Abstract
K-ras mutations have been identified in up to 95% of pancreatic cancers, implying their critical role in the molecular pathogenesis. Expression of K-ras oncogene in an immortalized human pancreatic ductal epithelial cell line, originally derived from normal pancreas (H6c7), induced the formation of carcinoma in mice. We hypothesized that K-ras oncogene correlates with increased non-mitochondrial-generated superoxide (O2·−), which could be involved in regulating cell growth contributing to tumor progression. In the H6c7 cell line and its derivatives, H6c7er-Kras+ (H6c7 cells expressing K-ras oncogene), and H6c7eR-KrasT (tumorigenic H6c7 cells expressing K-ras oncogene), there was an increase in hydroethidine fluorescence in cell lines that express K-ras. Western blots and activity assays for the antioxidant enzymes that detoxify O2·− were similar in these cell lines suggesting that the increase in hydroethidine fluorescence was not due to decreased antioxidant capacity. To determine a possible non-mitochondrial source of the increased levels of O2·−, Western analysis demonstrated the absence of NADPH oxidase-2 (NOX2) in H6c7 cells but present in the H6c7 cell lines expressing K-ras and other pancreatic cancer cell lines. Inhibition of NOX2 decreased hydroethidine fluorescence and clonogenic survival. Furthermore, in the cell lines with the K-ras oncogene, overexpression of superoxide dismutases, that detoxify non-mitochondrial sources of O2·−, and treatment with the small molecule O2·− scavenger Tempol, also decreased hydroethidine fluorescence, inhibited clonogenic survival and inhibited growth of tumor xenografts. Thus, O2·− produced by NOX2 in pancreatic cancer cells with K-ras, may regulate pancreatic cancer cell growth.
INTRODUCTION
Reactive oxygen species (ROS) have been linked to pancreatic cancer (1). ROS are generated during normal aerobic metabolism; increased flux of these species are produced during various forms of oxidative stress. The net intracellular concentration of ROS is the result of the production of ROS and the ability of antioxidants to remove them. A large increase (21- to 97-fold) in levels of nitrotyrosine, a footprint of the reactive nitrogen species peroxynitrite (formed by the reaction of superoxide with nitric oxide), has been demonstrated in pancreatic cancer specimens compared to normal pancreas (1). One reason why pancreatic cancer is so aggressive and unresponsive to treatment is its resistance to apoptosis, which may be linked to the increase in ROS levels. Vaquero and colleagues have recently demonstrated that ROS are pro-survival, antiapoptotic factors in pancreatic cancer (2).
Increases in intracellular production of superoxide (O2·−) in pancreatic cancers may influence downstream propagation of mitogenic signaling. Fibroblasts transfected with the viral ras oncogene have increased O2·− production and the generated O2·− may act as a second messenger molecule to promote cell proliferation (3). Santillo et al. (4) confirmed this demonstrating that in K-ras transformed thyroid cells, ROS is increased leading to activation of signal transduction pathways. Based on these observations it is hypothesized that K-ras may activate the NADPH oxidase (NOX) system to produce O2·− that leads to cell proliferation. Similar results have been found in human keratinocytes (5). In ras transformed keratinocytes, increased O2·− production was demonstrated and this increased production could be blocked efficiently by superoxide dismutase (SOD). Although K-ras is found in 95% of pancreatic cancers, no studies to date have demonstrated this same mechanism in pancreatic ductal epithelial cells, the cell of origin in pancreatic adenocarcinoma.
We hypothesized that K-ras oncogene in pancreatic cancer correlates to increases in non-mitochondrial-generated O2·−, which could be involved in regulating cell growth contributing to pancreatic tumor progression. This model could explain increased susceptibility of pancreatic cancer cells to scavenging of non-mitochondrial-generated superoxide. Overexpression of extracellular superoxide dismutase (EcSOD, located in the extracellular space) and copper/zinc dismutase (CuZnSOD, located in the cytosol) had even greater inhibitory effects on pancreatic tumor growth when compared to MnSOD (located in the mitochondria), suggesting that scavenging non-mitochondrial sources of O2·− may prove beneficial for suppression of pancreatic cancer growth (6,7). In addition, scavenging the O2·− radical with superoxide dismutases or a small molecule scavenger that act on or near the cell membrane would inhibit growth in these tumors.
MATERIALS AND METHODS
Cell Culture
We used an immortalized cell line derived from normal pancreatic ductal epithelial with near normal genotype and phenotype of pancreatic duct epithelial cells HPV16-E6E7 (H6c7); the isogenic cell line that expresses K-rasG12V H6c7eR-Kras+ (Kras+), and the isogenic cell line that also expresses K-rasG12V and forms tumors H6c7eR-KrasT (KrasT) (8). These cell lines were maintained in keratinocyte serum free media and supplemented with epidermal growth factor and bovine pituitary extract (9). Initial work with these cells demonstrated that they did not form colonies. Thus, feeder cells were used and prepared by growing B1 mouse fibroblast cells in DMEM containing 10% FBS and 1% penicillin and streptomycin plus MEM nonessential amino acids. Cells were then plated in 100-mm dishes and grown to 80% confluence before being irradiated for a total dose of 30 Gy. The cells were harvested and preserved in growth media containing 10% DMSO and aliquots were frozen in liquid nitrogen. Twenty-four hours before clonogenic assay, irradiated cells were thawed and diluted in keratinocyte serum free media. Cells were then seeded at 400 cells per well in 6-well plates as described (10).
Human pancreatic cancer cells were purchased from American Type Culture Collection (Manassas, VA). MIA PaCa-2 cells are undifferentiated human primary pancreatic adenocarcinoma cells and are maintained at 37°C in Dulbecco modified Eagle medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum and 2.5% horse serum. AsPC-1 are grown in RPMI 1640 medium contains 20% FBS and 1.0 mM sodium pyruvate.
Measurement of ROS
Intracellular generation of O2·− was assessed using hydroethidine fluorescence. The level of presumably intracellular peroxide was also determined using the oxidation sensitive [C-400; 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate(DCFH); Molecular Probes, Eugene, OR] and its oxidized form [C-369; 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate] dissolved in DMSO. The oxidized probe was utilized to control for changes in uptake, ester cleavage, and efflux so that differences in fluorescence can definitively be attributed to changes in oxidation of the probe (11). Briefly, cells were incubated with 10 μM of hydroethidine for 40 min or 10 μM of DCFH in HBSS for 20 min in the dark at 37°C with 5% CO2. Cells were then washed with PBS, harvested, and analyzed using a FACScan flow cytometer as described (11).
Electron paramagnetic resonance (EPR) was performed in cells treated with 4-hydroxy-TEMPO (Tempol) to determine relative superoxide levels. EPR spectra were acquired and peak heights were quantified and compared in cells treated with Tempol (0, 0.1, 1.0, 10 mM). The cells were washed twice with HBSS and treated with 50 mM DMPO in PBS. The samples were transferred to a quartz flat cell and spectra were recorded using a Bruker EMX spectrometer with the following settings: receiver gain, 1.0 × 106; modulation amplitude, 1.0 G, modulation frequency, 100 kHz. EPR was performed in plasma from mice with and without Tempol in their drinking water. EPR spectra were quantified by comparing to Tempol standard solutions in PBS to determine absolute levels of Tempol.
