Abstract
Astrocytes play a critical role in regulation of extracellular neurotransmitter levels in the central nervous system. This function is particularly prominent for the excitatory amino acid glutamate, with estimates that 80–90% of extracellular glutamate uptake in brain is through astrocytic glutamate transporters. This uptake has significance both in regulation of the potential toxic accumulation of extracellular glutamate, and in normal resupply of inhibitory and excitatory synapses with neurotransmitter. This resupply of neurotransmitter is accomplished by astroglial uptake of glutamate, transformation of glutamate to glutamine by the astrocytic enzyme glutamine synthetase, and shuttling of glutamine back to excitatory and inhibitory neurons via specialized transporters. Once in neurons, glutamine is enzymatically converted back to glutamate, which is utilized for synaptic transmission, either directly, or following decarboxylation to GABA. Many neurologic and psychiatric conditions, particularly epilepsy, are accompanied by the development of reactive gliosis, a pathology characterized by anatomical and biochemical plasticity in astrocytes, accompanied by proliferation of these cells. Among the biochemical changes evident in reactive astrocytes is a downregulation of several of the important regulators of the glutamine-glutamate cycle, including glutamine synthetase, and possibly also glutamate transporters. This downregulation may have significance in contributing both to the aberrant excitability and to the altered neuropathology characterizing epilepsy. In the present review, we provide an overview of the normal function of astrocytes in regulating extracellular glutamate homeostasis, neurotransmitter supply, and excitotoxicity. We further discuss the potential role reactive gliosis may play in the pathophysiology of epilepsy.
1. INTRODUCTION
Of the many important functions subserved by astrocytes, regulation of extracellular neurotransmitter levels in the central nervous system is a major component. This is particularly true for the primary small molecule neurotransmitters responsible for fast excitatory and inhibitory signaling in the central nervous system: glutamate and GABA, respectively. Extracellular accumulation of these amino acids, particularly glutamate, has deleterious effects on function and survival of neurons. Maintaining low concentrations of glutamate in the extracellular environment is primarily accomplished through astrocytic mechanisms (Rothstein et al. 1996; Tanaka et al. 1997).
A second, related role played by astrocytes is in the regulation of synaptic function, including resupply of synapses with neurotransmitter. Together with action potential initiation and propagation, synaptic transmission is the primary currency of nervous system function. Action potentials invade the nerve terminal, initiate presynaptic voltage-dependent calcium influx, which in turn results in the release of neurotransmitter from synaptic vesicles. This neurotransmitter diffuses across the synaptic cleft, and initiates a response in the postsynaptic neuron, with the nature of the response dependent both on the identity of the neurotransmitter and the composition of the receptors binding the signaling molecule. Once a synaptic response has been generated, termination of the signal is an absolute requirement for high fidelity encoding of information. The nature of the processes regulating termination of synaptic function varies depending on the identity of the synapse, but the primary mediators are diffusion of neurotransmitter away from the synapse, and uptake and removal of neurotransmitter from the extracellular space through specialized transporters (Danbolt 2001; Rusakov and Kullmann 1998; Tzingounis and Wadiche 2007). These processes are followed by enzymatic inactivation of transmitter and shuttling (recycling) of its major chemical components back to the synapse for economical synthesis and packaging of new transmitter. Uptake, degradation, and recycling of neurotransmitter are primarily accomplished by astrocytes. Many of these processes appear to be altered in the epileptic brain. In this article, we will first review the role of astrocytes in the regulation of extracellular glutamate under physiological conditions. Then the involvement of these cells in the glutamate homeostasis in epilepsy will be discussed.
2. ASTROCYTIC REGULATION OF GLUTAMATE HOMEOSTASIS UNDER NORMAL CONDITIONS
Approximately 5 – 15 mmol of free glutamate per kg wet weight is present in the brain (Schousboe 1981). This is more than 1,000 fold above the concentration required to activate extracellular glutamate receptors. Excessive activation of glutamate receptors can lead to excitation (Fonnum 1984), seizures (Ben-Ari 1985; Nadler and Cuthbertson 1980; Olney et al. 1972) and neuronal death (Choi and Hartley 1993; Meldrum 1991; Meldrum 1986; Olney et al. 1986; Olney et al. 1972). Most of brain glutamate is therefore contained in the intracellular compartment, and several mechanisms are in place to carefully control the extracellular glutamate levels. Some of these mechanisms will be elucidated below.
2.1 Synthesis and release of glutamate into the extracellular space
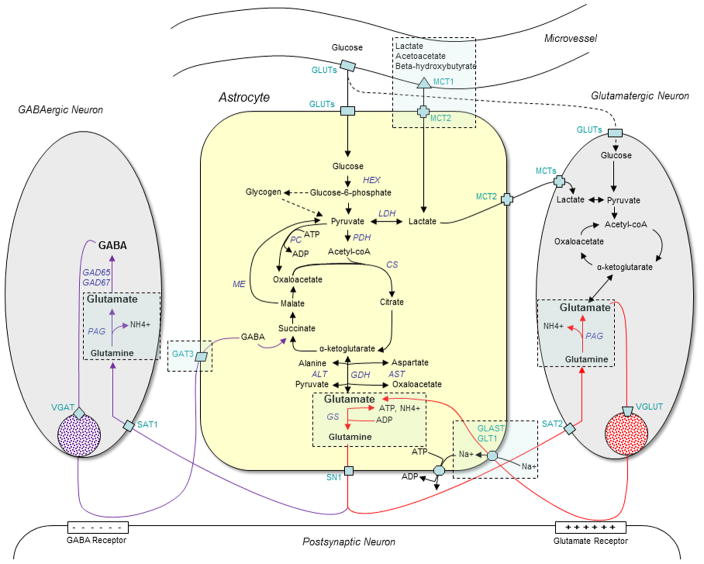
Blood-derived (plasma) glutamate does not readily cross the blood-brain-barrier. Only 0.67 nmol plasma glutamate/(min × g) has been estimated to enter the brain (Hawkins et al. 1995; Smith 2000). This rate is 5- to 10-fold less than that of most large neutral and basic amino acids such as glutamine, valine and arginine (Smith 2000). Thus, a large proportion of brain glutamate is synthesized from plasma glucose (Lajtha et al. 1959), which enters the brain via a family of glucose transporter molecules (GLUTs, Fig. 1) present on endothelial cells, astrocytes, neurons, and possibly other cells in the brain (Qutub and Hunt 2005). Glucose first enters the astrocyte compartment of the brain and is either converted to glycogen via the glycogen synthetase reaction (Lomako et al. 1993) or to pyruvate via glycolysis. Some of the pyruvate is converted to lactate by lactate dehydrogenase (LDH, Fig. 1) and some enters the TCA cycle as acetyl coenzyme A via pyruvate dehydrogenase (PDH, Fig. 1). The TCA cycle intermediate alpha-ketoglutarate then gives rise to glutamate via glutamate dehydrogenase (GDH, Fig. 1).
Figure 1.
This diagram depicts some of the major metabolic pathways and tissue compartments involved in the normal homeostasis of brain glutamate, as discussed in the first section of this review. The dashed frames indicate areas being affected in epilepsy (discussed in the later sections of the review). In brief, metabolic fuels from the blood are believed to primarily enter the astrocyte compartment for initial metabolism, which leads to the synthesis of glutamate from alpha-ketoglutarate. Glutamate is then converted to glutamine via glutamine synthetase (GS) in astrocytes. Glutamine from astrocytes is an important substrate for glutamate and GABA in neurons. GABA and glutamate released from neurons may be taken up by astrocytes and incorporated into the astrocyte metabolism with glutamine as one of the metabolic products. The glutamine-glutamate-GABA metabolic pathways just described are sometimes referred to as the “glutamine-glutamate cycle” (red arrows) and the “glutamine-glutamate-GABA cycle” (purple arrows). Abbreviations: ADP, adenosine diphosphate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ATP, adenosine triphosphate; CS, citrate synthase; GABA, γ-aminobutyric acid; coA, coenzyme A; GAT3, GABA transporter subtype 1; GAD65/67, glutamate decarboxylase isoforms 65/67; GDH, glutamate dehydrogenase; GLUTs, glucose transporters; HEX, hexokinase; LD, lactate dehydrogenase; MCT1/2, monocarboxylate transporter subtypes 1/2; ME, malic enzyme; PAG, phosphate activated glutaminase; PDH, pyruvate dehydrogenase; SAT1/2, system A transporter subtypes 1/2; SN1, system N transporter subtype 1; VGAT, vesicular GABA transporter; VGLUT, vesicular glutamate transporter.
