Abstract
Enhancer of zeste homologue 2 (EZH2) is the catalytic subunit of Polycomb repressive complex 2 that catalyzes the trimethylation of histone H3 on Lys 27, and represses gene transcription. EZH2 enhances cancer-cell proliferation and regulates stem cell maintenance and differentiation. Here, we demonstrate that EZH2 is highly expressed in medulloblastoma, a highly malignant brain tumor of childhood, and this altered expression is correlated with genomic gain of chromosome 7 in a subset of medulloblastoma. Inhibition of EZH2 by RNAi suppresses medulloblastoma tumor cell growth. We show that 3-deazaneplanocin A, a chemical inhibitor of EZH2, can suppress medulloblastoma cell growth partially by inducing apoptosis. Suppression of EZH2 expression diminishes the ability of tumor cells to form spheres in culture and strongly represses the ability of known oncogenes to transform neural stem cells. These findings establish a role of EZH2 in medulloblastoma and identify EZH2 as a potential therapeutic target especially in high-risk tumors.
Keywords: EZH2, Medulloblastoma, Polycomb repressive complex 2, histone lysine methylation, DZNEP
Introduction
Medulloblastoma is the most common malignant pediatric brain tumor, affecting young children with a peak incidence at 7 years1. Current therapy involves a combination of surgery, radiation, and conventional chemotherapeutic approaches. These therapies present considerable risks of neurocognitive and other systemic adverse effects2, 3. Despite aggressive therapy, 5-year survival4 rates are currently approximately 70%. In addition, high-risk patients with Myc amplification and metastatic disease continue to have substantially worse outcomes4. To improve therapy, a better understanding of the molecular and cellular biology is necessary. Importantly, medulloblastoma is a genetically heterogeneous tumor that can be sub-classified into four to five distinct genomic groups5,6.
Among the key steps critical for tumorigenesis is the aberrant regulation of chromatin maintenance and modification7. We have previously shown that many tumor suppressor genes are silenced by histone deacetylation and reversal of this process by inhibition of histone deacetylase8 enzymes can inhibit medulloblastoma cell growth. More recently, single nucleotide polymorphism analysis revealed highly focal genetic events in genes targeting histone lysine methylation, particularly that of histone H3 lysine 9 (H3K9)9. Furthermore, a recent landmark study of the genomic landscape and genetic variation in medulloblastoma revealed inactivating mutations in the histone lysine methyltransferases MLL2 and MLL3 in 16% of tumor samples evaluated, further suggesting a critical role for regulation of histone lysine methylation in medulloblastoma pathogenesis10. To explore the role of histone lysine methylation further, we examined expression of histone lysine methylases in two independent cohorts of medulloblastoma patients. We identified enhancer of zeste homologue 2 (EZH2) as a potential mediator of medulloblastoma cell growth.
EZH2 is the catalytically active component of the Polycomb repressor complex 2 (PRC2) that is responsible for methylating lysine 27 of histone H3 and contributes to chromatin compaction11, 12. Thus EZH2 acts as a transcriptional repressor in the context of the PRC2 complex.13 PRC2 is involved in diverse biological processes, including differentiation, maintaining cell identity, and proliferation13. Importantly, EZH2 is critical for normal development and its deletion is embryonic lethal14.
Over-expression of EZH2 correlates with tumor cell proliferation and invasive growth15. Further, strong EZH2 expression is associated with features of aggressive tumor subgroups, as well as clinical progression, drug resistance, and reduced survival15, 16 Recent studies in prostate cancer revealed chromosomal rearrangements leading to fusion proteins. that directly activate EZH2 and that disruption of EZH2 inhibits prostate cancer tumorigenicity.17, 18 EZH2 expression increases with tumor grade in adult and pediatric brain tumors, and is a poor prognostic factor. In glioblastoma, EZH2 inhibits differentiation, and is crucial for tumor stem cell maintenance19, 20.
Based on its role in tumorigenesis and stem cell maintenance, we investigated the effects of targeting EZH2 in medulloblastoma cells. We show that inhibition of EZH2 by RNAi or chemical inhibitors potently suppresses medulloblastoma cell growth and inhibits tumor sphere formation. Further, we demonstrate that EZH2 is critical for transformation of tumor stem cells in vitro.
Materials and methods
Patient tumor samples and Gene Expression Microarray Analysis
Patient tumor samples were evaluated for gene expression using Affymetrix U133 Plus 2.0 Gene Chip microarrays as previously described by us21. Briefly, samples were collected at the time of surgery and snap-frozen in liquid nitrogen. Ribonucleic acid was extracted from each sample using an RNeasy kit (Qiagen) and hybridized to HG-U133 Plus 2.0 GeneChips (Affymetrix) according to the manufacturer’s instructions. All tissues are obtained under an IRB approved protocol. All medulloblastoma tumor samples were obtained from patients < 16 years of age. Cerebellum samples (ages 7-16) were obtained from the Pediatric Co-operative Human Tissue Network (Columbus, OH) as approved by the IRB. Samples for the MB100 data set were obtained as detailed previously5.
