Abstract
Atypical eletrodermal and cardiovascular response patterns in psychopathic individuals are thought to be biological indicators of fearless and disinhibition. This study investigated the relationship between psychopathic traits and these autonomic response patterns using a count-down task in 843 children (aged 9–10 years). Heart rate (HR) and non-specific skin conductance responses (NS-SCRs) were recorded while participants anticipated and reacted to 105 dB signaled or unsignaled white-noise bursts. Using multilevel regression models, both larger HR acceleration and fewer NS-SCR were found to be significantly associated with psychopathic traits during anticipation of signaled white-noise bursts. However, two divergent patterns appeared for HR and SCR: (1) larger HR acceleration was specific to the callousness-disinhibition factor of psychopathic traits while reduced NS-SCR was only associated with the manipulative-deceitfulness factor; (2) the negative association between the manipulative-deceitfulness factor and NS-SCR was only found in boys but not in girls. These findings replicated what has been found in psychopathic adults, suggesting that autonomic deficits present in children at risk may predispose them to later psychopathy. The divergent findings across psychopathic facets and sexes raised the possibility of different etiologies underlying psychopathy, which may in turn suggest multiple treatment strategies for boys and girls.
Keywords: Psychopathy, Children, Heart rate, Skin conductance, Countdown
Psychopathy, or psychopathic personality, can be costly and harmful to society due to the behavioral and criminal consequences that accompany it. Characterized by a cluster of stable personality traits including superficial emotion, impulsivity, manipulativeness and antisocial behavior, the concept of psychopathy has been extended downward to children and adolescents (Farrington 2005; Lynam 1997). The callous-unemotional traits (an important component of psychopathy) in younger samples have also been found to show robust predictive validity to later antisocial personality disorder, violence, aggression, and delinquency (see review by Frick and White 2008). Therefore, an earlier identification of psychopathic personality traits in development, prior to the influence of harmful sequelae (e.g., time spent in prison, substance use), is important because it may shed light on more successful early intervention (Frick et al. 2000) and therefore a reduction of the costs of antisocial behavior to individuals and society. Given the importance of juvenile psychopathy, many efforts are devoted to understanding its etiology and developmental antecedents. For example, the autonomic physiology of psychopathy which has been studied extensively in adults (See review by Arnett 1997; Gao et al. 2009; Hare 1978, 1982; Ogloff and Wong 1990; Patrick et al. 2007; Verona et al. 2004) also begins to witness an increase of attention in adolescents or children recently (Blair 1999; Fung et al. 2005; Herpertz et al. 2001; Isen et al. 2010).
One consistent finding in the adult psychopathy literature is that psychopaths tend to show autonomic hyporeactivity in response to aversive stimuli compared to non-psychopathic individuals (Gao and Raine 2010; Lorber 2004). One of the paradigms used to evaluate physiological responses to the anticipation and receipt of noxious stimuli (e.g. a white noise burst or an electrical shock) is the countdown task (Hare and Craigen 1974). This task is usually composed of signaled trials only, but may sometimes also include un-signaled trials. In the signaled condition, subjects receive a visual countdown of numbers on a screen from 12 to 0, as an indication of when the aversive stimulus will occur. In the un-signaled condition, no visual countdown is provided; hence the subject has no knowledge of when the aversive stimulus will come. These un-signaled trials are used as a comparison condition in order to evaluate the efficacy of the decreasing numbers in the signaled trials in eliciting anticipatory fear of the unpleasant event (Fung et al. 2005).
This hyporeactivity in psychopaths has been shown by reduced skin conductance responses (SCRs) when anticipating the aversive stimuli in the countdown task (Hare et al. 1978; Ogloff and Wong 1990). Skin conductance is used to assess autonomic nervous system functioning and controlled mainly by the sympathetic nervous system. It is believed to be under considerable control of the amygdala as evidenced by lesion studies in which patients with amygdala dysfunction/deformation showed impaired SCR in emotion-related tasks (Bechara et al. 1995; LaBar et al. 1995). This skin conductance hyporeactivity may index characteristics such as emotional deficits and impulsivity that underlie or heighten the psychopathic individual’s propensity for antisocial behavior and negative interpersonal relationships (Fung et al. 2005).
Coupled with the hyporeactivity in SCR, psychopathic individuals also display an abnormal change in heart rate (HR) in anticipation of an aversive event (Raine 1997). In the countdown paradigm, HR typically shows a very stable and robust tri-phasic response pattern (Hugdahl 1995), whereby an initial deceleration period at the beginning of the countdown period (D1 component), is followed by an acceleration (A component), and then a deceleration at the end of the countdown period (D2 component). Although also exhibiting a tri-phasic pattern on average, adult psychopaths tend to demonstrate greater anticipatory HR acceleration in anticipation of the aversive stimuli (e.g. a larger increase in HR during the A component), whereas their D1 and D2 components are not different from the nonpsychopathic controls (Hare 1982; Hare et al. 1978; Ogloff and Wong 1990). As another measure of autonomic nervous system functioning, HR is controlled by both the sympathetic and parasympathetic nervous systems. Although the neural basis of HR is not as well understood as that for SCR, the phase-dependent HR changes are generally thought to be the result of temporal dynamics between the vagus and the central system (Somsen et al. 2004). This may include, for example, the Fight/Flight regulation system in which the amygdala, the periaqueductal gray matter, and the ventrome-dial hypothalamus are centrally involved (Beauchaine et al. 2001). The post-orienting HR acceleration is associated with greater Fight/Flight reactivity via the mediation of vagal withdrawal (Porges 1995), and a chronically attenuated cardiac vagal tone may mark a reduced threshold for acute anger and panic episodes (Beauchaine et al. 2001), which thus facilitate the tendency for fight or aggression.
To our knowledge, only one study to date has examined the anticipatory SCR in adolescents with psychopathic traits in the countdown paradigm. Fung et al. (2005) reported significantly reduced non-specific skin conductance responses (NS-SCR) in 16-year-old boys with psychopathic traits in anticipation of a signaled 105-dB white noise. However, no female subjects were included and HR data were not assessed in that study. Therefore, the primary goal of the current study was to examine whether the same autonomic impairments in the adult psychopaths (i.e. reduced SCRs and greater HR acceleration in anticipation to aversive stimuli) can be observed in a community sample of 9–10 year old children. Based on previous literature, it was hypothesized that (1) all participants would show an average tri-phasic response pattern of HR under the signaled condition of the countdown task, and (2) when anticipating aversive stimuli children with psychopathic traits would demonstrate reduced NS-SCR and a greater HR acceleration (i.e. during the A component).
