Abstract
GABAA receptors are a family of ligand-gated ion channels which are essential for the regulation of central nervous system function. Benzodiazepines – which target GABAA receptors containing the α1, α2, α3, or α5 subunits non-selectively – have been in clinical use for decades and are still among the most widely prescribed drugs for the treatment of insomnia and anxiety disorders. However, their use is limited by side effects and the risk of drug dependence. In the past decade, the identification of separable key functions of GABAA receptor subtypes suggests that receptor subtype-selective compounds could overcome the limitations of classical benzodiazepines and, furthermore, might be valuable for novel indications, such as analgesia, depression, schizophrenia, cognitive enhancement and stroke.
Introduction
GABAA receptors are the molecular targets of benzodiazepines. In this Review, we provide an overview on advances in our understanding of the physiological and pharmacological roles of GABAA receptor subtypes, their potential applications to drug development and an update on the clinical development of GABAA receptor subtype-selective compounds, thus complementing other more historically oriented1, 2 or specialized3–7 recent reviews.
The term benzodiazepine refers to a chemical structure consisting of a fusion of a benzene ring and a diazepine ring, in which the two N atoms are mostly located in positions 1 and 4 (1,4-benzodiazepines). In the 1950s, it was discovered by serendipity that benzodiazepines have a variety of therapeutically useful actions, including anxiolysis, sedation, seizure suppression and muscle relaxation. As sedative-hypnotic (sleep-inducing) drugs, they have essentially replaced the barbiturates owing to a substantially improved therapeutic index. Benzodiazepines mediate their action via a modulatory binding site (the benzodiazepine site) on most (although not all) GABAA receptors8 (Box 1). In contrast to barbiturates, GABAA receptor modulation by benzodiazepine site agonists is self-limiting: the conductance of the channel in the presence of GABA and benzodiazepines is not higher than the conductance that can be achieved with high concentrations of GABA alone. Moreover, also in contrast to barbiturates, benzodiazepines do not open the chloride channel in the absence of GABA. Limitations of current benzodiazepines include that the pharmacological effects cited above are not clearly separable by dosing. For example although the anxiolytic actions are observed at lower doses than the sedative actions, sedation is still a problem when benzodiazepines are used as daytime anxiolytics, and therefore novel anxioselective, non-sedating compounds would be desirable. Furthermore, benzodiazepines have addictive properties and thus abuse liability, and this limits their long-term use. In addition to the development of addiction, physical dependence and tolerance are also areas of concern.
Box 1. GABAA receptors.
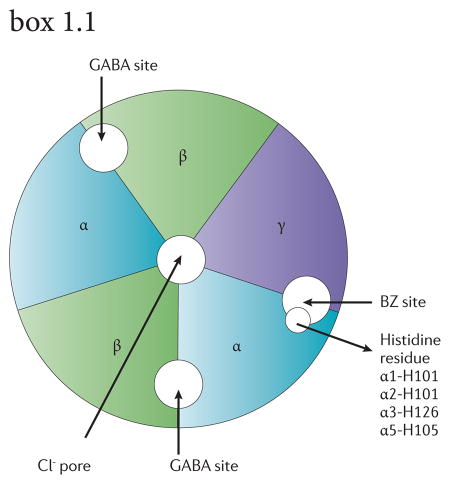
GABAA receptors are heteropentamers made up from 19 known subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π, and ρ1-3)18, 86 with an integral channel that is permeable to Cl− ions (see figure 1). It is noteworthy that homopentameric ρ receptors are insensitive to bicuculline and baclofen and have been referred to as GABAC receptors87; however, the Nomenclature Committee of the International Union of Pharmacology (IUPHAR) does not recommend this nomenclature86. GABA-induced chloride influx hyperpolarizes the postsynaptic neurons. Many GABAA receptors contain two α subunits, two β subunits and one γ subunit with two GABA binding sites formed by α and β subunits. The binding site for benzodiazepines is formed by one of the α subunits α1, α2, α3, and α5 and a γ subunit, typically the γ2 subunit, which is present in approximately 90% of GABAA receptors. GABAA receptors containing the α4 or α6 subunit do not bind clinically used classical benzodiazepines. Histidine to arginine mutations at a conserved residue in the α subunits functionally abolish the benzodiazepine binding site.
The subunit combination α1β2γ2 represents approximately 60% of all GABAA receptors, α2β3γ2 approximately 15–20%, α3βnγ2 approximately 10–15%, α4βnγ or α4βnδ approximately 5%, α5β2γ2 less than 5%, and α6β2/3γ2 also less than 5%88. It is noteworthy that some GABAA receptors may also contain two different α subunits89. In recombinant receptors, the α subunit adjacent to the γ2 subunit determines the sensitivity to benzodiazepines90.
In addition to benzodiazepines, the GABAA receptor is also the major target for the clinically used hypnotic drugs zolpidem, zopiclone, (S)-zopiclone, and zaleplone, for barbiturates, and for many general anesthetics91. GABAA receptors are a major target for the actions of the clinically used intravenous anesthetics etomidate and propofol, and β3(N265M) mice cannot be immobilized using these drugs, suggesting an essential role of β3-containing GABAA receptors for immobilization92. Clinically used volatile anesthetics like isoflurane, enflurane, and sevoflurane presumably act via a multitude of targets, GABAA receptors being only one of them. Their contribution to the hypnotic and immobilizing action of volatile anesthetics is limited92–94.
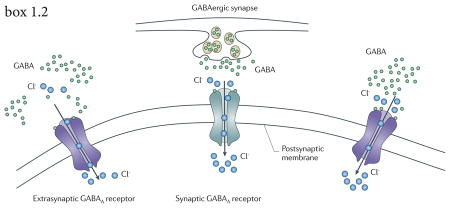
GABAA receptor-mediated events have two effects on the postsynaptic membrane: an increase of the postsynaptic membrane conductance (shunting inhibition) and a change in the membrane potential due to movement of Cl− ions through the membrane (hyperpolarizing inhibition). Synaptic receptors which detect millimolar concentrations of GABA mediate fast inhibitory postsynaptic potentials (IPSPs) whilst extrasynaptic receptors which detect micromolar concentrations of GABA mediate slower IPSPs and also tonic conductances (see figure 2). Tonic and phasic conductances underlie different physiological and behavioral processes.
Human mutations in GABAA receptors subunits
While GABAergic agents have been used to treat a variety of disorders, only a limited number of mutations have been found in GABAA receptor subunit genes. These include point mutations in the α1 and γ2 subunits in patients with genetic epilepsies95. Genetic association studies indicate single nucleotide polymorphisms (SNPs) in the gene encoding the α2 subunit in alcohol dependence96, 97 and illicit drug dependence98–100. However, the functional consequences of this genomic variation are not fully understood. Furthermore, the gene encoding the β1 subunit of the GABAA receptor has been linked to alcohol dependence101 and also to bipolar disorder102. Association signals have also been detected for the genes encoding the α4, α5, β3 and ρ1 subunits102. The genes encoding the α1, α6, β2 and π subunits have been linked to schizophrenia103.
Benzodiazepines have been shown to bind to specific sites in the CNS 9, 10, which later turned out to be modulatory sites on the GABAA receptor (see Box 1). The functions of individual GABAA receptor subtypes have been elucidated mainly in genetically modified mice in which individual GABAA receptor α subunits have been rendered insensitive to diazepam. Histidine to arginine point mutations at a conserved residue in the α1, α2, α3 or α5 subunit abolish binding of diazepam, while the action of the physiological neurotransmitter GABA is preserved11–14 (Box 1). In α1(H101R) mice, the sedative and anterograde amnestic action of diazepam were absent and its anticonvulsant action was reduced, but its anxiolytic-like action was present11. In α2(H101R) mice, the anxiolytic-like action of diazepam was absent and its myorelaxant action (which is observed at higher doses than the anxiolytic-like action) was reduced, while the sedative action was present13, 15. In α3(H126R) mice and in α5(H105R) mice, the myorelaxant action of diazepam was reduced, while sedative and anxiolytic-like actions were present13–15. These experiments demonstrated that the sedative, anterograde amnestic and in part the anticonvulsant actions of diazepam are mediated by α1-containing GABAA receptors, that the anxiolytic-like and to a large part the myorelaxant actions are mediated by α2-containing GABAA receptors, and that the myorelaxant action is mediated in part by α3- and α5-containing GABAA receptors. Moreover, the development of tolerance to the sedative action of benzodiazepines has been linked to α5-containing GABAA receptors16, and their addictive properties to α1-containing GABAA receptors17. While experiments with the histidine to arginine mutated mouse lines clearly define a role for the mutated GABAA receptor α subunit if a response to diazepam is absent, they do not formally exclude a contribution of other α subunits to the response in question. E.g., the observation that diazepam does not sedate α1(H101R) mice indicates that diazepam acting on α2-, α3- and α5-containing GABAA receptors is not sedative, but this does not exclude the possibility that one of the three diazepam-sensitive α subunits in these mice has a sedative effect and another one a stimulant effect, so they cancel each other out. In the last decade important advances in the understanding of functions of GABAA receptor subtypes have been made which have helped to identify GABAA receptor subtypes as potential therapeutic targets and to define GABAA receptor subtypes to be avoided as they have been linked to unwanted side effects. Accordingly, drug discovery has been directed towards identifying compounds that do not interact with these receptor subtypes. This was achieved either by binding selectivity or by selective modulation at a given receptor subtype (Box 2).