Reagents
DZNep was kindly provided by Laboratory of Medicinal Chemistry, Center for Cancer Research, National Cancer Institute at Frederick (National Institutes of Health, Frederick, MA). Antibodies used for Western blot analysis were from the following sources: trimethyl-histone H3 Lys27 (H3K27me3) was purchased from Cell Signaling Technology, Danvers, MA; actin was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA).
Cells and cell culture
Daoy medulloblastoma cells were obtained from American Type Culture Collection (Rockville, MD) and ONS-76 cells were a kind gift of JT. Rutka. C17.2 mouse neural stem cells were a kind gift from EY. Snyder. Daoy and ONS-76 cells were cultured in Dulbecco’s modified Eagle medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Gibco) according to the supplier’s recommendations.
Transfection of Daoy and ONS-76 cells with shEZH2
Daoy and ONS-76 medulloblastoma cells, and C17.2 mouse neural stem cells were transfected with control and shEZH2 plasmids (Sigma, St. Louis, MO) in 6-well plates using Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
Colony formation assay
Control, treated, or transfected cells were seeded into 6-well plates in triplicate at a density of 500 cells/well in 3 ml medium containing 10% FBS. Cells were grown for 7 days in 37°C humidified atmosphere containing 95% air and 5% CO2. The cell clones were stained for 15 min with a solution containing 0.5% crystal violet and 25% methanol, followed by three rinses with tap water to remove excess dye. The colony numbers (>50 cells per colony) were counted using a precise electronic counter (Heathrow Scientific, Vernon Hills, IL) and inverted microscope.
Quantification of apoptosis
Transfected and treated cells were checked for apoptosis using the Nexin assay on the Guava EasyCyte Plus System (Millipore, Billerica, MA). The assay was performed according to the manufacturer’s instructions.
Flow cytometric analysis
Flow cytometric analysis was performed to define the cell cycle distribution. Cells were seeded in 6-well plates (1 × 105 cells/well) and treated with DZNep or transfected with 1 μg control or shEZH2 plasmid using Lipofectamine 2000 (Invitrogen). Cells were harvested 24 hours later by trypsinization and fixed with 70% ethanol overnight. Collected cells were treated with 250 μl cell cycle reagent (Millipore) and evaluated per the manufacturer’s recommendations.
Soft agar assay for colony formation
The effect of shEZH2 transfection and DZNep treatment on the ability of C17.2 mouse neural stem cells to form colonies in soft agar was examined by studying anchorage-independent growth of the malignant cells. Soft agar plates were prepared using 6-well tissue culture dishes. The 6-well plate was first coated with base agar (1%). Cells (8000) per dish were mixed in 50-ml sterile tubes with equal volumes of Dulbecco’s modified Eagle medium containing 20% FBS and 1.6% agar (0.8% final). The mixture was immediately aliquoted in triplicate and the agar allowed to set. The plates were incubated in a humidified chamber at 37°C for 14 days. Individual colonies of >50 cells were counted using an inverted microscope and precise electronic counter (Heathrow Scientific).
Western blotting
Protein expression levels were determined by Western blotting. Cells were lysed in 1X RIPA buffer (Thermo Scientific, Rockford, IL) containing protease inhibitor cocktail (Roche, Indianapolis, IN), 1 mM sodium vanadate, and 0.1 mM sodium molibdate. The lysates were centrifuged at full speed in a microcentrifuge for 20 minutes and the supernatants collected for protein concentration determination by the Bradford reagent (Sigma). Equal amounts of cell lysates were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and Western blot analysis was performed with specific antibodies as described in the figure legends.
Cell proliferation
Cell proliferation (cell index) was checked by the xCelligence Real-Time Cell Analyzer instrument (Roche). Cells were seeded in triplicate at 6 × 103 cells/well in the E-Plate 96, a specialized 96-well plate used with the Real-Time Cell Analyzer instrument. Each of the 96 wells on the E-Plate 96 contains an integral sensor electrode array so that cells inside each well can be monitored and assayed. Cells were treated with different concentrations of DZNep (0.5, 2.5, and 5 μM) 6 hours later. Cell growth was monitored for 5 days.
Luciferase reporter analysis
Luciferase assays were performed using the Cignal Finder Pathway Reporter Assays (SA Biosciences, Frederick, MD) following the manufacturer’s instructions. Cells were seeded into 96-well plates containing luciferase reporters and control or shEZH2 vectors using Surefect transfection reagent. Cells were incubated for 24 hours. Luciferase activity was measured using the Dual Luciferase Assay system (Promega, Madison, WI) on a Glomax multi luminometer (Promega). Firefly luciferase was the experimental reporter and Renilla luciferase was the normalizing reporter.
