Abstract
We retrospectively investigated the ability of adalimumab (ADA) to reduce disease activity, improve physical function, and retard the progression of structural damage in 167 patients with rheumatoid arthritis. Clinical and functional outcomes were compared between patients with or without prior biologic treatment and those with or without concomitant methotrexate (MTX) treatment. At week 52, 38.3% achieved clinical remission: 42.4 and 28.6% of patients achieved remission in those without and with previous biologics, respectively, while 42.7 and 12.5% of patients achieved remission in those with and without concomitant MTX, respectively. ADA treatment significantly reduced the rate of radiographic progression from 27.1 ± 46.0 (median 13.6; 25th–75th percentiles 8.3 to 28.9) at baseline to 0.8 ± 5.0 (median 0.0; 25th–75th percentiles −0.9 to 2.0) at week 52 (P < 0.0001). Radiographic progression was absent in 59.8% of patients. Sixty adverse events (34.21/100 patient-years) were reported, 16 of which were serious (9.12/100 patient-years). ADA therapy is highly effective for reducing disease activity, improving physical function, and limiting radiographic progression. It is generally safe and well tolerated by Japanese RA patients in routine clinical practice.
Keywords: Adalimumab, Japanese, Retrospective study, Radiographic outcome, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is characterized by progressive inflammatory synovitis and subsequent articular matrix degradation, which may result in joint destruction [1]. Disability and premature death result if the aggressive form of the disease goes untreated [2]. Over the last decade, management of RA has evolved radically because of the development of aggressive therapies for early stages of the disease and the advent of molecular targeted therapies [3, 4]. Although the pathophysiology of RA is not completely understood, tumor necrosis factor (TNF) plays a critical role in mediating the inflammatory synovitis, articular matrix degradation, and bony erosions in RA. Hence, TNF is recognized to be an important molecular target for directed biologic intervention [5].
Adalimumab (ADA) is a fully human immunoglobulin G1 (IgG1) monoclonal antibody with a high specificity for TNF-α [6]. ADA’s efficacy and safety are well established both with and without concomitant methotrexate (MTX) treatment, based on randomized controlled clinical trials with RA patients conducted in Western countries [7–11]. In Japan, ADA was approved in 2008, making it the third TNF blocker to earn approval. Infliximab (a chimeric monoclonal antibody to TNFα) [12] and etanercept (a recombinant human TNF receptor-Fc fusion protein) [13] were the first two TNF blockers to be approved. Recently, these biological agents have been reported to be effective and safe for Japanese RA patients encountered during routine clinical practice [14–17]. For ADA, the CHANGE study served as the bridging study for extrapolating data obtained for patients of Western origin to Japanese patients, in whom only the effects of monotherapy had previously been investigated [18]. However, the overseas clinical data obtained so far suggest that ADA monotherapy has only limited effectiveness compared to combination therapies with DMARDs, and in particular MTX.
Therefore, it is of clinical importance to further investigate the effects of ADA, particularly when it is administered concomitantly with MTX to Japanese RA patients. This study aimed to retrospectively investigate the clinical, functional, and radiographic responses to ADA as well as safety in Japanese RA patients encountered in routine clinical practice. This is the first study to evaluate the radiographic response to ADA in Japanese RA patients.
Patients and methods
Patients
Patients with available baseline components for the 28-joint Disease Activity Score based on erythrocyte sedimentation rate (DAS28-ESR) who started treatment with ADA between July 15, 2008 and June 15, 2009 at the following 4 medical institutions were enrolled in this study: (1) the Division of Rheumatology and Clinical Immunology, Department of Internal Medicine, Saitama Medical Center, Saitama Medical University, Saitama; (2) the Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Keio University, Tokyo; (3) the First Department of Internal Medicine of the School of Medicine, University of Occupational and Environmental Health, Kitakyushu; and (4) the Institute of Rheumatology, Tokyo Women’s Medical University, Tokyo. All of the patients satisfied the classification criteria of the American College of Rheumatology [19]. Information on patient characteristics was obtained from medical records and pooled for retrospective analyses; the demographic data included age, gender, disease duration, concomitant medications, co-morbidity, and other variables. For subanalyses, patients were divided into subsets based on whether they had or had not received the following: (1) previous biologic treatment; (2) concomitant MTX treatment at baseline.
This study was a retrospective observational study using anonymized information, and it conformed to the standard anti-TNF treatment guideline proposed by the Japan College of Rheumatology (JCR). Written consent was obtained from the patients according to the Declaration of Helsinki.
ADA treatment
ADA treatment was started in accordance with the Japan College of Rheumatology guidelines for adalimumab therapy [20]. We administered 40 mg ADA every other week, in keeping with the dosage instructions on the Japanese drug label. Concomitant use of MTX, disease-modifying antirheumatic drugs (DMARDs) other than MTX, and/or oral steroids was at the discretion of the attending physician. Dose adjustment was carried out according to standard medical practice for controlling disease activity.
Clinical efficacy
Disease activity was assessed using the DAS28-ESR [21]. Functional disability was assessed using the disability index of the Health Assessment Questionnaire (HAQ-DI) [22]. Radiographs of the hands/wrists and feet at baseline and at week 52 were available for 71 patients. The images were scored using van der Heijde’s modified Sharp method [23] independently by 2 readers.
Safety
Safety was assessed based on the adverse events reported by patients as well as on the findings of physical examinations and standard clinical laboratory tests recorded from the start of July 15, 2008 through to the data cut-off date of June 15, 2010. All adverse events were summarized according to the Medical Dictionary for Regulatory Activities system organ class (MedDRA SOC) and reported as events per 100 patient-years. Adverse events judged to be serious by the attending physicians were individually listed.
Retention rate
Kaplan–Meier analysis was used to estimate retention rates during the first 52 weeks; 2 patients were excluded because their exact discontinuation dates were unknown. Reasons for discontinuation were categorized for all patients who withdrew at any time, even after 52 weeks.