Box 2. Binding and functional selectivity.
Ligands at the benzodiazepine binding site (BZ site) of the GABAA receptor are allosteric modulators. They modify the efficacy and/or affinity of agonists, e.g. GABA, and thus regulate their activity. The direction of the modulation can be positive, negative or neutral, and is achieved by stabilizing different conformations of the receptor. Allosteric modulators of the GABAA receptor are frequently referred to as BZ site agonists or BZ site inverse agonists, in part because it was originally assumed that the benzodiazepine binding site is an independent receptor9, 104. More precisely, they might be referred to as positive allosteric modulators (PAMs) or negative allosteric modulators (NAMs), respectively. Prototypic ligands for a PAM, a NAM and a BZ site antagonist are diazepam, β-CCM (methyl beta-carboline-3-carboxylate) and flumazenil (Ro 15-1788), respectively.
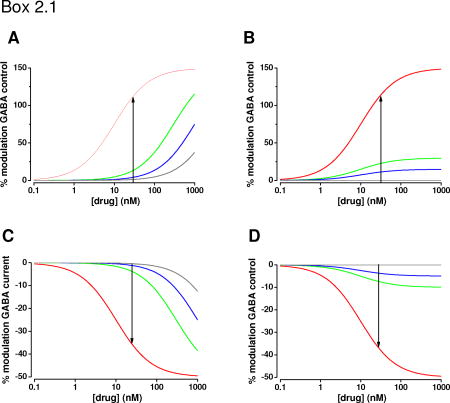
Selectivity of a ligand for a specific GABAA receptor subtype can be obtained either by binding, i.e. by forming a receptor-ligand complex, or efficacy by eliciting a biological response after binding to the receptor. These two properties define its potency profile. Since binding experiments cannot reveal the full potency profile of a ligand, subtype selectivity is also assessed in electrophysiological experiments performed with e.g. human embryonic kidney 293 cells or Xenopus oocytes heterologously expressing GABAA receptor subtypes (Fig. 3A). The affinity-selective positive allosteric modulator (agonist) represented in (A) has different affinities for the red, green, blue and grey receptor subtypes (shifts in the concentration-response curves). The efficacy-selective positive allosteric modulator (agonist) in (B) has the same affinities for all receptor subtypes (no shifts in the concentration response curves). However, its maximum efficacy at the red receptor subtype is much higher than that at the other receptor subtypes. Although these positive allosteric modulators (agonists) have very different potency profiles, at a given therapeutic concentration (30 nM, vertical arrows in A and B) their efficacies measured at the different receptor subtypes are similar. This also holds true for the affinity- (C) and efficacy-selective (D) negative allosteric modulators (inverse agonists).
In this Review, we provide a brief summary of GABAA receptor structure and function, and discuss recent progress in drug development efforts to address the sedative side effects and addictive properties of classical benzodiazepines using GABAA receptor subtype-selective compounds. Finally, we highlight the emerging potential of such compounds in novel indications, including in psychiatric disorders.
GABAA receptors
An estimated 20–30% of the neurons in the CNS are GABAergic. Activation of neuronal GABA receptors typically results in hyperpolarization, and thus GABA appears to be the major inhibitory neurotransmitter in the CNS. Two pharmacologically distinct classes of GABA receptors have been identified: GABAA receptors (Figure 1, Box 1) are pentameric ligand-gated chloride channels whose activation typically leads to an influx of chloride, and GABAB receptors are heterodimeric Gi/Go-protein-coupled receptors which activate potassium channels and inhibit calcium channels18, 19. The diversity in the GABAA receptor system with 19 known subunit genes is much larger than in the GABAB system with 3 known subunit genes. Both classes of GABA receptors modulate emotions, cognition, pain, and muscle tone and are targets of clinically used drugs. GABAA receptors are allosterically modulated by benzodiazepines, which are used for their sedative, anxiolytic, anticonvulsant and muscle relaxant actions. Baclofen, an agonist at the GABAB receptor, is used to relieve muscle spasticity.
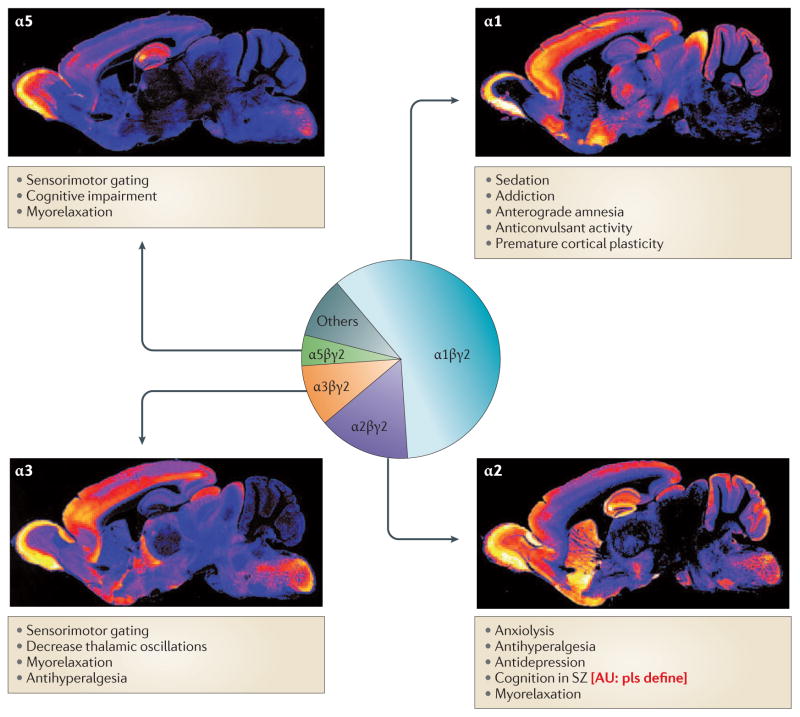
Figure 1. Pharmacological effects and distribution of GABAA receptor α subunits in the mouse brain.
The pie chart represents the approximate abundance of the GABAA receptor subtypes that are known to exist in vivo. α1 is expressed in cortex, thalamus, pallidum and hippocampus. α2 is expressed in hippocampus, cortex, striatum, and nucleus accumbens (not shown). α3 is expressed in the cortex and the reticular nucleus of the thalamus, and α5 in the hippocampus and in deep layers of the cortex. The anti-hyperalgesic actions are mediated by spinal GABAA receptors. Data in references 42, 105–107.
Immunohistochemical pictures are courtesy of Dr. Jean-Marc Fritschy, University of Zurich, and have been published in ref. 88. [CE: waiting to see if we need to apply for permission to use these]
Benzodiazepines have sedative-hypnotic properties. While these properties are useful for the treatment of insomnia, they are undesirable side effects when benzodiazepines are used for most other purposes, e.g. for daytime anxiolysis. Moreover, sedative effects would also be a major obstacle for the use of benzodiazepine site ligands for novel therapeutic indications. For indications other than insomnia, it is thus important to identify – and avoid - the receptor subtype(s) mediating the sedative action of benzodiazepines.
In addition to benzodiazepines, other compounds have been developed which bind to the same site or to an overlapping site as benzodiazepines. Zolpidem, an imidazopyridine, has a high affinity for α1-, a 20-fold lower affinity at α2- and α3-, and no affinity at α5-containing GABAA receptors20, and is therefore frequently referred to as being α1-selective. It is used clinically for the treatment of insomnia. This suggests that α1-containing GABAA receptors are important targets for the sedative-hypnotic action of zolpidem. Indeed, in α1(H101R) mice the motor sedative action of zolpidem was abolished, demonstrating that the sedative action of zolpidem is mediated by α1-containing GABAA receptors21. In α1(H101R) mice, diazepam increases sleep continuity – i.e., it reduces the number of brief awakenings derived from EEG recordings - while its motor sedative effect is absent, indicating that motor sedation is mediated by α1-containing GABAA receptors, but enhancement of sleep continuity is independent of α1-containing GABAA receptors 22. The significance of this finding for the design of hypnotic drugs is currently unclear.
The GABA analogue gaboxadol (=THIP, Fig. 2A) acts at the GABA site of the GABAA receptors. In mice, it induces sedation largely via α4-containing GABAA receptors23 but development was stopped in 2007 when phase III trials revealed unexpected side effects including hallucinations and disorientation.
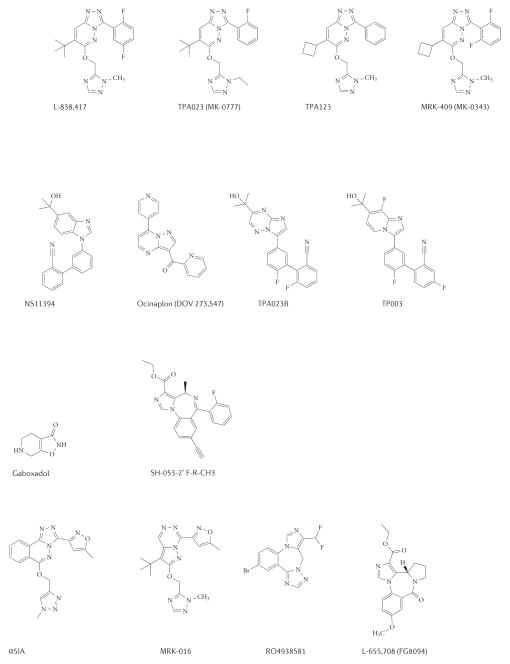
Figure 2. Structures of allosteric GABAA receptor modulators.