Results
EZH2 is overexpressed in primary medulloblastoma tissues and cell lines
We first evaluated the expression of EZH2 in a cohort of 21 classic medulloblastoma patient tissues and seven anaplastic medulloblastoma tissues (LCA). Compared to normal pediatric cerebellum, expression of EZH2 was significantly increased in both sets of tumors (Figure 1A). Anaplastic pathology correlates closely with myc amplification and poor prognosis. Next, we examined the expression of EZH2 in a recently described independent cohort of 120 medulloblastoma samples5. These samples were divided into 4 sub groups (Shh, Wnt, C &D) based on the genomic signatures 5. Expression of EZH2 was significantly elevated (>2-fold) relative to fetal cerebellar levels in ~20% of medulloblastoma tissues (Figure 1B). Interestingly, when we examined medulloblastoma in a genomic subgroup-specific fashion, EZH2 is extensively up-regulated in Group D tumors (40%) followed by Group C tumors (15%; Figure 1B). Both these subgroups (recently renamed as group 3 and 4 in the new consensus classification) are associated with worse outcomes. Because EZH2 is encoded on chromosome 7q we evaluated copy number variation of chromosome 7 and 7q in 81 medulloblastoma patients for whom we also had subgroup data. Group C and Group D (group 3 and 4 in the new consensus classification) tumors had an approximately 30% incidence of chromosome 7 or 7q gain (Figure 1C). This event was not found (0%) in WNT tumors and at a lower frequency (11.5%) for SHH tumors. While no focal EZH2 amplifications exist within our MDT-MB-100 dataset, there is a statistically significant correlation between the presence of broad (chr7 or 7q arm) gain and EZH2 expression (P<3.30E-4, Mann-Whitney U-rank test across all medulloblastoma samples). We can further break down this association in a subgroup-specific manner and observe the same trends holding true for both Group C and Group D molecular subgroups (Supplementary Fig1A). WNT tumors are not indicated in this analysis as no chr7 or 7q amplifications were observed.
Figure 1.
EZH2 gene expression microarray data in medulloblastoma. A. Expression of EZH2 mRNA in normal cerebellum specimens (CB), classic medulloblastoma (MED) patient samples (n = 21), and large cell anaplastic medulloblastoma (LCA MED)(n = 7). B. EZH2 mRNA expression in medulloblastoma samples sorted by genomic categories. C. Quantification of genomic gain of chromosome 7 in medulloblastoma samples. D. Expression of EZH2 mRNA in common medulloblastoma cell lines is increased consistent with patient tumor samples.
To further interrogate the relationship between copy number status and expression of EZH2, we plotted the relationship of increasing copy number (>2.5) against expression. There is a positive correlation across all chr7 or 7q amplified medulloblastomas (R2=0.133) with the highest correlation coefficient observed in the GroupD subgroup (R2=0.167) Supplementary Fig1B).
We next examined the expression of EZH2 in a panel of medulloblastoma cell lines that are commonly used to study medulloblastoma biology. All cell lines expressed increased levels of EZH2 compared to normal cerebellum (Figure 1D).
EZH2 knockdown by shRNA decreases medulloblastoma cell growth
Because EZH2 expression is increased in medulloblastoma, we examined the effects of inhibiting EZH2 gene expression on proliferation of medulloblastoma cells. We used two individual shRNA vectors to knockdown EZH2 in both Daoy and ONS-76 medulloblastoma cell lines. We chose these cell lines because they are well characterized by us and others and form medulloblastoma tumors in vivo22, 23. Transduction of EZH2 shRNA resulted in significantly fewer cells as measured by the ViaCount Assay at 72 hours post-transfection (Figure 2A). To examine the impact of long-term EZH2 inhibition, we performed colony formation analysis. Inhibition of EZH2 by shRNA significantly decreased the number of colonies formed by both Daoy (p < 0.001) and ONS-76 cells (p < 0.01) compared to the PSIF control vector (Figure 2B). EZH2 knockdown was verified by q-RT-PCR and Western blotting (Figure S2).
Figure 2.
Effect of EZH2 depletion on cell viability and clonogenic index. A. Daoy and ONS-76 cells were transfected with plasmid vector control pSIF and shEZH2 plasmid. Cell viability was evaluated by Guava EasyCyte Plus flow cytometer using ViaCount reagent. Viability was normalized to untransfected cells. EZH2 knockdown significantly impaired cell viability in vitro in both cell lines (p < 0.001). B. Colony focus assay was done in both Daoy and ONS-76 cells lines after plasmid transfection. Colony numbers were significantly reduced after transfection with shEZH2 in both cell lines (p < 0.001 in Daoy cells and p < 0.01 in ONS-76 cells). Colony counts were done in triplicate of 3 independents experiments.
