 |
| 25 | 10.31 | > 10 | nd | nd | nd |
| 26 | 36.7 | 7.9 ± 0.1 | nd | nd | nd |
| 27 | 11.3 | 6.6 ± 0.1 | nd | nd | nd |
| 28 | 2.54 | 4.0 ± 0.3 | 5.63 + 2.40 | 0.24 + 0.02 | 23 |
| 34 | 1.99 | 2.3 ± 0.1 | 4.34 ± 0.05 | 0.23 + 0.01 | 19 |
| 35 | 1.05 | 0.5 ± 0.0 | 0.44 ± 0.03 | 0.07 ± 0.01 | 6 |
| 36 | 6.52 | > 10 | nd | nd | nd |
| Nfxe | 1.71 ± 0.06 | 0.13 ± 0.04 | 13 | ||
| Bnzf | 21.80 ± 1.00 | 2.20 ± 0.30 | 10 | ||
| Melarsg | 0.0034 ± 0.0001 | 0.0034 ± 0.0001 | 1 | ||
STIB 900 trypomastigotes;
bloodstream form wild type T. b. brucei (Lister 427; clone 221a) parasites;
bloodstream form T. b. brucei parasites, engineered to overexpress type I nitroreductase in the presence (+tet) or absence (−tet) of tetracycline;
not determined;
nifurtimox (positive control);
benznidazole (positive control);
melarsoprol (negative control).
It is observed that parasites overexpressing tetracycline-inducible TbNTR were more susceptible to all such compounds (3, 4, 6, 13, 20, 23, 28, 34 and 35) as compared to wild-type parasites, with −tet/+tet (non-induced/induced) ratio ranging from 5 to 31 (Table 2). This implies that the major growth inhibitory activity of these compounds is via type I NTR activation. It is also observed in Table 2 that the least active compounds against wild type T. b. brucei (6, 23, 28 and 34) showed a greater −tet/+tet ratio than the most active compounds 13 and 35.
Selected compounds from Table 1 were tested as substrates of purified type I TbNTR. As shown in Fig. 1, all of the tested compounds were preferred substrates of the nitroreductase and there is, in general, a good correlation between enzymatic activity and activity against T. b. rhodesiense.
Fig. 1.
Activity of recombinant TbNTR toward different amides/sulfonamides and nifurtimox (Nfx).
To exclude the possibility that these compounds may exert some of their anti-trypanosomal activity via trypanothione reductase (TR) inhibition,8,27 we have tested selected compounds (3, 6, 10, 15, 16, 21, 23) against this enzyme. None of the compounds showed an inhibitory activity against TR at concentrations < 100 μM (unpublished results, private communication with Dr. Mary O’Sullivan, Canisius College, Buffalo, NY).
CONCLUSIONS
From the above results and discussion, it is concluded that, like the 3-nitrotriazole-based aromatic and aliphatic amines, 3-nitrotriazole-based amides and sulfonamides exert exceptional in vitro antichagasic and anti-HAT activities. All tested compounds satisfy the Lipinski rule of 5 and at least 19 of them (3–8, 10–13, 16, 22, 23, 27–29, 34–36) have been identified (Table 1) as potential candidates for in vivo studies in T. cruzi infected mice. All of the 19 compounds have demonstrated significant antichagasic activity at low to intermediate nmolar concentrations and selectivity > 200. In addition, all of them were 2–56 fold more potent as antichagasic agents than benznidazole (Table 1). Compounds 4, 13, 23, 24 and 29 also deserve further in vivo investigation as anti-HAT agents, whereas compounds 17–19 should be used as initial scaffolds for further investigation of anti-leishmania drugs.
EXPERIMINTAL
All starting materials and solvents were purchased from Sigma-Aldrich (Milwaukee, WI), were of research-grade quality and used without further purification. Solvents used were anhydrous and the reactions were carried out under a nitrogen atmosphere and exclusion of moisture. Melting points were determined by using a Mel-Temp II Laboratory Devices apparatus (Holliston, MA) and are uncorrected. Elemental Analyses were obtained by Midwest Microlab, LLC (Indianapolis, IN). Proton NMR spectra were obtained on a Varian Inova-500 or a Bruker Avance-III-500 spectrometer at 500 MHz and are referenced to Me4Si or to the corresponding protonated solvent, if the solvent was not CDCl3. High-resolution electrospray ionization (HRESIMS) mass spectra were obtained on a Agilent 6210 LC-TOF mass spectrometer at 11000 resolution. Thin-layer chromatography was carried out on aluminum oxide N/UV254 or polygram silica gel G/UV254 coated plates (0.2 mm, Analtech, Newark, DE). Chromatography was carried out on preparative TLC alumina GF (1000 microns) or silicagel GF (1500 microns) plates (Analtech). All of the amides/sulfonamides were purified by preparative TLC chromatography on silicagel GF plates (≥ 95% purity). The results from elemental analysis for C, H and N were within 0.4 of the theoretical value.
The synthesis of compound 1 has been described before.22
General synthetic procedure of arylamides/sulfonamides and urea 20
For compounds 2–13 and 21–36: The appropriate commercially available arylcarbonyl/arylsulfonyl chloride (1.24 mmol) was dissolved in 2–3 mL dry dichloromethane and added dropwise to a solution of 3-nitro-1H-1,2,4-triazolyl-alkylamine23 (1.24 mmol) and triethylamine (2.48 mmol) in 6–8 mL of dry dichloromethane, at room temperature and under an inert atmosphere. In three cases (1, 2, 21), 3-(2-nitro-1H-imidazolyl)-propylamine23 (1.24 mmol) was used. The reaction mixture was worked up after 12 h of stirring at room temperature. For compounds 3, 5, 22, 26, 30 and 32 the hydrochloride salt of 2-(3-nitro-1H-1,2,4-triazole)ethylamine (instead of the free amine) and 4 eq of triethyl amine were used. In this case, the reaction mixture was a suspension and the yields of the final product were not very good.