Another emerging issue that has acquired increasing attention in understanding the etiology of psychopathy is the multiple dimensions of this concept. As mentioned above, psychopathy is characterized by a cluster of distinct facets: callous-unemotional affective traits, arrogant-deceitful interpersonal traits, and impulsive-irresponsible behavioral traits (Hare 1991; Lynam 1997). Besides these core traits, some instruments also incorporate the behavioral deviance (externalizing) feature, e.g., the conduct problems, juvenile delinquency, and criminal versatility as a supplemental facet (Frick and Hare 2001; Hare and Neumann 2006). In an effort to integrate different conceptualizations, Patrick et al. (2009) suggested a triarchic model of psychopathy, which summarized the distinct facets of psychopathy in three new constructs: Boldness, Meanness, and Disinhibition.
Regardless of variations across different measuring instruments or conceptualizations, a recurring theme is that different facets of psychopathy, despite interrelating with each other, might be etiologically different and show divergent correlations with various external criterion measures. For example, there is some evidence showing that arrogant-deceitful interpersonal traits correlate negatively with internalizing problems, while the impulsive-irresponsible behavioral traits positively correlate with sensation seeking, high neuroticism, and externalizing deviancy (Blonigen et al. 2005; Hall et al. 2004; Hicks and Patrick 2006). The autonomic hyporeactivity (e.g., reduced SCR, and diminished startle blink) in response to aversive stimuli has been suggested to specifically reflect the fearlessness or emotional callousness traits of psychopaths (Fowles 1993; Patrick 1994). Therefore, a secondary goal of this study was to address the question of whether increased HR acceleration and/or reduced NS-SCR would apply to child psychopathy unitarily or differentially to its specific facets.
Finally, recent research has suggested that there may be sex differences in physiological correlates of children with conduct problems or psychopathic traits. For example, Beauchaine et al. (2008) found that aggressive girls exhibited greater baseline skin conductance than controls but showed no difference in pre-ejection period (PEP, a cardiovascular measure controlled solely by the SNS), whereas aggressive boys demonstrated lower baseline PEP than controls but no difference in skin conductance. Using the same sample as in the current study, Isen et al. (2010) found reduced SC orienting responses in boys with high psychopathy scores but not in girls. Since these studies used either neutral or positive stimuli, it is unclear what pattern of sex differences would appear in anticipation of aversive stimuli in the countdown task. Although sex differences were examined in the present analyses, no priori hypothesis was made for the degree or direction of differences in the autonomic response patterns in relation to psychopathic traits.
Method
Participants
Participants comprised a subgroup from the ongoing University of Southern California Twin Study of Risk Factors for Antisocial Behavior (USC-RFAB, See Baker et al. 2006 for a detailed description). In brief, this is a longitudinal study beginning from 2000 and now approaching the end of fourth wave data collection. Twins were mainly recruited through public schools in the Great Los Angeles area around 2000–2001 and the ethnic distribution was comparable to that of the southern California urban community.
The present analyses were based on psychophysiological and survey data obtained from the first wave of assessment, when the children were 9–10 years old (mean age=9.6, SD=0.58). The whole sample includes 1,219 children (605 sets of twins and triplets). Assessment of psychopathic traits was provided by caregivers, who were predominantly (91.4%) biological mothers. The remaining 8.6% were mostly the biological father (5.7%) and the other 2.9% were grandmother, stepmother, foster mother and other male or female relatives. Each child’s ethnicity was determined by the ethnicity of his/her biological mother and father, as reported by the primary caregiver. The ethnicity breakdown of the sample was as follows: 36.74% Hispanic, 27.4% Caucasian, 14.02% Black, 4.35% Asian, 0.16% Native American and 17.32% mixed. Both sexes were represented approximately equally: 48.83% were boys and 51.17% were girls. Statistical methods used to correct for the dependent (paired) nature of the sample are described below.
Measures
Childhood Psychopathy Scale
Children’s psychopathic traits were assessed using Lynam’s (1997) Childhood Psychopathy Scale (CPS). This measure, based on Hare’s PCL-R (1991), has been shown to have strong psychometric properties. The full scale contains 58 age-appropriate questions rated on a two-point scale:(Yes = 2 or No = 1) and taps into 14 of 20 PCL-R criteria: Glibness, Grandiosity, Boredom Susceptibility, Untruthfulness, Manipulation, Lack of Guilt, Poverty of Affect, Callousness, Impulsiveness, Parasitic Lifestyle, Behavioral Dyscontrol, Lack of Planning, Unreliability, and Failure to Accept Responsibility. A higher-order factor analysis with oblique rotation on these 14 subscales revealed two broad factors that are not structured according to Hare’s original conceptualization (Bezdjian et al. 2011). The first factor tapped into the affective and impulsive behavioral traits in Hare’s structure (thus referred to as Callous/Disinhibited), while the second factor focused more on the interpersonal aspect of psychopathy involving deceit and manipulation of others (thus referred as Manipulative/Deceitful).
In the present study, the CPS was completed separately for each child by one of their caregivers. The composite factor scores were obtained by averaging the mean of corresponding subscales loading on each factor. The correlation between the two composite factor scores was 0.46. The internal consistency for the Callous/Disinhibited (C/D) and Manipulative/Deceitful (M/D) were 0.80 and 0.78 respectively. The CPS total score was also calculated as the mean of all 58 items and its internal consistency was good (Cronbach’s α=0.84). The six-month test–retest correlation was high (r =0.87).
Procedures
The electrodermal (skin conductance) and cardiovascular (heart rate) measures were recorded continuously during the psychophysiological testing session. The psychophysiological protocol lasted approximately 2 h and included a total of 12 tasks in the following order: 3-min resting period 1, orienting, oddball, Go/NoGo, mismatch negativity, Pendulum, startle, countdown, movies, embarrassing question, P50, and resting period 2. Each task lasted 3–6 min and the inter-task interval was about 2–5 min. In the current study, data from the count-down were used.