It has been postulated that lactate is the main metabolic substrate for neurons while glucose is predominantly utilized by astrocytes (Magistretti et al. 1993; Pellerin et al. 1998). According to this theory, plasma glucose is primarily taken up by astrocytes and converted to lactate, which is shuttled to neurons via monocarboxylate transporter molecules (MCTs, Fig. 1) (Pellerin et al. 2005). Lactate in neurons is subsequently converted to pyruvate by lactate dehydrogenase (LDH). The rest of the metabolic pathway from pyruvate to glutamate remains the same as in astrocytes. Glutamate in neurons is concentrated in synaptic vesicles via vesicular glutamate transporters (VGLUT) (Bellocchio et al. 1998; Fremeau et al. 2002; Fremeau et al. 2001; Takamori et al. 2002) and released into the extracellular space in response to neuronal depolarization (Fig. 1).
It is important to note that the synthesis of glutamate from glucose or lactate with subsequent release of glutamate from the cells will deplete the TCA cycle of its intermediates. Replenishment of TCA cycle intermediates, i.e. anaplerosis, is therefore necessary for continued ATP synthesis. Astrocytes are uniquely capable of anaplerosis due to their preferential expression of pyruvate carboxylase (PC, Fig. 1), which converts pyruvate to oxaloacetate (Shank et al. 1985). Because pyruvate carboxylase is not present in neurons, these cells are incapable of anaplerosis and therefore critically dependent on astrocytes for glutamate synthesis. The glutamine-glutamate cycle pathway, which transfers glutamine from astrocytes to neurons, is pivotal for the synthesis of glutamate in neurons (Benjamin and Quastel 1975; van den Berg and Garfinkel 1971). The pathway will be discussed in detail in Section 2.3.
While neurons have been considered the main source of extracellular glutamate in the brain, an increasing number of studies suggest that also astrocytes can release glutamate. The groundbreaking work of Kimelberg and colleagues demonstrated that astrocytes in culture release glutamate in response to osmotic swelling (Kimelberg et al. 1990; Kimelberg et al. 1995). Other investigators have confirmed and extended these findings to include release of glutamate from astrocytes via a variety of Ca2+-dependent and -independent mechanisms (Araque et al. 2000; Attwell 1994; Bezzi et al. 1998; Hamilton and Attwell 2010; Innocenti et al. 2000; Parpura et al. 1994; Santello et al. 2011). However, most of these studies were performed in vitro. Thus, it remains somewhat controversial whether glutamate release by astrocytes also occurs in the intact brain, and if so, whether such release is physiologically important or is implicated in pathological conditions.
2.2 Extracellular glutamate uptake
Astrocytes are critically important for the clearance of extracellular glutamate due to the abundant expression of high affinity glutamate transporter proteins (excitatory amino acid transporters, EAATs) on their cell surface (Danbolt 2001). The EAAT transporter subtypes GLAST (EAAT1) and GLT1 (EAAT2) are preferentially expressed on astrocytes (Fig. 1). EAATs move glutamate from the extracellular space using the electrochemical Na+ and K+ gradients across the plasma membranes as the driving force. Due to the massive release of glutamate during neuronal depolarization and the large concentration gradient of glutamate between the extra- and intracellular space, it has been estimated that nearly 90% of the brain’s glucose demand is used to maintain the electrochemical gradients necessary for glutamate uptake and neuronal depolarization (Magistretti et al. 1999).
2.3 Metabolic clearance and recycling of glutamate – the glutamine-glutamate (-GABA) cycle
Once taken up by astrocytes, glutamate can be directed towards several different routes. (1) Possibly release into the extracellular space as a gliotransmitter (Araque et al. 2000; Attwell 1994; Bezzi et al. 1998; Hamilton and Attwell 2010; Innocenti et al. 2000; Kimelberg et al. 1990; Kimelberg et al. 1995; Parpura et al. 1994; Santello et al. 2011). (2) Re-entry into the TCA cycle via the GDH reaction for use as a metabolic fuel (Fig. 1) (Dennis et al. 1977). (3) Enzymatic conversion to glutamine by glutamine synthetase (GS, Fig. 1) (Martinez-Hernandez et al. 1977). The latter route will be described in detail because it is critically important for the synthesis of neuronal glutamate, via the glutamine-glutamate cycle pathway, and for the synthesis of GABA, via the glutamine-glutamate-GABA cycle pathway.
GS and the glutamine-glutamate cycle
GS is abundantly present in the liver and brain and is critically important for glutamate metabolism and ammonia detoxification. The enzyme is preferentially located in the cytoplasm of astrocytes in the normal adult brain and catalyzes the following reaction:
A portion of glutamine formed in this reaction leaves the cytoplasm of astrocytes via glutamine transporter proteins present on the plasma membrane of the cells, most notably the system N transporter 1 (SN1, Fig. 1) (Chaudhry et al. 2002). Glutamine is then funneled into GABAergic and glutamatergic neurons via the system A type glutamine transporters, SAT1 (Solbu et al. 2010) and SAT2 (Jenstad et al. 2009), respectively (Fig. 1). Once in neurons, glutamine is converted to glutamate via the mitochondrial enzyme phosphate activated glutaminase (PAG, Fig. 1) (Kvamme et al. 2001; Svenneby 1970). Glutamate can then be packaged into synaptic vesicles by vesicular glutamate transporter molecules (VGLUT, Fig. 1) (Bellocchio et al. 1998; Fremeau et al. 2002; Fremeau et al. 2001; Takamori et al. 2002), released into the extracellular space, taken up by astrocytes and converted back to glutamine by GS, thus completing the metabolic cycle.
The glutamine-glutamate-GABA cycle
GABAergic neurons are characterized by their expression of the glutamate decarboxylase isoenzymes GAD65, which is concentrated in the nerve terminals and GAD67, which is present throughout the cytoplasm (Bu et al. 1992). Glutamate formed in GABAergic neurons via the PAG reaction described above (Kvamme et al. 2001; Svenneby 1970) is further converted to GABA via GAD65 or GAD67. GABA may then be used for a variety of purposes such as a trophic factor for synaptogenesis, protection after neuronal injury, source of energy, regulator of redox potential after oxidative stress, and neurotransmission (Lamigeon et al. 2001; Pinal and Tobin 1998; Waagepetersen et al. 1999). GABA used for neurotransmission may be packaged in synaptic vesicles via the vesicular GABA transporter protein (VGAT, Fig. 1) (Jin et al. 2003). GABA released from neurons in response to neuronal depolarization can then be taken up from the extracellular space via GABA transporters that are present on the plasma membrane of neurons and astroglial cells, including astrocytes (Eulenburg and Gomeza 2010). The GABA transporter subtype GAT3 (slc6a11) is preferentially present on astrocytes, while GAT1 and GAT2 are mainly expressed on neurons. Once taken up by neurons and astroglial cells, a portion of GABA enters the TCA cycle as succinate, which in turn may be converted to glutamate via the GDH reaction (Dennis et al. 1977) and subsequently to GABA via GAD65/67. Some of the GABA taken up by neurons and astrocytes is not metabolized and may be released back into the extracellular space by both cell types. Because the synthesis of GABA from succinate with subsequent release of the neurotransmitters will deplete the TCA cycle of its intermediates, transfer of glutamine from astrocytes to GABAergic neurons is therefore necessary. This has led to the concept of a glutamine-glutamate-GABA cycle pathway (Fig. 1).
3. ASTROCYTIC REGULATION OF SYNAPTIC NEUROTRANSMITTER RECYCLING AND SYNAPTIC FUNCTION
In addition to a) contributing to the synthesis of glutamate (see discussion above), b) helping terminate synaptic responses, c) sequestering synapses to maintain signal identity and prevent spillover of neurotransmitter, and d) buffering extracellular glutamate levels (see discussion above), astroglial uptake of glutamate plays an integral role in recycling of neurotransmitter pools. This is primarily mediated by shuttling of transporter-derived glutamate through the glutamine-glutamate cycle, and local transport of glutamine back to presynaptic terminals of neurons. In these terminals, glutamine is then converted back to glutamate by phosphate activated glutaminase. Glutamate is then available directly for neurotransmitter supply in excitatory synapses, or is converted to GABA in inhibitory synapses (Fig. 1). This astroglial glutamine-glutamate cycle is critical in maintaining the ability of synapses to exhibit sustained neurotransmitter release.