EZH2 knockdown inhibits medulloblastoma tumor sphere formation
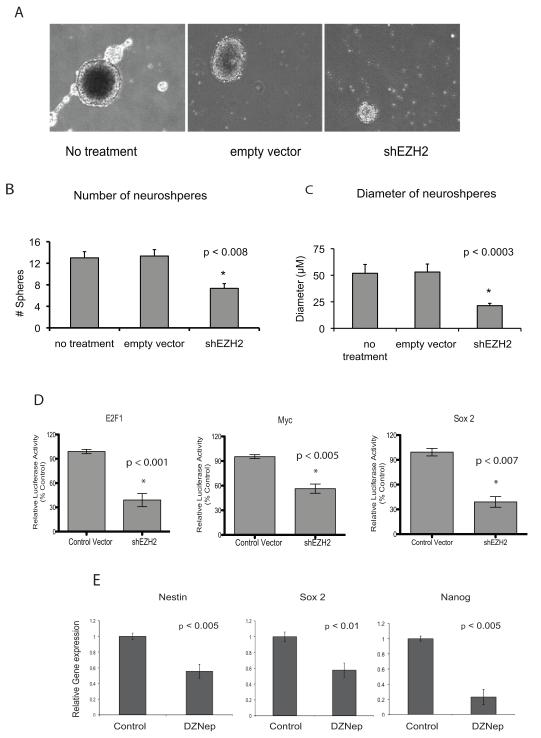
EZH2 regulates stem cell plasticity and has been implicated in the maintenance of tumor initiating cells in glioblastoma20. Thus as a preliminary step we next examined the effects of EZH2 knockdown on the ability of medulloblastoma cells to form tumor spheres. Daoy cells form tumor spheres when cultured in stem cell media (Neurobasal with epidermal growth factor and leukemia inhibitory factor). These tumor spheres upregulate expression of many stem and progenitor markers including Nestin, SOX2 and Nanog as shown in Figure S3B and as previously described for CD133 and CD4424. Knock-down of EZH2 potently inhibited the ability of Daoy cells to form tumor spheres (Figure 3A and B) and the spheres formed were significantly smaller (Figure 3A and C). Furthermore inhibition of EZH2 inhibited the ability of Daoy cells to form spheres from single cell suspensions (Figure 3A). To examine whether EZH2 inhibition altered tumor cell-associated signaling pathways in medulloblastoma tumor spheres, we performed functional analysis using luciferase reporter assays (Cignal Cancer Pathway Finder; SA Biosciences). Inhibition of EZH2 resulted in significantly decreased E2F1 activity indicating an impairment of Cyclin D-Rb responsive signaling (Figure 3D). Similarly, both Myc activity and Sox2 activity were significantly attenuated in EZH2 knockdown Daoy cell tumor spheres (Figure 3D) further indicating decreased tumor cell plasticity. Finally using a chemical inhibitor of EZH2 as an alternative method to decrease EZH2 activity we found that expression of Nestin, Nanog and Sox 2 were all significantly decreased in tumor spheres with decreased EZH2 activity (Figure 3E).
Figure 3.
Functional relevance of EZH2 in tumorigenesis and signaling pathways. A. Inhibition of EZH2 activity reduces the tumor sphere forming ability of medulloblastoma cells. B. The number of spheres is significantly decreased in cells with shEZH2 knockdown compared to controls (p < 0.008). In addition the sphere diameter (C) was significantly smaller in cells with shEZH2 knockdown compared to controls (p < 0.0003). D. EZH2 knockdown by shRNA leads to significantly decreased E2F1, Myc, and Sox2 activity (p < 0.001, p < 0.005 and p <0.007 respectively). Luciferase activity was measured by the Dual Luciferase Assay system (Promega). E. Inhibition of EZH2 leads to significantly decreased expression of progenitor cell markers Nestin, Nanog, Sox2 and Myc in Daoy tumor spheres.
Pharmacologic inhibition of EZH2 decreases medulloblastoma cell growth and attenuates H3K27 methylation
Recently, 3-deazaneplanocin A (DZNep) was discovered to selectively inhibit H3K27 trimethylation via inhibition of PRC225. We evaluated whether DZNep would alter medulloblastoma cell growth. Daoy and ONS-76 medulloblastoma cells were treated with varying concentrations of DZNep and cell growth was evaluated on the xCelligence Real-Time Cell Analyzer (Roche) system. DZNep treatment inhibited medulloblastoma cell growth and altered the growth rate of cells (Figure 4A). It also abrogated clonogenicity of medulloblastoma cells (Figure 4B). DZNep decreased H3K27me3 as detected by Western blotting. Interestingly the combination of shRNA mediated knockdown and DZNep treatment was co-operative in inhibiting the methylation of H3K27 (Figure 4C).
Figure 4.