Countdown Task
This task was composed of three signaled trials and two un-signaled trials. The starting points for each signaled trial were set to be the 7th, 94th, and 188th second respectively, and were set to be the 48th and 139th second for the two un-signaled trials. For each of the three (signaled) trials, the children watched the number 12 on the computer screen as a hint of starting trial. The children were told that the number would decrease by 1 each second and that a loud noise (1 s burst of 105 dB white noise) would be delivered when the number reached zero. For the un-signaled trials, no numeric countdown was visually presented prior to the noise burst and thus the participants were unaware of when the trial began or the noise occurred. They received the unexpected white-noise burst after 12 s of watching the blank computer screen. These un-signaled trials were used as a comparison condition in order to evaluate the efficacy of the countdown in the signaled trials in eliciting anticipatory fear (Fung et al. 2005). Participants were unaware of the number of trials or the alternating nature of the trials. The mean inter-trial interval was 45 s (range=40–50s). The whole task lasted about 233 s.
Therefore, we examined skin conductance and HR in the following two periods of primary interest: (a) signaled anticipation (12 s before white-noise burst in signaled condition), (b) pseudo anticipation (12 s before white-noise burst in unsignaled condition). For the purpose of analysis, however, this 12 s period for HR was divided into several components, which will be addressed in more detail in a later section.
Psychophysiological Data Attainment
All psychophysiological data were collected with equipment and software from the James Long Company (1999; Caroga Lake, New York). A 38 channel Isolated Bioelectric Amplifier was used and physiological data were recorded online directly into a data acquisition computer. The amplification rates and high-pass filter (HPF) and low-pass filter (LPF) settings were as follows: electrocardiogram (gain=0.25× 1,000 voltages, HPF=0.1 Hz, LPF=1,000 Hz), and skin conductance (gain = 0.1 V per microsiemens, HPF = none/ DC, LPF=10 Hz).
Heart Rate
The channels of the grounded electrocardiograph were recorded through self adhesive ECG electrodes. Disposable electrodes (Electro-Cap International Inc., Eaton, OH) were attached between the 1st and 2nd ribs on either side of the chest. Alligator clips with a 48″ lead wire were attached to tabs on the electrodes. Before attaching the electrodes, target skin areas were cleaned with a cotton pad and isopropyl rubbing alcohol (70% by volume). HR data were determined by using the Inter-beat Interval (IBI) analysis software program (James Long Company). The IBI was measured as time elapsed, in milliseconds, between two successive R peaks in the ECG. That is, the time between two consecutive R-waves provides an accurate measure of the frequency with which the heart beats. The IBI was then converted to Beats per Minute (BPM=(60×103)/IBI). R-waves were sampled every 10th of a second.
Skin Conductance
Skin conductance activity was recorded from bipolar leads on the distal phalanges of the index and middle fingers of the non dominant hand using silver-silver chloride electrodes (8 mm in diameter). A water soluble lubricant (K-Y lubricating jelly) was used, supported by an adhesive electrode collar (8 mm in diameter) that maintained full contact with the skin. Waterproof tape was then wrapped around the finger and electrode to further secure it. Participants were required to keep the hand still during testing
A skin conductance response (SCR) was defined as an increase in conductivity exceeding 0.05 microsiemens (μS 10−6 s) in amplitude and the number of NS-SCRs was scored manually based on visual inspection of the continuous wave form and reported in the following analyses. Testers who were trained to score SCRs were blind to the conditions of the task and hypothesis of the study.
Of the 1,219 children participants, 1,185 (97%) came to the laboratory for assessment. Due to equipment problems, the psychophysiology data collected before September 2001 (n=204) was unusable. These subjects did not differ significantly from the remaining subjects on psychopathic traits (including CPS Total, CPS C/D and CPS M/D) and sex distribution, but included a significantly larger proportion of Caucasians (33%) compared to the rest of sample (25%), χ2=16.74, p<0.01. After excluding data from these subjects and those with excessive movement artifacts, usable data were available for 843 subjects (47.71% boys) on the HR measure and 758 subjects (45.33% boys) on the NS-SCR measure.
Statistical Analysis
Because each pair of twins was nested within the family, multilevel (hierarchical linear modeling; HLM) analyses were conducted using PROC MIXED in SAS. The advantage of the multilevel approach is the simultaneous estimation of both within-family and between-family effects. PROC MIXED employed maximum likelihood estimation to fit a multilevel model separately for SCR and HR measures in the countdown task. The relationships among psychopathic traits (CPS), sex of each subject and either HR or SCR were examined. The decomposition of the variance-covariance matrix in HR analyses followed the mixed-effects biometric approach developed by McArdle (2006), whereas for NS-SCR, the decomposition was relatively straightforward with the variance-covariance set to be unstructured. The method used to determine degrees of freedom for evaluation fixed effects was SATTERTH for both measures.
NS-SCR
A two-level mixed-model analysis was conducted with NS-SCR as the dependent variable. The level-1 model was a regular regression model for each individual, with CPS score, sex, and the interaction between these two terms entered as the predictors. The level-2 model expressed each regression parameter from the level-1 model as a fixed effect plus a random effect (r) unique to each family. The two-level regression model predicting NS-SCR (Yij) for the jth twin in the ith family is shown in the following equations:
The two-level model thus takes into account the clustered nature of twin pairs, by estimating the between-family regression coefficients (β0i, β1i, and β2i) while controlling for within-family variation. The same multilevel model was run separately for the two phases mentioned above: (a) signaled anticipation (12 s before white-noise burst in signaled condition), (b) pseudo anticipation (12 s before white-noise burst in un-signaled condition).
HR Change Scores
HR responses were first determined as change scores by subtracting the mean HR during the 5-s period preceding each trial (i.e., the baseline HR level) from the HR at each second during the 12-s period before the white-noise burst. These HR change scores were then averaged across trials, on a second by second basis separately for signaled (3 trials), and un-signaled (2 trials) conditions. Since the temporal changes of HR throughout each countdown trial are well-known to be non-linear (i.e., the tri-phasic response form), the 12-s period was decomposed into the acceleration and deceleration phases based on the findings of Hare et al. (1978). Specifically, HR acceleration (A component) was indexed by seconds 2 to 7, and D2 by seconds 8 to 12. D1 was abandoned from the analyses because: (1) this phase was not the interest of the current study; and (2) only 1 s was left in this phase. The mean HR change at each second, averaged across the three signaled trials is provided in Table 4 in the Appendix.