Surprisingly, under control, basal conditions, astrocytic glutamate uptake-mediated regulation of local synaptic neurotransmitter supply appears to primarily support the continued function of inhibitory, GABAergic synapses. These synapses fail within minutes following pharmacological blockade of any step of the glutamine-glutamate cycle, both in hippocampus (Fricke et al. 2007; Liang et al. 2006) and in thalamus (Yang and Cox 2011). Further supporting this concept, studies of inhibitory and excitatory synaptic function in hippocampus following selective induction of gliosis, with an associated downregulation in GS, demonstrated that there was a pronounced deficit in inhibitory, but not excitatory, synaptic function (Ortinski et al. 2010). Other studies have also demonstrated that excitatory, glutamatergic synapses maintain basal neurotransmitter release for hours in the absence of glutamine cycle function (Kam and Nicoll 2007; Ortinski et al. 2010).
Metabolic studies support this linkage between the glutamine-glutamate cycle and production of GABA in inhibitory synapses (Battaglioli and Martin 1991; Rae et al. 2003). Glutamate may be the primary substrate for neurotransmitter refilling inhibitory synaptic vesicles, since GAD65 sits in a macromolecular complex with vesicular GABA transporters in inhibitory terminals (Jin et al. 2003). This glutamate primarily derives from the glutamine-glutamate cycle, although some may come from direct glutamate uptake in inhibitory terminals (Mathews and Diamond 2003; Sepkuty et al. 2002).
4. ASTROCYTIC REGULATION OF GLUTAMATE HOMEOSTASIS IN EPILEPSY
Proliferation of reactive astrocytes (gliosis) is a common feature of temporal lobe epilepsy (TLE), which is one of the most prevalent forms of localization-related epilepsies in humans (Cohen-Gadol et al. 2004; Eid et al. 2008b; Gloor 1991; Jabs et al. 2008; Petroff et al. 2002; Seifert et al. 2010; Seifert et al. 2006). TLE is characterized by spontaneous recurrent seizures that involve mesial temporal lobe structures, such as the hippocampal formation, amygdala and entorhinal cortex (Spencer 2002; Spencer and Spencer 1994). Even though many patients with TLE are refractory to antiepileptic drugs, some can be successfully treated with surgical resection of the anteromedial temporal lobe. Up to 2/3 of patients treated surgically for TLE exhibit mesial temporal sclerosis (de Lanerolle et al. 2003). This pathology is recognized by pattered neuronal loss and proliferation of reactive astrocytes in mesial temporal lobe structures, particularly in CA1, CA3 and the dentate hilus of the hippocampal formation, in layer III of the entorhinal cortex, and in the amygdala (Bouchet 1825; Du et al. 1993; Gloor 1991; Sommer 1880).
The gliosis in TLE is correlated with an increased interictal concentration and slowed clearance of extracellular glutamate in the hippocampal formation (Cavus et al. 2005; Petroff et al. 2004). Studies in laboratory animals have shown that glutamate and glutamate analogues cause seizures and neuron loss similar to human TLE, suggesting that glutamate is a crucial element in the pathophysiology of this condition (Ben-Ari 1985; Fremeau et al. 2002; Nadler and Cuthbertson 1980; Olney et al. 1972). Indeed, interictal extracellular glutamate is elevated fivefold more in the epileptogenic vs. the non-epileptogenic human hippocampus, measured in vivo by simultaneous depth electrode EEG and microdialysis (Cavus et al. 2005). Moreover, extracellular glutamate increases six-fold above the interictal level in the hippocampus during the seizure (30-times higher than normal), and remains markedly elevated (12-times higher than normal) for several hours after the cessation of seizure activity (During and Spencer 1993). Notably, the interictal extracellular glutamate concentration is considerably higher in patients with hippocampal sclerosis than in patients without this pathology, despite the 60–80% neuronal loss and doubling of astroglial density in the sclerotic hippocampus (Kim et al. 2004; Petroff et al. 2004; Petroff et al. 2003). However, the high concentration of extracellular glutamate in the gliotic and neuron-depleted hippocampus is paradoxical because neurons are normally the main source of extracellular glutamate whereas astrocytes are extremely efficient in clearing glutamate from the extracellular space (Danbolt 2001). We postulate that the proliferated (reactive) astrocytes in TLE have several unique characteristics that contribute to the perturbed glutamate homeostasis and triggering of seizures in TLE. Some of these characteristics will be discussed below.
4.1 Metabolic fuels, the ketogenic diet and astrocytes
It is well established that metabolic fuels can either prevent or trigger epileptic seizures. For example, prolonged fasting or consistent intake of a low carbohydrate, high fat – ketogenic – diet leads to significant reductions in the frequency of many types of epileptic seizures (Stafstrom et al. 2007). However, if patients on a strict ketogenic diet eat a carbohydrate rich food, there is often a dramatic and rapid resurgence of their seizures (Huttenlocher 1976; Schwartz et al. 1989). While the mechanisms underlying these effects are not completely understood, there is evidence to suggest that astrocytes and the glutamine-glutamate-GABA metabolic cycle may be involved (Stafstrom et al. 2007).
The perivascular astrocyte end feet are positioned between the endothelial cells of blood vessels and the rest of the brain (Peters et al. 1991); thus, blood-derived metabolic fuels normally enter the endothelial cell and astrocyte compartments first. The importance of these compartments for fuel metabolism is underscored by the dense expression of glucose transporters (GLUTs) and monocarboxylate transporters (MCTs) on endothelial cells and astrocytes (Bergersen 2007; Halestrap and Price 1999; Pellerin et al. 2005; Qutub and Hunt 2005). Recent studies have shown that one of the main transporter molecules of ketone bodies across the blood brain barrier, MCT1, is perturbed in patients with TLE as well as in several animal models of the condition (Lauritzen et al. 2011; Lauritzen et al. 2012). MCT1 is deficient on endothelial cells of microvessels in sclerotic vs. nonsclerotic hippocampal formations in patients with TLE (Lauritzen et al. 2011). Similar findings are also evident in three different rodent models of TLE vs. control animals without epilepsy (Lauritzen et al. 2012). Furthermore, the loss of MCT1 on endothelial cells in TLE is accompanied by an upregulation of MCT1 on reactive astrocytes, particularly on astrocyte processes not facing the endothelial cell domain. The blood-brain-barrier, particularly its expression of MCT1 appears to be a rate limiting step in the utilization of circulating ketone bodies by the brain (Morris 2005). It is therefore possible that the sclerotic hippocampal formation in TLE is deficient in ketone bodies under euglycemic conditions and that this deficiency may promote excitability due to the relative abundance of carbohydrate fuels. Treatment with a ketogenic diet not only increases the concentration gradient of ketone bodies across the blood brain barrier but also increases the expression of MCT1 on brain microvessels, thereby allowing a larger flux of ketone bodies into the brain(Leino et al. 2001).
Ketone bodies increase the brain energy stores, stabilize the neuronal membrane potential, enhance GABA-mediated inhibition and possibly decrease glutamate-mediated excitation. Studies have shown that a change in brain fuel consumption from glucose to ketone bodies results in a nearly 50% increase in mitochondrial density (Bough 2008) and an elevated interictal phosphocreatine/creatine ratio (Pan et al. 1999). These changes are likely to improve the mitochondrial capacity for ATP production, thereby allowing neurons and astrocytes to more readily restore the Na+/K+ transporters, thus stabilizing the membrane potential and increasing the capacity for extracellular glutamate uptake via EAATs (Bough 2008). Furthermore, ketone bodies rather than glucose may increase the energy stores preferentially in GABAergic neurons (Williamson et al. 2005) thus resulting in more sustained GABA-mediated inhibition (Cantello et al. 2007; Yudkoff et al. 2004). In addition, beta-hydroxybutyrate inhibits the enzymatic breakdown of GABA in astrocytes (Suzuki et al. 2009).
4.2 Astrocyte transport of glutamate, glutamine and GABA in epilepsy
The expression of the two major high-affinity glutamate transporter proteins on astrocytes – GLT1 and GLAST – has been investigated in brain tissue from various seizure models and from patients with localization-related epilepsies. Tanaka et al showed that transgenic mice lacking GLT1 develop seizures (Tanaka et al. 1997). A deficiency in GLAST increases the duration of generalized seizures in amygdala kindled rats (Watanabe et al. 1999). Investigations of patients with TLE have indicated that GLT1 and GLAST are decreased in the epileptogenic hippocampal formation (Mathern et al. 1999; Proper et al. 2002). However, other investigators have failed to detect alterations in astroglial glutamate transporters in human TLE and in laboratory models of seizures. For example, in patients undergoing resective brain surgery for the treatment of TLE, no differences in the expression of GLT1 or GLAST were present between sclerotic and non-sclerotic hippocampal formations (Eid et al. 2004; Tessler et al. 1999). Furthermore, kindled rats had decreases in the neuronal glutamate transporter EAAC1, but not in GLT1 or GLAST (Akbar et al. 1997; Miller et al. 1997; Simantov et al. 1999). The discrepancies among these studies may be due to several factors. Poor specificity of some antibody sources could lead to false positive or false negative staining patterns that would obscure real differences in EAAT expression. Western blotting, which is used in some of the studies, does not have the level of spatial resolution as immunocytochemistry approaches. Thus changes in the cellular and ultrastructural distribution of EAATs without a change in the overall concentration of transporter protein may not be detected by Western blotting. Finally, expression of EAAT protein is not necessarily proportional to its function, as mutations could preserve antigenicity while altering transport kinetics.