A. Effect of DZNep treatment on cell proliferation. Pharmacologic depletion of EZH2 reduces medulloblastoma cell proliferation and altered the growth rate of cells. Daoy and ONS-76 medulloblastoma cells were treated with 0.5, 2.5, and 5 μM of DZNep. Both cell lines showed a dose-dependent growth inhibition with DZNep treatment. B. Colony formation assay. DZNep efficiently decreased the clonogenic potential at concentrations starting from 0.5 μM (p < 0.005). The numbers of colonies decreased with increasing DZNep dose. At the highest concentration of DZNep (5 μM), colony formation was reduced up to 30% in ONS-76 cells and over 70% in Daoy cells compared to untreated controls. C. Effect of combination treatment with shEZH2 and DZNep on H3K27me3 levels. Daoy and ONS-76 cell lines were first treated with shEZH2 followed by treatment with either 0.5 μM or 5 μM DZNep. For comparison, these cells were treated with either shEZH2 or DZNep alone. Western blot analysis was performed with 50 μg total cell lysates from each sample, using antibodies directed against H3K27me3 (Cell Signaling. 1:1000) and actin (Santa Cruz, 1:1000). Cells treated with either shEZH2 or DZNep (5 μM) alone showed a decrease in H3K27me3 levels. Interestingly, treatment with both knocked down H3K27me3 levels even further.
Inhibition of EZH2 induces a G2 cell cycle arrest and apoptosis in medulloblastoma cells
To further examine the impact of EZH2 suppression in Daoy and ONS-76 cells, we performed cell cycle analysis. In Daoy cells, inhibition of EZH2 did not significantly impact cell cycle compartments (Figure S4). In ONS76 cells inhibition of EZH2 by shRNA or DZNep alone induces G2 arrest and dual inhibition co-operates to further increase the number of cells in G2 phase (Figure S4). The differences between the two cell lines maybe due to the fact that Daoy are p53 mutated while ONS cells are p53 wild type. It will be interesting to further examine the interaction of p53 and EZH2.
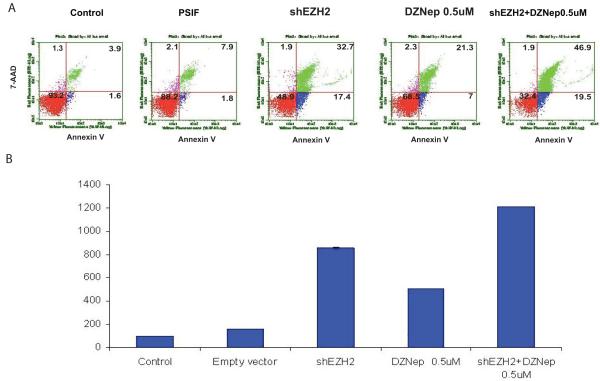
To determine if the growth suppression and inhibition of clonogenicity was associated with cell death, we analyzed apoptosis by flow cytometry. Both Daoy and ONS-76 cells underwent greater apoptosis when treated with DZNep or a combination of shRNA and DZNep. Daoy cells treated with shRNA or DZNep showed a 3-5 fold increase in apoptosis compared to both untreated cells or control vector transfected cells (Figure 5 A and B). Similarly ONS 76 cells also demonstrated an increase in apoptosis when transfected with EZH2 shRNA or treated with DZNep (Figure S5A). Analysis of additional medulloblastoma cell lines show that inhibition of EZH2 results in increased apoptosis as well (Figure S5B).
Figure.5.
Knockdown of EZH2 induces apoptosis. Daoy cells were treated with control plasmid (pSIF), shEZH2 plasmid, or DZNep (0.5 μM). Following treatment, cells were stained for Annexin, a cell surface marker of apoptosis. Annexin-positive cells were measured by Guava Nexin Assay. DZNep significantly induced apoptosis in non-transfected and shEZH2-transfected Daoy cells (n=4, p < 0.0005).
Abrogation of EZH2 inhibits transformation of neural stem cells in vitro
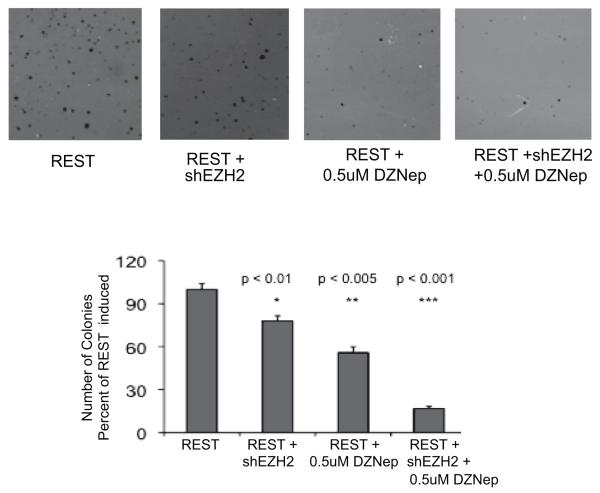
Medulloblastoma can be modeled by transforming neural stem cells in vitro. The C17.2 cell line is immortalized with myc and can be propagated in cell culture using serum free media supplemented with epidermal growth factor and leukemia inhibitory factor26. C17.2 cells can be transformed using oncogenes such as repressor element 1silencing transcription factor (REST) or Bmi127, 28. REST-transformed C17.2 cells form medulloblastoma tumors when implanted orthotopically in murine models27. Because this is a well-defined system, we chose to evaluate the role of EZH2 in C17.2 cells. We transformed C17.2 cells using REST and evaluated their ability to form anchorage-independent colonies on agar. REST alone enables C17.2 cells to grow in agar while unmodified C17.2 cells do not form colonies (Figure 6). Inhibition of EZH2 strongly attenuates the ability of REST to transform C17.2 cells as detected by anchorage-independent growth on agar (Figure 6). Cells transfected with REST and shEZH2 formed fewer colonies than REST alone (p < 0.01). The combined inhibition of EZH2 with shRNA and DZNep decreased soft agar colonies by 74% (p < 0.001).