In addition to examining the effects of Sex and CPS score on HR changes, the effects of Time were also incorporated into the analysis model. A three-level mixed model was therefore used, whereby the level-1 model represented a linear individual growth model within each component (A and D2 of the countdown trial, prior to the stimulus), while the level-2 model expressed the parameters from the individual growth model as predicted by person-level covariates Sex and CPS. The level-3 model expressed each parameter from the level-2 model as a fixed effect plus a random effect unique (r) to each family. The average HR change score at each of the kth seconds for the jth twin in the ith family (Yijk) was used as the dependent variable in the model. The three-level mixed regression model for each component of the countdown trials is summarized in equation form as follows:
This three-level model was run separately for two components of the signaled condition (A and D2). The same analysis procedure was used in the un-signaled condition, to make the models relatively simple and to be comparable to prior literature (Fung et al. 2005). Prior to multilevel analysis, both HR and SCR was firstly ranked (PROC RANK; Blom 1958) and then normalized (PROC STANDARD) in SAS to decrease the skewness and kurtosis in distribution. After transformation, the skewness and kurtosis of both measures were close to 0.
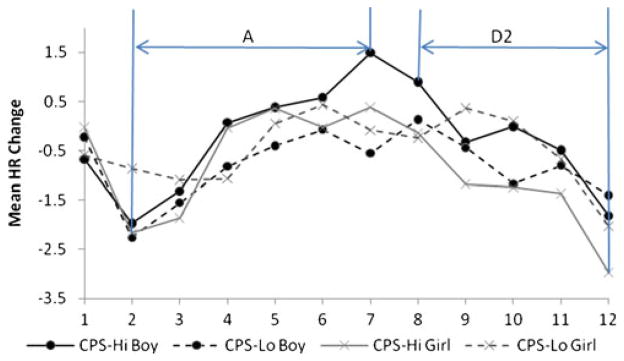
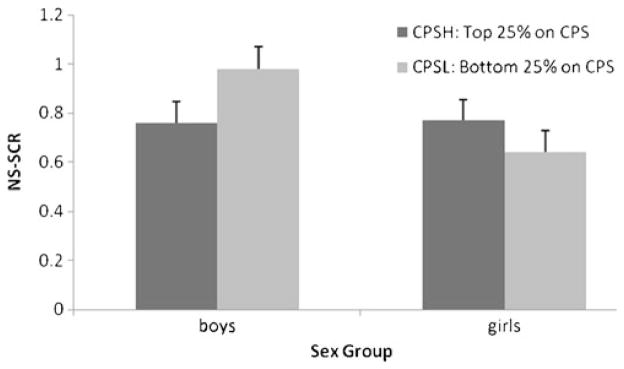
All participants with complete psychophysiological and CPS data were included in the regression analyses. In addition, two groups were identified to illustrate the autonomic changes and interaction effects (Figs. 1 and 2): CPS-Hi group (n=206) who scored above the 75% quartile on the CPS, and CPS-Lo group (n=201) scoring below the 25% quartile on the CPS. Although the high and low CPS groups did not differ on ethnicity (χ2=5.17, p>0.05), the sex distribution was significantly different (χ2=15.07, p<0.01), with more boys (n=128) than girls (n=78) in the CPS-Hi group, and fewer boys (n=73) than girls (n=128) in the CPS-Lo group. The autonomic changes during the countdown task were thus plotted separately for boys and girls.
Fig. 1.
Mean heart rate change at A and D2 phases during signaled condition for CPS-Hi and CPS-Lo groups across sex
Fig. 2.
Mean number of NS-SCRs during countdown under signaled condition for CPS-Hi and CPS-Lo groups across sex
Results
Descriptive Statisitics
An independent sample t test was conducted to examine if the gender difference was significant on the CPS total score and its two factors. Boys demonstrated significantly higher levels than girls on CPS total (t=3.32, p<0.01; M=1.24, SD=0.12 for boys; M=1.21, SD=0.11 for girls) and CPS C/D (t=4.34, p<0.01; M=1.25, SD=0.17 for boys; M= 1.20, SD=0.15 for girls), but did not differ from girls on CPS M/D (t=1.25, p>0.05; M=1.29, SD=0.16 for boys; M=1.28, SD=0.17 for girls). When it came to the ethnic difference, a significant effect was only found for the CPS M/D (F=4.28, p<0.01). Hispanic (M=0.26, SD=0.13) and Asians (M=0.24, SD=0.11) demonstrated lower levels in this trait compared to Blacks (M=0.30, SD=0.16) and Caucasians (M=0.32, SD=0.16).
Boys and girls differed significantly on both mean NS-SCR (t=2.52, p<0.05) and mean HR (t=−2.45, p< 0.05). Compared to girls (M=0.69, SD=0.33 for NS-SCR; M=83.99, SD=11.77 for HR), boys showed more NS-SCR (M=0.79, SD=0.35) but smaller HR (M= 86.97, SD=9.85). There was a significant ethnic difference on the mean NS-SCR (F=6.12, p<0.01) but not on the mean HR (F=0.69, p>0.05). In general, Blacks tended to have smaller NS-SCR (M=0.56, SD=0.37) than the other groups.
The correlations between the key physiological variables and CPS score (including the total score and subscales) were calculated based on a single twin randomly selected from each pair. The HR acceleration was obtained by calculating the difference between mean HR at Second 2 (i.e. the beginning point of phase A) and Second 7 (i.e. the ending point of phase A). The results are displayed in Table 1. No significant correlations were found for either HR acceleration or NS-SCR under the unsignaled condition. For the signaled condition, the HR acceleration was positively associated with the CPS C/D factor across both boys (r=0.19, p<0.05) and girls (r=0.14, p<0.05), while NS-SCR was only found negatively associated with the CPS M/D in boys (r=−0.21, p<0.05). In addition, the HR acceleration was also positively correlated with the CPS total score (r=0.15, p<0.05) in boys.
Table 1.