Western analysis
Immunoreactive protein corresponding to antioxidant enzymes was identified and quantified from total cell protein. Total protein was electrophoresed in a 4–20 % Tris-HCl Ready Gel (Bio-Rad, Hercules, CA). The proteins were then electotransferred to polyvinylidine difluoride (PVDF) membrane. After blocking in 5% fat-free milk for 1 h, the sheets were washed and then treated with antibodies to either MnSOD (1:1000), CuZnSOD (1:5000), EcSOD (1:4000), NOX1 (1:500), NOX2 (1:1000), NOX3 (1:1000), and NOX4 (1:650) overnight. Polyclonal rabbit-anti-human antibodies to MnSOD and CuZnSOD were purchased from Cell Signaling Technology (Danvers, MA) (12). The EcSOD antibody was prepared and characterized by Dr. James Crapo (National Jewish Medical and Research Center, Denver, CO). NOX1, 2, 3, and 4 antibodies were purchased from Abcam (Cambridge, MA). The blots were incubated with horseradish peroxidase-conjugated goat-anti-rabbit IgG (1:10,000, Millipore, Billerica, MA) for 1 h at room temperature. The washed blots were then treated with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL) and exposed to Classic Blue Autoradiography Film (MIDSCI, St. Louis, MO). All western blots were performed in duplicate.
Adenovirus Gene Transfer
The adenovirus constructs used were replication-defective, E1- and partial E3-deleted recombinant adenovirus (13, 14). Inserted into the E1 region of the adenovirus genome were the human CuZnSOD, EcSOD, or GPx gene, which are driven by a cytomegalovirus promoter (Viraquest, North Libery, IA). For the vector control, we used the same adenovirus with no gene added (an empty vector) (AdEmpty) or with the green fluorescence protein added (AdGFP). In addition, we used an adenoviral vector expressing siRNA against NOX2 (AdsiNOX2), which was prepared and characterized by Dr. Robin Davisson (15) and constructed, purified and provided by The University of Iowa Gene Vector Core.
Approximately 106 cells were plated in complete media in a 100-cm2 dish and allowed to attach for 24 h. Cells were then washed three times in serum- and antibiotic-free media. The AdCuZnSOD, AdEcSOD, AdGPx, AdsiNOX2 or AdEmpty constructs, suspended in 3% sucrose, were then applied to cells suspended in 4 ml of serum-and antibiotic-free media at 0, 10, 25, 50, and 100 MOI (multiplicity of infection). Cells were incubated with the adenovirus constructs for 24 h. Media was then replaced with 10 ml of complete media for an additional 24 h before cells were harvested.
Fluorescence Analysis
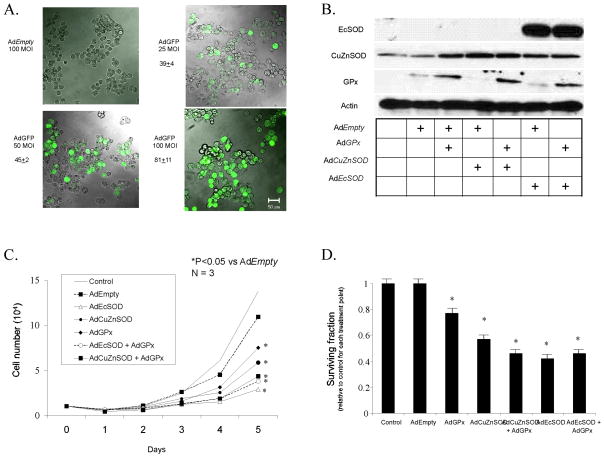
MIA PaCa-2 cells were seeded in 8-well chamber slides (Thermo Fisher Scientific, Rochester, NY). Cells were infected with 25, 50 and 100 MOI of AdGFP in serum-free DMEM for 24 h, and then incubated with full media for an additional 24 h. AdEmpty (100 MOI) was used as a control. Cells were fixed with 4% para-formaldehyde for 15 min at room temperature and examined with a fluorescence microscope (Olympus BX-51). For each field, GFP expressing cells and non-expressing cells were counted and the ratio of GFP positive cells was calculated.
Cell Growth
After 48 h of adenoviral infection, or treatment with Tempol for one hour, cells were trypsinized, counted, and reseeded at a density of 1 × 104 in 24-well plates with 1.5 mL of complete media. For the growth analysis, cells were trypsinized and then counted daily for 1 week using a Coulter counter. Cell population doubling time in hours (DT) was determined using the following equation:
where to = time at which exponential growth began, t = time in hours, Nt = cell number at time t, and No = initial cell number (12).
Clonogenic survival
AdCuZnSOD, AdEcSOD, AdGPx, AdsiNOX2, AdEmpty-transduced cells (100 MOI) or Tempol treated cells were plated in triplicate into 60-mm dishes in complete media. The dishes were maintained in the incubator for 10 – 14 days to allow colony formation. The colonies were then fixed with 70% ethanol, stained with Coomassie blue (10% acetic acid, 50% methanol and 0.1 % Coomassie Blue G-250), and those colonies containing greater than 50 cells were scored.
Nude Mice
Thirty-day-old athymic nude mice were obtained from Harlan Sprague-Dawley (Indianapolis, IN). The nude mice protocol was reviewed and approved by the Animal Care and Use Committee of the University of Iowa and were in compliance with The U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals (NIH). Each experimental group consisted of 5 to 8 mice. MIA PaCa-2 tumor cells were delivered subcutaneously into the flank region of nude mice with a 1-cc tuberculin syringe equipped with a 25-gauge needle. The tumors were allowed to grow until they reached between 3 mm to 4 mm in greatest dimension (2 weeks), at which time they were treated with adenovirus in the first series of in vivo experiments. The adenovirus constructs were delivered through two injections sites in the tumor by means of a 25-gauge needle attached to a 1-cc tuberculin syringe. Previous studies from our laboratory used single intratumoral injections of the adenovirus constructs (7). However, in this study AdCuZnSOD, AdEcSOD or AdEmpty constructs (5 × 107 PFU in 50 μL of 3% sucrose) were delivered to the tumor on days 0, 7, and 14 for a total of 3 injections. Control tumors received 50 μL of 3% sucrose. Tumor size was measured every three to four days by means of a vernier caliper, and tumor volume was estimated according to the following formula: tumor volume = π/6 × L × W2, where L is the greatest dimension of the tumor, and W is the dimension of the tumor in the perpendicular direction (16). Animals were killed by CO2 asphyxiation when the tumors reached a predetermined size of 1000 mm3 as this was considered the time to sacrifice. In separate experiments, pre-established tumors were injected with an AdGFP construct (5 × 107 PFU) and fluorescence microscopy was used to determine in vivo gene transfer. Forty-eight hours after AdGFP infection, nude mice with tumor xenografts were sacrificed and the tumors were collected and fixed in 10% formalin solution. After treatment with gradient sucrose solution as cryoprotectant, the samples were frozen in Tissue-Tek OCT compound (EMS, Hatfield, PA) at −80°C. The frozen blocks were cut in 10 micrometer thick sections on a Microm 505 cyrostat set at −25°C and slides were mounted with DAPI (4′, 6-Diamidino-2-phenylindole) containing Vectashield fluorescent mounting media. Visualization and documentation were performed with an Olympus BX-51 fluorescence microscope (Olympus, Melville, NY). (DAPI) was visualized with the UV filter cube and GFP signal (green) was collected with an FITC filter cube.
In the second series of in vivo experiments, mice with 3–4 mm tumors were divided into three groups: Controls, Tempol 10 mM in the drinking water, and Tempol 20 mM in the drinking water. Tumor size was again measured every three to four days by means of a vernier caliper, to estimate tumor volume. Animals were killed when the tumors reached a predetermined size of 1000 mm3. To determine Tempol levels in mice, tumors harvested from mice were homogenized with a known amount of pH 6.5 PBS, either 750 μL or 1 mL, depending on tumor size. Homogenates were spun down and supernatant was removed. Enough 10 mM KFeCN was added to the supernatant to make it 1 mM. Samples were incubated at room temp for 10 minutes to allow the KFeCN to oxidize all the Tempol to the radical form. Samples were analyzed for Tempol using a Bruker EMX EPR spectrometer with a TM110 cavity. Samples were run at room temperature with the following parameters: center field 3480.9 G, sweep width 60 G, microwave frequency 9.766 GHz, power 20 mW, receiver gain 2 × 105, modulation frequency 100 kHz, modulation amplitude 2 G, time constant 163.84 ms, conversion time 20.48 ms, resolution 1024 points, and number of scans 20. Tempol was quantified using a 3-carboxy-proxyl standard.