For urea 20, the commercially available 3,5-bis(trifluoromethyl)phenyl isocyanate (1.1 mmol) was added dropwise to a dichloromethane solution of 3-nitro-1H-1,2,4-triazolyl-propylamine (1.1 mmol), at room temperature and under an inert atmosphere. The urea was formed immediately at 100% yield, as a white precipitate.
For amides 14–19, 3-nitro-1,2,4-triazole (0.9–1.0 mmol) was stirred under an inert atmosphere and exclusion of moisture with 1.2 eq of KOH in acetonitrile under mild heating (ca. 40 °C) and then this suspension was slowly added to an acetonitrile solution of the appropriate α-chloroacetamide24 (37a–f).
α-Chloroacetamides 37b–f were synthesized at room temperature by adding a dichloromethane solution of an appropriate amine (2.79 mmol) and triethylamine (3.07 mmol) to a dichloromethane solution of α-chloroacetyl chloride (3.07 mmol), according to the literature24.
N-[3-(2-Nitro-1H-imidazol-1-yl)propyl]-4-(trifluoromethoxy)benzamide (2)
Off white powder (54%): mp 68–70 °C; 1H NMR (500 MHz, CDCl3) δ: 7.84 (d, J=9.0 Hz, 2H), 7.31 (br s, 2H), 7.30 (s, 1H), 7.18 (s, 1H), 6.40 (br s, 1H), 4.54 (t, J=7.0 Hz, 2H), 3.57 (m, 2H), 2.21 (m, 2H). HRESIMS calcd for C14H14F3N4O4 and C14H13F3N4NaO4 m/z [M+H]+ and [M+Na]+ 359.0962, 381.0781, found 359.0962, 381.0784.
N-[2-(3-Nitro-1H-1,2,4-triazol-1-yl)ethyl]-4-(trifluoromethyl)benzamide (3)
White powder (65%): mp 155–157 °C; 1H NMR (500 MHz, CDCl3) δ: 8.24 (s, 1H), 7.86 (br s, 1H), 7.72 (d, J=8.0 Hz, 2H), 7.46 (d, J=8.0 Hz, 2H), 4.43 (t, J=5.0 Hz, 2H), 3.76 (m, 2H). HRESIMS calcd for C12H11F3N5O3 m/z [M+H]+ 330.0809, found 330.0815.
N-[4-(3-Nitro-1H-1,2,4-triazol-1-yl)butyl]-4-(trifluoromethyl)benzamide (4)
White powder (62%): mp 78–79 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.69 (s, 1H), 8.07 (d, J=8.0 Hz, 2H), 8.04 (br s, 1H), 7.81 (d, J=8.0 Hz, 2H), 4.49 (t, J=7.0 Hz, 2H), 3.49 (m, 2H), 2.06 (m, 2H), 1.69 (m, 2H). HRESIMS calcd for C14H15F3N5O3 m/z [M+H]+ 358.1122, found 358.1131.
N-[2-(3-Nitro-1H-1,2,4-triazol-1-yl)ethyl]-4-(trifluoromethoxy)benzamide (5)
White powder (65%): mp 108–109 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.70 (s, 1H), 8.17 (br s, 1H), 7.95 (d, J=8.5 Hz, 2H), 7.41 (d, J=8.5 Hz, 2H), 4.65 (t, J=5.5 Hz, 2H), 3.93 (t, J=5.5 Hz, 2H). HRESIMS calcd for C12H11F3N5O4 m/z [M+H]+ 346.0758, found 346.0765.
N-[4-(3-Nitro-1H-1,2,4-triazol-1-yl)butyl]-4-(trifluoromethoxy)benzamide (6)
White powder (72%): mp 64–65 °C; 1H NMR (500 MHz, CDCl3) δ: 8.26 (s, 1H), 7.82 (d, J=8.5 Hz, 2H), 7.27 (d, J=8.0 Hz, 2H), 6.44 (br s, 1H), 4.39 (t, J=7.0 Hz, 2H), 3.53 (m, 2H), 2.06 (quintet, J=7.0 Hz, 2H), 1.69 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C14H15F3N5O4 m/z [M+H]+ 374.1071, found 374.1075.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-3-(trifluoromethyl)benzamide (7)
White powder (70%): mp 81–83 °C; 1H NMR (500 MHz, CDCl3) δ: 8.45 (s, 1H), 8.05 (s, 1H), 7.98 (d, J=7.5 Hz, 1H), 7.81 (d, J=8.0 Hz, 1H), 6.63 (t, J= 8.0 Hz, 1H), 6.55 (br s, 1H), 4.43 (t, J=6.5 Hz, 2H), 3.58 (m, 2H), 2.32 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C13H13F3N5O3 m/z [M+H]+ 344.0965, found 344.0969.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-3,5-bis(trifluoromethyl)benzamide (8)
White powder (83%): mp 152–153 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.72 (s, 1H), 8.52 (s, 2H), 8.46 (br s, 1H), 8.25 (s, 1H), 4.57 (t, J=7.0 Hz, 2H), 3.59 (q, J=6.5 Hz, 2H), 2.35 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C14H12F6N5O3 m/z [M+H]+ 412.0839 found 412.0844.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-6-(trifluoromethyl)pyridine-3-carboxamide (9)
White powder (71%): mp 92–94 °C; 1H NMR (500 MHz, CDCl3) δ: 9.09 (s, 1H), 8.39 (s, 1H), 8.33 (d, J=8.5 Hz, 1H), 7.82 (d, J=8.0 Hz, 1H), 6.73 (br s, 1H), 4.43 (t, J=6.5 Hz, 2H), 3.60 (q, J=6.5 Hz, 2H), 3.42 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C12H12F3N6O3 and C12H11F3N6NaO3 m/z [M+H]+ and [M+Na]+ 345.0917, 367.0737, found 345.0929, 367.0745. Calculated analysis for C12H11F3N6O3: C, 41.87; H, 3.22 ; N, 24.41. Found: C, 41.93; H, 3.38; N, 24.17.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]quinoline-2-carboxamide (10)
Off white powder (70%): mp 135–137 °C; 1H NMR (500 MHz, CD3OD) δ: 8.68 (s, 1H), 8.46 (d, J=8.5 Hz, 1H), 8.17 (t, J=9.0 Hz, 2H), 8.0 (d, J=8.5 Hz, 1H), 7.83 (t, J=8.5 Hz, 1H), 7.69 (t, J=8.0 Hz, 1H), 4.46 (t, J=8.0 Hz, 2H), 3.58 (t, J=6.5 Hz, 2H), 2.34 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C15H15N6O3 and C15H14N6NaO3 m/z [M+H]+ and [M+Na]+ 327.1200, 349.1020, found 327.1209, 349.1026.