Correlations between HR/NS-SCR and subscales of CPS across different genders
| HR acceleration
|
NS-SCR
|
|||
|---|---|---|---|---|
| Boys (n=195) | Girls (n=217) | Boys (n=168) | Girls (n=205) | |
| Signaled | ||||
| CPS Total | 0.15* | 0.09 | −0.15 | 0.09 |
| CPS C/D | 0.19* | 0.14* | −0.07 | 0.07 |
| CPS M/D | 0.08 | 0.03 | −0.21* | 0.06 |
| Unsignaled | ||||
| CPS Total | 0.00 | 0.01 | 0.01 | 0.01 |
| CPS C/D | 0.03 | 0.04 | 0.06 | 0.02 |
| CPS M/D | −0.04 | −0.04 | −0.07 | −0.03 |
Correlations were based on a single twin within each family. HR acceleration was obtained by minus the mean HR at Second 2 from the mean HR at Second 7.
p<0.05
CPS Total Score
HR Change
For HR responses in the signaled condition, preliminary analyses were conducted first to decide whether the full model or a reduced model without the three-way interaction (TIME × CPS × SEX) fit the data better. Chi-square change was used as the index of the model difference test. If the switch from full model to the reduced model resulted in a significant chi-square change, the full model was retained. Otherwise, the reduced model was accepted. Results revealed that the reduced model was acceptable for both the A (Δχ2=1.1, df=1) and D2 ((Δχ2=1.3, df=1) components. Regression coefficients in the Level-1 model were thus inspected within the reduced model, in order to investigate the main effects of CPS, Time, Sex, and the two-way interactions between CPS, Time, and Sex within each phase. Level-1 regression coefficients (see Table 2) indicated that the only significant effect in the A component was the interaction between Time and CPS score (β=0.70, SE=0.32, p<0.05), indicating that the higher the CPS score, the faster the HR accelerated.
Table 2.
Level-1 unstandardized regression coefficients predicting heart rate changes within each condition
| Effect | Signaled
|
Unsignaled
|
||
|---|---|---|---|---|
| A | D2 | A | D2 | |
| Intercept | 3.03 (4.26) | −0.62 (6.74) | −5.78 (4.38) | −8.61 (7.68) |
| Time | −0.40 (0.44) | 0.02 (0.51) | 0.15 (0.47) | 0.10 (0.56) |
| CPS | −3.83 (3.35) | 4.20 (5.15) | 4.45 (3.44) | 6.71 (5.45) |
| Sex | −0.98 (2.53) | 2.21 (3.14) | 4.47 (2.60) | 5.35 (3.22) |
| Time* CPS | 0.70 (0.32)* | −0.37 (0.38) | −0.11 (0.34) | −0.09 (0.41) |
| Time* Sex | −0.04 (0.08) | −0.03 (0.10) | 0.00 (0.09) | 0.04 (1.10) |
| CPS* Sex | 0.77 (2.03) | −0.92 (2.44) | −3.57 (2.08) | −4.39 (2.47) |
A is the phase from second 2 to second 7; D2 is the phase from second 8 to second 12, and responsivity is the 4 s phase after the white-noise burst.
p<0.05
The same multilevel regression analyses were conducted for HR changes during the un-signaled condition (see Table 2). No significant result was found for either A or D2 component. The mean HR changes under signaled condition in CPS-Hi group and CPS-Lo group are plotted separately for boys and girls in Fig. 1.
NS-SCR
Regression analyses revealed only significant effects in NS-SCRs during the signaled anticipation phase (see Table 3), in which the main effects of CPS (β=−14.20, SE=2.96, p<0.01) and Sex (β=−11.87, SE=3.90, p<0.01) were both significant. Moreover, the interaction between CPS and Sex was also significant (β=8.17, SE=3.17, p<0.01), indicating that the relationship between NS-SCR and CPS score differed across sexes. To decompose this interaction, the simple main effect of the CPS score was tested within each sex under the multilevel model. For boys, CPS showed a significant inverse relationship to NS-SCR (β=−5.77, SE=2.10, p<0.01), such that boys with higher psychopathic traits had fewer NS-SCRs. In contrast, girls scoring high on the CPS showed a trend to respond more than those scoring low on the CPS, but this trend was not significant (β=2.22, SE=2.41, p=0.36). The mean NS-SCR of the CPS-Hi and CPS-Lo groups across boys and girls under signaled condition are shown in the Fig. 2.
Table 3.
Level-1 unstandardized regression coefficients predicting non-specific SCR within each condition
| Effect | Signaled Anticipation | Pseudo Anticipation |
|---|---|---|
| Intercept | 20.35 (5.96)** | −4.98 (6.34) |
| CPS | −14.20 (4.77)** | 3.92 (5.12) |
| Sex | −11.87 (3.90)** | 2.55 (4.09) |
| CPS*Sex | 8.17 (3.17)* | −2.00 (3.43) |
p<0.01
Two-Factor Structure
To address the second goal of the current study, similar multilevel regression analyses were conducted on the CPS C/D and M/D factors separately. In consideration of the moderate correlation between these two factors, each factor was entered into the model with the other factor as a covariate. These analyses were only conducted to the phases or periods when the main or interaction effects involving CPS total score was significant. Therefore, for HR, only the A component under signaled condition, and for NS-SCR only the signaled anticipation period were subject to the two-factor analysis.
HR Change
For the signaled A phase, the interaction between CPS C/D and time (β=0.52, SE=0.25, p<0.05) was significant after controlling for CPS M/D, which indicated that the higher on the CPS C/D score, the faster that HR accelerated. No significant effect was found for CPS M/D after controlling for CPS C/D. As in the analysis of the CPS total score, the interaction between each CPS factor and Sex was not significant either.
NS-SCR
After controlling for CPS C/D for the signaled anticipation, the main effect of CPS M/D (β=−9.61, SE= 3.46, p<0.01), Sex (β=−9.02, SE=2.77, p<0.01), and the interaction between CPS M/D and Sex (β=5.65, SE=2.16, p<0.01) were also significant, while in the model of CPS C/D, only the main effect of sex (β=−5.77, SE=2.98, p=0.053) was found marginally significant after controlling for CPS M/D. A further decomposition of the interaction between CPS M/D and Sex indicated that boys with higher score on the CPS M/D tended to respond less than those with lower scores (β=−3.69, SE=1.76, p<0.05) after controlling for CPS C/D, while this trend was not significant in girls.