Impaired uptake of glutamate by astrocytes may also slow the synthesis of glutathione and leave the brain vulnerable to oxidative damage. The glutamate/cystine antiporter requires high astrocytic concentrations of glutamate to drive cystine (one of the precursors of glutathione) into the cells (Albrecht et al. 2010). Moreover, glutamate is required for the synthesis of glutathione via the formation of gamma-glutamylcysteine from glutamate and cysteine.
There are no studies on the involvement of astrocytic glutamine transporters in epilepsy and only one report on the astrocytic GABA transporter GAT3 in this condition. The latter study showed that GAT3 is increased on protoplasmic (nonreactive) astrocytes in the sclerotic vs. the non-sclerotic hippocampal formation in patients with TLE (Lee et al. 2006). This increase is mainly confined to astrocytes in areas of high neuronal density, such as the subiculum, the CA2 and the dentate granule cell layer. The pathological significance of the increased GAT3 expression is not understood.
4.3 Decreased GS and GDH in the epileptogenic temporal lobe
At least two glutamate metabolizing enzymes with preferential expression in astrocytes – GS and GDH – are altered in the brain in TLE (Eid et al. 2004; Malthankar-Phatak et al. 2006; van der Hel et al. 2005). Studies have shown that the protein expression and activity of GS in the hippocampal formation is significantly lower in TLE patients with hippocampal sclerosis than in TLE patients without sclerosis and in non-epilepsy control subjects (Eid et al. 2004; van der Hel et al. 2005). The loss of GS is particularly pronounced in areas of the hippocampal formation with astroglial proliferation, even though astrocytes normally contain high levels of the enzyme(Martinez-Hernandez et al. 1977). Because GS converts glutamate in astrocytes to glutamine, it has been postulated that the deficiency in GS would cause an increase in glutamate in astrocytes negative for the enzyme (Eid et al. 2004). This increase in glutamate would slow the cycling of astrocytic glutamate to glutamine, and by mass action lead to accumulation of astrocytic and extracellular glutamate. The known stoichiometry of glutamate transport across the astroglial plasma membrane suggests that a rapid metabolism of intracellular glutamate is a prerequisite for efficient glutamate clearance from the extracellular space (Otis and Jahr 1998).
The deficiency in GS in TLE would also impair the detoxification of ammonia in the hippocampus. Although the functional significance of increased ammonia locally in the hippocampus is not known, it is likely that the ammonia increase has important pathophysiological effects. Ammonia is a neurotoxin that is involved in the pathophysiology of hepatic encephalopathy, which is characterized by hyperammonemia, brain edema, altered consciousness and seizures (Butterworth 2010). The mechanism of ammonia-associated neurotoxicity is not fully understood, but may involve excitatory and inhibitory neurotransmitter pathways (Leke et al. 2011), ion transport and glutamate uptake (Kelly et al. 2009), swelling of astrocytes (Vaquero et al. 2003) and oxidative stress (Gorg et al. 2010). Thus, the loss of GS in TLE may also lead to ammonia-induced toxic effects with increased extracellular glutamate concentrations (Kelly et al. 2009) and seizures as possible sequelae.
It can be argued that the downregulation of GS in TLE is an epiphenomenon and not a causative factor for this condition. Thus, the hypothesis that a deficiency in hippocampal GS leads to recurrent seizures and neuropathological features typical of TLE is currently being validated. In one such validation study, sustained brain GS deficiency was created by continuous (~28 days) microinfusion of methionine sulfoximine (MSO: 0.625 to 2.5 μg/hr) into the hippocampus in rats (Eid et al. 2008a). This treatment led to a deficiency in hippocampal GS activity by 82 to 97% versus saline. The majority (>95%) of the MSO-treated animals exhibited recurrent seizures that continued for several weeks. Some of the MSO-treated animals exhibited neuropathological features that were similar to mesial temporal sclerosis, such as hippocampal atrophy and patterned loss of hippocampal neurons. This and other studies support the idea that a deficiency in GS can cause TLE (Eid et al. 2008a; Folbergrova et al. 1969; Wang et al. 2009).
If a deficiency in GS causes TLE, then restoration of the enzyme in the brain may represent a novel therapeutic approach for this condition. Knowledge about the mechanism underlying the enzyme deficiency in TLE is likely to facilitate the development of such a therapeutic approach. Although not fully understood, several mechanisms have been proposed to underlie the loss of GS in TLE. Firstly, the expression of GS is regulated by cAMP analogues and by corticosteroids (Jackson et al. 1995; Stanimirovic et al. 1999; Vardimon et al. 1999). The inductive effect of glucocorticoids is depressed by c-Jun, which is upregulated after epileptic seizures (Beer et al. 1998; Vardimon et al. 1999). Thus, interference with a glucocorticoid-dependent stimulatory pathway, possibly through c-Jun or cytokines, might underlie the loss of GS in TLE (Huang and O’Banion 1998). C-Jun mediates the decrease in GS induced by basic fibroblast growth factor (Kruchkova et al. 2001), which is known to have a seizure-inducing effect in rats (Liu and Holmes 1997). Secondly, in the pentylentetrazole model of epilepsy, brain regions showing increased expression of heat shock protein in astroglial cells also had reduced activity of GS (Bidmon et al. 2008). The reduction in enzyme activity was correlated with nitration of the enzyme, suggesting that a nitrosative stress response may underlie the loss of GS in epilepsy. Thirdly, studies of the triple transgenic Alzheimer’s disease mouse model have shown an age-dependent decrease in GS expression in astrocytes in the hippocampal formation (Olabarria et al. 2011). The decrease in GS is associated with the presence of Aβ deposits showing a decrease of 48% as opposed to 23% in areas free of Aβ. Patients with Alzheimer’s disease exhibit an increased prevalence of epilepsy, and several studies have indicated that some of the mechanisms underlying Alzheimer’s disease may also be implicated in TLE (Amatniek et al. 2006; Hesdorffer et al. 1996; Palop et al. 2007; Palop and Mucke 2009; Romanelli et al. 1990). However, whether Aβ deposits occur in patients with TLE and results in a loss of GS remains to be established. Finally, a component of the loss of GS activity evident in animal models of epilepsy (and potentially in patients) may be due to altered subcellular trafficking of this enzyme, with a shift from the protoplasmic (synapse apposing) portions of astrocytes, and an aggregation in the cell bodies (Papageorgiou et al. 2011). This may remove GS from close apposition with synapses, and uncouple the glutamine-glutamate cycle in this critical microdomain.
The activity of brain GDH is also decreased in patients with TLE and concomitant hippocampal sclerosis vs. patients without hippocampal sclerosis (Malthankar-Phatak et al. 2006). The GDH activity is significantly decreased in the temporal neocortex in these patients, and a non-significant decrease in GDH activity is also evident in the sclerotic hippocampal formation. Because GDH, which is mainly present in astrocytes, catalyzes the reversible conversion of glutamate to alpha-ketoglutarate (Fig. 1), it has been proposed that glutamate is increased in astrocytes with low GDH activity. This increase in glutamate could also lead to accumulation of extracellular glutamate in the hippocampal formation in TLE. However, whether the decreased GDH is involved in the mechanism of epilepsy in TLE remains to be established.
4.4 In vitro studies of the glutamine-glutamate cycle: Effects on excitability during pathological activity
The preferential role of the glutamine cycle in maintaining inhibition under basal activity conditions appears quite different when high activity levels are ongoing. In this context, glutamine plays a significant role in maintaining excitatory transmission during pathological, sustained activity, such as spontaneous and evoked epileptiform discharges in both thalamus (Bryant et al. 2009) and neocortex (Tani et al. 2010). Blockade of the glutamine-glutamate cycle was shown to inhibit epileptiform activity in hippocampus (Bacci et al. 2002). These studies were conducted in slices prepared from control animals. Glutamine has also been shown to induce epileptiform activity in brain slices prepared from an animal model of epilepsy, presumably in a context of significant gliosis (Sandow et al. 2009). At first glance, these data appear paradoxical, and certainly conflict with the underlying hypothesis that compromise in the glutamine-glutamate cycle may contribute to epilepsy induction.