N-[4-(3-Nitro-1H-1,2,4-triazol-1-yl)butyl]quinoline-2-carboxamide (11)
Off white powder (67%): mp 124–126 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.75 (br s, 1H), 8.70 (s, 1H), 8.52 (d, J=8.5 Hz, 1H), 8.24 (d, J=8.5 Hz, 1H), 8.06 (t, J=9.5 Hz, 2H), 7.84 (t, J=7.0 Hz, 1H), 7.70 (t, J=7.0 Hz, 1H), 4.52 (t, J=7.0 Hz, 2H), 3.58 (q, J=6.5 Hz, 2H), 2.09 (m, 2H),1.76 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C16H17N6O3 m/z [M+H]+ 341.1357, found 341.1369.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]quinoxaline-2-carboxamide (12)
Off white powder (66%): mp 143–144 °C; 1H NMR (500 MHz, CDCl3) δ: 9.67 (s, 1H), 8.47 (s, 1H), 8.22 (d, J=8.0 Hz, 1H), 8.21 (br s, 1H), 8.12 (d, J=7.5 Hz, 1H), 7.90 (m, 2H), 4.45 (t, J=6.5 Hz, 2H), 3.66 (q, J=6.5 Hz, 2H), 2.39 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C14H14N7O3 m/z [M+H]+ 328.1153, found 328.1166. Calculated analysis for C14H13N7O3: C, 51.38; H, 4.0; N, 29.96. Found: C, 51.29; H, 4.17; N, 29.68.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-4-phenylbenzamide (13)
White powder (96%): mp 177–179 °C; 1H NMR (500 MHz, CDCl3) δ: 8.48 (s, 1H), 7.85 (d, J=8.5 Hz, 2H), 7.69 (d, J=7.0 Hz, 2H), 7.48 (t, J=7.5 Hz, 2H), 7.40 (t, J=7.5 Hz, 1H), 6.49 (br t, 1H), 4.43 (t, J=6.5 Hz, 2H), 3.57 (q, J=6.5 Hz, 2H), 2.30 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C18H18N5O3 and C18H17N5NaO3 m/z [M+H]+ and [M+Na]+ 352.1404, 374.1224, found 352.1406, 374.1222. Calculated analysis for C18H17N5O3: C, 61.53; H, 4.88; N, 19.93. Found: C, 61.79; H, 4.96; N, 19.58.
N-Benzyl-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (14)
Off white powder (40%): mp 103–106 °C; 1H NMR (500 MHz, CDCl3) δ: 8.39 (s, 1H), 7.38-7.28 (m, 5H), 6.26 (br s, 1H), 4.98 (s, 2H), 4.50 (d, J=5.5 Hz, 2H). HRESIMS calcd for C11H12N5O3 m/z [M+H]+ 262.0935, found 262.0935.
2-(3-Nitro-1H-1,2,4-triazol-1-yl)-N-{[4-(trifluoromethyl)phenyl]methyl}acetamide (15)
White microcrystal (78%): mp 168–170 °C; 1H NMR (500 MHz, CDCl3) δ: 8.37 (s, 1H), 7.62 (d, J=8.0 Hz, 2H), 7.40 (d, J=8.0 Hz, 2H), 6.35 (br s, 1H), 4.99 (s, 2H), 4.56 (d, J=6.0 Hz, 2H). HRESIMS calcd for C12H11F3N5O3 and C12H10F3N5NaO3 m/z [M+H]+ and [M+Na]+ 330.0809, 352.0628, found 330.0814, 352.0632.
1-(4-Benzylpiperidin-1-yl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)ethan-1-one (16)
White powder (84%): mp 129–131 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.57 (s, 1H), 7.31-7.18 (m, 5H), 5.47 (dt, J=19.0, 16.5 Hz, 2H), 4.44 (d J=13 Hz, 1H), 3.97 (d, J=13.5 Hz, 1H), 3.16 (t, J=13.5 Hz, 1H), 2.64 (t, J=13.0 Hz, 1H), 2.60 (d, J=7.0 Hz, 2H), 1.88 (m, 1H), 1.76 (d, J=13.0 Hz, 1H), 1.69 (d, J=13.0 Hz, 1H), 1.36-1.32 (dq, J=12.5, 4.5 Hz, 1H), 1.16-1.13 (dq, J=12.0, 4.0 Hz, 1H). HRESIMS calcd for C16H20N5O3 m/z [M+H]+ 330.1561, found 330.1576. Calculated analysis for C16H19N5O3: C, 58.35; H, 5.82; N, 21.26. Found: C, 58.27; H, 5.83; N, 21.30.
N-(6-methyl-1,3-benzothiazol-2-yl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (17)
Off white powder (59%): mp 230 °C (dec); 1H NMR (500 MHz, CD3COCD3) δ: 8.81 (s, 1H), 7.74 (s, 1H), 7.63 (d, J=8.0 Hz, 1H), 7.28 (d, J=8.0 Hz, 1H), 5.68 (s, 2H), 2.44 (s, 3H). HRESIMS calcd for C12H11N6O3S m/z [M+H]+ 319.0608, found 319.0617.