Statistical Analysis
A single factor ANOVA, followed by post-hoc Tukey test, was used to determine statistical differences between means. All means were calculated from three experiments, and error bars represent standard error of the mean (SEM). All Western blots were repeated at least twice. For the in vivo studies, the statistical analyses focused on the effects of different treatments on cancer progression. The primary outcome of interest was tumor growth over time. Tumor sizes were measured throughout the experiments, resulting in repeated measurements across time for each mouse. Linear mixed effects regression models were used to estimate and compare the group-specific tumor growth curves. In the growth curve analyses, statistically significant global tests of equality across groups were followed up with pairwise comparisons to identify specific group differences. All tests were two-sided and carried out at the 5% level of significance. Analyses were performed with the SAS and R statistical software package.
RESULTS
Expression of K-ras correlates with increased levels of reactive oxygen species
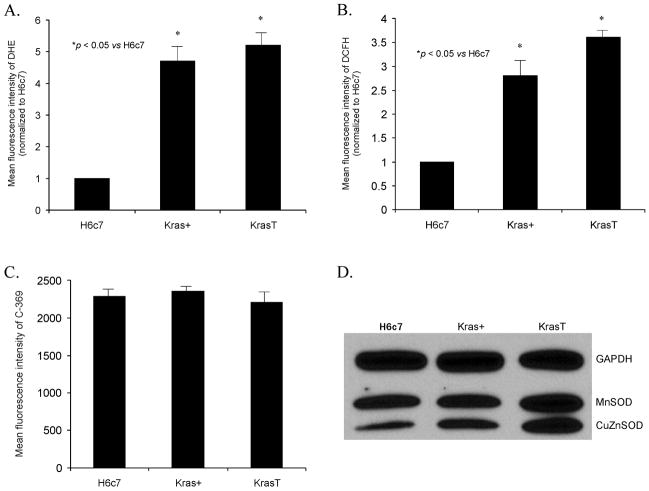
To determine if K-ras increases ROS, hydroethidine and DCFH fluorescence, and antioxidant enzyme levels were determined in the H6c7 cell line and its derivatives. Figure 1A demonstrates an increase in hydroethidine fluorescence in the H6c7eR-Kras+ (Kras+) and H6c7eR-KrasT (KrasT) cell lines when compared to the H6c7 cells. In addition, there was a concomitant increase in DCFH fluorescence in the same Kras+ and KrasT cell lines (Figure 1B). The mean fluorescence intensity of cells labeled with the oxidized probe (C-369) was unchanged between the H6c7, Kras+ and KrasT cell lines (Figure 1C). These results suggest that K-ras overexpression induces increased levels of O2·− and hydroperoxides in the H6c7 cells.
Figure 1. Expression of K-ras oncogene leads to increased levels of ROS in human pancreatic pancreatic ductal epthelial cells.
A. H6c7 cells that express K-ras oncogene (Kras+ and KrasT) demonstrate a 4.5 to 5-fold increase in hydroethidine fluorescence compared to the H6c7 cell line. Cells were incubated and then stained with hydroethidine (DHE). Mean fluorescence intensity was measured via flow cytometry, corrected for background fluorescence levels, and normalized to the H6c7 cell line. *p < 0.05 vs H6c7 cells, means ± SEM, n = 3.
B. H6c7 cells that express K-ras oncogene (Kras+ and KrasT) demonstrate a 2.5 to 3.0-fold increase in DCFH fluorescence compared to the parental (H6c7) cell line. Cells were incubated in various treatment groups and then stained with DCFH and analyzed as described in A. *p < 0.05 vs H6c7 cells, means ± SEM, n = 3.
C. MFI was similar in H6c7 cells, Kras+ cells and KrasT cells when labeled with the non-oxidation sensitive dye C-369, demonstrating that changes in MFI in panel B are indicative of changes in steady-state levels of dye oxidation.
D. Western blots for the antioxidant enzymes CuZnSOD and MnSOD were determined. Expression of K-ras oncogene did not result in decreases in antioxidant protein to account for the increases in DHE or DCFH fluorescence.
To determine if the K-ras-induced increases in ROS were due to changes in the antioxidant profile of these cell lines, MnSOD and CuZnSOD protein and activity were determined. Figure 1D demonstrates immunoreactive protein content in the three cell lines. There were similar levels of MnSOD immunoreactive protein in the three cell lines and increases in MnSOD and CuZnSOD protein in the Kras+ and KrasT cell lines as demonstrated by Western blotting (Figure 1D). Taken together, these data suggest that the increase in the oxidation of hydroethidine observed in the Kras+ and KrasT cells (possibly via superoxide) is not due to a decrease in antioxidant enzyme capacity that detoxify O2·−.
Overexpression of SOD inhibits growth of cells expressing K-ras
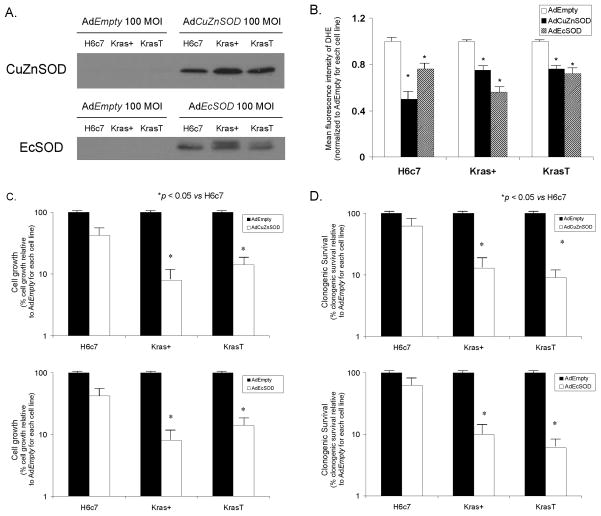
If K-ras oncogene correlates with increased O2·− levels, we then wanted to determine if scavenging of nonmitochondrial sources of O2·− results in growth inhibition in cells expressing K-ras. The H6c7, Kras+ and KrasT cells were infected with adenoviral vectors containing the cDNA for CuZnSOD (AdCuZnSOD) or EcSOD (AdEcSOD) (100 MOI) or the AdEmpty vector (100 MOI) and cell growth and clonogenic survival were determined. Figure 2A demonstrates the increase in immunoreactive protein for CuZnSOD or EcSOD after infecting the H6c7, Kras+ and KrasT with AdCuZnSOD or AdEcSOD, respectively, compared to those cells infected with the AdEmpty vector. Due to the abundance of SOD protein with adenoviral infection (Supplementary Figure 1), the exposure for this Western blot was decreased compared to the Western blot in Figure 1D. Since EcSOD is an extracellular protein, we also determined the presence of the protein in the culture media; we found that there was also increased immunoreactive protein in media of cells infected with AdEcSOD, as previously seen in our laboratory (Supplementary Figure 1) (7). If the antioxidant enzymes CuZnSOD and EcSOD inhibit growth of pancreatic cancer by scavenging of superoxide, then we would predict a decrease in hydroethidine fluorescence upon overexpression of these antioxidant enzymes. In the H6c7, Kras+ and KrasT cells infected with the AdCuZnSOD and AdEcSOD vectors, hydroethidine fluorescence decreased compared to the same cell lines infected with the AdEmpty vector (Figure 2B). This further suggests that the increase in hydroethidine fluorescence seen in Figure 1 is due in part to non-mitochondrial production of O2·−.
Figure 2. Overexpression of CuZnSOD or EcSOD decreases hydroethidine fluorescence, cell growth and clonogenic survival in cell lines expressing K-ras.