A. Preclinical and clinically tested, binding or functionally subtype-selective positive allosteric modulators (agonists). B. Preclinical and clinically tested, binding or functionally subtype-selective negative allosteric modulators (inverse agonists).
Receptor subtype selection to reduce sedative effects
The development of non-sedating benzodiazepine anxiolytics was unsuccessful for decades at least in part because it was unknown whether the sedative-hypnotic and anxiolytic actions are pharmacologically separable. After identifying which GABAA receptor subtype(s) mediate these actions, selective compounds have to be screened for and optimized. Selectivity can be achieved at the level of binding and at the level of efficacy (Box 2). Initial screens for binding-selective compounds were unsuccessful, but useful efficacy-selective (i.e., functionally selective) compounds have been identified.
As described above, α2-containing GABAA receptors have been found to mediate the anxiolytic-like action of diazepam13, while α1-containing GABAA receptors mediate the sedative action of diazepam11. Thus, one would predict that an α2-selective compound with no activity at α1-containing GABAA receptors would be a non-sedating anxiolytic. The contribution of α3-containing GABAA receptors to anxiolysis is less clear and controversial. While experiments with point-mutated mice are consistent with α3 being neither required nor sufficient for anxiolysis13, the experimental compound TP003 (Fig. 2A), which has been described to have a selective efficacy at recombinant α3-containing GABAA receptors in vitro, is anxiolytic in the elevated plus maze in rats24 and in a conflict test in monkeys25, where it also lacked the hyperphagic effect of unselective benzodiazepines25. However, its selectivity for α3-containing GABAA receptors has not been demonstrated in vivo. L-838,417 (Fig. 2A), which is a partial positive allosteric modulator (partial agonist) at α2-, α3-, and α5-containing GABAA receptors and an antagonist at α1-containing GABAA receptors12 (Fig. 3B) displays a pharmacological profile as a non-sedating anxiolytic in mice12 and primates26. However, unfavorable pharmacokinetic properties precluded further development27.
Figure 3. GABA-evoked currents in human embryonic kidney 293 (HEK293)- cells.
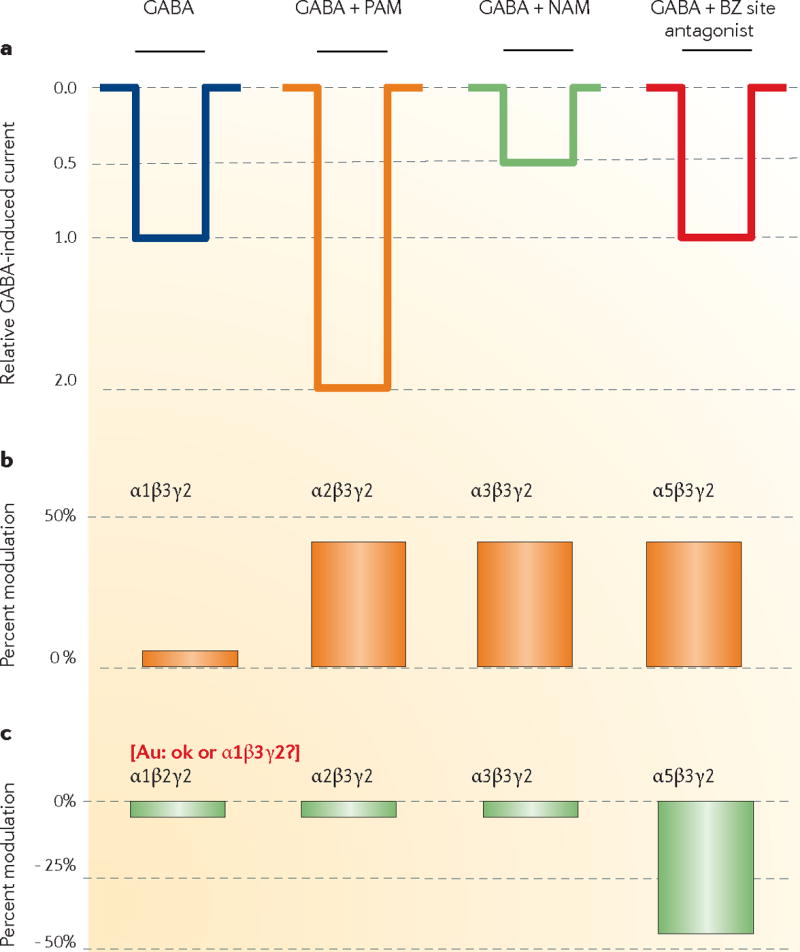
(A) GABA is applied to a cell expressing a GABAA receptor subtype for the time indicated by the black horizontal bar resulting in a current symbolized by the blue trace. When GABA is applied in the presence of a positive allosteric modulator (PAM, BZ site agonist), the current is enhanced (orange trace). Negative allosteric modulators (NAM, BZ site inverse agonists) and neutral allosteric modulators (BZ site antagonists) either decrease (green trace) or have no effect (red trace) on the GABA-induced current. A subtype-selective modulation of the current is observed when GABA is applied in the presence of L-838,417 (B) or RO4938581 (C) to cells expressing various GABAA receptors. Percent modulation of GABA-induced chloride currents is shown. The lack of modulation of α1-containing GABAA receptors by L-838,417 is thought to be the basis for the lack of a sedative action of this compound in animals, whereas its partial positive allosteric modulatory (agonistic) action at α2-containing GABAA receptors (and potentially α3-containing GABAA receptors) is thought to be responsible for its anxiolytic-like action. Similarly, the lack of modulation of α1-containing GABAA receptors by RO4938581 is thought to be – at least in part - the basis for the lack of a pro-convulsive potential of this drug; the cognition-enhancing effects are hypothesized to be mediated via its negative allosteric modulation (inverse agonism) at α5-containing GABAA receptors. These illustrative traces are based on data in 12, 77.
TPA023 (also called MK-0777) (Fig. 2A), an α2/α3-selective positive allosteric modulator (partial agonist, efficacy 0% at α1, 11% at α2, 21% at α3, and 5% at α5, compared with chlordiazepoxide), has also been demonstrated to be anxiolytic but not sedative in rodents28. TPA023 was evaluated in three separate Phase II studies in Generalized Anxiety Disorder (GAD). These studies were terminated early due to preclinical toxicity in long term dosing studies (cataract)2, therefore there were not enough data available for within-trial comparisons4. Combining the data from these separate studies revealed that TPA023 provided a significantly greater reduction of the Hamilton Anxiety Rating Scale (HAM-A) score relative to baseline, consistent with TPA023 having anxiolytic-like activity4. Since TPA023 was not sedative in these Phase II trials even at a receptor occupancy of >50%4, an occupancy at which diazepam has sedative effects in mice29, these data provide proof-of-principle that non-sedating anxiolysis can be achieved in humans by targeting the α2/α3 GABAA receptor subtypes.
Another experimental compound, ocinaplon (DOV 273,547, Fig. 2A), was anxiolytic but not sedative in rodents and in humans (Phase I/II studies with 127 and 60 patients, respectively)30,31. Surprisingly, in recombinant receptors expressed in Xenopus oocytes it modulated α1-, α2-, α3-, and α5-containing GABAA receptors without subtype-specificity30. The reason for this discrepancy between in vitro electrophysiological data obtained with recombinant receptors and in vivo observations is currently unknown. Ocinaplon was not developed further due to hepatic toxicity issues, but it can also be viewed as proof-of-principle that non-sedating anxiolytics targeting the GABAA receptor can be developed.
MRK-409 (MK-0343) (Fig. 2A), which is structurally related to TPA023 and L-838,417, is a positive allosteric modulator (agonist) with higher efficacy at α3-containing GABAA receptors but no binding selectivity. Its efficacy is 18% at α1, 23% at α2, 45% at α3, and 18% at α5, compared with chlordiazepoxide32. MRK-409 displayed an anxioselective profile in rats and primates but produced sedation in man at relatively low levels of occupancy (<10%)4. This indicates that while a low efficacy at α1-containing GABAA receptors may not be overtly sedating in rodents or primates, it apparently sedates humans, and therefore even a small residual efficacy at α1-containing GABAA receptors should raise caution32. Another high affinity compound, TPA023B (Fig. 2A), which like MRK-409 is a positive allosteric modulator (partial agonist) at the α2-, α3-, and α5-, but is an antagonist at the α1-containing GABAA receptor was well tolerated in man33. This demonstrates that experiments with rodents and primates may not predict accurately whether a compound is sedative in humans.
Receptor subtype selection to reduce abuse potential
In a community-based study of benzodiazepine prescription patterns, only 1.6% of patients receiving benzodiazepine prescriptions had prescriptions for long time periods with high doses, indicating an abuse of or a dependence on benzodiazepines34. However, in alcohol and drug-dependent outpatients, benzodiazepine dependence was found to be prevalent35. It has been estimated that approximately 0.1%–0.2% of the adult population abuse or are dependent upon benzodiazepines34, which might translate into approximately 300,000–600,000 people in the United States, highlighting the need for novel compounds with a reduced potential for addiction.