Statistical analysis
Patient baseline characteristics were summarized using mean (standard deviation), median (interquartile range), or n (%), as appropriate, for the entire patient population and for patient subgroups stratified by previous use of biological agents (previous biologics + or −) and concomitant use of MTX (concomitant MTX + or −). Demographic and baseline characteristics were analyzed using the Mann–Whitney U test for continuous variables and Pearson’s chi-square test for discrete variables for the previous biologics (+) versus (−) and the concomitant MTX (+) versus (−) groups. For patients who withdrew before week 52, the last observation carried forward (LOCF) method, including baseline values, was employed to evaluate all efficacy parameters other than the radiographic endpoint. Missing radiographic values at week 52 were determined by linear extrapolation using data at baseline and at the last observation point (where available) if the patients had received ADA treatment for at least 180 days. Patients who withdrew before the 180th day of treatment were not considered in the calculation. The Wilcoxon signed rank test was used to detect statistically significant differences in disease activity and functional outcomes between baseline and week 52. The impact of previous biologic treatment or concomitant MTX treatment on the patient’s response to ADA was examined using Pearson’s chi-square test. Kaplan–Meier analysis was used to estimate retention rates during the first 52 weeks, and the difference in retention curves was examined by means of a log-rank test. All reported P values are two-sided and not adjusted for multiple testing. P values <0.05 were considered significant. Data were analyzed with StatView for Windows Version 5.0 (SAS Institute Inc., Cary, NC, USA).
Endpoints
Co-primary endpoints were the percentages of patients achieving remission, as defined by a DAS28-ESR of <2.6 at week 52, and of patients with no radiographic progression, as defined by a change in the total Sharp score (TSS) ≤0.5 from baseline to week 52. Other endpoints include the proportion of patients achieving functional remission (HAQ score ≤0.5) and safety.
Results
Baseline characteristics of the patients
A total of 167 patients for whom ADA therapy was initiated between June 2008 and June 2009 at the 4 medical institutions had all of the DAS28-ESR components at baseline. Baseline demographic and disease characteristics are summarized in Table 1. The mean age of all 167 patients included in this study was 58.4 years, and the majority of the subjects were women (82.6%). The mean duration of disease was 9.0 ± 9.5 years. The baseline mean DAS28-ESR and HAQ scores were 5.3 ± 1.3 (n = 167) and 1.24 ± 0.78 (n = 149), respectively. The initial mean TSS was 89.7 ± 83.1 (median 65.5; 25th–75th percentiles 36.0–115.0) (n = 87), and yearly progression before the initiation of ADA therapy was estimated to be 27.1 ± 46.0 (median 13.6; 25th–75th percentiles 8.3–28.9) (n = 87). Among the 167 patients, 118 (70.7%) were naïve to biologic treatment, whereas 49 (29.3%) had been treated with biologics prior to ADA. In total, 143 (85.6%) received concomitant MTX and 69 (41.3%) received concomitant oral steroid, with mean doses of 8.5 ± 2.9 mg/week and 4.8 ± 2.7 mg/day (prednisolone equivalents), respectively, at the beginning of ADA treatment. A comparison of the baseline demographics for different patient subgroups is provided in Table 1. When compared within subsets, patients who had received previous biologic therapy (+) were younger (P < 0.05) and had a more severe disease by stage (P < 0.05), a longer duration of disease (P < 0.05), and a higher rate and dose of concomitant prednisolone (P < 0.05 for both) than patients who had not received previous biologic therapy (−). The duration of disease was longer in the concomitant MTX (−) group than in the concomitant MTX (+) group (P < 0.05). Moreover, a higher proportion of the patients received concomitant prednisolone in the concomitant MTX (−) group than in the concomitant MTX (+) group (P < 0.05). The baseline yearly radiographic progression was greater in the previous biologics (−) group (28.9 ± 50.2) (median 13.2; 25th–75th percentiles 7.9–31.0) than in the previous biologics (+) group (18.3 ± 10.7) (median 14.0; 25th–75th percentiles 11.2–26.5), while it was greater in the concomitant MTX (+) group (28.7 ± 48.0) (median 14.0; 25th–75th percentiles 8.5–30.9) than in the concomitant MTX (−) group (11.1 ± 7.1) (median 10.2; 25th–75th percentiles 7.1–14.4). There were no differences in other baseline demographic and disease characteristics between the previous biologics (+) and (−) groups and between the concomitant MTX (+) and (−) groups.
Table 1.