N-(6-chloro-1,3-benzothiazol-2-yl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (18)
Off white powder (58%): mp 245–248 °C (dec); 1H NMR (500 MHz, CD3COCD3) δ: 8.81 (s, 1H), 8.06 (s, 1H), 7.74 (d, J=8.5 Hz, 1H), 7.04 (dd J=8.5, 2.0 Hz, 1H), 5.71 (s, 2H). HRESIMS calcd for C11H8ClN6O3S m/z [M+H]+ 339.0062, 341.0034, found 339.0072, 341.0045.
N-(5-chloro-1,3-benzoxazol-2-yl)-2-(3-nitro-1H-1,2,4-triazol-1-yl)acetamide (19)
Off white powder (45%): mp 208–210 °C (dec); 1H NMR (500 MHz, CD3COCD3) δ: 8.76 (s, 1H), 7.60 (s, 1H), 7.59 (d, J=9.0 Hz, 1H), 7.34 (dd, J=8.5, 2.0 Hz, 1H), 5.80 (s, 1H). HRESIMS calcd for C11H6ClN6O4 m/z [M−H]− 321.0145, 323.0119, found 321.0147, 323.0143.
1-[3,5-bis(trifluoromethyl)phenyl]-3-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]urea (20)
White powder (95%): mp 151–152 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.71 (s, 1H), 8.68 (br s, 1H), 8.15 (s, 2H), 7.54 (s, 1H), 6.31 (br s, 1H), 4.51 (t, J=6.5 Hz, 2H), 3.35 (m, 2H), 2.21 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C14H13F6N6O3 m/z [M+H]+ 427.0948, found 427.0954.
N-[3-(2-Nitro-1H-imidazol-1-yl)propyl]-4-(trifluoromethyl)benzene-1-sulfonamide (21)
White powder (56%): mp 129–131 °C; 1H NMR (500 MHz, CDCl3) δ: 7.99 (d, J=8.0 Hz, 2H), 7.82 (d, J=8.5 Hz, 2H), 7.24 (s, 1H), 7.19 (s, 1H), 4.77 (br t, 1H), 4.57 (t, J=7.0 Hz, 2H), 3.06 (q, J=6.5 Hz, 2H), 2.12 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C13H14F3N4O4S and C13H13F3N4NaO4S m/z [M+H]+, [M+Na]+ 379.0682, 401.0502 found 379.0685, 401.0506.
N-[2-(3-Nitro-1H-1,2,4-triazol-1-yl)ethyl]-4-(trifluoromethyl)benzene-1-sulfonamide (22)
White powder (35%): mp 155–156 °C; 1H NMR (500 MHz, CDCl3 + several drops of CD3COCD3) δ: 8.51 (s, 1H), 7.99 (d, J=8.5 Hz, 2H), 7.82 (d, J=8.5 Hz, 2H), 7.04 (br s, 1H), 4.56 (t, J=6.0 Hz, 2H), 3.56 (m, 2H). HRESIMS calcd for C11H11F3N5O4S m/z [M+H]+ 366.0478, found 366.0481.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-4-(trifluoromethyl) benzene-1-sulfonamide (23)
White powder (88%): mp 67–68 °C; 1H NMR (500 MHz, CDCl3) δ: 8.36 (s, 1H), 7.97 (d, J=8.5 Hz, 2H), 7.81 (d, J=8.5 Hz, 2H), 5.01 (br t, 1H), 4.51 (t, J=6.5 Hz, 2H), 3.03 (q, J=6.5 Hz, 2H), 2.23 (quintet, J=6.0 Hz, 2H). HRESIMS calcd for C12H13F3N5O4S m/z [M+H]+ 380.0635, found 380.0635.
N-[4-(3-Nitro-1H-1,2,4-triazol-1-yl)butyl]-4-(trifluoromethyl) benzene-1-sulfonamide (24)
White powder (49%): mp 83–85 °C; 1H NMR (500 MHz, CD3OD) δ: 8.59 (s, 1H), 8.03 (d, J=8.0 Hz, 2H), 7.89 (d, J=8.0 Hz, 2H), 4.32 (t, J=7.0 Hz, 2H), 2.94 (t, J=6.5 Hz, 2H), 1.96 (quintet, J=7.5 Hz, 2H), 1.51 (quintet, J=7.5 Hz, 2H). HRESIMS calcd for C13H15F3N5O4S m/z [M+H]+ 394.0791, found 394.0796.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-2,5-bis(trifluoromethyl)benzene-1-sulfonamide (25)
White powder (85%): mp 131–133 °C; 1H NMR (500 MHz, CDCl3) δ: 8.42 (s, 1H), 8.31 (s, 1H), 8.06 (d, J=8.0 Hz, 1H), 8.00 (d, J=8.0 Hz, 2H), 5.10 (br s, 1H), 4.49 (t, J=6.5 Hz, 2H), 3.07 (m, 2H), 2.25 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C13H12F6N5O4S and C13H11F6N5NaO4S m/z [M+H]+ and [M+Na]+ 448.0509, 470.0328, found 448.0496, 470.0310.
N-[2-(3-nitro-1H-1,2,4-triazol-1-yl)ethyl]-3,5-bis(trifluoromethyl)benzene-1-sulfonamide (26)
White powder (40%): mp 164–165 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.67 (s, 1H), 8.40 (s, 2H), 8.37 (s, 1H), 4.60 (t, J=5.5 Hz, 2H), 3.67 (t, J=5.5 Hz, 2H). HRESIMS calcd for C12H10F6N5O4S and C12H9F6N5NaO4S m/z [M+H]+ and [M+Na]+ 434.0352, 456.0172, found 434.0358, 456.0178.