A. The H6c7 cell line and its derivatives, Kras+, and KrasT, were infected with either AdEmpty, AdCuZnSOD, or AdEcSOD at 100 MOI. AdCuZnSOD 100 MOI increased CuZnSOD protein in all of the cell lines compared to controls and cells infected with AdEmpty vector (100 MOI). AdEcSOD 100 MOI also increased EcSOD protein in all of the cell lines compared to controls and cells infected with the AdEmpty vector (100 MOI). There are two bands noted on the EcSOD Western blot. The top band (32 kDa) is the EcSOD protein with heparin binding domain and the bottom band (29.5 kDa) corresponds to the EcSOD protein without heparin binding domain.
B. Intracellular hydroethidine fluorescence decreased in H6c7, Kras+, and KrasT cells infected with AdCuZnSOD, or AdEcSOD. Intracellular superoxide levels as measured by DHE decreased significantly 24–48 hours after infection with AdCuZnSOD, or AdEcSOD 100 MOI compared to the same cells infected with the AdEmpty vector. *p < 0.05 vs AdEmpty, means ± SEM, n = 3.
C. Cell growth was decreased after infection with the AdCuZnSOD and AdEcSOD vectors. The H6c7 cell line and its derivatives, Kras+, and KrasT, were transduced with AdEmpty, AdCuZnSOD or AdEcSOD at 100 MOI. AdCuZnSOD and AdEcSOD demonstrated reductions in cell growth in cells expressing the K-ras oncogene. Mean in vitro cell growth on day 6 is shown. Each point represents the mean values, n = 3. * p < 0.05 vs 100 MOI AdEmpty.
D. Clonogenic survival. CuZnSOD and EcSOD overexpression decreased clonogenic survival only in the cell lines with expression of K-ras oncogene. Dark bars represent the surviving fraction of cells treated with the AdEmpty vector (100 MOI), while the open bars represent the surviving fraction treated with AdCuZnSOD or AdEcSOD. Each point represents the mean values, with p < 0.05 vs. AdEmpty, n = 3.
Tumor cell growth characteristics were used to evaluate the effect of the overexpression of CuZnSOD, and EcSOD in cell culture. The growth rate and plating efficiency were therefore examined. H6c7, Kras+ and KrasT cells infected with AdCuZnSOD and AdEcSOD (Figure 2C) demonstrated slower in vitro growth compared to parental cells and cells infected with the AdEmpty vector. The Kras+ and KrasT cell growth significantly decreased with AdCuZnSOD (100 MOI), when compared to the H6c7 cells or 100 MOI AdEmpty cells (Figure 2C). Similar results were seen in the KrasT cells with overexpression of CuZnSOD. Slower in vitro growth was also demonstrated in the Kras+ and KrasT using the AdEcSOD vector when compared to the H6c7 cells (Figure 2C). In addition, AdCuZnSOD and AdEcSOD demonstrated decreases in clonogenic survival in the Kras+ and KrasT cells when compared to the H6c7 cells (Figure 2D). These results suggest that H6c7 cells that express K-ras oncogene and have increased levels of O2·− are more sensitive to O2·− scavenging antioxidants compared to cells not expressing K-ras. Additionally, these results suggest that K-ras-induced O2·− production plays a major role in cell survival, and therefore scavenging of O2·− with CuZnSOD and EcSOD results in significant growth inhibition in vitro.
Inhibition of NOX2 alters the malignant phenotype
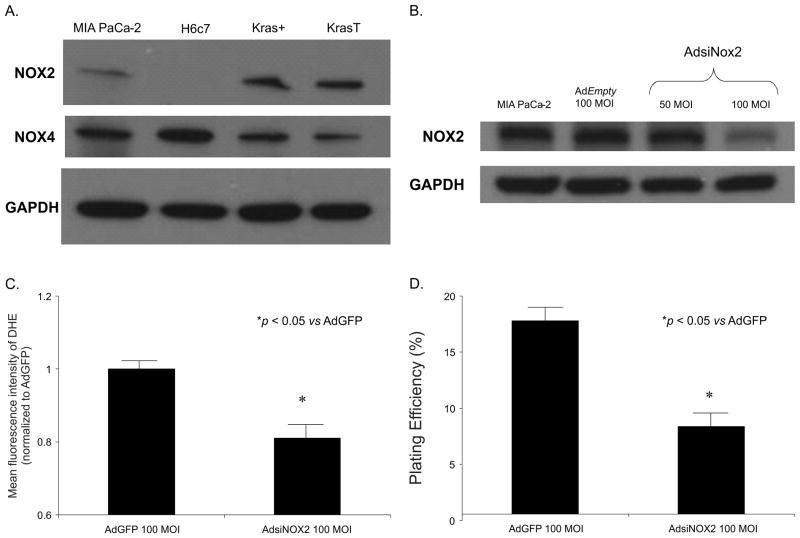
Scavenging the superoxide radical with superoxide dismutases that act on or near the cell membrane inhibit growth of the pancreatic cancer cells (7). It has been hypothesized that K-ras activates the NADPH oxidase (NOX) system to produce O2·− that leads to cell proliferation (2). Western analysis was used to assess the expression of the NOX proteins in the H6c7, Kras+, KrasT and MIA PaCa-2 cell lines. NOX1 and NOX3 were not detected in the H6c7, Kras+, KrasT and MIA PaCa-2 cell lines (data not shown). NOX4 protein was somewhat decreased in MIA PaCa-2, Kras+ and KrasT cells compared to the H6c7 cells. Most notably, NOX2 was absent in the H6c7 cell line but present in the Kras+, KrasT and MIA PaCa-2 (which expresses mutant K-ras) cell lines (Figure 3A). These results suggest that K-ras expression may induce NOX2 expression in pancreatic cancer. To determine the role of NOX2 in pancreatic cancer cells we used the AdsiNOX2 vector (15) and determined protein levels, hydroethidine fluorescence and clonogenic survival. In MIA PaCa-2 cells, AdsiNOX2 (100 MOI) significantly decreased immunoreactive protein (Figure 3B). In addition, there was a decrease in hydroethidine fluorescence (Figure 3C) and clonogenic survival (Figure 3D), which was also demonstrated in a similar pattern as with SOD overexpression as seen in Figure 2. Thus, NOX2 is absent in pancreatic ductal epithelial cells (H6c7) but present in the same cells that express K-ras (Kras+ and KrasT) and in human pancreatic cancer cells (MIA PaCa-2). Overall, these results suggest that in pancreatic cancer cells, K-ras may induce O2·− production via NOX2, leading to cell survival and tumor promotion. Additionally, NOX2 may be a nonmitochondrial source of O2·− in pancreatic cancer cells and that either inhibiting this enzyme or scavenging O2·− produced by this enzyme with SOD, will inhibit the malignant phenotype.
Figure 3. NOX2 is involved in superoxide production in pancreatic cancer cells.
A. Western blot analysis for NOX2 demonstrates expression in MIA PaCa-2, Kras+ and KrasT cells but no expression in the H6c7 pancreatic ductal epithelial cell line. NOX4 was present in all cell lines tested. GAPDH was used as a loading control
B. An adenoviral vector expressing siRNA against NOX2 (AdsiNOX2) was transfected into the MIA PaCa-2 pancreatic cancer cell line. At 100 MOI, there was a significant decrease in immunoreactive protein when compared to both the parental cell line and cells transfected the adenoviral control vector AdGFP (100 MOI).
C. MIA PaCa-2 cells infected with the AdsiNOX2 vector demonstrate a significant decrease in hydroethidine fluorescence when compared to the same cell line infected with the AdGFP vector. Mean fluorescence intensity was measured via flow cytometry, corrected for background fluorescence levels, and normalized to the cells infected with AdGFP. *p < 0.05 vs AdGFP cells, means ± SEM, n = 3.
D. AdsiNOX2 infection (100 MOI) in MIA PaCa-2 cells decreased plating efficiency when compared to the same cell line infected with the AdGFP vector (100 MOI). Each point represents the mean values, with p < 0.05 vs. AdGFP, n = 3.