Baseline characteristics of patients
| Variables | Total (n = 167) |
Previous biologics | Concomitant MTX | ||||
|---|---|---|---|---|---|---|---|
| (+) (n = 49) |
(−) (n = 118) |
P value | (+) (n = 143) |
(−) (n = 24) |
P value | ||
| Age (years) | 58.4 ± 13.0 | 55.1 ± 11.5 | 59.7 ± 13.4 | <0.05 | 58.2 ± 12.9 | 59.1 ± 14.1 | 0.5560 |
| Gender, n (% female) | 138 (82.6) | 43 (87.8) | 95 (80.5) | 0.2603 | 118 (82.5) | 20 (83.3) | 0.9222 |
| Disease duration (years) | 9.0 ± 9.5 | 9.9 ± 8.1 | 8.7 ± 10.0 | <0.05 | 8.6 ± 9.5 | 11.8 ± 8.9 | <0.05 |
| Stage (I/II/III/IV %) | (15.0/33.5/18.6/32.9) | (10.2/24.5/16.3/49.0) | (16.9/37.3/19.5/26.3) | <0.05 | (16.1/34.3/18.9/30.8) | (8.3/29.2/16.7/45.8) | 0.4836 |
| Class (I/II/III/IV %) | (11.4/74.3/14.4/0.0) | (12.2/69.4/18.4/0.0) | (11.0/76.3/12.7/0.0) | 0.5953 | (11.2/72.7/16.1/0.0) | (12.5/83.3/4.2/0.0) | 0.3052 |
| Prior use of biologics, n (%) | 49 (29.3) | 49 (100.0) | 0 (0.0) | – | 39 (27.3) | 10 (41.7) | 0.1518 |
| RF positive, n (%) | 158 (94.6) | 46 (93.9) | 112 (94.9) | 0.7868 | 136 (95.1) | 22 (91.7) | 0.4900 |
| MTX use, n (%) | 143 (85.6) | 39 (79.6) | 104 (88.1) | 0.1518 | 143 (100.0) | 0 (0.0) | – |
| MTX dose (mg/week) | 8.5 ± 2.9 | 9.9 ± 8.1 | 8.1 ± 3.0 | 0.2153 | 8.5 ± 2.9 | 0.0 ± 0.0 | – |
| Oral steroid use, n (%) | 69 (41.3) | 26 (53.1) | 43 (36.4) | <0.05 | 54 (37.8) | 15 (62.5) | <0.05 |
| Oral steroid dose (mg/daya) | 4.8 ± 2.7 | 5.7 ± 2.6 | 4.2 ± 2.6 | <0.05 | 4.7 ± 2.6 | 4.9 ± 3.1 | 0.9590 |
| MMP-3 (ng/mLb) | 297.6 ± 344.3 | 292.4 ± 250.7 | 299.8 ± 377.5 | 0.2757 | 312.3 ± 366.1 | 208.1 ± 127.9 | 0.7895 |
| SJC, 0-28 | 6.5 ± 5.6 | 6.2 ± 6.2 | 6.6 ± 5.4 | 0.2307 | 6.3 ± 4.9 | 7.6 ± 8.8 | 0.6004 |
| TJC, 0-28 | 7.3 ± 6.9 | 6.7 ± 6.8 | 7.6 ± 6.9 | 0.3585 | 7.4 ± 6.5 | 7.2 ± 9.1 | 0.1809 |
| ESR (mm/h) | 54.0 ± 31.3 | 54.4 ± 28.8 | 53.8 ± 32.4 | 0.7544 | 54.0 ± 31.4 | 53.6 ± 31.2 | 0.9582 |
| CRP (mg/dL) | 2.8 ± 3.9 | 2.9 ± 3.4 | 2.8 ± 4.1 | 0.4068 | 2.9 ± 4.1 | 2.3 ± 2.5 | 0.7391 |
| GH, VAS 0–100 mm | 50.7 ± 25.1 | 56.2 ± 24.5 | 48.4 ± 25.1 | 0.0932 | 49.6 ± 25.1 | 57.3 ± 25.1 | 0.1192 |
| DAS28-ESR | 5.3 ± 1.3 | 5.3 ± 1.2 | 5.3 ± 1.3 | 0.8398 | 5.3 ± 1.3 | 5.2 ± 1.5 | 0.6598 |
| HAQ-DIc | 1.24 ± 0.78 | 1.24 ± 0.85 | 1.25 ± 0.76 | 0.8833 | 1.24 ± 0.78 | 1.27 ± 0.84 | 0.8360 |
| TSSd | 89.7 ± 83.1 | 98.8 ± 66.0 | 87.9 ± 86.6 | 0.2757 | 88.9 ± 80.5 | 98.3 ± 112.5 | 0.6648 |
| Median (IQR) | 65.5 (36.0–115.0) | 73.5 (52.5–141.5) | 65.3 (32.6–109.6) | 66.5 (39.8–113.3) | 44.3 (22.0–153.5) | ||
| Estimated YP (∆TSS)d | 27.1 ± 46.0 | 18.3 ± 10.7 | 28.9 ± 50.2 | 0.2795 | 28.7 ± 48.0 | 11.1 ± 7.1 | 0.1542 |
| Median (IQR) | 13.6 (8.3–28.9) | 14.0 (11.2–26.5) | 13.2 (7.9–31.0) | 14.0 (8.5–30.9) | 10.2 (7.1–14.4) | ||
Mean ± SD unless otherwise indicated
Demographic and baseline characteristics were analyzed by the Mann–Whitney U test for continuous variables and Pearson’s chi-square test for discrete variables for previous biologics (+) versus (−) and concomitant MTX (+) versus (−)
RF rheumatoid factor, MTX, methotrexate, MMP-3 matrix metalloproteinase 3, SJC swollen joint count, TJC tender joint count, ESR erythrocyte sedimentation rate, CRP C-reactive protein, GH patient’s global assessment of disease activity, VAS visual analogue scale, DAS disease activity score, HAQ-DI health assessment questionnaire disability index, TSS total Sharp score, YP yearly progression, IQR interquartile range
aPrednisolone equivalents
bTotal n = 163; previous biologics (+) n = 48; previous biologics (−) n = 115; concomitant MTX (+) n = 140; concomitant MTX (−) n = 23
cTotal n = 149; previous biologics (+) n = 41; previous biologics (−) n = 108; concomitant MTX (+) n = 131; concomitant MTX (−) n = 18
dTotal n = 87; previous biologics (+) n = 15; previous biologics (−) n = 72; concomitant MTX (+) n = 79; concomitant MTX (−) n = 8
Clinical efficacy of ADA
DAS28-ESR
Overall, the mean DAS28-ESR score decreased from 5.3 ± 1.3 at baseline to 3.5 ± 1.5 at week 52 (P < 0.0001 vs. baseline) (Fig. 1). In the previous biologics (+) and (−) groups, the mean DAS28-ESR scores decreased from 5.3 ± 1.2 to 4.0 ± 1.7 and from 5.3 ± 1.3 to 3.3 ± 1.4, respectively. Although the decreases were statistically significant in both previous biologics (+) and (−) groups, it was more substantial in the previous biologics (−) group (P < 0.0001 vs. baseline) than the previous biologics (+) group (P < 0.05 vs. baseline). Similarly, in the concomitant MTX (+) and (−) groups, the DAS28-ESR scores decreased from 5.3 ± 1.3 to 3.3 ± 1.4 (P < 0.0001 vs. baseline) and from 5.2 ± 1.5 to 4.6 ± 1.5 (P < 0.05 vs. baseline), respectively. In all groups, rapid improvement was achieved during the first 4 weeks of ADA treatment.