Several possible explanations may exist for this apparent contradiction. First, it must be recognized that glutamine is present in high concentrations in the extracellular space (0.5–2 mM), and glutamine regulation of synaptic transmission is highly compartmentalized, and this compartmentalization may be only partly preserved in functional studies in slices. Regulation of normal transcellular glutamine shuttling may be subject to significant dilution by the rapid exchange of extracellular milieu necessary to maintain viability of brain slices in vitro. This would depend on the relative accessibility of synaptic compartments to slice perfusion, an area that is not well understood. The potential for glutamine dilution in slice studies has the potential to compromise glutamine cycle-dependent aspects of synaptic function in both basal and active states, and this may vary in differing brain areas. Second, and interacting with point one above, is that glutamine regulation of excitability is complex, and will differ in depleted compared to normal situations. In significantly depleted slices, the normal dichotomy in regulation of inhibition and excitation described above may be quite different, and supply of exogenous glutamine may assume a primary role in restoring excitatory neurotransmitter supply, and blockade of the glutamine cycle may further deplete excitatory synapses, leading to their failure as has been seen in studies of effects on pathological activity (Bacci et al. 2002; Bryant et al. 2009; Sandow et al. 2009; Tani et al. 2010). In disinhibited slices, this effect of glutamine will occur in isolation and assume an exaggerated importance (Tani et al. 2010).
Finally, a significant experimental artifact associated with supply of exogenous glutamine can be contamination of commercial glutamine supplies with glutamate, which can be in the range of 0.1–0.5%. Supply of millimolar quantities of the relatively inert amino acid glutamine may be accompanied by 10–50 micromolar quantities of glutamate, sufficient to activate glutamate receptors and potentially contribute to initiation of pathologic, epileptiform discharges. This potential for additional, artifactual effects of glutamate-glutamate cycle modulators extends to methionine sulfoximine (MSO), one of the primary antagonists of GS utilized in experimental studies. In addition to being an antagonist, MSO is also a convulsant (Eid et al. 2008a), and its convulsant activity may be unrelated or only partially related to its effects on GS, since it has direct excitatory actions on neurons (Kam and Nicoll 2007).
4.5 In vitro studies of gliosis in dysregulation of inhibitory synaptic function and circuit excitability in epilepsy
Accompanying the development of epilepsy, astrocytes exhibit significant changes in morphology, biochemistry, and function. Early after an epileptogenic injury, astrocytic cell bodies exhibit significant hypertrophy, associated with increased expression of cytoskeletal proteins such as vimentin and GFAP (Pekny and Nilsson 2005). These alterations exhibit significant regional differences, and it is currently unclear whether reactive gliosis is a single entity, or may involve multiple, distinct cells with differing properties. Accompanying these anatomic alterations are a series of phenotypic changes in reactive astrocytes, including the reduced expression of GS, and possibly also glutamate transporters (Eid et al. 2008a; Eid et al. 2004). GS is the keystone enzyme of the glutamine-glutamate cycle, and astrocytic glutamate transporters are also critical contributors to function of this transcellular pathway. In addition to altering glutamate homeostasis (discussed below), reactive gliosis may alter circuit excitability by disrupting the normal coupling between the amount of excitatory activity and inhibitory synapse neurotransmitter supply inherent in the role played by the glutamine-glutamate cycle in differential regulation of neurotransmitter supply (discussed above). This may contribute to aberrant excitability and seizure initiation characteristic of the epileptic brain.
Analysis of this possible outcome of gliosis in epilepsy is complicated by the broad spectrum of anatomic, physiologic, and biochemical changes evident in the brain of patients and animal models of epilepsy. In particular, expression of postsynaptic GABA receptors in hippocampal neurons is significantly altered in epilepsy, a significant confound in studies examining effects of gliosis on presynaptic GABA release (Brooks-Kayal et al. 1998; Cohen et al. 2003; Coulter 2001; Gibbs et al. 1997; Rice et al. 1996). One approach to simplify determination of the consequences of gliosis on brain excitability is to induce this phenomenon in isolation. One recent study examined the consequences of specific, virally-induced gliosis in the hippocampus on inhibitory and excitatory synaptic function, as well as circuit excitability (Ortinski et al. 2010). Injection of high titer, astrocytes-specific AAVs driving expression of GFP into the hippocampus was found to have little or no effect on the intrinsic excitability and anatomy of CA1 pyramidal neurons and interneurons, but had significant effects on astrocytes. These effects included anatomic alterations in astrocytes consistent with gliosis, including hypertrophy, increased expression of vimentin and GFAP, as well as reduced expression of GS.
In neurons in regions with significant numbers of reactive, GFAP positive astrocytes, there was a specific deficit in inhibitory synaptic function, but little change in excitatory synaptic function, either in response to single stimuli or high frequency trains of stimuli. The deficits in inhibition could be mimicked in control tissue by blockade of the glutamine-glutamate cycle, were reversed by supply of exogenous glutamine, and occluded the effects of glutamine cycle antagonists evident in control tissue, all consistent with inhibitory deficits being generated by deficits in this cycle accompanying the development of reactive gliosis. These data utilizing experimental strategies to induce gliosis confirm predictions derived from studies in control tissue: loss of glutamine-glutamate cycle activity has significant effects on inhibitory but not excitatory synaptic responses, and occurs in a setting of reactive gliosis accompanied by downregulation of GS.
Virally-induced gliosis was also found to generate circuit hyperexcitability in area CA1, specifically in the regulation of direct cortical inputs innervating CA1 neurons on the distal apical dendritic tuft (Ortinski et al. 2010). This pathway is important in cognitive tasks dependent on the hippocampus (Brun et al. 2002; Buzsaki et al. 1995), and normally regulated powerfully by feed-forward GABAergic inhibition (Ang et al. 2005; Empson and Heinemann 1995; Soltesz 1995) This regulation is significantly compromised in animal models of epilepsy (Ang et al. 2006; Denslow et al. 2001; Wozny et al. 2005). In slices encompassing broad areas of virally-induced gliosis, we found a similar loss in regulation of direct cortical inputs to CA1 to that seen in animals with epilepsy. Stimulation of the temporo-ammonic pathway normally induces a spatially constrained EPSP in the distal apical tuft, and proximal dendritic and somatic inhibition in control animals (Ang et al. 2006), but generated powerful, broad excitation in animals with gliosis. This could be reversed by application of exogenous glutamine, so was likely generated by glutamine-glutamate cycle deficits accompanying the development of reactive astrocytosis.
5. CONCLUSIONS & FUTURE DIRECTIONS
Astrocytic regulation of glutamate homeostasis is a critical aspect of brain function, with significance at many levels. Perhaps foremost, astrocyte regulation limits the accumulation of toxic levels of glutamate in the extracellular space. The astrocytic glutamine-glutamate cycle also supplies neurotransmitter precursors for re-use in excitatory and inhibitory synapses, a crucial part of the local, synaptic economy which allows the supply of neurotransmitter to be regulated by the degree of ongoing activity. In conditions such as epilepsy, these astrocytic functions are corrupted, due to induction of a complex set of alterations in astroglia termed reactivity. Part of this reactive process is downregulation of the keystone enzyme in the glutamine-glutamate cycle, GS, and possibly also astrocyte-specific glutamate transporters. Because of this, development of reactivity in epilepsy is accompanied by significant static and dynamic changes in extracellular glutamate accumulation, as well as the potential for corrupted synaptic function, particularly loss of inhibitory synaptic efficacy. These alterations could contribute to the neuropathology and hyperexcitibility evident in epilepsy. In addition to contributing to the pathophysiology of epilepsy, these deficits in astroglial function suggest the potential for new therapeutic strategies targeting astrocytes, including metabolic treatments, which could help control seizures and blunt neuropathological consequences of this condition.