Tempol inhibits the malignant phenotype
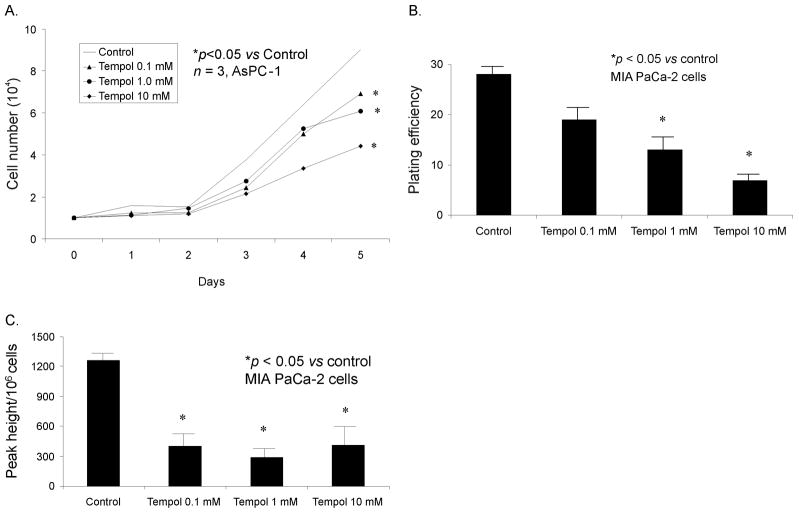
To determine if there was a pharmacological approach to scavenging superoxide in pancreatic cancer, we used Tempol, a nitroxide compound that is low molecular weight, membrane permeable, and a stable free radical that is EPR detectable (16) and has been demonstrated to possess antioxidant activity and protect cells against oxidative stress (17,18,19). Several mechanisms have been proposed to account for nitroxide antioxidant activity, including SOD mimetic activity, which will catalytically remove O2·− (19). MIA PaCa-2 and AsPC-1 human pancreatic cancer cells were treated with Tempol (0.1, 1.0, or 10 mM) for 1 h and clonogenic survival and cell growth were determined. Tempol inhibited growth (Figure 4A) and decreased clonogenic survival (Figure 4B) in both cell lines in a dose-dependent fashion. If Tempol inhibits growth of pancreatic cancer by scavenging O2·−, we hypothesized that there would be a concomitant decrease in superoxide levels after Tempol administration. Superoxide levels were measured using EPR, demonstrating a significantly decreased superoxide signal with Tempol (0.1–10 mM) (Figure 4C). Thus, pharmacological treatment of human pancreatic cancer cells with Tempol inhibits the in vitro malignant phenotype with a concomitant decrease in superoxide levels.
Figure 4. Tempol inhibits pancreatic cancer cell growth.
A. For cell growth, cells were treated with Tempol (0, 0.1, 1.0, and 10 mM) for 1 h. Mean in vitro cell growth of AsPC-1 cells are shown.
B. For clonogenic survival, cells were treated with Tempol (0, 0.1, 1 and 10 mM) for 1 h, 400 cells were plated in each well of 6-well plates and incubated at 37°C for 14 days. Colonies were fixed and stained with Coomassie blue; only colonies with more than 50 cells were counted. Each point was determined in triplicate from the same culture. * p < 0.01 vs control.
C. Electron paramagnetic resonance was performed in MIA PaCa-2 cells treated with Tempol to determine superoxide levels. EPR spectra were acquired and peak heights were quantified and compared in cells treated with Tempol (0.1–10 mM). DMPO-OH signals from MIA PaCa-2 cells treated with Tempol. In the spectral analysis the Tempol signals were removed to see the DMPO-OH signal better.
The role of superoxide in the tumor suppressive effects of SOD overexpression
We then wanted to further explore the role of O2·− in the tumor suppressive effect seen with EcSOD and CuZnSOD. The mechanisms by which SOD suppresses tumor cell growth are unknown; however, change in the cellular redox status, especially change attributable to accumulation of H2O2 or other hydroperoxides, is a possible reason to explain the suppression of tumor growth observed in SOD-overexpressing cells (20). In human glioma cells, GPx overexpression reversed the tumor cell growth inhibition caused by MnSOD overexpression, suggesting that the increased flux of H2O2 by MnSOD (not the decreased O2·−) led to the tumor suppressive effects (20). In pancreatic cancer cell lines, GPx alone has been shown to have a tumor suppressive effect (21). Previous studies from our laboratory have demonstrated decreases in O2·− levels and increases in H2O2 with EcSOD and CuZnSOD overexpression (7). However, we have not yet determined if the tumor suppression with EcSOD or CuZnSOD is due to decreases in O2·− levels or increases in H2O2 levels. First, we demonstrated a dose-dependent increase in GFP fluorescence with increasing viral titer of the AdGFP construct resulting in greater than 80% of the MIA PaCa-2 pancreatic cancer cells expressed the transgene (Figure 5A). Next, to determine the effector molecule in CuZnSOD and EcSOD tumor suppression, GPx was also overexpressed in MIA PaCa-2 pancreatic tumor cells. Expression of CuZnSOD, EcSOD, and GPx immunoreactive protein was demonstrated using Western analysis (Figure 5B). As we have previously observed (7, 21), AdCuZnSOD, AdEcSOD, and AdGPx alone (50 MOI) decreased cell growth compared to the AdEmpty vector (Figure 5C). However, the combination of CuZnSOD + GPx overexpression or EcSOD + GPx overexpression did not increase the tumor suppressive effects of the SODs alone (Figure 5C). To further confirm this finding, we performed a clonogenic assay using the same groups as in the growth curve. Overexpression of GPx, CuZnSOD, and EcSOD decreased clonogenic survival. The decrease in clonogenic survival induced by CuZnSOD or EcSOD was not reversed when combined with AdGPx (Figure 5D). Combined, these results suggest tumor suppression by CuZnSOD or EcSOD is due to the decreased levels of O2·− and not due to accumulation of H2O2 or other hydroperoxides.
Figure 5. Superoxide is a growth signal in pancreatic cancer.
A. Fluorescence Analysis. MIA PaCa-2 cells were seeded in 8-well chamber slides (Thermo Fisher Scientific, Rochester, NY). Cells were infected with 25, 50 and 100 MOI of AdGFP in serum-free DMEM for 24 h, and then incubated with full media for an additional 24 h. AdEmpty (100 MOI) was used as a control. Cells were fixed with 4% para-formaldehyde for 15 min at room temperature and examined with a fluorescence microscope (Olympus BX-51). For each field, GFP expressing cells and non-expressing cells were counted and the ratio of GFP positive cells was calculated. Fluorescence photomicrographs of MIA PaCa-2 cells infected with increasing viral titer of the AdEmpty or AdGFP constructs demonstrate that there is no fluorescence in the group of cells that received the AdEmpty vector. However, increasing doses of the AdGFP construct increases the percentage of cells that stain positive for GFP.
B. Western analysis of MIA PaCa-2 cells infected with the AdEmpty, AdGPx, AdCuZnSOD and AdEcSOD or combinations. To equalize the viral load of the combined virus in these experiments, the AdEmpty vector was given. For example, the 50 MOI AdGPx was given along with 50 MOI AdEmpty to equal the viral load of the combination of AdGPx (50 MOI) + AdCuZnSOD or AdEcSOD (50 MOI). Lane assignments: 1 = Control; 2 = AdEmpty; 3 = AdEmpty + AdGPx; 4 = AdEmpty + AdCuZnSOD; 5 = AdGPx + AdCuZnSOD; 6 = AdEmpty + AdEcSOD; 7 = AdGPx + AdEcSOD.