Fig. 1.
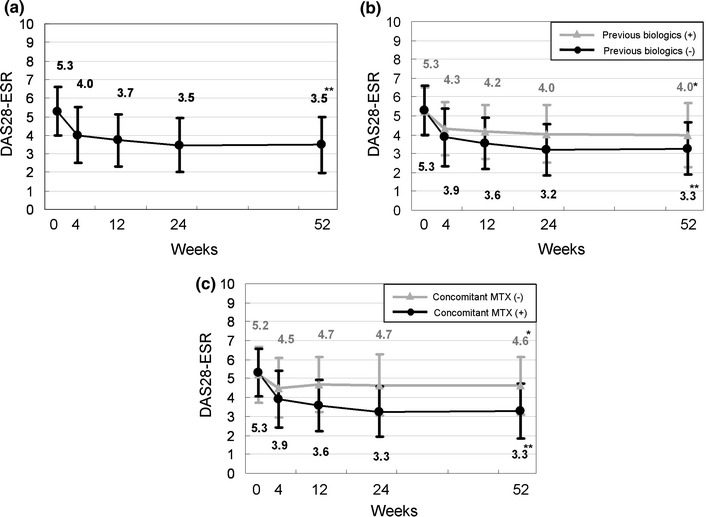
Time course of the disease activity score over 52 weeks following the initiation of adalimumab treatment. Data were analyzed by the last observation carried forward (LOCF) method. Points and bars represent means and standard deviations, respectively. a All patients (n = 167), b previous biologics (+) (n = 49) and (−) (n = 118), c concomitant MTX (+) (n = 143) and (−) (n = 24). *P < 0.05 and **P < 0.0001 versus baseline by the Wilcoxon signed rank test
Figure 2 shows the percentages of patients who achieved different disease statuses (high, DAS28 > 5.1; moderate, 3.2 ≤ DAS28 ≤ 5.1; low, 2.6 ≤ DAS28 < 3.2; and remission, DAS28 < 2.6) over the time course of treatment. The percentages of patients who achieved clinical remission using the criterion of DAS28 < 2.6 were 31.7% at week 24 and 38.3% at week 52. At week 52, 28.6 and 42.4% of patients in the previous biologics (+) and (−) groups, respectively, achieved remission. The difference in the remission rate was more pronounced between the concomitant MTX (+) and (−) groups (P < 0.01) than between the previous biologics (+) and (−) groups (P = 0.0948) at week 52. In the concomitant MTX (+) group, the proportion of patients who achieved remission increased over time and reached 42.7% at week 52, while in the concomitant MTX (−) group, the baseline value shifted steadily around 12.5% after 4 weeks.
Fig. 2.
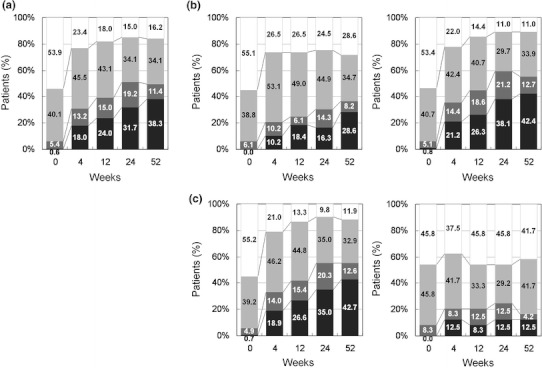
Time course of disease activity over 52 weeks following initiation of adalimumab treatment. Data were analyzed by the last observation carried forward (LOCF) method. a All patients (n = 167), b previous biologics (+, left) (n = 49) and (−, right) (n = 118), and c concomitant MTX (+, left) (n = 143) and (−, right) (n = 24). Disease activity was categorized as follows 
HAQ
The mean HAQ score of 1.24 ± 0.78 at baseline decreased to 0.92 ± 0.77 at week 52 (Fig. 3). The improvement was moderate but significant (P < 0.0001 vs. baseline). At week 4, the mean change was −0.22, which has been associated with meaningful clinical improvements and can be considered to represent the minimum clinically important difference (MCID) [24]. Although the baseline HAQ scores were comparable between the previous biologics (+) and (−) groups on average (1.24 ± 0.85 vs. 1.25 ± 0.76), patients without previous biologic therapy (−) showed a greater improvement than those with previous biologic treatment (+) (0.83 ± 0.72 vs. 1.16 ± 0.86) at week 52. In addition, the difference at week 52 was even more striking between the concomitant MTX treatment (+) and (−) groups (0.87 ± 0.75 vs. 1.29 ± 0.85). A significant improvement in the HAQ score as compared to baseline was detected only in the previous biologics (−) and concomitant MTX (+) groups (P < 0.0001 for both groups).
Fig. 3.