Confounder Analysis
Given the heterogeneity in ethnicity of the current sample and the resulting significant ethnic difference on mean SCR, ethnicity was taken into account as a potential confounder. While controlling for ethnic differences (entered as dummy-coded covariates), all significant findings in the mixed-model regression analyses remained the same.
Discussion
This study aimed to extend previous findings of impaired autonomic reactivity in psychopathic adults in anticipation of an aversive event to a community sample of 9–10 year old boys and girls. The main findings are threefold: (1) consistent with our prediction based on findings in adults, in the signaled condition, the mean HR reactivity of all subjects showed a tri-phasic pattern in anticipation of the noxious stimulus (white-noise burst); no clear pattern was found, however, for the 12 s duration before the un-signaled white-noise, since this period actually resembles a baseline condition; (2) the subjects with higher psychopathic traits showed larger HR acceleration compared to the controls and this pattern applied only to the callous/disinhibited factor of the CPS; (3) there was a negative association between the psychopathic traits and NS-SCR but only specific to the manipulative/deceitful CPS factor; in addition, this association was only significant in boys but not in girls.
The finding of both larger HR acceleration and reduced NS-SCR in individuals with psychopathic traits compared to controls, which was previously reported in adult males (Hare 1982; Hare and Craigen 1974; Hare et al. 1978; Ogloff and Wong 1990), was basically replicated in community children sample aged 9–10 of the current study. However, several divergences appeared between the HR acceleration and reduced NS-SCR.
First, the reduced NS-SCR was only associated with the manipulative/deceitful aspect of psychopathic personality. At first glance, this finding was paradoxical because diminished autonomic responses to aversive stimuli have been considered to reflect fearlessness or emotional callousness traits of psychopaths (Fowles 1993; Patrick 1994). The triarchic model (Patrick et al. 2009), however, proposes that the boldness (a broad personality construct including the manipulative and deceitful interpersonal traits) should be a more benign and pure expression of the fearlessness traits than meanness (another construct containing the callousness affective traits) which reflects an overlay of both fearlessness and disinhibition. From this perspective, the reduced NS-SCR could be still viewed a reflection of fearlessness traits in psychopathic individuals. In contrast, the larger HR acceleration was found to be significantly correlated with the C/D factor of CPS, suggesting that HR acceleration may be a biological marker of both reduced affective regulatory control (difficult temperament) and behavioral disinhibition underlying psychopathic traits.
The inhibitory control and the emotional state of fear are thought to be two important trait constructs that can help bridge psychopathological phenotypes with neurobiological processes (Patrick and Bernat 2010). For example, variations in the emotional state of fear is posited to reflect individual differences in brain’s defensive motivation system, while inhibitory control tends to reflect functioning of brain systems that modulates affective and behavioral response in the service of distal goals (Patrick and Bernat 2010). As extreme states of these two constructs, fearlessness and disinhibition may intensify the propensity of individuals with psychopathic traits to be more tolerable of fear or punishment, and more apt to pursuing aversive situations and involving in antisocial activities compared to normal controls.
Another important finding of this study is the sex difference in the relationship between psychopathic traits and psychophysiological responses when anticipating the aversive stimuli. Different from the co-occurrence of attenuated NS-SCR and larger HR acceleration prior to the aversive stimulus in boys with psychopathic traits, only the latter was found in girls with psychopathic traits. No significant difference was found between the group with high psychopathic traits and the control group for girls in NS-SCR.
There is some evidence showing that females’ skin conductance is more responsive to changes in the environment compared to males. Venables and Mitchell (1996) found that in females’ NS-SCR varies with the season of year and time of day of testing; females showed more NS-SCR in the morning in the cool season and in the afternoon in the hot season. This pattern of seasonal and time-of-day effects was not found in males. In addition, females showed significantly higher SC levels than males overall. These patterns were stable across age groups from 5 to 25 years. These external correlates of SC in females may have led to greater measurement error, which could have in turn attenuated any relationship to psychopathic traits in the present study.
Alternatively, there may be sex differences in relative influences of the sympathetic nervous system (SNS) and parasympathetic nervous system (PNS). Beauchaine et al. (2008) found that aggressive boys aged 8–12 years with conduct problems exhibited lower baseline respiratory sinus arrhythmia (RSA, a PNS-linked cardiac activity) and less PEP reactivity (a SNS-linked cardiac activity) to reward than the control group. However, no difference in RSA was observed for girls who were high versus those low on aggression. In addition, only aggressive girls with conduct problems demonstrated greater baseline skin conductance than controls. Although our finding that girls with psychopathic traits showed abnormal HR acceleration (controlled by both PNS and SNS) but normal NS-SCR (SNS only) is not completely in line with Beauchaine et al.’s findings, it is reasonable to infer that psychopathic traits may be associated differently with parasympathetic and sympathetic dominance across different sexes.
Given the scarcity of research on sex differences in physiological correlates of psychopathy, these results should be interpreted with caution until these are replicated in future studies. Nonetheless, the findings overall lend some support to the notion of sex differences in vulnerability and risk for psychopathy. Alternatively, this sex difference in the psychopathy-psychophysiology association may be partly due to the issue of assessment of these personality traits, e. g., girls with psychopathic personality traits may engage in different types of antisocial behaviors (e.g. early use of alcohol and drugs, early sexual activity, violations of rules, and poor academic achievement) and therefore receive lower psychopathy scores. A similar conceptualization was put up by Rutherford et al. (1995) on diagnosis of CD. Several studies from behavior genetics research also indicated that biological markers of vulnerability for psychopathology in males and females could be driven by different influences of genes and environments or at least to different extents even if under the same genetic and environmental influences. As an important biological marker for impaired cognitive processing in psychopathology, for example, the P300 amplitude appeared to be largely heritable among boys (Carlson and Iacono 2006; van Beijsterveldt et al. 2001; Yoon et al. 2006), whereas for girls, the shared environmental factors contributed more or equally compared to the genetic factors (Van Beijsterveldt et al. 2001; Yoon et al. 2006).