N-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]-3,5-bis(trifluoromethyl)benzene-1-sulfonamide (27)
White microcrystals (62%): mp 132–134 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.64 (s, 1H), 8.41 (s, 2H), 8.38 (s, 1H), 7.15 (br s, 1H), 4.53 (t, J=7.0 Hz, 2H), 3.15 (t, J=6.5 Hz, 2H), 2.22 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C13H12F6N5O4S m/z [M+H]+ 448.0509, found 448,0495.
4-Methyl-N-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]benzene-1-sulfonamide (28)
White microcrystals (81%): mp 122–124 °C; 1H NMR (500 MHz, CD3COD) δ: 8.57 (s, 1H), 7.70 (d, J=8.5 Hz, 2H), 7.37 (d, J=8.5 Hz, 2H), 4.41 (t, J=6.5 Hz, 2H), 3.31 (t, J=6.5 Hz, 2H), 2.42 (s, 3H), 2.08 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C12H16N5O4S and C12H15N5NaO4S m/z [M+H]+ and [M+Na]+ 326.0918, 348.0737, found 326.0917, 348.0734. Calculated analysis for C12H15N5O4S: C, 44.30; H, 4.65; N, 21.53; S, 9.85. Found: C, 44.51; H, 4.81; N, 21.22; S, 9.89.
N-[4-(3-nitro-1H-1,2,4-triazol-1-yl)butyl]-4-(trifluoromethoxy)benzene-1-sulfonamide (29)
White powder (42%): mp 66–68 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.64 (s, 1H), 7.99 (d, J=8.5 Hz, 2H), 7.55 (d, J=8.0 Hz, 2H), 6.68 (br s, 1H), 4.42 (t, J=7.0 Hz, 2H), 3.01 (t, J=6.5 Hz, 2H), 2.01 (m, 2H), 1.59 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C13H15F3N5O5S m/z [M+H]+ 410.0741, found 410.0744.
N-[2-(3-Nitro-1H-1,2,4-triazol-1-yl)ethyl]-1-phenylmethanesulfonamide (30)
White powder (31%): mp 165–166 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.62 (s, 1H), 7.42-7.35 (m, 5H), 6.42 (br s, 1H), 4.50 (t, J=5.5 Hz, 2H), 4.37 (s, 2H), 3.58 (t, J=5.5 Hz, 2H). HRESIMS calcd for C11H14N5O4S and C11H13N5NaO4S m/z [M+H]+ and [M+Na]+ 312.0761, 334.0580, found 312.0773, 334.0594.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-1-phenylmethanesulfonamide (31)
White microcrystals (45%): mp 104–106 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.62 (s, 1H), 7.43-7.35 (m, 5H), 6.25 (br s, 1H), 4.49 (t, J=7.0 Hz, 2H), 4.35 (s, 2H), 3.13 (m, 2H), 2.17 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C12H16N5O4S and C12H15N5NaO4S m/z [M+H]+ and [M+Na]+ 326.0918, 348.0737, found 326.0923, 348.0737.
1-Methyl-N-[2-(3-nitro-1H-1,2,4-triazol-1-yl)ethyl]-1H-imidazole-2-sulfonamide (32)
White powder (24%): mp 170–172 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.72 (s, 1H), 7.45 (br s, 1H), 7.30 (s, 1H), 7.02 (s, 1H), 4.63 (t, J=6.0 Hz, 2H), 3.90 (s, 3H), 3.79 (t, J=6.0 Hz, 2H). HRESIMS calcd for C8H12N7O4S and C8H11N7NaO4S m/z [M+H]+ and [M+Na]+ 302.0666, 324.0485, found 302.0664, 324.0480.
1-Methyl-N-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]-1H-imidazole-2-sulfonamide (33)
White powder (61%): mp 106–109 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.68 (s, 1H), 7.28 (s, 1H), 6.99 (s, 1H), 4.57 (t, J=7.0 Hz, 2H), 3.92 (s, 3H), 3.28 (t, J=6.5 Hz, 2H). 2.25 (quintet, J=7.0 Hz, 2H). HRESIMS calcd for C9H14N7O4S and C9H13N7NaO4S m/z [M+H]+ and [M+Na]+ 316.0822, 338.0642, found 316.0832, 338.0649. Calculated analysis for C9H13N7O4S: C, 34.28; H, 4.16; N, 31.10; S, 10.17. Found: C, 34.32; H, 4.27; N, 30.83; S, 9.85.
5-Chloro-N-[3-(3-nitro-1H-1,2,4-triazol-1-yl)propyl]thiophene-2-sulfonamide (34)
White powder (75%): mp 104–105 °C; 1H NMR (500 MHz, CD3COCD3) δ: 8.65 (s, 1H), 7.47 (d, J=4.0 Hz, 1H), 7.16 (d, J=4.0 Hz, 1H), 7.01 (br s, 1H), 4.53 (t, J=7.0 Hz, 2H), 3.13 (t, J=6.5 Hz, 2H), 2.23 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C9H11ClN5O4S2 and C9H10ClN5NaO4S2 m/z [M+H]+ and [M+Na]+ 351.9935, 373.9755, found 351.9930, 353.9899, 373.9751, 375.9721.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]-4-phenylbenzene-1-sulfonamide (35)
White powder (60%): mp 132–133 °C; 1H NMR (500 MHz, CDCl3) δ: 8.36 (s, 1H), 7.88 (d, J=8.5 Hz, 2H), 7.73 (d, J=8.0 Hz, 2H), 7.59 (d, J=7.0 Hz, 2H), 7.49 (t, J=7.5 Hz, 2H), 7.43 (t, J=7.0 Hz, 1H), 4.76 (t, J=6.0 Hz, 1H), 4.51 (t, J=6.5 Hz, 2H), 2.80 (q, J=6.5 Hz, 2H), 2.21 (quintet, J=6.5 Hz, 2H). HRESIMS calcd for C17H18N5O4S and C17H17N5NaO4S m/z [M+H]+ and [M+Na]+ 388.1074, 410.0893, found 388.1070, 410.0887.