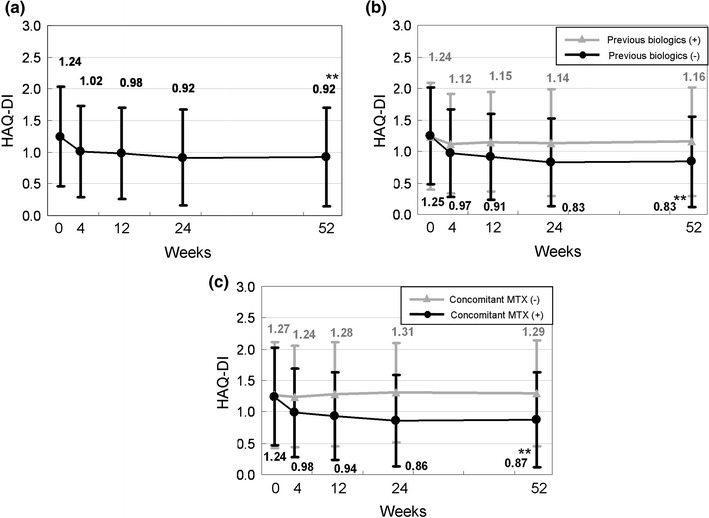
Time course of Health Assessment Questionnaire—Disability Index (HAQ-DI) over 52 weeks following the initiation of adalimumab treatment. Data were analyzed by the last observation carried forward (LOCF) method. Points and bars represent the mean and standard deviation, respectively. a All patients (n = 149), b previous biologics (+) (n = 41) and (−) (n = 108), c concomitant MTX (+) (n = 131) and (−) (n = 18). **P < 0.0001 versus baseline by the Wilcoxon signed rank test
Figure 4 shows the time course of HAQ-DI categorized by increments of 0.5 units from 0.0 to 3.0. At baseline, 23.5% of all patients had HAQ scores ≤0.5, suggesting that about a quarter of the patients had normal function at the time of entry. At week 52, the percentage increased to 43.0%. Although in general the functional profile was consistently better in the previous biologics (−) group at all the time points, there was no difference in the percentage of patients with a HAQ score of ≤0.5 from the previous biologic (+) group at week 52 (44.4 vs. 39.0%, P = 0.5506). In the concomitant MTX (+) group, the proportion of patients with a HAQ score of ≤0.5 at baseline (22.9%) increased steadily and almost doubled to 45.0% at week 52. In contrast, there was no increase in the proportion of patients who did not receive concomitant MTX (−) at week 52 when compared to the baseline, though it was not significantly different from the concomitant MTX (+) group (P = 0.1654) at week 52.
Fig. 4.
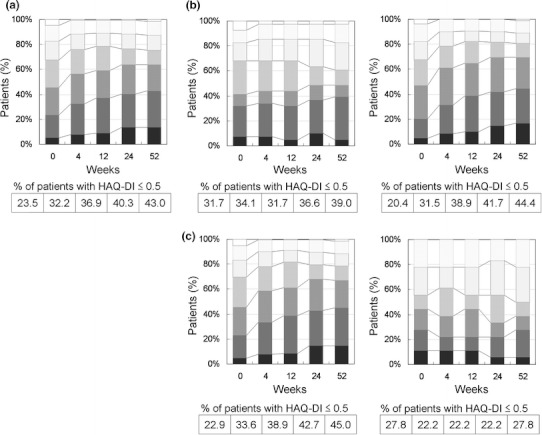
Time course of the Health Assessment Questionnaire—Disability Index (HAQ-DI) over 52 weeks following the initiation of adalimumab treatment. Data were analyzed by the last observation carried forward (LOCF) method. a All patients (n = 149), b previous biologics (+, left) (n = 41) and (−, right) (n = 108), and c concomitant MTX (+, left) (n = 131) and (−, right) (n = 18). HAQ-DI was categorized as follows 
Radiographic outcomes
Radiographic data at both the baseline and week 52 were available for 71 patients. Linear imputation was employed to determine missing data at week 52 for 16 patients who received ADA treatment for at least 180 days. A total of 87 patients were, therefore, subject to an evaluation of radiographic response to ADA. The mean estimated yearly progression was 27.1 ± 46.0 (median 13.6; 25th–75th percentiles 8.3–28.9) at baseline (Fig. 5), which is indicative of a great risk of further joint damage. After 52 weeks of ADA treatment, the mean change was significantly reduced to 0.8 ± 5.0 (median 0.0; 25th–75th percentiles −0.9 to 2.0) (P < 0.0001) (Fig. 5). It is particularly worth noting that ADA also suppressed the most aggressive progression in individuals with baseline changes of >100 TSS units/year. The results clearly indicate the ability of ADA to prevent further joint damage as assessed by a reduction in the rate of radiographic disease progression. A cumulative probability plot of changes in TSS was used to illustrate these findings (Fig. 6) [29]. The percentage of patients with no radiographic progression (as defined by a change in TSS of ≤0.5 units) over 52 weeks was 59.8%. However, there were 4 patients with a change in TSS of >10 despite ADA treatment (range 11.0–26.5), 2 of whom discontinued treatment before 52 weeks, and their radiographic data were therefore imputed.
Fig. 5.
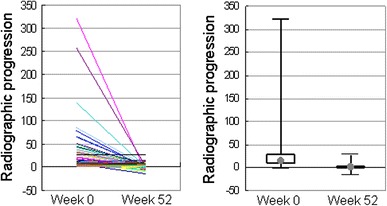
Yearly progression of TSS in individual patients at weeks 0 and 52 of adalimumab treatment (n = 87). Radiographic images were available for 71 of 167 patients at weeks 0 and 52. Linear imputation was used for missing data at week 52 for 16 patients who received adalimumab treatment for at least 180 days. Right points and boxes represent the median (13.6 at week 0 and 0.0 at week 52) and the interquartile range (8.3–28.9 at week 0 and −0.9 to 2.0 at week 52), respectively. Median reduction in the yearly radiographic progression was 100%. The reduction was statistically significant by the Wilcoxon signed rank test (P < 0.0001)
Fig. 6.
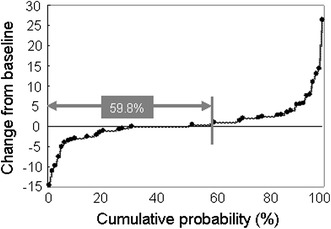
Cumulative probability plot of change in the total modified Sharp score from baseline to week 52 (n = 87). Radiographic images were available for 71 of 167 patients at baseline and week 52. Linear imputation was used for missing data at week 52 for 16 patients who received adalimumab treatment for at least 180 days. In 52 out of the 87 patients (59.8%), the yearly radiographic progression was ≤0.5
Safety
The overall exposure time to ADA used for the safety evaluation was conservatively estimated to be 175.4 patient-years (as of June 15, 2010), using the last visit records for the 2 patients whose exact discontinuation dates were unknown. ADA was generally well tolerated. A total of 60 adverse events (34.21/100 patient-years) were reported (Table 2). The most frequently reported adverse event (SOC) was general disorders and administration site conditions, which were observed at a frequency of 11.40/100 patient-years. ADA therapy was also associated with incidences of infections and infestations at a rate of 10.26/100 patient-years.