One limitation of the present study is that this sample was composed of community-recruited children, most of who are not at risk for psychopathology, including psychopathy. Moreover, it is limited to the small number of trials (only 3 signaled anticipation and 2 unsignaled trials), which increases the chance of measurement error. However, the disparity of results between signaled condition and unsignaled condition across both measures of HR and NS-SCR supported the validity of the experimental design to some extent. Another limitation of the current study is the heavy reliance on a single information source (caregiver) in report of psychopathic traits. With the advent of new approaches to integrating data from multiple-informants (Baker et al. 2007; Kraemer et al. 2003), it is worth exploring these autonomic response deficits from a multi-rater perspective in future studies. Finally, given the inconsistent sex effects observed in reduced SCRs and larger HR acceleration, future research would benefit from incorporating RSA and PEP measures so that the effects of PNS and SNS can be further differentiated.
In conclusion, the multiple dimension of psychopathy was supported by divergent relationships of two factors with the autonomic profile in the current study. Sex differences of reduced NS-SCR with respect to psychopathic traits as opposed to HR provided support for distinct pathways to psychology across boys and girls. Taken together, these results demonstrated the existence of similar autonomic activity deficits in children with psychopathic traits as in psychopathic adults, and the continuity of the response pattern from childhood to adulthood supports the existence of a juvenile form of psychopathy. The establishment of psychopathy as a valid disorder with biological roots in childhood could have significant implications for its treatment and intervention. The possibility of different psycho-physiological correlates of psychopathic traits in males and females, particularly in the SCR profiles, needs further investigation. Increasing our understanding of the different mechanisms across psychopathic facets and gender may potentially largely improve treatment efficacy.
Acknowledgments
This study was supported by grants from NIMH to Laura A. Baker (R01 MH58354) and Adrian Raine (K02 MH01114-08). We would like to thank the Southern California Twin Project staff for their assistance in collecting data, and the twins and their families for participation.
Appendix
Table 4.
Mean Heart rate change at each second across the signaled anticipation phase, averaged across three signaled countdown trials
| Time | Mean Heart Rate change | Standard Deviation |
|---|---|---|
| Second 1 | −0.33 | 4.96 |
| Second 2 | −1.81 | 5.08 |
| Second 3 | −1.45 | 5.26 |
| Second 4 | −0.44 | 5.65 |
| Second 5 | 0.07 | 5.82 |
| Second 6 | 0.18 | 5.59 |
| Second 7 | 0.28 | 5.49 |
| Second 8 | 0.23 | 5.83 |
| Second 9 | −0.35 | 5.96 |
| Second 10 | −0.71 | 6.16 |
| Second 11 | −1.32 | 6.16 |
| Second 12 | −2.16 | 6.48 |
Contributor Information
Pan Wang, Email: panwang@usc.edu, Department of Psychology (SGM 501), University of Southern California, 3620 S. McClintock Ave., Los Angeles, CA 90089-1061, USA.
Laura A. Baker, Department of Psychology (SGM 501), University of Southern California, 3620 S. McClintock Ave., Los Angeles, CA 90089-1061, USA
Yu Gao, Department of Psychology, Brooklyn College of the City University of New York, Brooklyn, USA.
Adrian Raine, Departments of Criminology, Psychiatry, and Psychology, University of Pennsylvania, Philadelphia, USA.
Dora Isabel Lozano, Universidad Autónoma de Ciudad Juárez, Chihuahua, Mexico.
References
- Arnett PA. Autonomic responsivity in psychopaths: a critical review and theoretical proposal. Clinical Psychology Review. 1997;17(8):903–936. doi: 10.1016/s0272-7358(97)00045-7. [DOI] [PubMed] [Google Scholar]
- Baker LA, Barton M, Lozano DI, Raine A, Fowler JH. The Southern California Twin Register at the University of Southern California: II. Twin Research and Human Genetics: The Official Journal of The International Society for Twin Studies. 2006;9(6):933–940. doi: 10.1375/183242706779462912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Jacobson KC, Raine A, Lozano DI, Bezdjian S. Genetic and environmental bases of childhood antisocial behavior: a multi-informant twin study. Journal of Abnormal Psychology. 2007;116(2):219–235. doi: 10.1037/0021-843X.116.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Hong J, Marsh P. Sex differences in autonomic correlates of conduct problems and aggression. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(7):788–796. doi: 10.1097/CHI.0b013e318172ef4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110(4):610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269(5227):1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bezdjian S, Raine A, Baker LA, Lynam DR. Psychopathic personality in children: genetic and environmental contributions. Psychological Medicine. 2011;41(3):589–600. doi: 10.1017/S0033291710000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. Responsiveness to distress cues in the child with psychopathic tendencies. Personality and Individual Differences. 1999;27(1):135–145. [Google Scholar]
- Blom G. Statistical estimates and transformed beta-variables. New York: Wiley; 1958. [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, Iacono WG. Psychopathic personality traits: heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine. 2005;35(05):637–648. doi: 10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43(5):470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Farrington DP. The importance of child and adolescent psychopathy. Journal of Abnormal Child Psychology. 2005;33(4):489–497. doi: 10.1007/s10802-005-5729-8. [DOI] [PubMed] [Google Scholar]
- Fowles DC. Electrodermal activity and antisocial behavior: Empirical findings and theoretical issues. In: Roy J-C, Boucsein W, Fowles DC, Gruzelier JH, editors. Progress in electrodermal research. New York: Plenum Press; 1993. pp. 223–237. [Google Scholar]
- Frick PJ, Bodin SD, Barry CT. Psychopathic traits and conduct problems in community and clinic-referred samples of children: further development of the psychopathy screening device. Psychological Assessment. 2000;12(4):382–393. [PubMed] [Google Scholar]
- Frick PJ, Hare RD. Antisocial process screening device. Toronto: Multi-Health Systems; 2001. [Google Scholar]