All addictive drugs increase dopamine levels in the mesolimbic dopamine system36, and it has been suggested that addictive drugs hijack the reward system. Benzodiazepines increase the firing of dopaminergic neurons in the VTA by decreasing the activity of GABAergic interneurons (Fig. 4). Dopaminergic neurons in the VTA express α3-containing GABAA receptors; in contrast, the GABAergic interneurons in the VTA express α1-containing GABAA receptors17. Benzodiazepines inhibit the interneurons via their α1-containing GABAA receptors, which results in disinhibition of the dopaminergic neurons. Unselective benzodiazepines also modulate the α3-containing GABAA receptors on dopaminergic neurons in the VTA, but the disinhibition via α1-containing GABAA receptors on interneurons seems to be the predominant effect. This disinhibition triggers drug-evoked synaptic plasticity in excitatory glutamatergic afferents onto dopaminergic neurons in the VTA and underlies drug reinforcements17, 37. In mice, even a single dose of benzodiazepines has been shown to elicit such neuroplastic changes37.
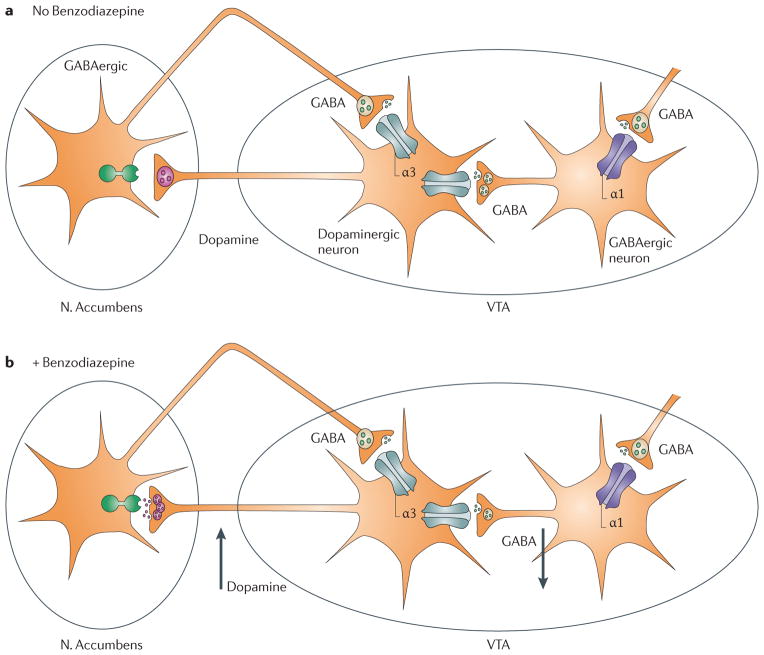
Figure 4. GABAA receptor subtypes in the mesolimbic dopaminergic systems involved in pathways of addiction.
GABAergic neurons in the ventral tegmental area (VTA) express the α1 subunit, whereas dopaminergic neurons in the VTA predominantly express the α3 subunit. Binding of benzodiazepines to the α1-containing GABAA receptors on GABAergic VTA neurons leads to a reduction of the activity of these cells, and thus reduced release of GABA, which results in a disinhibition of the dopaminergic VTA neurons and a resulting increase in DA release in the ventral striatum. In principle, benzodiazepines likely have functionally opposing actions via the α1-containing GABAA receptors on GABAergic neurons and on α3-containing GABAA receptors on the dopaminergic neurons of the VTA. However, the effect on the α1-containing GABAA receptors on the dopaminergic neuron is functionally predominant.
In α1(H101R) mice, the disinhibition is absent, clearly demonstrating a crucial role of α1-containing GABAA receptors17. Furthermore, in an oral self-administration experiment, wild type mice and α3(H126R) mice preferred midazolam, whereas α1(H101R) mice showed no preference for midazolam, indicating that α1-containing GABAA receptors are essential for midazolam self-administration17. Thus, subunit-selective benzodiazepines which do not positively modulate α1-containing GABAA receptors may be less addictive. In self-administration experiments with rhesus monkeys under a progressive ratio schedule of intravenous drug delivery, the breakpoint, i.e. the highest response requirement completed, a measure of how hard an animal worked to obtain the drug, was higher for zolpidem, midazolam, and diazepam, which all modulate α1-containing GABAA receptors, than for L-838,417, which is an antagonist at α1-containing GABAA receptors and a partial positive allosteric modulator (partial agonist) at α2-, α3-, and α5-containing GABAA receptors26. This finding is consistent with the idea that α1-containing GABAA receptors play an important role in the addictive properties of benzodiazepines. Furthermore, in self-administration experiments in baboons, a withdrawal syndrome was observed following cessation of TPA123 (Fig. 2A; efficacy at α1: 23%, α2: 35%, α3: 43%, α5: 19% when compared with chlordiazepoxide) self-administration38. In contrast to TPA123, only a mild withdrawal syndrome developed following cessation of self-administration of TPA023, which has no efficacy at α1-containing GABAA receptors (efficacy at α1: 0%, α2: 11%, α3: 21%, α5: 5%, compared to chlordiazepoxide)38. These findings are consistent with an important role of α1-containing GABAA receptors for reinforcement. However, it cannot be ruled out at this point that the differences in reinforcement are due to differences in efficacy at α2-, α3-, or α5-containing GABAA receptors.
Novel indications for GABAA receptor subtype-selective compounds
Recently gained knowledge on the physiological and pharmacological functions of GABAA receptor subtypes has made it possible to target GABAA receptor subtypes for indications that are unrelated to the current uses of benzodiazepines. For these indications, the use of subtype-selective allosteric modulators acting via the benzodiazepine site represents a novel approach compared to currently established pharmacological therapies.
Analgesia
Benzodiazepines are generally not considered to be analgesic agents. They lack clear efficacy when given systemically in humans39. In particular, sedative actions have been found to limit the usefulness of GABAergic agents as analgesics39, although this limitation can be overcome experimentally by administering the drugs intrathecally. Central GABAA receptors, such as those in the periaqueductal gray (PAG), an area known to be involved in the regulation of descending antinociceptive tracts, are pro-nociceptive at supraspinal sites40. In contrast, GABAA receptors in the spinal cord have anti-hyperalgesic actions3. When diazepam is administered intrathecally in α2(H101R) and α3(H126R) mice, its anti-hyperalgesic action is significantly reduced in models of inflammatory pain and of neuropathic pain, demonstrating that spinal α2- and α3-containg GABAA receptors are mediating the anti-hyperalgesic actions of intrathecal diazepam41. Studies in α5(H105R) mice showed a minor role for spinal α5-containing GABAA receptors in a model of inflammatory pain41 and systemically applied L-838,417 has an anti-hyperalgesic action in wild type rats in models of inflammatory and neuropathic pain41. As mentioned previously, L-838,417 is an α2-, α3-, and α5-partial positive allosteric modulator (partial agonist) and an α1-antagonist12. As α2-, α3-, and α5 subunits are the predominant α subunits in the spinal cord, and the PAG predominantly expresses the α1 subunit42, L-838,417 is likely to positively modulate anti-hyperalgesic spinal GABAA receptors, whilst blocking central pro-algesic GABAA receptors. Potentially both actions contribute to its anti-hyperalgesic effects. Interestingly, functional magnetic resonance imaging in rats demonstrated that L-838,417 (after stimulation of an inflamed hind paw with noxious heat) reduced the activity of brain areas related to the sensory and associative-emotional components of pain, e.g. medial thalamus, contralateral primary sensory cortex, cingulate cortex, frontal association cortex, limbic system (including amygdala, entorhinal cortex, and hippocampus)41. Furthermore, in contrast to morphine, over a 10 day treatment period no tolerance develops to L-838,41741.
These findings suggest that α2/α3-selective or α2/α3/α5-selective positive allosteric modulators (agonists) may represent a novel class of analgesic drugs, e.g., in conditions associated with inflammatory pain or with neuropathic pain, either alone or in combination with existing analgesics. There is an overlap between pain and emotion-reward-motivation brain circuitry; psychiatric disorders are commonly associated with alterations in pain processing and chronic pain may impair emotional and neurocognitive functions43. Thus, the dual actions of α2/α3-selective positive allosteric modulators (agonists) on emotions and pain may be particularly useful therapeutically.
The compound NS11394 (Fig. 2A), a partial positive allosteric modulator (partial agonist) with a functional selectivity profile α5 [maximal potentiation relative to diazepam: α5 (78%)> α3(56%) > α2(22%) > α1(7.8%)], is anti-hyperalgesic in rat models of inflammatory and neuropathic pain44, as well as anxiolytic and only minimally sedative45. Likewise, the compound HZ-166, a partial positive allosteric modulator (partial agonist) with selectivity for α2- and α3-containing GABAA receptors, had anti-hyperalgesic action in mouse models of neuropathic and inflammatory pain46. At doses producing maximal anti-hyperalgesia, HZ-166 did not induce sedation and motor impairment46. Furthermore, there was no development of tolerance over a 9-day chronic treatment period46. TPA023, an α2/α3-selective partial positive allosteric modulator (partial agonist) which is anxiolytic in humans, like NS113934 attenuated formalin-induced nocifensive behavior, and both compounds reversed hind paw mechanical hypersensitivity and weight bearing deficits in carageenan-inflamed and nerve-injured rats47. Diazepam was ineffective in these models.