Table 2.
Adverse events
| MedDRA SOC | Number of events | Events/100 patient-years |
|---|---|---|
| Total | 60 | 34.21 |
| Infections and infestations | 18 | 10.26 |
| Respiratory, thoracic, and mediastinal disorders | 5 | 2.85 |
| General disorders and administration site conditions | 20 | 11.40 |
| Hepatobiliary disorders | 3 | 1.71 |
| Gastrointestinal disorders | 5 | 2.85 |
| Skin and subcutaneous tissue disorders | 2 | 1.14 |
| Blood and lymphatic system disorders | 1 | 0.57 |
| Eye disorders | 1 | 0.57 |
| Neoplasms (benign, malignant, and unspecified) | 1 | 0.57 |
| Injury, poisoning, and procedural complications | 1 | 0.57 |
| Investigations | 3 | 1.71 |
MedDRA SOC Medical Dictionary for Regulatory Activities system organ class
Serious adverse events are individually depicted in Table 3. A total of 16 serious adverse events were observed at a rate of 9.12/100 patient-years. Other than the injection site reactions, infections such as Pneumocystis jiroveci pneumonia, tuberculosis, nontuberculous mycobacteriosis, and cellulitis were the most frequent serious adverse events. In one patient, perforated colon diverticulum was detected. In another patient, malignant lymphoma was diagnosed. There were no deaths in this study.
Table 3.
Serious adverse events
| Adverse events | Number of events | Events/100 patient-years |
|---|---|---|
| Total | 16 | 9.12 |
| Injection site reactionsa | 3 | 1.71 |
| Interstitial pneumonitis | 2 | 1.14 |
| Pneumocystis jiroveci pneumonia | 1 | 0.57 |
| Pneumonia | 1 | 0.57 |
| Miliary tuberculosis | 1 | 0.57 |
| Nontuberculous mycobacteriosis | 1 | 0.57 |
| Cellulitis | 1 | 0.57 |
| Malignant lymphoma | 1 | 0.57 |
| Lymphoproliferative disorder | 1 | 0.57 |
| Perforated colon diverticulum | 1 | 0.57 |
| Generalized rash | 1 | 0.57 |
| Generalized urticaria | 1 | 0.57 |
| Left fibula fracture | 1 | 0.57 |
Serious adverse events as judged by the attending physicians
aInjection site reactions include erythema, itching, hemorrhage, pain, and swelling
Retention rate
In this study, the median duration of ADA treatment was estimated to be 55.9 weeks, with a minimum of 2 weeks and a maximum of 100 weeks (n = 167). At week 52, 69.7% of the 165 patients were still undergoing ADA therapy (Fig. 7). A greater percentage of patients in the previous biologics (−) group adhered to the treatment (77.6%) than patients in the previous biologics (+) group (51.0%) during the 52-week period (P < 0.0001). Similarly, the retention rate in the concomitant MTX (+) group (73.0%) was significantly higher than that in the concomitant MTX (−) group (50.0%) (P < 0.05).
Fig. 7.
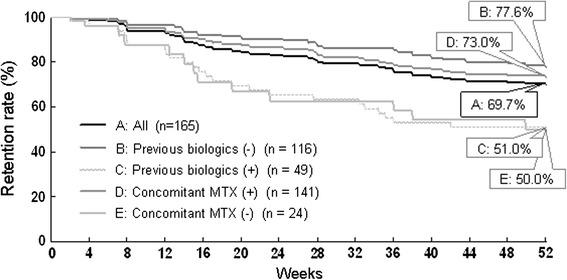
Retention rates of adalimumab treatment over 52 weeks (Kaplan–Meier plots). Two patients were excluded from the plots because of an unknown date of discontinuation. P < 0.0001 between previous biologics (+) versus (−), and P = 0.0109 between concomitant MTX (+) versus (−) by the log-rank test
Reasons for withdrawals, including those that occurred after 52 weeks of ADA treatment, are summarized in Table 4. The most common reason for discontinuation was lack of efficacy (n = 24), followed by adverse events (n = 16). Adverse events that led to discontinuation were Pneumocystis jiroveci pneumonia (n = 1), miliary tuberculosis (n = 1), interstitial pneumonitis (n = 2), interstitial pneumonitis/common colds (n = 1), generalized rash/nontuberculous mycobacteriosis/upper respiratory inflammation (n = 1), cellulitis/injection site reaction (n = 1), lymphoproliferative disorder (n = 1), perforated colon diverticulum/injection site reaction (n = 1), pancytopenia (n = 1), malignant lymphoma (n = 1), gastrointestinal disorder/injection site reaction (n = 1), generalized urticaria/injection site reaction (n = 1), and injection site reaction (n = 3). Note that 5 patients withdrew after maintaining remission status (DAS28-ESR < 2.6) for more than 24 weeks. The median ADA treatment duration in those 5 patients was 38 weeks (range 28–52 weeks).
Table 4.
Reasons for discontinuation
| Variables | All (n = 167) | Previous biologics | Concomitant MTX | ||
|---|---|---|---|---|---|
| (+) (n = 49) | (–) (n = 119) | (+) (n = 144) | (–) (n = 24) | ||
| Total | 55 | 25 | 30 | 42 | 13 |
| Lack of efficacy | 24 | 14 | 10 | 16 | 8 |
| Adverse events | 16 | 9 | 7 | 13 | 3 |
| Efficacy | 5 | 0 | 5 | 4 | 1 |
| Other reasonsa | 10 | 2 | 8 | 9 | 1 |
Two drop-outs with unknown discontinuation date were included. Those who discontinued after 52 weeks of treatment were also included
aOther reasons include patient’s choice and eye surgery
Discussion
The present study was carried out to retrospectively analyze the efficacy and safety of ADA in Japanese patients with RA. The study included 167 patients with all individual DAS28-ESR components at baseline. Further, 149 of these had baseline HAQ-DI, and 87 had evaluable radiographic data. For our subjects, ADA therapy provided significant clinical, functional, and radiographic benefits during routine clinical care while also demonstrating generally acceptable safety and tolerability.