- Frick PJ, White SF. Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry. 2008;49(4):359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Fung MT, Raine A, Loeber R, Lynam DR, Steinhauer SR, Venables PH, et al. Reduced electrodermal activity in psychopathy-prone adolescents. Journal of Abnormal Psychology. 2005;114(2):187–196. doi: 10.1037/0021-843X.114.2.187. [DOI] [PubMed] [Google Scholar]
- Gao Y, Glenn AL, Schug RA, Yang Y, Raine A. The neurobiology of psychopathy: a neurodevelopmental perspective. Canadian Journal of Psychiatry. 2009;54(12):813–823. doi: 10.1177/070674370905401204. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A. Successful and unsuccessful psychopaths: a neurobiological model. Behavioral Sciences & the Law. 2010;28(2):194–210. doi: 10.1002/bsl.924. [DOI] [PubMed] [Google Scholar]
- Hall JR, Benning SD, Patrick CJ. Criterion-related validity of the three-factor model of psychopathy. Assessment. 2004;11(1):4–16. doi: 10.1177/1073191103261466. [DOI] [PubMed] [Google Scholar]
- Hare RD. Electrodermal and cardiovascular correlates of psychopathy. In: Hare RD, Schalling D, editors. Psychopathic behavior: approaches to research. Chichester: Wiley; 1978. pp. 107–143. [Google Scholar]
- Hare RD. Psychopathy and physiological activity during anticipation of an aversive stimulus in a distraction paradigm. Psychophysiology. 1982;19(3):266–271. doi: 10.1111/j.1469-8986.1982.tb02559.x. [DOI] [PubMed] [Google Scholar]
- Hare RD. Manual for the revised psychopathy checklist. Toronto: Multi-Health Systems; 1991. [Google Scholar]
- Hare RD, Craigen D. Psychopathy and physiological activity in a mixed motive game situation. Psychophysiology. 1974;11(2):197–206. doi: 10.1111/j.1469-8986.1974.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Hare RD, Frazelle J, Cox DN. Psychopathy and physiological responses to threat of an aversive stimulus. Psychophysiology. 1978;15(2):165–172. doi: 10.1111/j.1469-8986.1978.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Hare RD, Neumann CS. The PCL-R assessment of psychopathy: Development, structural properties, and new directions. In: Patrick CJ, editor. Handbook of psychopathy. New York: Guilford Press; 2006. pp. 58–88. [Google Scholar]
- Herpertz SC, Werth U, Lukas G, Qunaibi M, Schuerkens A, Kunert HJ, et al. Emotion in criminal offenders with psychopathy and borderline personality disorder. Archives of General Psychiatry. 2001;58(8):737–745. doi: 10.1001/archpsyc.58.8.737. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Patrick CJ. Psychopathy and negative emotionality: analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. Journal of Abnormal Psychology. 2006;115(2):276–287. doi: 10.1037/0021-843X.115.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K. Psychophysiology: the mind-body perspective. Cambridge: Harvard University Press; 1995. [Google Scholar]
- Isen J, Raine A, Baker L, Dawson M, Bezdjian S, Lozano DI. Sex-specific association between psychopathic traits and electrodermal reactivity in children. Journal of Abnormal Psychology. 2010;119(1):216. doi: 10.1037/a0017777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Measelle JR, Ablow JC, Essex MJ, Boyce WT, Kupfer DJ. A new approach to integrating data from multiple informants in psychiatric assessment and research: mixing and matching contexts and perspectives. The American Journal of Psychiatry. 2003;160(9):1566–1577. doi: 10.1176/appi.ajp.160.9.1566. [DOI] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. The Journal of Neuroscience. 1995;15(10):6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychological Bulletin. 2004;130(4):531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lynam DR. Pursuing the psychopath: capturing the fledgling psychopath in a nomological net. Journal of Abnormal Psychology. 1997;106(3):425–438. doi: 10.1037//0021-843x.106.3.425. [DOI] [PubMed] [Google Scholar]
- McArdle JJ. Latent curve analyses of longitudinal twin data using a mixed-effects biometric approach. Twin Research and Human Genetics. 2006;9(3):343–359. doi: 10.1375/183242706777591263. [DOI] [PubMed] [Google Scholar]
- Ogloff JRP, Wong S. Electrodermal and cardiovascular evidence of a coping response in psychopaths. Criminal Justice and Behavior. 1990;17(2):231–245. [Google Scholar]
- Patrick CJ. Emotion and psychopathy: startling new insights. Psychophysiology. 1994;31(4):319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM. Neuroscientific foundations of psychopathology. In: Millon RFKT, Simonsen E, editors. Contemporary directions in psychopathology: scientific foundations of the DSM-V and ICD-II. New York: Guilford Press; 2010. pp. 419–452. [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology. 2009;21:913–938. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Hicks BM, Nichol PE, Krueger RF. A bifactor approach to modeling the structure of the psychopathy checklist-revised. Journal of Personality Disorders. 2007;21(2):118–141. doi: 10.1521/pedi.2007.21.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Raine A. The psychopathology of crime: criminal behavior as a clinical disorder. London: Academic; 1997. [Google Scholar]
- Rutherford MJ, Alterman AI, Cacciola JS, Snider EC. Gender differences in diagnosing antisocial personality disorder in methadone patients. The American Journal of Psychiatry. 1995;152(9):1309–1316. doi: 10.1176/ajp.152.9.1309. [DOI] [PubMed] [Google Scholar]
- Somsen RJM, Jennings JR, Van der Molen MW. The cardiac cycle time effect revisited: temporal dynamics of the central vagal modulation of heart rate in human reaction time tasks. Psychophysiology. 2004;41(6):941–953. doi: 10.1111/j.1469-8986.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CEM, van Baal GCM, Molenaar PCM, Boomsma DI, de Geus EJC. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behavior Genetics. 2001;31(6):533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- Venables PH, Mitchell DA. The effects of age, sex and time of testing on skin conductance activity. Biological Psychology. 1996;43(2):87–101. doi: 10.1016/0301-0511(96)05183-6. [DOI] [PubMed] [Google Scholar]
- Verona E, Patrick CJ, Curtin JJ, Bradley MM, Lang PJ. Psychopathy and physiological response to emotionally evocative sounds. Journal of Abnormal Psychology. 2004;113(1):99–108. doi: 10.1037/0021-843X.113.1.99. [DOI] [PubMed] [Google Scholar]
- Yoon HH, Iacono WG, Malone SM, McGue M. Using the brain P300 response to identify novel phenotypes reflecting genetic vulnerability for adolescent substance misuse. Addictive Behaviors. 2006;31(6):1067–1087. doi: 10.1016/j.addbeh.2006.03.036. [DOI] [PubMed] [Google Scholar]