Acknowledgments
The authors are supported by grants from the National Institutes of Health (NIH): P01 NS054900 and P20 MH071705 to DAC and NS058674 and NS070824 to TE. This work was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
References
- Akbar MT, Torp R, Danbolt NC, Levy LM, Meldrum BS, Ottersen OP. Expression of glial glutamate transporters GLT-1 and GLAST is unchanged in the hippocampus in fully kindled rats. Neuroscience. 1997;78:351–9. doi: 10.1016/s0306-4522(96)00570-2. [DOI] [PubMed] [Google Scholar]
- Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–82. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, Bell K, Albert M, Brandt J, Stern Y. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47:867–72. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci. 2005;25:9567–80. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–6. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. J Neurosci. 2000;20:666–73. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D. Glia and neurons in dialogue. Nature. 1994;369:707–708. doi: 10.1038/369707a0. [DOI] [PubMed] [Google Scholar]
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol. 2002;88:2302–10. doi: 10.1152/jn.00665.2001. [DOI] [PubMed] [Google Scholar]
- Battaglioli G, Martin DL. GABA synthesis in brain slices is dependent on glutamine produced in astrocytes. Neurochem Res. 1991;16:151–6. doi: 10.1007/BF00965703. [DOI] [PubMed] [Google Scholar]
- Beer J, Mielke K, Zipp M, Zimmermann M, Herdegen T. Expression of c-jun, junB, c-fos, fra-1 and fra-2 mRNA in the rat brain following seizure activity and axotomy. Brain Res. 1998;794:255–66. doi: 10.1016/s0006-8993(98)00233-9. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–59. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- Benjamin AM, Quastel JH. Metabolism of amino acids and ammonia in rat brain cortex slices in vitro: a possible role of ammonia in brain function. J Neurochem. 1975;25:197–206. doi: 10.1111/j.1471-4159.1975.tb06953.x. [DOI] [PubMed] [Google Scholar]
- Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience. 2007;145:11–9. doi: 10.1016/j.neuroscience.2006.11.062. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–5. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bidmon HJ, Gorg B, Palomero-Gallagher N, Schleicher A, Haussinger D, Speckmann EJ, Zilles K. Glutamine synthetase becomes nitrated and its activity is reduced during repetitive seizure activity in the pentylentetrazole model of epilepsy. Epilepsia. 2008;49:1733–48. doi: 10.1111/j.1528-1167.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- Bouchet C. De l’épilepsie considérée dans ses rapports avec l’aliénation mentale. Recherche sur la nature et le siége de ces deux maladies. Arch Gén Méd. 1825;9:510–42. [Google Scholar]
- Bough K. Energy metabolism as part of the anticonvulsant mechanism of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):91–3. doi: 10.1111/j.1528-1167.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–72. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–6. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Bryant AS, Li B, Beenhakker MP, Huguenard JR. Maintenance of thalamic epileptiform activity depends on the astrocytic glutamate-glutamine cycle. J Neurophysiol. 2009;102:2880–8. doi: 10.1152/jn.00476.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB, Evans GA, Tobin AJ. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci U S A. 1992;89:2115–9. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF. Altered glial-neuronal crosstalk: cornerstone in the pathogenesis of hepatic encephalopathy. Neurochem Int. 2010;57:383–8. doi: 10.1016/j.neuint.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Penttonen M, Bragin A, Nadasdy Z, Chrobak JJ. Possible physiological role of the perforant path-CA1 projection. Hippocampus. 1995;5:141–6. doi: 10.1002/hipo.450050210. [DOI] [PubMed] [Google Scholar]
- Cantello R, Varrasi C, Tarletti R, Cecchin M, D’Andrea F, Veggiotti P, Bellomo G, Monaco F. Ketogenic diet: electrophysiological effects on the normal human cortex. Epilepsia. 2007;48:1756–63. doi: 10.1111/j.1528-1167.2007.01156.x. [DOI] [PubMed] [Google Scholar]
- Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol. 2005;57:226–35. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol. 2002;157:349–55. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Hartley DM. Calcium and glutamate-induced cortical neuronal death. In: Waxman SG, editor. Molecular and Cellular Approaches to the Treatment of Neurologic Disease. New York: Raven Press; 1993. [PubMed] [Google Scholar]
- Cohen-Gadol AA, Pan JW, Kim JH, Spencer DD, Hetherington HH. Mesial temporal lobe epilepsy: a proton magnetic resonance spectroscopy study and a histopathological analysis. J Neurosurg. 2004;101:613–20. doi: 10.3171/jns.2004.101.4.0613. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABA(A) receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–16. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DA. Epilepsy-associated plasticity in gamma-aminobutyric acid receptor expression, function, and inhibitory synaptic properties. Int Rev Neurobiol. 2001;45:237–52. doi: 10.1016/s0074-7742(01)45013-6. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Williamson A, Spencer SS, Zaveri HP, Eid T, Spencer DD. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: Evidence for distinctive patient subcategories. Epilepsia. 2003;44:677–687. doi: 10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- Dennis SC, Lai JC, Clark JB. Comparative studies on glutamate metabolism in synpatic and non-synaptic rat brain mitochondria. Biochem J. 1977;164:727–36. doi: 10.1042/bj1640727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow MJ, Eid T, Du F, Schwarcz R, Lothman EW, Steward O. Disruption of inhibition in area CA1 of the hippocampus in a rat model of temporal lobe epilepsy. J Neurophysiol. 2001;86:2231–45. doi: 10.1152/jn.2001.86.5.2231. [DOI] [PubMed] [Google Scholar]
- Du F, Whetsell WO, Jr, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–33. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–10. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Eid T, Ghosh A, Wang Y, Beckstrom H, Zaveri HP, Lee TS, Lai JC, Malthankar-Phatak GH, de Lanerolle NC. Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain. 2008a;131:2061–70. doi: 10.1093/brain/awn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Eid T, Williamson A, Lee TS, Petroff OA, de Lanerolle NC. Glutamate and astrocytes--key players in human mesial temporal lobe epilepsy? Epilepsia. 2008b;49(Suppl 2):42–52. doi: 10.1111/j.1528-1167.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- Empson RM, Heinemann U. The perforant path projection to hippocampal area CA1 in the rat hippocampal-entorhinal cortex combined slice. J Physiol. 1995;484 (Pt 3):707–20. doi: 10.1113/jphysiol.1995.sp020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulenburg V, Gomeza J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res Rev. 2010;63:103–12. doi: 10.1016/j.brainresrev.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Folbergrova J, Passonneau JV, Lowry OH, Schulz DW. Glycogen, ammonia and related metabolities in the brain during seizures evoked by methionine sulphoximine. J Neurochem. 1969;16:191–203. doi: 10.1111/j.1471-4159.1969.tb05937.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Burman J, Qureshi T, Tran CH, Proctor J, Johnson J, Zhang H, Sulzer D, Copenhagen DR, Storm-Mathisen J, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proc Natl Acad Sci U S A. 2002;99:14488–93. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–60. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fricke MN, Jones-Davis DM, Mathews GC. Glutamine uptake by System A transporters maintains neurotransmitter GABA synthesis and inhibitory synaptic transmission. J Neurochem. 2007;102:1895–904. doi: 10.1111/j.1471-4159.2007.04649.x. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABA(A) receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–38. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Gloor P. Mesial temporal sclerosis: Historical background and an overview from a modern perspective. In: Luders H, editor. Epilepsy Surgery. New York: Raven Press; 1991. pp. 689–703. [Google Scholar]
- Gorg B, Qvartskhava N, Bidmon HJ, Palomero-Gallagher N, Kircheis G, Zilles K, Haussinger D. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52:256–65. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–38. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, DeJoseph MR, Hawkins PA. Regional brain glutamate transport in rats at normal and raised concentrations of circulating glutamate. Cell Tissue Res. 1995;281:207–14. doi: 10.1007/BF00583389. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA, Annegers JF, Kokmen E, Rocca WA. Dementia and adult-onset unprovoked seizures. Neurology. 1996;46:727–30. doi: 10.1212/wnl.46.3.727. [DOI] [PubMed] [Google Scholar]