C. Cell growth. MIA PaCa-2 cells transduced with 50 MOI AdGPx, 50 MOI AdCuZnSOD (± 50 MOI AdGPx), or 50 MOI AdEcSOD (± 50 MOI AdGPx), demonstrated significant reductions in growth compared to the parental cells and those infected with AdEmpty. No significant changes were seen with AdEmpty transfer compared with parental cells. Mean in vitro cell growth of MIA PaCa-2 cells are shown. Each point represents the mean values, n = 3. * p < 0.001 vs AdEmtpy.
D. Clonogenic survival. MIA PaCa-2 cells transduced with 50 MOI AdCuZnSOD, AdEcSOD, AdGPx, AdCuZnSOD + AdGPx, or AdEcSOD +AdGPx demonstrated reductions in clonogenic survival compared to AdEmpty transduced cells. As in the cell growth experiments, the AdEmpty vector was given to equalize the viral load of the combined virus. Values are mean plating efficiency ± SEM of MIA PaCa-2, n = 3. *p < 0.05 vs AdEmpty.
Scavenging superoxide inhibits in vivo tumor growth
We have demonstrated a significant tumor growth inhibition with a single, intratumoral injection of an adenoviral vector that contains one of the various forms of SOD (6, 7). Since our previous studies demonstrated that CuZnSOD and EcSOD clearly had greater tumor suppression in this animal model with one injection of the adenoviral vector, we wanted to determine if we could enhance tumor growth inhibition with multiple doses of the adenoviral vector. In addition to determining if we could enhance this effect, we wanted to determine the biodistribution of gene expression using the technique of intratumoral injections of these adenoviral vectors. Biodistribution of gene expression using AdGFP intratumoral injections demonstrated green fluorescence throughout the tumor, most notably present along presumed needle injection tracks (Figure 6A). Further inspection of the histological sections demonstrated no fluorescence in cells from tumors that underwent the AdEmpty injections (Figure 6B). However, cells that are GFP positive were demonstrated in several areas of the tumor sections that received the AdGFP injections, which we interpreted to be clusters of gene expression throughout the tumor (Figure 6B). Unlike previous studies from our laboratory (7), we used small doses of virus (5 × 107 PFU), given in multiple injections in preestablished pancreatic tumors. When the AdCuZnSOD or AdEcSOD constructs were given, a slower growth in tumor was observed in comparison to the control group as well as the AdEmpty injected group (Figure 6C). Table 1 provides statistical summaries of tumor volumes. The sample sizes (n) given in the table are the total number of available within each group. Mixed linear regression analysis of the tumor growth curves demonstrated that their rate of growth differed significantly between the groups (p < 0.0001). Pairwise group comparisons indicated significant differences for Controls vs. AdCuZnSOD (p < 0.05), AdEmpty vs. AdCuZnSOD (p < 0.05), and AdEmpty vs. AdEcSOD (p < 0.05). Thus, scavenging of superoxide with the specific SODs that act near the cell membrane, inhibit in vivo tumor growth further suggesting that non-mitochondrial superoxide is a growth signal in pancreatic cancer.
Figure 6. Intratumoral injections of AdCuZnSOD and AdEcSOD inhibit growth.
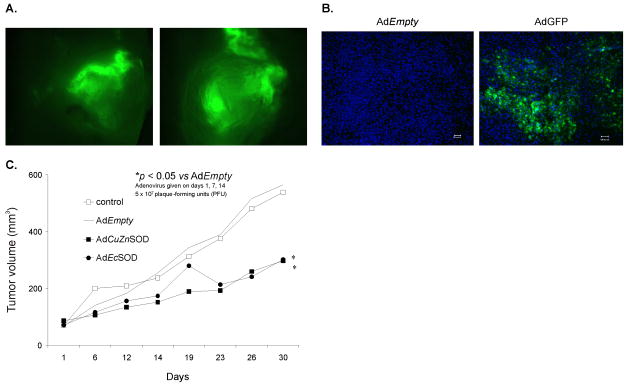
A. Detection of transgene expression and biodistribution in whole tumor xenografts. MIA PaCa-2 cells (2 × 106) were injected subcutaneously into the flank region of nude mice and allowed the tumors to reach 4–5 mm in diameter. Tumors were then injected with AdGFP or AdEmpty adenoviral vectors. Two representative tumors demonstrate GFP expression which is clearly visible by the green fluorescence and heterogeneously distributed throughout the tumor.
B. Histological sections detecting transgene expression and biodistribution in MIA PaCa-2 human pancreatic tumor xenograft in vivo after intratumoral injection of an AdGFP construct (1 × 109 PFU). AdEmpty was used as the control. Low power fields of fluorescence microscopy on sections from tumors injected with AdEmpty and AdGFP respectively. Sections were counterstained with DAPI. GFP expressing cells are clearly visible by their green fluorescence and are widely distributed throughout the tumor. Bar = 100 microns.
C. AdCuZnSOD or AdEcSOD injections decreased MIA PaCa-2 tumor growth in nude mice. The AdCuZnSOD and AdEcSOD groups had significantly slower tumor growth when compared to the AdEmpty group (p < 0.05, n = 6–8/group). MIA PaCa-2 tumor cells (2 × 106) were delivered subcutaneously into the flank region of nude mice. Controls received serum-free media in similar volumes. 5 × 107 PFUs of the AdCuZnSOD, AdEcSOD, or AdEmpty constructs were delivered to the tumor on days 1, 7 and 14 of the experiment. On day 30 there was nearly a 2-fold decrease in tumor growth in animals receiving the AdEcSOD vector or AdCuZnSOD when compared to treatment with the AdEmpty vector.
Table 1.
Summary statistics for the tumor volume measurements in the mixed linear regression analysis. The sample sizes (n) given in the table are the total number of available within each group. Mixed linear regression analysis of the tumor growth curves demonstrated that their rate of growth differed significantly between the groups (p < 0.0001).
| Groups | n | Mean Tumor Size (mm3) |
|---|---|---|
| Controls | 97 | 469 |
| AdEmpty | 77 | 445 |
| AdCuZnSOD | 127 | 311 |
| AdEcSOD | 143 | 375 |
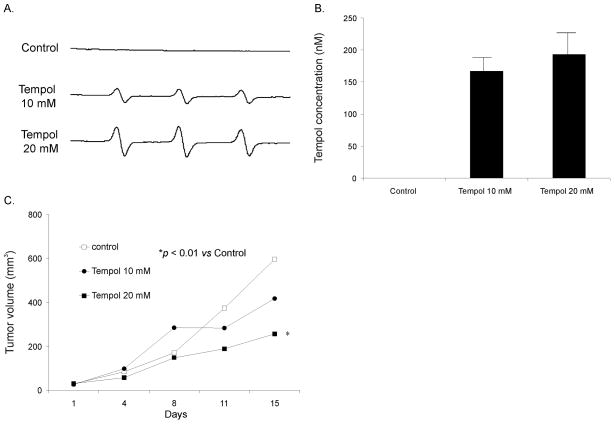
At this time, gene therapy for malignant disease is not yet feasible. So to determine if we could mimic the results see with EcSOD and CuZnSOD with a pharmacological approach, we used Tempol to reduce tumor growth. Animals were divided into three groups with the two treatment groups of mice receiving either 10 mM or 20 mM Tempol in their drinking water, while controls received the normal water supply. Electron paramagnetic resonance was performed in plasma in mice with and without Tempol in their drinking water (Figure 7A). EPR spectra were acquired and peak heights were quantified and compared against Tempol standard solutions in PBS to determine absolute levels of Tempol as seen in Figure 7B. There was no signal for Tempol in mice that did not receive Tempol in their drinking water. However, EPR demonstrated increased Tempol concentrations in plasma of mice treated with Tempol.
Figure 7. Tempol inhibits the growth of pancreatic tumor xenografts.
A. Representative EPR spectra obtained from plasma in mice after they were sacrificed demonstrating the Tempol signal in mice that received Tempol (10 or 20 mM) in their drinking water. Mice that did not receive Tempol in the drinking water had no EPR signal.