N-[3-(3-Nitro-1H-1,2,4-triazol-1-yl)propyl]quinoline-8-sulfonamide (36)
Off white powder (63%): mp 142–143 °C; 1H NMR (500 MHz, CDCl3) δ: 9.04 (d, J=4.0 Hz, 1H), 8.40 (s, 2H), 8.32 (d, J=8.0 Hz, 1H), 8.10 (d, J=8.5 Hz, 1H), 7.68 (t, J=7.5 Hz, 1H), 7.62-7.59 (m, 1H), 6.61 (br t, J=6.0 Hz, 1H), 4.55 (t, J=6.0 Hz, 2H), 2.80 (m, 2H), 2.18 (m, 2H). HRESIMS calcd for C14H15N6O4S and C14H14N6NaO4S m/z [M+H]+ and [M+Na]+ 363.0870, 385.0689, found 363.0883, 385.0680.
N-Benzyl-2-chloroacetamide (37a)
This was commercially available by Aldrich.
2-Chloro-N-{[4-(trifluoromethyl)pheny]methyl}acetamide (37b)
Pink-white crystallic powder28 (89%): mp 87–88 °C; 1H NMR (500 MHz, CDCl3) δ: 7.62 (d, J=7.5 Hz, 2H), 7.42 (d, J=8.0 Hz, 2H), 6.98 (br s, 1H), 4.57 (d, J=6.0 Hz, 2H), 4.14 (s, 2H). HRESIMS calcd for C10H10ClF3NO m/z [M+H]+ 252.0398, 254.0370, found 252.0407, 254.0378.
1-(4-benzylpiperidin-1-yl)-2-chloroethan-1-one (37c)
Yellowish oil29 (91%): 1H NMR (500 MHz, CDCl3) δ: 7.32-7.14 (m, 5H), 4.55 (d, J=13.0 Hz, 1H), 4.07 (m, 2H), 3.83 (d, J=13.5 Hz, 1H), 3.05 (t, J=13.0 Hz, 1H), 2.61-2.55 (m, 3H), 1.81-1.74 (m, 3H), 1.20–1.29 (m, 2H). HRESIMS calcd for C14H19ClNO m/z [M+H]+ 252.1150, 254.1124, found 252.1161, 254.1134.
2-Chloro-N-(6-methyl-1,3-benzothiazol-2-yl)acetamide (37d)
Off white crystallic powder30 (100%): mp 190–191 °C; 1H NMR (500 MHz, CDCl3) δ: 9.74 (br s, 1H), 7.70 (d, J=8.5 Hz, 1H), 7.63 (s, 1H), 7.28 (d, J=8.5 Hz, 1H), 4.31 (s, 2H), 2.49 (s, 3H). HRESIMS calcd for C10H10ClN2OS m/z [M+H]+ 241.0197, 243.0168, found 241.0194, 243.0163.
2-Chloro-N-(6-chloro-1,3-benzothiazol-2-yl)acetamide (37e)
White microcrystallic powder30,31 (73%): mp 203–204 °C (dec); 1H NMR (500 MHz, CDCl3) δ: 9.71 (br s, 1H), 7.82 (s, 1H), 7.73 (d, J=9.0 Hz, 1H), 7.43 (dd, J=10.5, 6.5 Hz, 1H), 4.33 (s, 2H). HRESIMS calcd for C9H7Cl2N2OS m/z [M+H]+ 260.9651, 262.9621, found 260.9663, 262.9630.
2-Chloro-N-(5-chloro-1,3-benzoxazol-2-yl)acetamide (37f)
Light brownish powder (70%): mp 168–170 °C; 1H NMR (500 MHz, CDCl3) δ: 9.38 (br s, 1H), 7.80-7.30 (m, 3H), 4.38 (s, 2H). HRESIMS calcd for C9H7Cl2N2O2 and C9H6Cl2N2NaO2 m/z [M+H]+ and [M+Na]+ 244.9879 and 266.9699, found 244.9871 and 266.9700.
In vitro biological evaluation
In vitro activity against T. cruzi, T. b. rhodesiense, Leishmania donovani axenic amastigotes and cytotoxicity assessment using L6 cells (rat skeletal myoblasts) was determined using a 96-well plate format as previously described.25 Data were analyzed with the graphic program Softmax Pro (Molecular Devices, Sunnyvale, CA, USA), which calculated IC50 values by linear regression from the sigmoidal dose inhibition curves.
In vitro T. brucei brucei antiproliferating assays and susceptibility studies
T. b. brucei bloodstream form parasites were seeded at 1 × 103 ml−1 in 200 μL of growth medium containing different concentrations of a nitrotriazole or nifurtimox. Where appropriate, induction of the TbNTR was carried out by adding tetracycline (1 μg/mL). After incubation for 3 days at 37 °C, 20 μL of Alamar blue was added to each well and the plates incubated for a further 16 h. The cell density of each culture was determined as described before11 and the IC50 established.
Enzymatic activity studies
Recombinant TbNTR was prepared and assayed as previously described.16 The activity of purified his-tagged TbNTR was assessed spectrophotometrically at 340 nm using various nitrotriazole substrates (100 μM) and NADH (100 μM) and expressed as nmol NADH oxidized min−1 mg−1 of enzyme.
Acknowledgments
The authors thank Dr. Yuyang Wu for obtaining the NMR spectra of the compounds, and M. Cal, S. Sax and C. Stalder (Swiss TPH) for parasite assay results. This work was supported by an NIH Challenge Grant: 1R01AI082542 – 01, Subaward No: RU374-063/4693578.