The PREMIER study showed that when combination treatment with ADA and MTX is initiated early, it leads to superior clinical, functional, and radiographic outcomes as compared with treatment with MTX alone or ADA alone; adverse event profiles were comparable in all 3 arms [11]. The efficacy confirmed in the CHANGE study should be seen as such [18], since all the ADA-treated patients received ADA monotherapy. The results compared well to those of the DE011 monotherapy study conducted overseas [8]. The present HARMONY study is the first study to demonstrate the efficacy and safety of ADA therapy in combination with MTX in Japanese RA patients. An average of 8.5 mg/week MTX was used at baseline. This study clearly confirmed the superior effectiveness of combination therapy with MTX over ADA monotherapy. Indeed, the impact of concomitant MTX use was greater than that of a lack of history of biologic therapy in terms of both clinical and functional improvement (42.7% DAS28 remission and 45.0% normal function at week 52). Although a rapid response was evident in terms of both HAQ and DAS28 by week 4, the corresponding remission rates tended to increase even after week 24 until week 52, from 35.0 to 42.7% (DAS28-ESR < 2.6) and from 42.7 to 45.0% (HAQ-DI ≤0.5). Thus, it may be prudent to wait a further 24 weeks to see whether ADA can induce remission in a small portion of patients who responded to ADA at early time points. MTX reduced apparent ADA clearance after multiple dosing in 44% of patients with RA, thereby increasing systemic ADA trough levels [25]. This is because concomitant MTX use is considered to suppress levels of anti-ADA antibodies due to its immunosuppressive effect.
The radiographic outcome presented here is the first evidence of the ability of ADA to significantly limit radiographic progression in Japanese RA patients. Approximately 60% of patients exhibited no radiographic progression in HARMONY, which compares well with the results obtained in the PREMIER study (64 and 51% in the ADA + MTX and ADA monotherapy groups, respectively) [11]. Note that 26 out of the 87 evaluable patients (29.9%) exhibited ∆TSS ≤−0.5, indicating possible radiographic repair.
ADA treatment was generally well tolerated. No anaphylactoid reaction was reported, while injection site reactions occurred at a rate of 11.9% (20/167). This rate was far lower than that reported in the CHANGE study (30.8% in the 40 mg arm). The observed difference may possibly be due to the immunosuppressive effects of the concomitant use of MTX in favor of combination therapy.
Serious infections occurred at a rate of 2.85/100 patient-years (one event of each: Pneumocystis jiroveci pneumonia, pneumonia, military tuberculosis, cellulitis, and nontuberculous mycobacteriosis). Recently, the effectiveness and safety of biologic agents in Japanese patients were reviewed, and pneumonia, tuberculosis, Pneumocystis jiroveci pneumonia and interstitial pneumonitis were identified as important adverse reactions [26]; these were also observed in our study. Komano et al. [27] reported serious infections at a rate of 6.24/100 patient-years in Japanese patients treated with either infliximab or etanercept for up to 1 year. Although direct comparisons cannot be made among different studies, this may suggest that ADA therapy does not carry an increased risk for serious infections when compared to another anti-TNF therapy.
The overall retention rate observed in the present study (82.4% at 26 weeks and 69.7% at 52 weeks) falls within the range reported for infliximab (75.6% at 54 weeks) [15], etanercept (85.1% at 6 months) [17], and tocilizumab (79.5% at 24 weeks) [28] in daily clinical practice. However, it is not surprising that the retention rate varies among different biologics, as it is believed to be influenced by numerous factors other than efficacy and safety, such as co-morbidity, concomitant therapy, costs, launch timing, and availability of other therapies [29]. In the literature, it was indicated that the drug survival time of a second TNF inhibitor is shorter than a prior TNF inhibitor, while the survival of anti-TNF treatment was shown to be prolonged with concomitant use of MTX [30–32]. Our own findings in HARMONY resemble these published data, as shown by week 52 retention rates in the previous biologic (−) and concomitant MTX (+) groups of 77.6 and 73.0%, respectively.
In conclusion, this retrospective study has demonstrated that ADA therapy is highly efficacious at reducing disease activity, improving physical function, and limiting radiographic progression, and is generally safe and tolerable in Japanese RA patients encountered during routine clinical practice. Furthermore, the results of this study demonstrate that ADA in combination with MTX is associated with substantial improvements in clinical, functional, and radiographic responses and retention rate, meaning that this could potentially serve as a first-line treatment.
Acknowledgments
The authors thank all medical staff in all institutions for providing the data. This work was supported in part by a Research Grant-In-Aid for Scientific Research from the Ministry of Health, Labor and Welfare of Japan, and the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest
Dr. Takeuchi has received consulting fees, speaking fees, honoraria and/or research grant support from Mitsubishi-Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Pfizer Japan Inc., Abbott Japan Co., Ltd., Daiichi-Sankyo Co., Ltd., Janssen Pharmaceutical K. K., Astra-Zeneca K. K., Takeda Industrial Pharmaceutical Co., Ltd., Astellas Pharma Inc., and Bristol-Myers Squibb. Dr. Tanaka has received consulting fees, speaking fees, and/or honoraria from Mitsubishi-Tanabe Pharma Corporation, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Pfizer Japan Inc., Abbott Japan Co., Ltd., Daiichi-Sankyo Co., Ltd., Janssen Pharmaceutical K. K., Astra-Zeneca K. K., Takeda Industrial Pharmaceutical Co., Ltd., Astellas Pharma Inc., Asahikasei Pharma Corporation, and GlaxoSmithKline K. K., and has received research grant support from Mitsubishi-Tanabe Pharma Corporation, Bristol-Myers Squibb, Takeda Industrial Pharmaceutical Co., Ltd., MSD K. K., Astellas Pharma Inc., Eisai Co., Ltd., Chugai Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Daiichi-Sankyo Co., Ltd. Dr. Yamanaka has received research grants from Abbott Japan Co., Ltd., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd, Eisai Co., Ltd., Janssen Pharmaceutical K.K, Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co., Ltd, Pfizer Japan Inc., Takeda Industrial Pharmaceutical Co., Ltd., and UCB Japan Co., Ltd, and speakers honoraria/consulting fees from Abbott Japan Co., Ltd, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Takeda Industrial Pharmaceutical Co., Ltd., and UCB Japan Co., Ltd. Dr. Amano has received research grants from Chugai Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, and Astellas Pharmaceutical Co., Ltd. Dr. Kameda has received consulting fees, speaking fees, and honoraria from Mitsubishi-Tanabe Pharma Corporation, Eisai Co., Ltd., Pfizer Japan Inc., and Abbott Japan Co., Ltd.