- Huang TL, O’Banion MK. Interleukin-1 beta and tumor necrosis factor-alpha suppress dexamethasone induction of glutamine synthetase in primary mouse astrocytes. J Neurochem. 1998;71:1436–42. doi: 10.1046/j.1471-4159.1998.71041436.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res. 1976;10:536–40. doi: 10.1203/00006450-197605000-00006. [DOI] [PubMed] [Google Scholar]
- Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci. 2000;20:1800–8. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs R, Seifert G, Steinhauser C. Astrocytic function and its alteration in the epileptic brain. Epilepsia. 2008;49(Suppl 2):3–12. doi: 10.1111/j.1528-1167.2008.01488.x. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Zielke HR, Max SR. Effect of dibutyryl cyclic AMP and dexamethasone on glutamine synthetase gene expression in rat astrocytes in culture. Neurochem Res. 1995;20:201–7. doi: 10.1007/BF00970545. [DOI] [PubMed] [Google Scholar]
- Jenstad M, Quazi AZ, Zilberter M, Haglerod C, Berghuis P, Saddique N, Goiny M, Buntup D, Davanger S, FMSH, et al. System A transporter SAT2 mediates replenishment of dendritic glutamate pools controlling retrograde signaling by glutamate. Cereb Cortex. 2009;19:1092–106. doi: 10.1093/cercor/bhn151. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC, Kopke RD, Wu JY. Demonstration of functional coupling between gamma -aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci U S A. 2003;100:4293–8. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27:9192–200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T, Kafitz KW, Roderigo C, Rose CR. Ammonium-evoked alterations in intracellular sodium and pH reduce glial glutamate transport activity. Glia. 2009;57:921–34. doi: 10.1002/glia.20817. [DOI] [PubMed] [Google Scholar]
- Kim JH, Je S, Petroff OA, Spencer SS, Hwang JY, Spencer DD. Hippocampal glial density in temporal lobe epilepsy. Epilepsia. 2004;45:S33–S34. [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Rutledge E, Goderie S, Charniga C. Astrocytic swelling due to hypotonic or high K+ medium causes inhibition of glutamate and aspartate uptake and increases their release. J Cereb Blood Flow Metab. 1995;15:409–16. doi: 10.1038/jcbfm.1995.51. [DOI] [PubMed] [Google Scholar]
- Kruchkova Y, Ben-Dror I, Herschkovitz A, David M, Yayon A, Vardimon L. Basic fibroblast growth factor: a potential inhibitor of glutamine synthetase expression in injured neural tissue. J Neurochem. 2001;77:1641–9. doi: 10.1046/j.1471-4159.2001.00390.x. [DOI] [PubMed] [Google Scholar]
- Kvamme E, Roberg B, Torgner IA. Kinetics and localization of phosphate activated glutaminase. J Neurosci Res. 2001;66:951–958. doi: 10.1002/jnr.10041. [DOI] [PubMed] [Google Scholar]
- Lajtha A, Berl S, Waelsch H. Amino acid and protein metabolism of the brain. IV. The metabolism of glutamic acid. J Neurochem. 1959;3:322–32. doi: 10.1111/j.1471-4159.1959.tb12638.x. [DOI] [PubMed] [Google Scholar]
- Lamigeon C, Bellier JP, Sacchettoni S, Rujano M, Jacquemont B. Enhanced neuronal protection from oxidative stress by coculture with glutamic acid decarboxylase-expressing astrocytes. J Neurochem. 2001;77:598–606. doi: 10.1046/j.1471-4159.2001.00278.x. [DOI] [PubMed] [Google Scholar]
- Lauritzen F, de Lanerolle NC, Lee TS, Spencer DD, Kim JH, Bergersen LH, Eid T. Monocarboxylate transporter 1 is deficient on microvessels in the human epileptogenic hippocampus. Neurobiol Dis. 2011;41:577–84. doi: 10.1016/j.nbd.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen F, Perez EL, Melillo ER, Roh JM, Zaveri HP, Lee TS, Wang Y, Bergersen LH, Eid T. Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol Dis. 2012;45:165–176. doi: 10.1016/j.nbd.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TS, Bjornsen LP, Paz C, Kim JH, Spencer SS, Spencer DD, Eid T, de Lanerolle NC. GAT1 and GAT3 expression are differently localized in the human epileptogenic hippocampus. Acta Neuropathol (Berl) 2006;111:351–63. doi: 10.1007/s00401-005-0017-9. [DOI] [PubMed] [Google Scholar]
- Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int. 2001;38:519–527. doi: 10.1016/s0197-0186(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Leke R, Bak LK, Iversen P, Sorensen M, Keiding S, Vilstrup H, Ott P, Portela LV, Schousboe A, Waagepetersen HS. Synthesis of neurotransmitter GABA via the neuronal tricarboxylic acid cycle is elevated in rats with liver cirrhosis consistent with a high GABAergic tone in chronic hepatic encephalopathy. J Neurochem. 2011;117:824–32. doi: 10.1111/j.1471-4159.2011.07244.x. [DOI] [PubMed] [Google Scholar]
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–48. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Holmes GL. Basic fibroblast growth factor-induced seizures in rats. Neurosci Lett. 1997;233:85–8. doi: 10.1016/s0304-3940(97)00627-7. [DOI] [PubMed] [Google Scholar]
- Lomako J, Lomako WM, Whelan WJ, Dombro RS, Neary JT, Norenberg MD. Glycogen synthesis in the astrocyte: from glycogenin to proglycogen to glycogen. FASEB J. 1993;7:1386–93. doi: 10.1096/fasebj.7.14.8224611. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–7. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Sorg O, Yu N, Martin J-L, Pellerin L. Neurotransmitters regulate energy metabolism in astrocytes: implications for metabolic trafficking between neural cells. Dev Neurosci. 1993;15:306–312. doi: 10.1159/000111349. [DOI] [PubMed] [Google Scholar]
- Malthankar-Phatak GH, de Lanerolle N, Eid T, Spencer DD, Behar KL, Spencer SS, Kim JH, Lai JC. Differential glutamate dehydrogenase (GDH) activity profile in patients with temporal lobe epilepsy. Epilepsia. 2006;47:1292–9. doi: 10.1111/j.1528-1167.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–8. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, Nelson N, Leite JP, Chimelli L, Born DE, et al. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–72. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- Mathews GC, Diamond JS. Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. J Neurosci. 2003;23:2040–8. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum B. Excitotoxicity and epileptic brain damage. Epilepsy Res. 1991;10:55–61. doi: 10.1016/0920-1211(91)90095-w. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Cell damage in epilepsy and the role of calcium in cytotoxicity. Adv Neurol. 1986;44:849–55. [PubMed] [Google Scholar]
- Miller HP, Levey AI, Rothstein JD, Tzingounis AV, Conn PJ. Alterations in glutamate transporter protein levels in kindling-induced epilepsy. J Neurochem. 1997;68:1564–1570. doi: 10.1046/j.1471-4159.1997.68041564.x. [DOI] [PubMed] [Google Scholar]
- Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28:109–21. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- Nadler J, Cuthbertson G. Kainic acid neurotoxicity toward the hippocampal formation: dependence on specific excitatory pathways. Brain Res. 1980;195:47–56. doi: 10.1016/0006-8993(80)90865-3. [DOI] [PubMed] [Google Scholar]
- Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: mechanism for deficient glutamatergic transmission? Mol Neurodegener. 2011;6:55. doi: 10.1186/1750-1326-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Collins RC, Sloviter RS. Excitotoxic mechanims of epileptic brain damage. In: Delgado-Escueta AV, Ward AA Jr, Woodbury DM, Porter RJ, editors. Advances in neurology. New York: Raven Press; 1986. pp. 857–877. [PubMed] [Google Scholar]
- Olney JW, Sharpe LG, Feigin RD. Glutamate-induced brain damage in infant primates. J Neuropathol Exp Neurol. 1972;31:464–88. doi: 10.1097/00005072-197207000-00006. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13:584–91. doi: 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Jahr CE. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J Neurosci. 1998;18:7099–110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–40. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JW, Bebin EM, Chu WJ, Hetherington HP. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia. 1999;40:703–7. doi: 10.1111/j.1528-1157.1999.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Papageorgiou IE, Gabriel S, Fetani AF, Kann O, Heinemann U. Redistribution of astrocytic glutamine synthetase in the hippocampus of chronic epileptic rats. Glia. 2011;59:1706–18. doi: 10.1002/glia.21217. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–7. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–34. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bergersen LH, Halestrap AP, Pierre K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res. 2005;79:55–64. doi: 10.1002/jnr.20307. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci. 1998;20:291–9. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. Neurons and their supporting cells. New York: Oxford University Press; 1991. The fine structure of the nervous system. [Google Scholar]