ABBREVIATIONS USED
- T. cruzi
Trypanosoma cruzi
- T. brucei
Trypanosoma brucei
- HAT
human African trypanosomiasis
- Nfx
nifurtimox (4-(5-nitrofurfurylindenamino)-3-methylthio-morpholine-1,1-dioxide)
- Bnz
benznidazole (N-benzyl-2(2-nitro-1H-imidazol-1-yl)acetamide)
- NTR
type I nitroreductase
- TbNTR
T. brucei NTR
- DNDi
Drugs for Neglected Diseases initiative
- SI
selectivity index
- SARs
structure-activity relationships
- tet
tetracycline
References
- 1.Stuart K, Brun R, Croft S, Fairlamb A, Gütler RE, McKerrow J, Reed S, Tarleton R. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–1310. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Simarro PP, Cecchi G, Paone M, Franco JR, Diarra A, Ruiz JA, Fevre EM, Courtin F, Mattioli RC, Jannin JG. The Atlas of human African trypanosomiasis: a contribution to global mapping of neglected tropical diseases. Int J Health Geographics [Electronic Resource] 2010;9:57. doi: 10.1186/1476-072X-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Simarro PP, Diarra A, Ruiz Postigo JA, Franco JR, Jannin JG. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2008–2009: the way forward. PLoS Neglected Tropical Diseases [electronic resource] 2011;5(2):e1007. doi: 10.1371/journal.pntd.0001007. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lescure FX, Le Loup G, Freilij H, Develoux M, Paris L, Brutus L, Pialoux G. Chagas disease: changes in knowledge and management (review) Lancet Infect Dis. 2010;10(8):556–570. doi: 10.1016/S1473-3099(10)70098-0. [DOI] [PubMed] [Google Scholar]
- 3.(a) Moncayo A, Silveira AC. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Memorias do Instituto Oswaldo Cruz. 2009;104(Suppl 1):17–30. doi: 10.1590/s0074-02762009000900005. [DOI] [PubMed] [Google Scholar]; (b) Schmunis GA, Yadon ZE. Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]; (c) Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease (review) Lancet. 2010;375(9723):1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 4.(a) Murta SM, Gazzinelli RT, Brener Z, Romanha AJ. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol Biochem Parasitol. 1998;93:203–214. doi: 10.1016/s0166-6851(98)00037-1. [DOI] [PubMed] [Google Scholar]; (b) Rodriques Coura J, de Castro SL. A critical review on Chagas disease chemotherapy. Memorias do Instituto Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 5.(a) Docampo R, Moreno SNJ. Free radical metabolism of antiparasitic agents. Fed Proc. 1986;45:2471–2476. [PubMed] [Google Scholar]; (b) Soeiro MNC, Dantas AP, Daliry A, Silva CF, Batista DGJ, de Souza EM, Oliveira GM, Salomão K, Batista MM, Pacheco MGO, Silva PB, Santa-Rita RM, Menna-Barreto RFS, Boykin DW, de Castro SL. Experimental chemotherapy for Chagas disease: 15 years of research contribution from in vivo and in vitro studies. Memórias do Instituto Oswaldo Cruz. 2009;104:301–310. doi: 10.1590/s0074-02762009000900040. [DOI] [PubMed] [Google Scholar]
- 6.Docampo R. Sensitivity of parasite to free radical damage by antiparasitic drugs. Chem Biol Interact. 1990;73:1–27. doi: 10.1016/0009-2797(90)90106-w. [DOI] [PubMed] [Google Scholar]
- 7.Viode C, Bettache N, Cenas N, Krauth-Siegel RL, Chauviere G, Bakalara N, Perie J. Enzymatic reduction studies of nitroheterocycles. Biochem Pharmacol. 1999;57(5):549–557. doi: 10.1016/s0006-2952(98)00324-4. [DOI] [PubMed] [Google Scholar]
- 8.Blumenstiel K, Schoneck R, Yardley V, Croft SL, Krauth-Siegel RL. Nitrofuran drugs as common subversive substrates of Trypanosoma cruzi lipoamide dehydrogenase and trypanothione reductase. Biochem Pharmacol. 1999;58(11):1791–1799. doi: 10.1016/s0006-2952(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 9.Turrens JF. Oxidative stress and antioxidant defenses: a target for the treatment of diseases caused by parasitic protozoa. Mol Aspects Med. 2004;25:211–220. doi: 10.1016/j.mam.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Hall BS, Bot C, Wilkinson SR. Nifurtimox activation by trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J Biol Chem. 2011;286(15):13088–13095. doi: 10.1074/jbc.M111.230847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. PNAS. 2008;105(13):5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Alsford S, Eckert S, Baker N, Glover L, Sanchez-Flores A, Leung KF, Turner DJ, Field MC, Berriman M, Horn D. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature. 2012;482:232–236. doi: 10.1038/nature10771. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Baker N, Alsford S, Horn D. Genome-wide RNAi screens in African trypanosomes identify the nifurtimox activator NTR and the eflornithine transporter AAT6. Mol Biochem Parasitol. 2011;176:55–57. doi: 10.1016/j.molbiopara.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson SR, Bot C, Kelly JM, Hall BS. Trypanocidal activity of nitroaromatic prodrugs: current treatments and future perspectives. Curr Top Med Chem. 2011;11:2072–2084. doi: 10.2174/156802611796575894. [DOI] [PubMed] [Google Scholar]