It has recently been shown that partial negative allosteric modulators (partial inverse agonists) like the non-selective FG-7142 and the α5-selective α5IA-II also display anti-hyperalgesic actions in models of inflammatory and/or neuropathic pain47. The reasons for this are currently unclear. In any case, the results with NS11394, TPA023, and HZ-166 in rodents provide independent evidence for the potential usefulness of α1-sparing compounds as anti-hyperalgesic agents. It remains to be determined whether such effects will also be observed in humans.
Schizophrenia
Benzodiazepines are frequently used as adjunctive treatment to neuroleptics in patients with schizophrenia, although convincing evidence that their use has long-term antipsychotic or cognitive benefits is lacking.
α3 knockout mice and α5(H105R) partial knockout mice with a reduced expression of the α5 subunit display deficits in sensorimotor gating, as determined by prepulse inhibition of acoustic startle, supporting a potential involvement of α3- and α5-containing GABAA receptors in the pathophysiology of schizophrenia48, 49. α5 partial knockout mice also display deficits in latent inhibition, i.e. retarded conditioning to a stimulus that is repeatedly presented without any reinforcement contingencies49. Deficits in this form of learning have also been described in schizophrenia50. An important pathophysiological feature in schizophrenia is a hyperactivity of dopaminergic neurons in the ventral tegmental area (VTA), which express α3-containing GABAA receptors42, 48. Moreover, the hippocampus, where α5-containing GABAA receptors are expressed42 activates - via a circuit involving glutamatergic neurons in the hippocampus, GABAergic neurons in the nucleus accumbens and GABAergic neurons in the ventral pallidum - tonic firing of dopaminergic neurons in the midbrain51, 52. This framework explains why deficiency of α3- or α5-containing GABAA receptors can lead to increased firing of dopaminergic neurons, highlighting these receptors as potential targets for novel antipsychotic medications. Moreover, since the GABAergic neurons in the ventral pallidum primarily express α1-containing GABAA receptors42, one would predict that a compound with activity at α1-containing GABAA receptors might increase firing of dopaminergic neurons and thus that α1 might be a subtype to be avoided when developing an antipsychotic agent. Interestingly, imidazenil, which has some selectivity for α5-containing GABAA receptors over α1-containing GABAA receptors53 and is also a partial positive allosteric modulator (partial agonist) at α3-containing GABAA receptors54, reduces the behavioral deficits in mice which model symptoms of schizophrenia without producing sedation or tolerance liability53. Interestingly, the therapeutic potential of positive allosteric modulation of α5-containing GABAA receptors has been demonstrated in rat model of schizophrenia, generated by treatment of pregnant dams on gestational day 17 with the DNA-methylating agent methylazoxymethanol acetate (MAM). In MAM-treated rats, the α5-selective partial positive allosteric modulator (partial agonist) SH-053-2′F-R-CH3, administered systemically or locally into the ventral hippocampus, reduced the number of spontaneously active dopaminergic neurons in the ventral tegmental area to levels observed in saline-tretated animals55. Moreover, SH-053-2′F-R-CH3 reduced the increased locomotor response of MAM-treated animals to amphetamine55.
Before the functions of the different GABAA receptor subtypes were known the non-selective partial positive allosteric modulator (partial agonist) and anxiolytic bretazenil, was shown to be efficacious as monotherapy in approximately 40% of patients with acute episodes of schizophrenia of moderate to marked severity56 and devoid of extrapyramidal side effects, indicating that modulators of GABAA receptors can be useful in the treatment of schizophrenia. Since sedation is the most frequent adverse event reported (in 21 out of 66 patients)56, it is conceivable that a future subtype-selective compound lacking activity at α1-containing GABAA receptors may be useful in the treatment of schizophrenia.
Recent evidence suggests that α2/α3-selective positive allosteric modulators (agonists) might have therapeutic value for the cognitive impairments of schizophrenia. Altered activation of the dorsolateral prefrontal cortex (DLPFC) has been suggested to be specific to the disease process leading to the cognitive deficits of schizophrenia. In cortex and hippocampus, hypofunction of NMDA receptors on GABAergic interneurons, which form synapses with the axon initial segments (AIS) of cortical pyramidal neurons results in decreased inhibition of glutamatergic pyramidal neurons52. In postmortem brain from patients with schizophrenia, there is an upregulation of the α2 subunit in the AIS of these pyramidal neurons57, which is thought to represent a compensatory adaptation. This provided the rationale for a small clinical trial involving 15 chronic schizophrenic patients with the α2/α3-selective partial positive allosteric modulator (partial agonist), TPA023. Some cognitive functions were improved with TPA023 treatment58. In addition, EEG recordings revealed increased frontal γ band power. γ oscillations are generated by the feedback inhibition mediated by the GABAergic interneurons and abnormalities of γ oscillations have been proposed to underlie cognitive and negative symptoms of schizophrenia52. However, a follow-up study including sixty patients, with some of the same tests, did not confirm these results59. However, it should be noted that the efficacy of TPA023 is only 11% at α2-containing GABAA receptors (and 21% at α3-containing GABAA receptors) compared to the full positive allosteric modulator (agonist) chlordiazepoxide28 and thus compounds with higher efficacy at the α2-containing GABAA receptors might elicit stronger and more consistent effects. Further studies with such compounds are required to validate the usefulness of an α2/α3-selective positive allosteric modulator for the treatment of cognitive dysfunction in schizophrenia.
Depression
Currently available antidepressant medications act on the serotonergic and/or noradrenergic systems but the response rate (defined as >50% decrease in depression severity from baseline) is only approximately 60%60. Additionally, these drugs can take weeks or months to develop their antidepressant actions. Therefore, there is an urgent medical need for novel and faster-acting antidepressants. While there is tremendous interest in developing antidepressant agents that utilize different mechanisms of action, the development of such agents has not yet been successful61.
Recently, a GABAergic hypothesis of depression was proposed which posits a central role of the GABA system in the pathophysiology of depression62. Moreover, clinical studies have revealed that the benzodiazepines alprazolam and adinazolam elicit antidepressant responses similar to widely prescribed antidepressants in patients with major depressive disorder63, 64; the receptor subtype(s) mediating these responses are however unknown. In addition, heterozygous γ2 (Gabrg2) knockout mice display an anxiety-like phenotype in tests of unconditioned anxiety65 and a depressive-like phenotype in conflict- and despair-based tests66. These mice also have elevated baseline corticosterone concentrations67, a feature of major depression in humans. Since the γ2 subunit is associated with all known α subunits (α1–α6), these studies do not indicate which GABAA receptor subtype(s) as defined by the α subunit are responsible for this depressive-like phenotype in these mice.
As mentioned previously, the α2-containing GABAA receptors have been linked to anxiolysis, and given the high co-morbidity between anxiety and depression, it is likely that α2-containing GABAA receptors might also be involved in mood regulation. Indeed, α2 knockout mice display increased anxiety/depression-like behavior in the conflict-based novelty-suppressed feeding test, and increased depression-like behavior in the despair-based forced swim test and tail suspension tests68. These results point to a physiological antidepressant-like role of α2-containing GABAA receptors, suggesting that α2-containing GABAA receptors might also be a valid target for novel non-monoamine-based antidepressant drugs.
Cognitive enhancement
Several studies in animals and humans have suggested that classical benzodiazepines can impair learning and memory69, 70, 71. This raises the question whether negative allosteric modulators (inverse agonists) at the benzodiazepine site of GABAA receptors, i.e. compounds which inhibit GABA-induced chloride influx, might have cognition-enhancing actions. Non-selective negative allosteric modulators (inverse agonists) have various unwanted effects including anxiogenesis, and proconvulsant or convulsant activity. Thus, only subtype-selective compounds avoiding such actions may be suitable as cognition enhancers. As α5-containing GABAA receptors are predominantly expressed in the hippocampus (where they make up approximately 20% of all GABAA receptors72), a region important for learning and memory, the role of this receptor subtype in cognition has been investigated using mutant mice and subtype-selective ligands. In the hippocampus, these receptors do not colocalize with the postsynaptic marker gephyrin14 (and are therefore extrasynaptic (see also Box 1)) and mediate both tonic and slow phasic inhibition73, 74, which modulate cognitive functions. In α5(H105R) partial knockout mice delay fear conditioning, a hippocampal-independent task in which a tone is immediately followed by an electric shock or coterminates with a shock, is unaltered from wild type mice. However, trace fear conditioning, a hippocampal-dependent task in which the tone and shock are separated in time (e.g., 1 sec or 30 sec), is improved14, 75. The separation in time of tone and shock usually decreases the response, but this is not the case in the α5(H105R) partial knockout mice14, 75. Mice lacking the α5 subunit showed a significantly improved performance in a water maze model of spatial learning76. Based on such results, it was hypothesized that α5-containing GABAA receptors may represent a valuable target for memory-enhancing drugs, and several pharmaceutical companies started programmes aimed at identifying α5-selective compounds. Prototypes for drugs with binding selectivity are RO493858177 (Figs. 2B and 3C) and L-665,708 (FG8094, Fig. 2B)78, although the latter compound has a very low efficacy at α5-containing receptors. RO4938581 reversed scopolamine-induced working memory impairment and increased performance in the Morris water maze77. L-655,708 also enhanced performance in the Morris water maze during acquisition and in a probe trial79. Interestingly, α5IA (Fig. 2B), a functionally-selective compound, was able to reverse memory deficits induced by alcohol consumption in a small study involving human volunteers80 without showing signs of anxiogenesis. This is in line with the hypothesis that a compound selective for α5-containing GABAA receptors might also improve cognition in a clinically impaired population (e.g., Alzheimer’s disease). However, the development of this compound was stopped due to a metabolite producing renal toxicity which precluded α5IA from being dosed to humans over prolonged periods of time81. MRK-016 (Fig. 2B), a clinical candidate and like α5IA functionally selective, acted as a cognition enhancer without convulsant or proconvulsant and anxiogenic effects in animals82. Unfortunately, development of this compound was stopped due to poor tolerability in healthy normal elderly volunteers. A further delineation of the role of α5-containing GABAA receptors in cognitive processes in humans is needed. Importantly, none of these α5-containing receptor selective drugs displays the convulsant and anxiogenic activities of non-selective GABAA receptor negative allosteric modulators (inverse agonists) FG-7142, DMCM, or β-CCM, which are not used therapeutically10, 83. A recent study using α5(H105R) partial knockout mice found that α5-containing GABAA receptors are involved in processing the memory for the location of objects84, and urges caution as it might indicate that α5 negative allosteric modulators (inverse agonists) could negatively affect some cognitive functions.