B. EPR spectra were acquired and peak heights were quantified and compared against Tempol standard solutions in PBS to determine absolute levels of Tempol. EPR demonstrated significant peaks for Tempol in the plasma of mice receiving Tempol 10 mM or 20 mM in the drinking water. As expected, there were no detectable signals for Tempol in mice with no Tempol in the drinking water.
C. Statistical analysis focused on the effects of different treatments tumor progression. The primary outcome of interest was tumor growth over time. Mice were injected with tumor cells at the start of each study. They were then randomly assigned to a treatment group and followed until death or until the experiment was terminated. Tumor sizes (mm3) were periodically measured throughout the experiments, resulting in repeated measurements for each mouse. Linear mixed effects regression models were used to estimate and compare group-specific tumor growth curves. Pairwise comparisons were performed to identify specific group differences in the growth curves. All tests were two-sided and carried out at the 5% level of significance. Pairwise group comparisons were carried out to assess group differences. Significant differences were observed for Control vs. Tempol 20 mM (p < 0.01), and Tempol 10 mM vs. Tempol 20 mM (p < 0.01).
Tumor sizes (mm3) were periodically measured throughout the experiments, resulting in repeated measurements for each mouse. Linear mixed effects regression models were used to estimate and compare group-specific tumor growth curves. Mice receiving Tempol 20 mM in the drinking water had slower tumor growth in comparison to the control group as well as the group of mice receiving Tempol 10 mM in the drinking water (Figure 7C). For example on day 15 after the start of treatment, the control had mean tumor volumes of 596 mm3 while the Tempol (20 mM in the drinking water) group had a mean tumor volume of 257 mm3 (p < 0.05 vs. Controls, Figure 7C). Table 2 provides additional statistical summaries of tumor volumes. Sample sizes (n) given are the total number of measurements within each group. Regression analysis demonstrated that their rate of growth differed significantly between the groups (p < 0.0001). Pairwise group comparisons were carried out to assess group differences. Significant differences were observed for Tempol 20 mM vs. Controls (p < 0.01) and Tempol 20 mM vs. Tempol 10 mM (p < 0.01). Thus, pharmacological treatment with an agent that has superoxide scavenging properties appears to have an in vivo growth inhibitory effect in pre-established pancreatic tumors.
Table 2.
Summary statistics for the tumor volume measurements in the mixed linear regression analysis. Sample sizes (n) given are the total number of measurements within each group. Regression analysis demonstrated that their rate of growth differed significantly between the groups (p < 0.0001). Pairwise group comparisons were carried out to assess group differences.
| Groups | n | Mean Tumor Size (mm3) |
|---|---|---|
| Controls | 142 | 791 |
| Tempol 10 mM | 128 | 996 |
| Tempol 20 mM | 150 | 769 |
DISCUSSION
K-ras mutation results in constitutive activation of intracellular signaling pathways, leading to uncontrolled cellular proliferation. Mutations of the K-ras gene occur in 90% of cases with adenocarcinoma of the pancreas (22) but are less common in other cancer types. K-ras mutation has been found in intraductal pancreatic cancer, ductal hyperplasia, and even chronic pancreatitis (23), suggesting that this may be an early event in pancreatic carcinogenesis. Indeed, K-ras mutations have been reported to be 30% in early neoplasms but 90% in advanced cancers (24). Although the entire spectrum of downstream genes regulated by the K-ras activation is not clear, several Ras-mediated signaling pathways and their target proteins have been demonstrated to regulate pancreatic cancer growth and survival (25,26).
Our data correlate with other studies in the literature demonstrating the correlation between K-ras status and ROS. Romanowska and colleagues demonstrated that K-ras activity correlated well with superoxide levels and DNA strand breaks in lung cancer cells (27). The presence of K-ras transformation may also allow cells to be resistant to various forms of oxidative stress. Two-dimensional gel electrophoresis led to the identification of differentially expressed proteins involved in cellular detoxification and oxidative stress and increased expression of these enzymes was paralleled by an elevated tolerance of K-ras mutants to hydrogen peroxide and an altered redox status (28). The presence of K-ras may also allow pancreatic cancer cells to be resistant to ionizing radiation. K-ras knockdown by siRNA or by inhibition of prenyltransferase activity resulted in radiation sensitization both in vitro and in vivo (29). Thus, the altered intracellular and extracellular redox status may play a role in both cell growth and invasion. Overexpression of EcSOD inhibited growth and cellular invasion in prostate cancer cells, suggesting that O2·− production at or near the cell membrane or in the extracellular matrix is important for cell growth and invasion of some tumor types (13).
Numerous studies have demonstrated a potential link to K-ras and the NADPH oxidase proteins, most notably NOX1 and NOX4. K-ras has been shown to strongly induce NOX1 expression in a fibroblast cell line and knockdown of NOX1 blocked Ras-dependent anchorage independence and tumor formation (30). Transfection with an antisense oligonucleotide to NOX4 inhibited NOX activity and reactive oxygen species in pancreatic cancer (2). Our study differs from the study of Vaquero and colleagues (2) since they investigated growth stimulated increases in reactive oxygen species and NOX4. Our present study did not utilize growth factor stimulation, but an immortalized pancreatic ductal epithelial cell line (H6c7) originally derived from normal pancreas, the isogeneic cell lines derived that express K-ras, and human pancreatic cancer cells that express K-ras. Thus, a novel finding in our present study demonstrates presence of the NOX2 protein in pancreatic tumor cells and tumorigenic cells from the H6c7 cell line that express K-ras, but the absence of NOX2 in the non-tumorigenic pancreatic ductal epithelial cell line H6c7. Knockdown of NOX2 resulted in decreased O2·− levels and decreased clonogenic survival. Regulation of NOX2 involves the cytosolic subunits p47phox, p67phox, p40phox, as well as the small GTPase Rac1 (31). Interestingly, DNA microarray and RT-PCR has demonstrated that Rac1 is upregulated in pancreatic cancer (32). Additionally, activation of Rac1-dependent O2·− generation in pancreatic cancer cells that express mutant K-ras leads to pancreatic cancer cell proliferation (33).
Pancreatic ductal epithelial cells are the cell of origin for pancreatic ductal adenocarcinoma. Our finding that H6c7 pancreatic ductal epithelial cells that express K-ras and form tumors in mice have increased levels of O2·−, is consistent with the hypothesis that cancer cells, relative to normal cells, may demonstrate increased steady-state levels of reactive oxygen species including O2·−. Aykin-Burns et al. demonstrated increased oxidation of the fluorescent probe dihydroethidine in both human colon and breast cancer cells when compared with that in normal cells (34). In addition, the tumor cell lines tested were more sensitive to oxidation-induced cytotoxicity. Thus, cancer cells may demonstrate excess production of reactive oxygen species and may provide a biochemical target to exploit for therapy. Our study further adds evidence for a biochemical target to exploit for therapy by demonstrating that scavenging O2·− could have potential for treatment.
In summary, K-ras oncogene in pancreatic ductal epithelial cells correlates with increased non-mitochondrial-generated superoxide. NOX2 may be a source of non-mitochondrial O2·− in pancreatic cancer cells. Inhibiting NOX2 or scavenging O2·− with molecular biological techniques that increase SOD expression at or near the cell membrane, or pharmacologically with Tempol treatment, will inhibit the malignant phenotype.
Supplementary Material
Acknowledgments
Supported by NIH grants CA137230 and CA115438, the Medical Research Service, Department of Veterans Affairs, and the Susan L. Bader Foundation of Hope.