- Petroff OA, Cavus I, Kim JH, Spencer DD. Interictal extracellular glutamate concentrations are increased in hippocampal sclerosis. Ann Neurol. 2004;56:S43. [Google Scholar]
- Petroff OA, Errante LD, Kim JH, Spencer DD. N-acetyl-aspartate, total creatine, and myo-inositol in the epileptogenic human hippocampus. Neurology. 2003;60:1646–51. doi: 10.1212/01.wnl.0000068020.85450.8b. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43:703–10. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- Pinal CS, Tobin AJ. Uniqueness and redundancy in GABA production. Perspect Dev Neurobiol. 1998;5:109–18. [PubMed] [Google Scholar]
- Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH, van Veelen CW, van Rijen PC, van Nieuwenhuizen O, Gispen WH, et al. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain. 2002;125:32–43. doi: 10.1093/brain/awf001. [DOI] [PubMed] [Google Scholar]
- Qutub AA, Hunt CA. Glucose transport to the brain: a systems model. Brain Res Brain Res Rev. 2005;49:595–617. doi: 10.1016/j.brainresrev.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Rae C, Hare N, Bubb WA, McEwan SR, Broer A, McQuillan JA, Balcar VJ, Conigrave AD, Broer S. Inhibition of glutamine transport depletes glutamate and GABA neurotransmitter pools: further evidence for metabolic compartmentation. J Neurochem. 2003;85:503–14. doi: 10.1046/j.1471-4159.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- Rice A, Rafiq A, Shapiro SM, Jakoi ER, Coulter DA, DeLorenzo RJ. Long-lasting reduction of inhibitory function and gamma-aminobutyric acid type A receptor subunit mRNA expression in a model of temporal lobe epilepsy. Proc Natl Acad Sci U S A. 1996;93:9665–9. doi: 10.1073/pnas.93.18.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli MF, Morris JC, Ashkin K, Coben LA. Advanced Alzheimer’s disease is a risk factor for late-onset seizures. Arch Neurol. 1990;47:847–50. doi: 10.1001/archneur.1990.00530080029006. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kunci RW, Kanai Y, Schielke JP, Welty DF. Konckout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Kullmann DM. Extrasynaptic glutamate diffusion in the hippocampus: ultrastructural constraints, uptake, and receptor activation. J Neurosci. 1998;18:3158–70. doi: 10.1523/JNEUROSCI.18-09-03158.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow N, Zahn RK, Gabriel S, Heinemann U, Lehmann TN. Glutamine induces epileptiform discharges in superficial layers of the medial entorhinal cortex from pilocarpine-treated chronic epileptic rats in vitro. Epilepsia. 2009;50:849–58. doi: 10.1111/j.1528-1167.2008.01973.x. [DOI] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A. TNFalpha controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron. 2011;69:988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Schousboe A. Transport and metabolism of glutamate and GABA in neurons are glial cells. Int Rev Neurobiol. 1981;22:1–45. doi: 10.1016/s0074-7742(08)60289-5. [DOI] [PubMed] [Google Scholar]
- Schwartz RM, Boyes S, Aynsley-Green A. Metabolic effects of three ketogenic diets in the treatment of severe epilepsy. Dev Med Child Neurol. 1989;31:152–60. doi: 10.1111/j.1469-8749.1989.tb03973.x. [DOI] [PubMed] [Google Scholar]
- Seifert G, Carmignoto G, Steinhauser C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010;63:212–21. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22:6372–9. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank RP, Bennett GS, Freytag SO, Campbell GL. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–7. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- Simantov R, Crispino M, Hoe W, Broutman G, Tocco G, Rothstein JD, Baudry M. Changes in expression of neuronal and glial glutamate transporters in rat hippocampus following kainate-induced seizure activity. Brain Res Mol Brain Res. 1999;65:112–23. doi: 10.1016/s0169-328x(98)00349-0. [DOI] [PubMed] [Google Scholar]
- Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr. 2000;130:1016S–22S. doi: 10.1093/jn/130.4.1016S. [DOI] [PubMed] [Google Scholar]
- Solbu TT, Bjorkmo M, Berghuis P, Harkany T, Chaudhry FA. SAT1, A Glutamine Transporter, is Preferentially Expressed in GABAergic Neurons. Front Neuroanat. 2010;4:1. doi: 10.3389/neuro.05.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I. Brief history of cortico-hippocampal time with a special reference to the direct entorhinal input to CA1. Hippocampus. 1995;5:120–4. doi: 10.1002/hipo.450050206. [DOI] [PubMed] [Google Scholar]
- Sommer W. Erkrankung des Ammonshorns als aetiologisches Moment der Epilepsie. Arch Psychiatr Nervenkr. 1880;10:631–675. [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–27. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–7. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom C, Vining EPG, Rho JM. Ketogenic diet. In: Engel J, Pedley TA, editors. Epilepsy: a comprehensive textbook. Lippincott Williams & Wilkins; 2007. pp. 1377–1386. [Google Scholar]
- Stanimirovic DB, Ball R, Small DL, Muruganandam A. Developmental regulation of glutamate transporters and glutamine synthetase activity in astrocyte cultures differentiated in vitro. Int J Dev Neurosci. 1999;17:173–84. doi: 10.1016/s0736-5748(99)00028-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Takahashi H, Fukuda M, Hino H, Kobayashi K, Tanaka J, Ishii E. Beta-hydroxybutyrate alters GABA-transaminase activity in cultured astrocytes. Brain Res. 2009;1268:17–23. doi: 10.1016/j.brainres.2009.02.074. [DOI] [PubMed] [Google Scholar]
- Svenneby G. Pig brain glutaminase: purification and identification of different enzyme forms. J Neurochem. 1970;17:1591–9. doi: 10.1111/j.1471-4159.1970.tb03729.x. [DOI] [PubMed] [Google Scholar]
- Takamori S, Malherbe P, Broger C, Jahn R. Molecular cloning and functional characterization of human vesicular glutamate transporter 3. EMBO Rep. 2002;3:798–803. doi: 10.1093/embo-reports/kvf159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tani H, Dulla CG, Huguenard JR, Reimer RJ. Glutamine is required for persistent epileptiform activity in the disinhibited neocortical brain slice. J Neurosci. 2010;30:1288–300. doi: 10.1523/JNEUROSCI.0106-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessler S, Danbolt NC, Faull RL, Storm-Mathisen J, Emson PC. Expression of the glutamate transporters in human temporal lobe epilepsy. Neuroscience. 1999;88:1083–91. doi: 10.1016/s0306-4522(98)00301-7. [DOI] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–47. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- van den Berg CJ, Garfinkel D. A stimulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. Biochem J. 1971;123:211–8. doi: 10.1042/bj1230211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hel WS, Notenboom RG, Bos IW, van Rijen PC, van Veelen CW, de Graan PN. Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology. 2005;64:326–33. doi: 10.1212/01.WNL.0000149636.44660.99. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Chung C, Blei AT. Brain edema in acute liver failure. A window to the pathogenesis of hepatic encephalopathy. Ann Hepatol. 2003;2:12–22. [PubMed] [Google Scholar]
- Vardimon L, Ben-Dror I, Avisar N, Oren A, Shiftan L. Glucocorticoid control of glial gene expression. J Neurobiol. 1999;40:513–27. doi: 10.1002/(sici)1097-4695(19990915)40:4<513::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Waagepetersen HS, Sonnewald U, Schousboe A. The GABA paradox: multiple roles as metabolite, neurotransmitter, and neurodifferentiative agent. J Neurochem. 1999;73:1335–42. doi: 10.1046/j.1471-4159.1999.0731335.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zaveri HP, Lee TS, Eid T. The development of recurrent seizures after continuous intrahippocampal infusion of methionine sulfoximine in rats: a video-intracranial electroencephalographic study. Exp Neurol. 2009;220:293–302. doi: 10.1016/j.expneurol.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Morimoto K, Hirao T, Suwaki H, Watase K, Tanaka K. Amygdala-kindled and pentylenetetrazole-induced seizures in glutamate transporter GLAST-deficient mice. Brain Res. 1999;845:92–6. doi: 10.1016/s0006-8993(99)01945-9. [DOI] [PubMed] [Google Scholar]
- Williamson A, Patrylo PR, Pan J, Spencer DD, Hetherington H. Correlations between granule cell physiology and bioenergetics in human temporal lobe epilepsy. Brain. 2005;128:1199–208. doi: 10.1093/brain/awh444. [DOI] [PubMed] [Google Scholar]
- Wozny C, Gabriel S, Jandova K, Schulze K, Heinemann U, Behr J. Entorhinal cortex entrains epileptiform activity in CA1 in pilocarpine-treated rats. Neurobiol Dis. 2005;19:451–60. doi: 10.1016/j.nbd.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Yang S, Cox CL. Attenuation of inhibitory synaptic transmission by glial dysfunction in rat thalamus. Synapse. 2011;65:1298–308. doi: 10.1002/syn.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, brain glutamate metabolism and seizure control. Prostaglandins Leukot Essent Fatty Acids. 2004;70:277–85. doi: 10.1016/j.plefa.2003.07.005. [DOI] [PubMed] [Google Scholar]