Figure 6.
Anchorage-independent colony formation in soft agar. C17.2 cells were transfected with REST or a combination of REST and shEZH2, and the ability of cells to form colonies in soft agar measured. Unmodified C17.2 cells do not grow colonies while REST transformed C17.2 cells exhibit robust colony formation in soft agar. Inhibition of EZH2 reduced the ability of REST to transform cells (p < 0.01). Inhibition with shRNA and 0.5μM DZNep was most potent in inhibiting transformation of C17.2 cells (p < 0.001).
Discussion
Epigenetic modifications have been shown to be critical in cancer initiation and progression29. Importantly, histone methylation was recently identified to play a prominent role in cancer30. In particular histone H3 lysine 9 methylation and histone H3K27me3 are associated with gene silencing in cancer12. In medulloblastoma, there are considerable genetic alterations in histone lysine methylases that regulate methylation of H3K9. The role of histone lysine 27 methylases is less clear. The PRC2 methylates H3K27 via its catalytic subunit EZH2. Overexpression of EZH2 is found in a diverse range of cancers such as breast cancer and is associated with resistance to chemotherapy31, 32.
We sought to evaluate the role of EZH2 in medulloblastoma. Initial expression data demonstrated that EZH2 is indeed over-expressed in medulloblastoma. In fact, EZH2 expression correlated with more aggressive group C & D genomic subtypes and the expression can be partially attributed to genomic gain of chromosome 7. For the first time, we demonstrated that inhibition of EZH2 by RNAi potently decreases growth of medulloblastoma cell lines in vitro. To further elucidate the role of EZH2 in medulloblastoma, we examined its ability to regulate tumor sphere formation. Inhibition of EZH2 strongly suppressed the ability of medulloblastoma cells to form spheres in stem cell culture conditions. These data are consistent with previous reports indicating that EZH2 is a regulator of stem cell self-renewal and is associated with maintenance of tumor initiating cells in glioblastoma20. Interestingly, inhibition of EZH2 led to significantly decreased activity of RB, Myc, and Sox2 transcriptional pathways and decreased expression of Nestin, Nanog and Sox2, further supporting our hypothesis that EZH2 is a mediator of tumor stem cell maintenance in medulloblastoma. Given our initial data and the known roles of EZH2 in other tumors, further in depth functional analysis of the role of EZH2 in medulloblastoma stem cell maintenance is warranted.
Recently, a novel chemical inhibitor, DZNep, was found to suppress EZH2mediated histone H3 lysine-27 methylation. We show that treatment of medulloblastoma cells with DZNep inhibits their growth in both short and long-term assays and robustly represses methylation of histone H3 lysine-27. Moreover, the mechanism of DZNep activity is at least partially due to enhanced apoptosis in medulloblastoma cells. In addition, we demonstrated that EZH2 is required for transformation of normal neural stem cells by known oncogenes. Inhibition of EZH2 expression resulted in repression of REST-mediated oncogenic transformation of C17.2 neural stem cells.
Thus, in this study we demonstrated that EZH2 is a critical regulator of medulloblastoma cell growth, important for transformation of neural stem cells, and a potential therapeutic target in this devastating brain tumor of childhood.
Supplementary Material
Figure S1. Analysis of amplification of EZH2 in Medulloblastoma samples. A. Presence of a significant correlation between the presence of broad (chr7 or 7q arm) gain and EZH2 expression. B. The relationship of increasing copy number (>2.5) against expression of EZH2 in medulloblastoma.
Figure S2. Validation of EZH2 knock-down by RNAi. A. qRT-PCR for EZH2 mRNA in control and shEZH2-transfected Daoy and ONS-76 cells. B. Expression of EZH2 protein in control and shEZH2-transfected Daoy and ONS-76 cells.