References
- 1.MacMillan-Crow LA, Greendorfer JS, Vickers SM, Thompson JA. Tyrosine nitration of c-SRC tyrosine kinase in human pancreatic ductal adenocarcinoma. Arch Biochem Biophys. 2000;377:350–6. doi: 10.1006/abbi.2000.1799. [DOI] [PubMed] [Google Scholar]
- 2.Vaquero EC, Edderkaoui M, Pandol SJ, Gukovsky I, Gukovskaya AS. Reactive oxygen species produced by NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J Biol Chem. 2004;279:34643–54. doi: 10.1074/jbc.M400078200. [DOI] [PubMed] [Google Scholar]
- 3.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–51. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 4.Santillo M, Mondola P, Seru R, Annella T, Cassano S, Ciullo I, et al. Opposing functions of Ki- and Ha-Ras genes in the regulation of redox signals. Curr Biol. 2001;11:614–9. doi: 10.1016/s0960-9822(01)00159-2. [DOI] [PubMed] [Google Scholar]
- 5.Yang JQ, Li SJ, Domann FE, Buettner GR, Oberley LW. Superoxide generated in v-Ha-ras-transduced human keratinocyte HaCaT cells. Mol Carcinog. 1999;26:180–8. [PubMed] [Google Scholar]
- 6.Wydert C, Roling B, Liu J, Ritchie JM, Oberley LW, Cullen JJ. Suppression of the malignant phenotype in human pancreatic cancer cells by the overexpression of manganese superoxide dismutase. Mol Cancer Ther. 2003;2:361–9. [PubMed] [Google Scholar]
- 7.Teoh MLT, Sun W, Smith BJ, Oberley LW, Cullen JJ. Modulation of reactive oxygen species (ROS) in pancreatic cancer: Insight into tumor growth suppression by the superoxide dismutases. Clin Cancer Res. 2007;13:7441–50. doi: 10.1158/1078-0432.CCR-07-0851. [DOI] [PubMed] [Google Scholar]
- 8.Qian J, Niu J, Li M, Chiao PJ, Tsao MS. In vitro modeling of human pancreatic duct epithelial cell transformation defines gene expression changes induced by K-ras oncogenic activation in pancreatic carcinogenesis. Cancer Res. 2005;65:5045–53. doi: 10.1158/0008-5472.CAN-04-3208. [DOI] [PubMed] [Google Scholar]
- 9.Lewis A, Ough M, Du J, Tsao MS, Oberley LW, Cullen JJ. Targeting NAD(P)H:quinone oxidoreductase (NQO1) in pancreatic cancer. Mol Carcinog. 2005;43:215–24. doi: 10.1002/mc.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jessop NW, Hay RJ. Preparation, preservation, recovery and use of irradiated feeder layers in cell culture research. TCA Manual. 1979;5:1137–9. [Google Scholar]
- 11.Du J, Daniels DH, Asbury CA, Venkataraman S, Liu J, Spitz DR, et al. Mitochondrial production of reactive oxygen species mediate dicumarol-induced cytotoxicity in cancer cells. J Biol Chem. 2006;281:37416–26. doi: 10.1074/jbc.M605063200. [DOI] [PubMed] [Google Scholar]
- 12.Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, et al. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer Res. 2003;63:1297–303. [PubMed] [Google Scholar]
- 13.Chaiswing L, Zhong W, Cullen JJ, Oberley LW, Oberley TD. Extracellular redox state of human prostate cancer cells regulates biological and biochemical properties related to metastatic behavior. Cancer Res. 2008;68:5820–6. doi: 10.1158/0008-5472.CAN-08-0162. [DOI] [PubMed] [Google Scholar]
- 14.Sun W, Kalen AL, Smith BJ, Cullen JJ, Oberley LW. Enhancing the antitumor activity of adriamycin and ionizing radiation. Cancer Res. 2009;69:4294–300. doi: 10.1158/0008-5472.CAN-09-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson JR, Burmeister MA, Tian X, Zhou Y, Guruju MR, Stupinski JA, et al. Genetic silencing of Nox2 and Nox4 reveals differential roles of these NADPH Oxidase homologues in the vasopressor and dispogenic effects of brain angiotensin II. Hypertension. 2009;54:1106–14. doi: 10.1161/HYPERTENSIONAHA.109.140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gusy S, Mitchell JB, Ehleiter D, Haimovitz-Friedman A, Kasid U. Nitroxides Tempol and tempo induce divergent signal transduction pathways in MDA-MB 231 breast cancer cells. J Biol Chem. 1998;273:17871–8. doi: 10.1074/jbc.273.28.17871. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell JB, Samuni A, Krishna MC, DeGraff WG, Ahn MS, Samuni U, et al. Biologically active, metal-independent superoxide dismutase mimics. Biochemistry. 1990;29:2802–7. doi: 10.1021/bi00463a024. [DOI] [PubMed] [Google Scholar]
- 18.Samuni A, Min A, Krishna CM, Mitchell JB, Russo A. SOD-like activity of 5-membered nitroxide spin labels. Adv Exp Med Biol. 1990;264:85–92. doi: 10.1007/978-1-4684-5730-8_12. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JB, Degraff W, Kaufman D, Krishna MC, Samuni A, Findelstein E, et al. Mn(III)-desferrioxamine superoxide dismutase mimic: alternative modes of action. Arch Biochem Biophys. 1991;289:62–70. doi: 10.1016/0003-9861(91)90186-m. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Yan T, Yang JQ, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–39. [PubMed] [Google Scholar]
- 21.Liu J, Hinkhouse MM, Sun W, Weydert CJ, Ritchie JM, Oberley LW, et al. Redox regulation of pancreatic cancer cell growth: role of glutathione peroxidase in the suppression of the malignant phenotype. Hum Gene Ther. 2004;15:239–50. doi: 10.1089/104303404322886093. [DOI] [PubMed] [Google Scholar]
- 22.Uemura T, Hibi K, Kaneko T, Takeda S, Inoue S, Okochi O, et al. Detection of K-ras mutations in the plasma DNA of pancreatic cancer patients. J Gastroenterol. 2004;39:56–60. doi: 10.1007/s00535-003-1245-1. [DOI] [PubMed] [Google Scholar]
- 23.Maisonneuve P, Lowenfels AB. Chronic pancreatitis and pancreatic cancer. Dig Dis. 2002;20:32–7. doi: 10.1159/000063165. [DOI] [PubMed] [Google Scholar]
- 24.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Dephino RA. Genetic and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 25.Avruch J, Zhang XF, Kyriakis JM. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–83. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 26.Marshall MS. Ras target proteins in eukaryotic cells. FASEB J. 1995;9:1311–8. doi: 10.1096/fasebj.9.13.7557021. [DOI] [PubMed] [Google Scholar]
- 27.Romanowska M, Macaig A, Smith AL, Fields JR, Fornwald LW, Kikawa KD, et al. DNA damage, superoxide and mutant K-ras in lung adenocarcinoma cells. Free Radic Biol Med. 2007;43:1145–55. doi: 10.1016/j.freeradbiomed.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Recktenwald CV, Kellner R, Lichtenfels R, Seliger B. Altered detoxification status and increased resistance to oxidative stress by K-ras transformation. Cancer Res. 2008;68:10068–93. doi: 10.1158/0008-5472.CAN-08-0360. [DOI] [PubMed] [Google Scholar]
- 29.Brunner TB, Cengel KA, Hahn SM, Wu J, Fraker DL, McKenna WG, et al. Pancreatic cancer cell radiation survival and prenyltransferase inhibition: The role of K-ras. Cancer Res. 2005;65:8433–41. doi: 10.1158/0008-5472.CAN-05-0158. [DOI] [PubMed] [Google Scholar]
- 30.Mitushita J, Lambeth JD, Kanata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–5. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- 31.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–31. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crnogorac-Jurcevic T, Efthimiou E, Capelli P, Blaveri E, Baron A, Terris B, et al. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–46. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- 33.Du J, Liu J, Smith BJ, Tsao MS, Cullen JJ. Role of Rac-1 dependent NADPH oxidase in the growth of pancreatic cancer. Cancer Gene Ther. 2011;18:135–43. doi: 10.1038/cgt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.