- 13.Baliani A, Gerpe A, Aran VJ, Torres de Ortiz S, Serna E, Vera de Bilbao N, Sanabria L, Yaluff G, Nakayama H, Rojas de Arias A, Maya JD, Morello JA, Cerecetto H, Gonzalez M. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J Med Chem. 2005;48:5570–5579. doi: 10.1021/jm050177+. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez J, Aran VJ, Boiani L, Olea-Azar C, Lavaggi ML, Gonzalez M, Cerecetto H, Maya JD, Carrasco-Pozo C, Cosoy HS. New potent 5-nitroindazole derivatives as inhibitors of Trypanosoma cruzi growth: Synthesis, biological evaluation, and mechanism of action studies. Bioorg Med Chem. 2009;17:8186–8196. doi: 10.1016/j.bmc.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Boiani L, Gerpe A, Aran VJ, Torres de Ortiz S, Serna E, Vera de Bilbao N, Sanabria L, Yaluff G, Nakayama H, Rojas de Arias A, Maya JD, Morello JA, Cerecetto H, Gonzalez M. In vitro and in vivo antitrypanosomatid activity of 5-nitroindazoles. Eur J Med Chem. 2009;44:1034–1040. doi: 10.1016/j.ejmech.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Hall BS, Wu X, Hu L, Wilkinson SR. Exploiting the Drug-Activating Properties of a Novel Trypanosomal Nitroreductase. Antimicrob Agents Chemother. 2010;54:1193–1199. doi: 10.1128/AAC.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bot C, Hall BS, Bashir N, Taylor MC, Helsby NA, Wilkinson SR. Trypanocidal activity of aziridinyl nitrobenzamide prodrugs. Antimicrob Agents Chemother. 2010;54(10):4246–4252. doi: 10.1128/AAC.00800-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu L, Wu X, Han J, Chen L, Vass SO, Browne P, Hall BS, Bot C, Gobalakrishnapillai V, Searle PF, Knox RJ, Wilkinson SR. Synthesis and structure-activity relationships of nitrobenzyl phosphoramide mustards as nitroreductase-activated prodrugs. Bioorg Med Chem Lett. 2011;21(13):3986–3991. doi: 10.1016/j.bmcl.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulou MV, Bourdin Trunz B, Bloomer WD, McKenzie C, Wilkinson SR, Prasittichai C, Brun R, Kaiser M, Torreele E. Novel 3-nitro-1H-1,2,4-triazole-based aliphatic and aromatic amines as anti-Chagasic agents. J Med Chem. 2011;54(23):8214–8223. doi: 10.1021/jm201215n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenzweig HS, Papadopoulou MV, Bloomer WD. Interaction of strong DNA-intercalating bioreductive compounds with topoisomerases I and II. Oncol Res. 2005;15:219–231. doi: 10.3727/096504005776382288. [DOI] [PubMed] [Google Scholar]
- 21.(a) Bustamante JM, Evans A, Papadopoulou MV, Tarleton R. Use of CD8+ T central memory characteristics as immunologic evidence for treatment efficacy in mice infected with Trypanosoma cruzi. 12th Woods Hole Immunoparasitology Meeting; Woods Hole, Massachusetts. April 27–29, 2008. [Google Scholar]; (b) Canavaci AMC, Bustamante JM, Padilla AM, Brandan CMP, Simpson LJ, Xu D, Boehlke CL, Tarleton RL. In vitro and in vivo high-throughput assays for the testing of anti-Trypanosoma cruzi compounds. PLos Neglected Trop Dis. 2010;4(7):e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadopoulou MV, Ji M, Bloomer WD. Novel Fluorinated Hypoxia-targeted Compounds as Non-invasive Probes for Measuring Tumor-hypoxia by 19F-Magnetic Resonance Spectroscopy (19F-MRS) Anticancer Res. 2006;26(5):3253–3258. [PubMed] [Google Scholar]
- 23.Papadopoulou MV, Bloomer WD. Nitroheterocyclic-linked acridines as DNA-targeting bioreductive agents. Drugs of the Future. 1993;18:231–238. [Google Scholar]
- 24.Hernández-Núñez E, Tlahuext H, Moo-Puc R, Torres-Gomez H, Reyes-Martínez R, Cedillo-Rivera R, Nava-Zuazo C, Navarrate-Vazquez G. Synthesis and in vitro trichomonicidal, giardicidal and amebicidal activity of N-acetamide(sulfonamide)-2-methyl-4-nitro-1H-imidazoles. Eur J Med Chem. 2009;44:2975–2984. doi: 10.1016/j.ejmech.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Orhan I, Sener B, Kaiser M, Brun R, Tasdemir D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar Drugs. 2010;8:47–58. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nwaka S, Ramirez B, Brun R, Maes L, Douglas F. Ridley, Advancing drug innovation for neglected diseases—criteria for lead progression. PLoS Negl Trop Dis. 2009;3(8):e440. doi: 10.1371/journal.pntd.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonse S, Santelli-Rouvier C, Barbe J, Krauth-Siegel RL. Inhibition of Trypanosoma cruzi trypanothione reductase by acridines: Kinetic studies and structure-activity relationships. J Med Chem. 1999;42:5448–5454. doi: 10.1021/jm990386s. [DOI] [PubMed] [Google Scholar]
- 28.Henry M. Preparation of N-phenylalkyl-N-alkylchloroacetamides as herbicide safeners for use with chloracetanilides. US 5028256 A 19910702. From US. 1991 Language: English, Database: CAPLUS.
- 29.Contreras JM, Parrot I, Sippl W, Rival YM, Wermuth CG. Design, synthesis, and structure-activity relationships of a series of 3-[2-(1-benzylpiperidin-4-yl)ethylamino] pyridazine derivatives as acetylcholinesterase inhibitors. J Med Chem. 2001;44:2707–2718. doi: 10.1021/jm001088u. [DOI] [PubMed] [Google Scholar]
- 30.Bhargava PN, Ram P. The synthesis of local anaesthetics. Bull Chem Soc Japan. 1965;38(3):339–341. [Google Scholar]
- 31.Amir M, Asif S, Ali I, Zaheen Hassan M. Synthesis of benzothiazole derivatives having acetamido and carbothioamido pharmacophore as anticonvulsant agents. Med Chem Res. 2011 doi: 10.1007/s00044-011-9791-1. [DOI] [Google Scholar]