Figure S3. A. Quantification of tumor sphere formation potential of Daoy cells from single cell cultures. 20.000 ONS 76 cells were seeded in ultra low attachment 6 well plate and grown in Neurobasal medium. 3 days later when they formed spheres, cells were treated with DZNep 0.5uM. 48 hrs later cells were reseeded (1 cell, 10 cells, 20 cells, 50 cells) in 96 well plate and spheres counted after 7 days. EZH2 inhibition potently decreases the ability of Daoy cells to form spheres from single cell and low cell number cultures. B. Comparison of gene expression of progenitor cell markers in cells grown as standard adherent cells in serum containing media or as spheres in neurobasal serum-free media. Progenitor markers Nestin, Sox 2, Nanog and Myc all increase in tumor sphere cultures compared to parental adherent cells.
Figure S4. Analysis of cell cycle dynamics in control and shEZH2-transfected Daoy and ONS-76 cells. All flow cytometry experiments were performed in duplicate two separate times. Inhibition of EZH2 induces a G2 arrest in ONS 76 cells (p < 0.005) but not Daoy cells.
Figure S5. A. Inhibition of EZH2 induces apoptosis in ONS76 cells as measured by Annexin V expression. B. Summary data showing inhibition of EZH2 in a panel of medulloblastoma cell lines induces apoptosis similar to Daoy and ONS 76 cells.
Acknowledgements
We acknowledge the ongoing support of the Department of Pediatrics and Childrens Hospital Colorado.
303-724-267 Support disclosure: This work was supported by NIH KO8NS59790-3 (RV) and The Morgan Adams Foundation (RV, NKF)
Footnotes
Conflict of Interest: There are no conflicts of interest for any authors.
References
- 1.Dhall G. Medulloblastoma. J Child Neurol. 2009;24:1418–30. doi: 10.1177/0883073809341668. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Archives of Neurology. 2008;65:1419–24. doi: 10.1001/archneur.65.11.1419. [DOI] [PubMed] [Google Scholar]
- 3.Mulhern RK, Palmer SL, Merchant TE, Wallace D, Kocak M, Brouwers P, Krull K, Chintagumpala M, Stargatt R, Ashley DM, Tyc VL, Kun L, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–9. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 4.Dhall G, Grodman H, Ji L, Sands S, Gardner S, Dunkel IJ, McCowage GB, Diez B, Allen JC, Gopalan A, Cornelius AS, Termuhlen A, et al. Outcome of children less than three years old at diagnosis with non-metastatic medulloblastoma treated with chemotherapy on the “Head Start” I and II protocols. Pediatr Blood Cancer. 2008;50:1169–75. doi: 10.1002/pbc.21525. [DOI] [PubMed] [Google Scholar]
- 5.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 29:1408–14. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 29:1424–30. doi: 10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Vibhakar R, Foltz G, Yoon JG, Field L, Lee H, Ryu GY, Pierson J, Davidson B, Madan A. Dickkopf-1 is an epigenetically silenced candidate tumor suppressor gene in medulloblastoma. Neuro Oncol. 2007;9:135–44. doi: 10.1215/15228517-2006-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S, Mack S, Kongkham PN, Peacock J, Dubuc A, Ra YS, Zilberberg K, et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–72. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C, Gallia GL, Jallo GI, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 331:435–9. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 12.Tsang DP, Cheng AS. Epigenetic regulation of signaling pathways in cancer: role of the histone methyltransferase EZH2. J Gastroenterol Hepatol. 26:19–27. doi: 10.1111/j.1440-1746.2010.06447.x. [DOI] [PubMed] [Google Scholar]
- 13.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 469:343–9. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Mol Cell Biol. 2001;21:4330–6. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, Akslen LA. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–73. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 16.Rizzo S, Hersey JM, Mellor P, Dai W, Santos-Silva A, Liber D, Luk L, Titley I, Carden CP, Box G, Hudson DL, Kaye SB, et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol Cancer Ther. 10:325–35. doi: 10.1158/1535-7163.MCT-10-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, Gong Y, Cheng H, Laxman B, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 17:443–54. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crea F, Hurt EM, Mathews LA, Cabarcas SM, Sun L, Marquez VE, Danesi R, Farrar WL. Pharmacologic disruption of Polycomb Repressive Complex 2 inhibits tumorigenicity and tumor progression in prostate cancer. Mol Cancer. 10:40. doi: 10.1186/1476-4598-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crea F, Hurt EM, Farrar WL. Clinical significance of Polycomb gene expression in brain tumors. Mol Cancer. 9:265. doi: 10.1186/1476-4598-9-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suva ML, Riggi N, Janiszewska M, Radovanovic I, Provero P, Stehle JC, Baumer K, Le Bitoux MA, Marino D, Cironi L, Marquez VE, Clement V, et al. EZH2 is essential for glioblastoma cancer stem cell maintenance. Cancer Res. 2009;69:9211–8. doi: 10.1158/0008-5472.CAN-09-1622. [DOI] [PubMed] [Google Scholar]