Stroke
A recent study examined a role for tonic inhibition mediated by extrasynaptic GABAA receptors in stroke85. A focal cortical stroke was induced in mouse brain and neuronal excitability in the peri-infarct zone was measured electrophysiologically. The tonic neuronal inhibition was increased which could be due to impaired GABA transporter (GAT-3/GAT-4) function. In mice treated with the α5-selective negative allosteric modulators (inverse agonist) L-655,708 which reduces tonic inhibition, or in mice lacking α5- or δ-GABAA receptor subunits, the post-stroke recovery of motor function was improved. Best outcomes were obtained when the drug was administered three days after stroke. This result may provide new pharmacological targets for recovery from stroke in humans. While it still has to be demonstrated in humans whether α5-selective negative allosteric modulators (inverse agonists) can improve post-stroke recovery, the fact that in preclinical studies the drug was effective even three days after stroke is particularly noteworthy as this may indicate that such drug treatment might work even in a delayed time frame when options for early interventions have been missed.
Conclusion and future directions
The identification of physiological and pharmacological functions of GABAA receptor subtypes defined by their α subunits has renewed the interest in the GABAA receptor system as a target for the development of drugs with less side-effects than classical benzodiazepines (e.g. non-sedating anxiolytics) and the development of drugs with indications that are distinct from those of classical benzodiazepines (e.g. analgesics, cognition-enhancing drugs). A significant number of compounds have now been developed that display GABAA receptor subtype selectivity, either by affinity or efficacy, or both (Table 1). In animal models, subtype-selective drugs do not lose their efficacy for the desired actions, and separation of desired from adverse effects can readily be achieved. The example of a compound that reached clinical studies, MRK-409 (MK-0343), but whose development had to be stopped due to sedative effects in humans demonstrates that more preclinical efforts are needed to identify compounds with improved selectivity. This may be primarily achieved with higher affinity only at the desired receptor subtype. In addition to the development of non-sedative anxiolytics and cognition-enhancing drugs, recent scientific discoveries provide hope for the development of analgesic drugs for the treatment of chronic pain.
Table 1.
Subtype selective compounds for GABAA receptors
| Compound | Receptor subtype | Binding/Functional selectivity | Indication | Development status |
|---|---|---|---|---|
| L-838,417 | Partial agonist at α2, α3, α5 | Functional | Anxiolytic | Preclinical |
| TPA023 (MK-0777) | Partial agonist at α2, α3 | Functional | Anxiolytic, Schizophrenia | Phase 2 |
| TPA023B | Partial agonist at α2, α3 | Functional | Anxiolytic, Schizophrenia | Phase 1 |
| TPA123 | Partial agonist at α1, α2, α3, α5 | Functional | Anxiolytic | On hold |
| MRK-409 (MK-0343) | Partial agonist at α2, α3 | Functional | Anxiolytic | Phase 1/Halted |
| TP003 | Agonist at α3 | Functional | Anxiolytic | On hold |
| Ocinaplon | Partial agonist at α2, α 3, α5 | Functional | Anxiolytic | On hold |
| NS11394 | Full agonist at α1 | Functional | Anxiolytic | Preclinical |
| Agonist at α5 | ||||
| Partial agonist at α3, α5 | ||||
| MRK-016 | Full inverse agonist at α5 | Functional | Cognition enhancer | Phase 1/Halted |
| α5IA | Partial inverse agonist at α5 | Functional | Cognition enhancer | Phase 1/Halted |
| RO4938581 | Full inverse agonist at α5 | 17–40-fold binding selectivity for α5 | Cognition enhancer | Preclinical |
| L-655,708 (FG8094) | Very weak inverse agonist at α5 | 30–70-fold binding selectivity for α5 | Cognition enhancer | Preclinical |
| SH-053-2′F-R-CH3 | Full agonist at α5 | 8–10-fold binding selectivity for α5 | Schizophrenia ? | Preclinical |
| Partial agonist at α1, α2, α3 | ||||
| Gaboxadol | Supra-maximal agonist at α4β3δ | > 10-fold binding selectivity for α4 | Hypnotic | Phase 3/Halted |
At a glance summary.
GABAA receptors are a family of ligand gated channels which regulate central nervous system function. GABAA receptors subtypes are formed by co-assembly from 19 different subunits (α1–6, β1–3, γ1–3, δ, ε, π, θ, ρ1-3) in a pentameric structure.
Genetic approaches and development of GABAA receptor subtype-selective ligands have led to the identification of separable key functions of GABAA receptor subtypes.
GABAA receptors subtypes containing the α1, α2, α3 or α5, but not those containing the α4 or α6 subunit are sensitive to benzodiazepines which modulate GABAA receptor function.
In addition to their anxiolytic effect, which is mediated by α2- and potentially also by α3-containing GABAA receptors, benzodiazepines possess sedative properties which are mediated via α1-containing GABAA receptors.
GABAA receptor subtype-selective compounds might be valuable for novel indications such as analgesia, depression, schizophrenia, cognitive enhancement and stroke.
The most advanced compounds are currently being evaluated in clinical studies for anxiolytic and memory enhancing effects. These compounds target α2- and α3-containing GABAA receptors (positive allosteric modulation), and α5-subunit containing GABAA receptors (negative allosteric modulation), respectively, and avoid functional effects at α1-containing GABAA receptors.
Acknowledgments
Research by UR is supported by award numbers GM086448, MH080006, MH085149, DA027051, DA026578, and MH087829 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, the National Institute of Mental Health, and the National Institute on Drug Abuse, or the National Institutes of Health.
The authors gratefully thank Drs. Theresa Ballard-Yardy, Rodolfo Gasser, Maria-Clemencia Hernandez, Andrew W. Thomas and Gerhard Trube (all Roche Basel) for discussions, ideas and support.
Glossary
- Anterograde amnesia
Loss of memory for events occurring subsequent to the administration of a drug while memories from before the administration remain intact
- Ligand-gated chloride channels
Transmembrane proteins that open their channel pore in response to the binding of an appropriate ligand. The resulting influx of chloride through the opened pore results in hyperpolarization
- Allosteric modulation
Is achieved by a drug binding at a site distinct from the site required for activation of a protein. Positive allosteric modulation, which is also referred to as agonism occurs when the binding of the drug enhances the activity of the protein. In contrast, negative allosteric modulation, also referred as inverse agonism reduces its activity
- Anti-Hyperalgesic
Compound that reduces an increased sensitivity to noxious stimuli
- Nocifensive
Defensive response to pain
Biographies
Uwe Rudolph
Uwe Rudolph attended Medical School at the Freie Universitat Berlin, where he also completed a doctoral thesis characterizing biochemical properties of G proteins. As a postdoctoral fellow at Baylor College of Medicine in Houston, he applied gene targeting to G proteins. Since then, first at the University of Zurich and now at McLean Hospital in Belmont, his focus in on dissecting the functions of GABAA receptor subtypes. He is currently Director of the Laboratory of Genetic Neuropharmacology at McLean Hospital and Lecturer in the Department of Psychiatry at Harvard Medical School.
Frédéric Knoflach
Frédéric Knoflach earned his master of science at the ETH Zurich and completed a doctoral thesis at the Institute of Pharmacology and Toxicology of the University of Zurich examining electrophysiological and pharmacological properties of recombinant GABAA receptors. He joined Roche Basel as a Postdoctoral Fellow characterizing recombinant and native metabotropic glutamate (mGlu) receptors, subsequently leading a project on mGlu 1 receptor positive allosteric modulators. He is currently in the Roche Pharma Research and Early Development (pRED) Division and is involved in the preclinical development of subtype selective ligands for GABAA receptors.
Footnotes
Declaration of competing financial interests.
Uwe Rudolph is a full-time employee of McLean Hospital and a consultant for Sunovion. Frédéric Knoflach is a full-time employee of Roche.