References
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Hakoda M, Oiwa H, Kasagi F, Masunari N, Yamada M, Suzuki G, et al. Mortality of rheumatoid arthritis in Japan: a longitudinal cohort study. Ann Rheum Dis. 2005;64:1451–1455. doi: 10.1136/ard.2004.033761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 4.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 5.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 6.Salfeld J, Kaymakҫalan Z, Tracey D, Roberts A, Kamen R. Generation of fully human anti-TNF antibody D2E7 [abstract]. Arthritis Rheum. 1998;41(Suppl 9):S57.
- 7.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 8.Putte LB, Atkins C, Malaise M, Sany J, Russell AS, Riel PL, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 10.Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti-tumor necrosis factor-α monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (safety trial of adalimumab in rheumatoid arthritis) J Rheumatol. 2003;30:2563–2571. [PubMed] [Google Scholar]
- 11.Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, Vollenhoven R, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 12.Miyasaka N, Takeuchi T, Eguchi K. Proposed [corrected] Japanese Guidelines for the use of infliximab for rheumatoid arthritis. Mod Rheumatol. 2005;15:4–8. doi: 10.1007/s10165-004-0357-7. [DOI] [PubMed] [Google Scholar]
- 13.Miyasaka N, Takeuchi T, Eguchi K. Guidelines for the proper use of etanercept in Japan. Mod Rheumatol. 2006;16:63–67. doi: 10.1007/s10165-006-0457-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamanaka H, Tanaka Y, Sekiguchi N, Inoue E, Saito K, Kameda H, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan (RECONFIRM) Mod Rheumatol. 2007;17:28–32. doi: 10.1007/s10165-006-0532-0. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka Y, Takeuchi T, Inoue E, Saito K, Sekiguchi N, Sato E, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year clinical outcomes (RECONFIRM-2) Mod Rheumatol. 2008;18:146–152. doi: 10.1007/s10165-008-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi T, Yamanaka H, Inoue E, Nagasawa H, Nawata M, Ikari K, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year outcome of joint destruction (RECONFIRM-2J) Mod Rheumatol. 2008;18:447–454. doi: 10.1007/s10165-008-0077-5. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto N, Kawakami A, Fujikuwa K, Aramaki T, Kawashiri S, Tamai M, et al. Prediction of DAS28-ESR remission at 6 months by baseline variables in patients with rheumatoid arthritis treated with etanercept in Japanese population. Mod Rheumatol. 2009;19:488–492. doi: 10.1007/s10165-009-0187-8. [DOI] [PubMed] [Google Scholar]
- 18.Miyasaka N, the CHANGE Study Investigators Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol. 2008;18:252–262. doi: 10.1007/s10165-008-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Japan College of Rheumatology. Guidelines for adalimumab (in Japanese). 2008. http://www.ryumachi-jp.com/info/guideline_ADA.pdf
- 21.Prevoo ML, van’t Hof MA, Kuper HH, Leeuwen MA, Putte LB, Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 22.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 23.Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–263. [PubMed] [Google Scholar]
- 24.Goldsmith CH, Boers M, Bombardier C, Tugwell P. Criteria for clinically important changes in outcomes: development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee. J Rheumatol. 1993;20:561–565. [PubMed] [Google Scholar]
- 25.Abbott Laboratories. Prescribing information for Humira® (adalimumab). Chicago: Abbott Laboratories; 2010.
- 26.Takeuchi T, Kameda H. The Japanese experience with biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. 2010;6:644–652. doi: 10.1038/nrrheum.2010.154. [DOI] [PubMed] [Google Scholar]
- 27.Komano Y, Tanaka M, Nanki T, Koike R, Sakai R, Kameda H, et al. Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the registry of Japanese rheumatoid arthritis patients for long-term safety. J Rheumatol. 2011. doi:10.3899/jrheum.101009. [DOI] [PubMed]
- 28.Yamanaka H, Tanaka Y, Inoue E, Hoshi D, Momohara S, Hanami K, et al. Efficacy and tolerability of tocilizumab in rheumatoid arthritis patients seen in daily clinical practice in Japan: results from a retrospective study (REACTION study) Mod Rheumatol. 2011;21:122–133. doi: 10.1007/s10165-010-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Reino JJ, Carmona L, BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29. [DOI] [PMC free article] [PubMed]
- 30.Kristensen LE, Saxne T, Nilsson JA, Geborek P. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Ther. 2006;8:R174. doi: 10.1186/ar2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazici Y, Krasnokutsky S, Barnes JP, Hines PL, Wang J, Rosenblatt L. Changing patterns of tumor necrosis factor inhibitor use in 9074 patients with rheumatoid arthritis. J Rheumatol. 2009;36:907–913. doi: 10.3899/jrheum.080592. [DOI] [PubMed] [Google Scholar]
- 32.Soliman MM, Ashcroft DM, Watson KD, Lunt M, Symmons DPM, Hyrich KL, et al. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:583–589. doi: 10.1136/ard.2010.139774. [DOI] [PMC free article] [PubMed] [Google Scholar]