- 21.Birks DK, Donson AM, Patel PR, Dunham C, Muscat A, Algar EM, Ashley DM, Kleinschmidt-Demasters BK, Vibhakar R, Handler MH, Foreman NK. High expression of BMP pathway genes distinguishes a subset of atypical teratoid/rhabdoid tumors associated with shorter survival. Neuro Oncol. doi: 10.1093/neuonc/nor140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Sheikh A, Fan R, Birks D, Donson A, Foreman NK, Vibhakar R. Inhibition of Aurora Kinase A enhances chemosensitivity of medulloblastoma cell lines. Pediatr Blood Cancer. doi: 10.1002/pbc.22465. [DOI] [PubMed] [Google Scholar]
- 23.Stearns D, Chaudhry A, Abel TW, Burger PC, Dang CV, Eberhart CG. c-myc overexpression causes anaplasia in medulloblastoma. Cancer Res. 2006;66:673–81. doi: 10.1158/0008-5472.CAN-05-1580. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Lee KF, Lu Y, Clarke I, Shih D, Eberhart C, Collins VP, Van Meter T, Picard D, Zhou L, Boutros PC, Modena P, et al. Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitive neuroectodermal brain tumors. Cancer Cell. 2009;16:533–46. doi: 10.1016/j.ccr.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21:1050. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker MA, Anderson JK, Corliss DA, Abraria VE, Sidman RL, Park KI, Teng YD, Cotanche DA, Snyder EY. Expression profile of an operationally-defined neural stem cell clone. Exp Neurol. 2005;194:320–32. doi: 10.1016/j.expneurol.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Su X, Gopalakrishnan V, Stearns D, Aldape K, Lang FF, Fuller G, Snyder E, Eberhart CG, Majumder S. Abnormal expression of REST/NRSF and Myc in neural stem/progenitor cells causes cerebellar tumors by blocking neuronal differentiation. Mol Cell Biol. 2006;26:1666–78. doi: 10.1128/MCB.26.5.1666-1678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkataraman S, Alimova I, Fan R, Harris P, Foreman N, Vibhakar R. MicroRNA 128a increases intracellular ROS level by targeting Bmi-1 and inhibits medulloblastoma cancer cell growth by promoting senescence. PLoS ONE. 2010;5:e10748. doi: 10.1371/journal.pone.0010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lund AH, van Lohuizen M. Epigenetics and cancer. Genes Dev. 2004;18:2315–35. doi: 10.1101/gad.1232504. [DOI] [PubMed] [Google Scholar]
- 30.Albert M, Helin K. Histone methyltransferases in cancer. Semin Cell Dev Biol. 21:209–20. doi: 10.1016/j.semcdb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Abbosh PH, Montgomery JS, Starkey JA, Novotny M, Zuhowski EG, Egorin MJ, Moseman AP, Golas A, Brannon KM, Balch C, Huang TH, Nephew KP. Dominant-negative histone H3 lysine 27 mutant derepresses silenced tumor suppressor genes and reverses the drug-resistant phenotype in cancer cells. Cancer Res. 2006;66:5582–91. doi: 10.1158/0008-5472.CAN-05-3575. [DOI] [PubMed] [Google Scholar]
- 32.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Analysis of amplification of EZH2 in Medulloblastoma samples. A. Presence of a significant correlation between the presence of broad (chr7 or 7q arm) gain and EZH2 expression. B. The relationship of increasing copy number (>2.5) against expression of EZH2 in medulloblastoma.
Figure S2. Validation of EZH2 knock-down by RNAi. A. qRT-PCR for EZH2 mRNA in control and shEZH2-transfected Daoy and ONS-76 cells. B. Expression of EZH2 protein in control and shEZH2-transfected Daoy and ONS-76 cells.
Figure S3. A. Quantification of tumor sphere formation potential of Daoy cells from single cell cultures. 20.000 ONS 76 cells were seeded in ultra low attachment 6 well plate and grown in Neurobasal medium. 3 days later when they formed spheres, cells were treated with DZNep 0.5uM. 48 hrs later cells were reseeded (1 cell, 10 cells, 20 cells, 50 cells) in 96 well plate and spheres counted after 7 days. EZH2 inhibition potently decreases the ability of Daoy cells to form spheres from single cell and low cell number cultures. B. Comparison of gene expression of progenitor cell markers in cells grown as standard adherent cells in serum containing media or as spheres in neurobasal serum-free media. Progenitor markers Nestin, Sox 2, Nanog and Myc all increase in tumor sphere cultures compared to parental adherent cells.
Figure S4. Analysis of cell cycle dynamics in control and shEZH2-transfected Daoy and ONS-76 cells. All flow cytometry experiments were performed in duplicate two separate times. Inhibition of EZH2 induces a G2 arrest in ONS 76 cells (p < 0.005) but not Daoy cells.
Figure S5. A. Inhibition of EZH2 induces apoptosis in ONS76 cells as measured by Annexin V expression. B. Summary data showing inhibition of EZH2 in a panel of medulloblastoma cell lines induces apoptosis similar to Daoy and ONS 76 cells.