References
- 1.Froestl W. A historical perspective on GABAergic drugs. Future Med Chem. 2011;3:163–175. doi: 10.4155/fmc.10.285. [DOI] [PubMed] [Google Scholar]
- 2.Mohler H. The rise of a new GABA pharmacology. Neuropharmacol. 2011;60:1042–1049. doi: 10.1016/j.neuropharm.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Zeilhofer HU, Mohler H, Di Lio A. GABAergic analgesia: new insights from mutant mice and subtype-selective agonists. Trends Pharmacol Sci. 2009;30:397–402. doi: 10.1016/j.tips.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Atack JR. GABAA Receptor α2/α3 Subtype-Selective Modulators as Potential Nonsedating Anxiolytics. Curr Top Behav Neurosci. 2010;2:331–360. doi: 10.1007/7854_2009_30. This review summarizes evidence that α2/α3-selective compounds have anxiolytic but not sedative effects in humans, thus indicating that the pharmacological profile of subtype-selective compounds targeting α2- and α3-containing GABAA receptors is different from that of classical benzodiazepines, and that the preclinical identification of α2- and potentially α3-containing GABAA receptors as mediators of anxiolysis and of α1-containing GABAA receptors as mediators of sedation can be successfully translated into novel therapeutic approaches. [DOI] [PubMed] [Google Scholar]
- 5.Mirza NR, Munro G. The role of GABAA receptor subtypes as analgesic targets. Drug News Perspect. 2010;23:351–360. doi: 10.1358/dnp.2010.23.6.1489909. [DOI] [PubMed] [Google Scholar]
- 6.Vinkers CH, Mirza NR, Olivier B, Kahn RS. The inhibitory GABA system as a therapeutic target for cognitive symptoms in schizophrenia: investigational agents in the pipeline. Exp Opin Invest Drugs. 2010;19:1217–1233. doi: 10.1517/13543784.2010.513382. [DOI] [PubMed] [Google Scholar]
- 7.Tan KR, Rudolph U, Luscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;34:188–197. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoch P, et al. Co-localization of GABA receptors and benzodiazepine receptors in the brain shown by monoclonal antibodies. Nature. 1985;314:168–171. doi: 10.1038/314168a0. [DOI] [PubMed] [Google Scholar]
- 9.Mohler H, Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977;198:849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- 10.Braestrup C, Schmiechen R, Neef G, Nielsen M, Petersen EN. Interaction of convulsive ligands with benzodiazepine receptors. Science. 1982;216:1241–1243. doi: 10.1126/science.6281892. [DOI] [PubMed] [Google Scholar]
- 11.Rudolph U, et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 12.McKernan RM, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 13.Low K, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 14.Crestani F, et al. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crestani F, et al. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- 16.van Rijnsoever C, et al. Requirement of α5-GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J Neurosci. 2004;24:6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan KR, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. This study demonstrates that the addictive properties of benzodiazepines are critically dependent on α1-containing GABAA receptors on GABAergic neurons in the VTA. By potentiating these receptors, benzodiazepines disinhibit firing of dopamine neurons and trigger drug-evoked synaptic plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen RW, Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacol. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulrich D, Bettler B. GABAB receptors: synaptic functions and mechanisms of diversity. Curr Opin Neurobiol. 2007;17:298–303. doi: 10.1016/j.conb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Pritchett DB, Seeburg PH. γ-aminobutyric acidA receptor α5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 21.Crestani F, Martin JR, Mohler H, Rudolph U. Mechanism of action of the hypnotic zolpidem in vivo. Br J Pharmacol. 2000;131:1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: role of the α1 GABAA receptor subtype. Proc Natl Acad Sci USA. 2001;98:6464–6469. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandra D, et al. GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias R, et al. Evidence for a significant role of α3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer BD, et al. Contribution of GABAA receptors containing α3 subunits to the therapeutic-related and side effects of benzodiazepine-type drugs in monkeys. Psychopharmacol. 2010;215:311–319. doi: 10.1007/s00213-010-2142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci USA. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott-Stevens P, Atack JR, Sohal B, Worboys P. Rodent pharmacokinetics and receptor occupancy of the GABAA receptor subtype selective benzodiazepine site ligand L-838417. Biopharm & Drug Disp. 2005;26:13–20. doi: 10.1002/bdd.423. [DOI] [PubMed] [Google Scholar]
- 28.Atack JR, et al. TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluor ophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for α2- and α3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- 29.Facklam M, et al. Relationship between benzodiazepine receptor occupancy and functional effects in vivo of four ligands of differing intrinsic efficacies. J Pharmacol Exp Ther. 1992;261:1113–1121. [PubMed] [Google Scholar]
- 30.Lippa A, et al. Selective anxiolysis produced by ocinaplon, a GABAA receptor modulator. Proc Natl Acad Sci USA. 2005;102:7380–7385. doi: 10.1073/pnas.0502579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czobor P, Skolnick P, Beer B, Lippa A. A multicenter, placebo-controlled, double-blind, randomized study of efficacy and safety of ocinaplon (DOV 273,547) in generalized anxiety disorder. CNS Neurosci & Ther. 2010;16:63–75. doi: 10.1111/j.1755-5949.2009.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atack J, et al. MRK-409 (MK-0343), a GABAA receptor subtype-selective partial agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in humans. J Psychopharmacol. 2011;25:314–328. doi: 10.1177/0269881109354927. [DOI] [PubMed] [Google Scholar]
- 33.Atack J, et al. Preclinical and clinical pharmacology of TPA023B, a GABAA receptor α2/α3 subtype-selective partial agonist. J Psychopharmacol. 2011:329–344. doi: 10.1177/0269881109354928. [DOI] [PubMed] [Google Scholar]
- 34.Petitjean S, Ladewig D, Meier CR, Amrein R, Wiesbeck GA. Benzodiazepine prescribing to the Swiss adult population: results from a national survey of community pharmacies. Int Clin Psychopharmacol. 2007;22:292–298. doi: 10.1097/YIC.0b013e328105e0f2. [DOI] [PubMed] [Google Scholar]
- 35.Kan CC, Breteler MH, van der Ven AH, Timmermans MA, Zitman FG. Assessment of benzodiazepine dependence in alcohol and drug dependent outpatients: a research report. Subst Use Misuse. 2001;36:1085–1109. doi: 10.1081/ja-100104491. [DOI] [PubMed] [Google Scholar]
- 36.Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heikkinen AE, Moykkynen TP, Korpi ER. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacol. 2009;34:290–298. doi: 10.1038/npp.2008.89. This study shows that the benzodiazepine site agonists diazepam and zolpidem induce an increase in the AMPA/NMDA ratio in VTA dopamine neurons, i.e. they modulate the glutamatergic transmission of VTA dopamine neurons. [DOI] [PubMed] [Google Scholar]
- 38.Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at α1 and α2/3 subtypes. J Pharmacol Exp Ther. 2010;332:4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enna SJ, McCarson KE. The role of GABA in the mediation and perception of pain. Adv Pharmacol. 2006;54:1–27. doi: 10.1016/s1054-3589(06)54001-3. [DOI] [PubMed] [Google Scholar]
- 40.Harris JA, Westbrook RF. Effects of benzodiazepine microinjection into the amygdala or periaqueductal gray on the expression of conditioned fear and hypoalgesia in rats. Behav Neurosci. 1995;109:295–304. doi: 10.1037//0735-7044.109.2.295. [DOI] [PubMed] [Google Scholar]
- 41.Knabl J, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. This study demonstrates that pronounced analgesia can be achieved in rodent models of neuropathic pain and of inflammatory pain by specifically targeting spinal α2- and α3-containing GABAA receptors. The α2-, α3-, and α5-selective compound L-838,417 was highly effective and devoid of unwanted sedation, motor impariment, and tolerance development. In a fMRI study, L-838,417 reduced the activity of brain areas related to the associative-emitonal components of brain. [DOI] [PubMed] [Google Scholar]
- 42.Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 43.Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: why it is important to teach pain to psychiatrists. Arch Gen Psych. 2011;68:12–20. doi: 10.1001/archgenpsychiatry.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munro G, et al. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2008;327:969–981. doi: 10.1124/jpet.108.144568. This study reports that the compound NS11394 with an efficacy profile of α5>α3>α2>α1 has analgesic actions in rodent models of neuropathic and inflammatory pain at doses that are 20- to 40-fold lower than those inducing minor sedation or ataxia. [DOI] [PubMed] [Google Scholar]
- 45.Mirza NR, et al. NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitr ile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008;327:954–968. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- 46.Di Lio A, et al. HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacol. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munro G, Erichsen HE, Rae MG, Mirza NR. A question of balance - Positive versus negative allosteric modulation of GABAA recetpor subtypes as a driver of analgesic efficacy in rat models of inflammatory and neuropathic pain. Neuropharmacol. 2011;61:121–132. doi: 10.1016/j.neuropharm.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Yee BK, et al. A schizophrenia-related sensorimotor deficit links α3-containing GABAA receptors to a dopamine hyperfunction. Proc Natl Acad Sci USA. 2005;102:17154–17159. doi: 10.1073/pnas.0508752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hauser J, et al. Hippocampal α5 subunit-containing GABAA receptors modulate the expression of prepulse inhibition. Mol Psych. 2005;10:201–207. doi: 10.1038/sj.mp.4001554. [DOI] [PubMed] [Google Scholar]
- 50.Lubow RE. Construct validity of the animal latent inhibition model of selective attention deficits in schizophrenia. Schizo Bull. 2005;31:139–153. doi: 10.1093/schbul/sbi005. [DOI] [PubMed] [Google Scholar]
- 51.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Lisman JE, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guidotti A, et al. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacol. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- 54.Costa E, Guidotti A. Benzodiazepines on trial: a research strategy for their rehabilitation. Trends Pharmacol Sci. 1996;17:192–200. doi: 10.1016/0165-6147(96)10015-8. [DOI] [PubMed] [Google Scholar]
- 55.Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5 GABAAR-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacol. 2011 May 11; doi: 10.1038/npp.2011.76. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delini-Stula A, Berdah-Tordjman D. Antipsychotic effects of bretazenil, a partial benzodiazepine agonist in acute schizophrenia--a study group report. J Psychiatr Res. 1996;30:239–250. doi: 10.1016/0022-3956(96)00003-9. [DOI] [PubMed] [Google Scholar]
- 57.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. Excellent review on the the abnormalities in GABA neurons contributing to the working memory disturbances in the context of schizophrenia. [DOI] [PubMed] [Google Scholar]
- 58.Lewis DA, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buchanan R, et al. A Randomized Clinical Trial of MK-0777 for the Treatment of Cognitive Impairments in People with Schizophrenia. Biol Psychiatry. 2011;69:442–449. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. doi: 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 62.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2010;16:383–406. doi: 10.1038/mp.2010.120. A comprehensive review summarizing preclinical and clinical evidence supporting a central and causal role of GABAergic deficits in the etiology of depressive disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amsterdam JD, Hornig-Rohan M, Maislin G. Efficacy of alprazolam in reducing fluoxetine-induced jitteriness in patients with major depression. J Clin Psychiatry. 1994;55:394–400. [PubMed] [Google Scholar]
- 64.Petty F, Trivedi MH, Fulton M, Rush AJ. Benzodiazepines as antidepressants: does GABA play a role in depression? Biol Psychiatry. 1995;38:578–591. doi: 10.1016/0006-3223(95)00049-7. [DOI] [PubMed] [Google Scholar]
- 65.Crestani F, et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 66.Earnheart JC, et al. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen Q, et al. γ-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry. 2010;68:512–520. doi: 10.1016/j.biopsych.2010.04.024. This study shows that developmental deficits in GABAergic inhibition in the forebrain cause behavioral and endocrine abnormalities and selective antidepressant drug responsiveness to desipramine (vs. fluoxetine) reminiscent of melancholic depression in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vollenweider I, Smith KS, Keist R, Rudolph U. Antidepressant-like properties of α2-containing GABAA receptors. Behav Brain Res. 2011;217:77–80. doi: 10.1016/j.bbr.2010.10.009. This study shows that GABAergic inhibition acting via α2-containing GABAA receptors has an antidepressant-like effect in vivo, suggesting that these receptors represent a specific molecular substrate that can regulate depressive-like states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8:45–58. doi: 10.2174/1381612023396654. [DOI] [PubMed] [Google Scholar]
- 70.Arolfo MP, Brioni JD. Diazepam impairs place learning in the Morris water maze. Behav Neural Biol. 1991;55:131–136. doi: 10.1016/0163-1047(91)80133-y. [DOI] [PubMed] [Google Scholar]
- 71.Seabrook GR, Easter A, Dawson GR, Bowery BJ. Modulation of long-term potentiation in CA1 region of mouse hippocampal brain slices by GABAA receptor benzodiazepine site ligands. Neuropharmacol. 1997;36:823–830. doi: 10.1016/s0028-3908(97)00040-3. Nice demonstration of the role of GABAA receptors in modulating synaptic plasticity. [DOI] [PubMed] [Google Scholar]
- 72.Fritschy JM, Benke D, Johnson DK, Mohler H, Rudolph U. GABAA-receptor α-subunit is an essential prerequisite for receptor formation in vivo. Neurosci. 1997;81:1043–1053. doi: 10.1016/s0306-4522(97)00244-3. [DOI] [PubMed] [Google Scholar]
- 73.Prenosil GA, et al. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- 74.Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor α5 subunits contribute to GABAA, slow synaptic inhibition in mouse hippocampus. J Neurophysiol. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yee BK, et al. GABA receptors containing the α5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- 76.Collinson N, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ballard TM, et al. RO4938581, a novel cognitive enhancer acting at GABAA α5 subunit-containing receptors. Psychopharmacol. 2009;202:207–223. doi: 10.1007/s00213-008-1357-7. This study shows that RO4938581, an α5-selective inverse agonist, has cogonition-enhancing effects in rodents and monkeys. In rats, enhancement of cogncition was observed with approximately 30% receptor occupancy. [DOI] [PubMed] [Google Scholar]
- 78.Casula MA, et al. Identification of amino acid residues responsible for the α5 subunit binding selectivity of L-655,708, a benzodiazepine binding site ligand at the GABAA receptor. J Neurochem. 2001;77:445–451. doi: 10.1046/j.1471-4159.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 79.Atack JR, et al. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacol. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 80.Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR. Blockade of alcohol’s amnestic activity in humans by an α5 subtype benzodiazepine receptor inverse agonist. Neuropharmacol. 2007;53:810–820. doi: 10.1016/j.neuropharm.2007.08.008. The compound α5IA almost completely blocks alcohol-induced memory impairment. [DOI] [PubMed] [Google Scholar]
- 81.Atack J. Preclinical and clinical pharmacology of the GABAA receptor α5 subtype-selective inverse agonist α5IA. Pharmacol & Therap. 2010;125:11–26. doi: 10.1016/j.pharmthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 82.Atack JR, et al. In vitro and in vivo properties of 3-tert-butyl-7-(5-methylisoxazol-3-yl)-2-(1-methyl-1H-1,2,4-triazol-5-ylme thoxy)-pyrazolo[1,5-d]-[1,2,4]triazine (MRK-016), a GABAA receptor α5 subtype-selective inverse agonist. J Pharmacol Exp Ther. 2009;331:470–484. doi: 10.1124/jpet.109.157636. [DOI] [PubMed] [Google Scholar]
- 83.Dorow R, Horowski R, Paschelke G, Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983;2:98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- 84.Prut L, et al. A reduction in hippocampal GABAA receptor α5 subunits disrupts the memory for location of objects in mice. Genes Brain Behav. 2010;9:478–488. doi: 10.1111/j.1601-183X.2010.00575.x. [DOI] [PubMed] [Google Scholar]
- 85.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. This study demonstrates that GABA-mediated tonic inhibition in the peri-infart zone constrains plasticity, and that diminishing tonic inhibition promotes functional recovery after stroke. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bormann J. The ‘ABC’ of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 88.Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 89.Benke D, et al. Analysis of the presence and abundance of GABAA receptors containing two different types of alpha subunits in murine brain using point-mutated alpha subunits. J Biol Chem. 2004;279:43654–43660. doi: 10.1074/jbc.M407154200. [DOI] [PubMed] [Google Scholar]
- 90.Minier F, Sigel E. Positioning of the alpha-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA. 2004;101:7769–7774. doi: 10.1073/pnas.0400220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 92.Jurd R, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 93.Liao M, et al. β3-containing γ-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesthesia & Analgesia. 2005;101:412–418. doi: 10.1213/01.ANE.0000154196.86587.35. [DOI] [PubMed] [Google Scholar]
- 94.Lambert S, Arras M, Vogt KE, Rudolph U. Isoflurane-induced surgical tolerance mediated only in part by β3-containing GABAA receptors. Eur J Pharmacol. 2005;516:23–27. doi: 10.1016/j.ejphar.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 95.Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol. 2010;588:1861–1869. doi: 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- 97.Edenberg HJ, et al. Variations in GABRA2, encoding the α2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agrawal A, et al. Association of GABRA2 with drug dependence in the collaborative study of the genetics of alcoholism sample. Behav Genet. 2006;36:640–650. doi: 10.1007/s10519-006-9069-4. [DOI] [PubMed] [Google Scholar]
- 99.Dick DM, et al. Association between GABRA1 and drinking behaviors in the collaborative study on the genetics of alcoholism sample. Alcohol Clin Exp Res. 2006;30:1101–1110. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 100.Drgon T, D’Addario C, Uhl GR. Linkage disequilibrium, haplotype and association studies of a chromosome 4 GABA receptor gene cluster: candidate gene variants for addictions. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:854–860. doi: 10.1002/ajmg.b.30349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Porjesz B, et al. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biol Psychol. 2002;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 102.Craddock N, et al. Strong genetic evidence for a selective influence of GABAA receptors on a component of the bipolar disorder phenotype. Mol Psychiatry. 2010;15:146–153. doi: 10.1038/mp.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Petryshen TL, et al. Genetic investigation of chromosome 5q GABAA receptor subunit genes in schizophrenia. Mol Psychiatry. 2005;10:1074–1088. 1057. doi: 10.1038/sj.mp.4001739. [DOI] [PubMed] [Google Scholar]
- 104.Squires RF, Brastrup C. Benzodiazepine receptors in rat brain. Nature. 1977;266:732–734. doi: 10.1038/266732a0. [DOI] [PubMed] [Google Scholar]
- 105.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neurosci. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 106.Fagiolini M, et al. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 107.Sohal VS, Keist R, Rudolph U, Huguenard JR. Dynamic GABAA receptor subtype-specific modulation of the synchrony and duration of thalamic oscillations. J Neurosci. 2003;23:3649–3657. doi: 10.1523/JNEUROSCI.23-09-03649.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]