Abstract
The polypeptide hormone Islet Amyloid Polypeptide (IAPP, amylin) is responsible for islet amyloid formation in type-2 diabetes and in islet cell transplants, where it may contribute to graft failure. Human IAPP is extremely amyloidogenic and fewer inhibitors of IAPP amyloid formation have been reported than for the Alzheimer's Aβ peptide or for α-synuclein. The ability of a set of hydroxyflavones to inhibit IAPP amyloid formation was tested. Fluorescence detected thioflavin-T-binding assays are the most popular methods for measuring the kinetics of amyloid formation and for screening potential inhibitors; however, we show that they can lead to false positives with hydroxyflavones. Several of the compounds inhibit thioflavin-T fluorescence, but not amyloid formation; a result which highlights the hazards of relying solely on thioflavin-T assays to screen potential inhibitors. Transmission electron microscopy (TEM) and right-angle light scattering show that Morin hydrate (2′,3,4′,5,7-Pentahydroxyflavone) inhibits amyloid formation by human IAPP and disaggregates preformed IAPP amyloid fibers. In contrast, Myricetin, Kaempferol, and Quercetin, which differ only in hydroxyl groups on the B-ring, are not effective inhibitors. Morin hydrate represents a new type of IAPP amyloid inhibitor and the results with the other compounds highlight the importance of the substitution pattern on the B-ring.
Keywords: amylin; amyloid inhibitors; islet amyloid polypeptide; Morin hydrate; hydroxyflavone, thioflavin-T
Introduction
Amyloid formation plays an important role in a broad range of different human diseases including Alzheimer's disease, Parkinson's disease, and type-2 diabetes.1–3 The search for inhibitors of amyloid fiber formation is an active area of research and particular attention has been paid to flavanoids.4–27 Here, we examine the ability of a set of hydroxyflavones to inhibit amyloid formation by (islet amyloid polypeptide (IAPP, Amylin)).
IAPP is a member of the calcitonin-like family of peptides and is found in all mammalian species examined.28–31 The 37-residue polypeptide hormone is packaged in the insulin secretory granule and normally acts as an endocrine partner to insulin.30, 32, 33 IAPP aggregates to form islet amyloid in type-2 diabetes; a process which is widely believed to contribute to β-cell death in the disease.23, 28, 29, 32, 34–40 Recent studies also highlight a potentially important role for IAPP amyloid formation in the failure of islet cell grafts.41–43
There is widespread interest in developing inhibitors of amyloid formation and compounds which can disaggregate preformed amyloid. Much of this work has focused on the Aβ peptide of Alzheimer's disease or on α-synuclein, the protein which plays a central role in Parkinson's disease, but fewer inhibitors of IAPP amyloid formation have been reported.4–27 IAPP is extremely amyloidogenic and is thus a challenging target for inhibition studies. Certain hydroxyflavones have been reported to be effective inhibitors of amyloid formation by the Aβ polypeptide although there are conflicting reports about their effectiveness.44–46 Their ability to inhibit amyloid formation by IAPP has not yet been examined. Here, we test the ability of a set of four related hydroxyflavones, Morin hydrate (2′,3,4′,5,7-Pentahydroxyflavone), Myricetin (3,3′4′,5,5′,7-Hexahydroxyflavone), Kaempferol (3,4′5,7-Tetrahydroxyflavone), and Quercetin (3,3′,4′,5,6-Pentahydroxyflavone), to inhibit amyloid formation by IAPP and to disaggregate already assembled IAPP amyloid fibers. Morin hydrate is shown to be an effective inhibitor, whereas the other compounds are not as effective. We also show that these compounds can compromise standard fluorescence assays of amyloid formation and, in some cases, can lead to false positives in inhibitor screens.
Results
Hydroxyflavones can interfere with standard fluorescence assays of amyloid formation
The structures of the compounds examined in this study are shown in Figure 1 together with the primary structure of IAPP. The compounds share the same core structure; they are polyhydroxylated and consist of a 12-membered heterocyclic ring linked to the 6-membered aromatic B-ring (Fig. 1).
Figure 1.
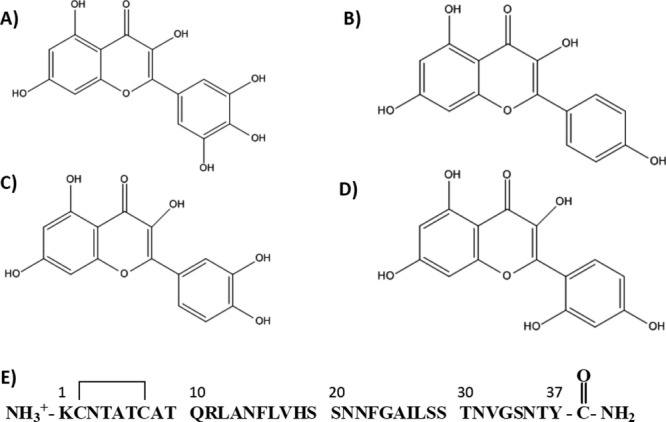
Structures of the compounds studied together with the primary sequence of IAPP. (A) Myricetin, (B) Kaempferol, (C) Quercertin, (D) Morin hydrate, and (E) primary structure of human IAPP. The peptide has an amidated C-terminus and a disulfide bond involving Cys-2 and Cys-7.
Amyloid formation by IAPP displays a lag phase during which no detectable amyloid fibrils are formed followed by a growth phase which leads to a steady state in which amyloid fibrils are in equilibrium with soluble peptide [Fig. 2(A)]. The rate of amyloid formation is typically measured using fluorescence-detected thioflavin-T-binding assays. Thioflavin-T is believed to bind to the surface of amyloid fibers in grooves formed by the alignment of side chains in the cross β-sheet structure. Binding to an amyloid fiber increases the fluorescence quantum yield of the dye by restricting the rotation of the benzaminic and benzothiozole rings and thus reducing self-quenching.47 Thioflavin-T assays are simple and convenient, but problems may arise in the studies of inhibitors because compounds which appear to inhibit amyloid formation could just be inhibitors of thioflavin-T fluorescence. This may occur because they absorb strongly which leads to inner filter effects, or because they quench the bound thioflavin-T fluorescence, or because they inhibit thioflavin-T binding to amyloid.48, 49
Figure 2.
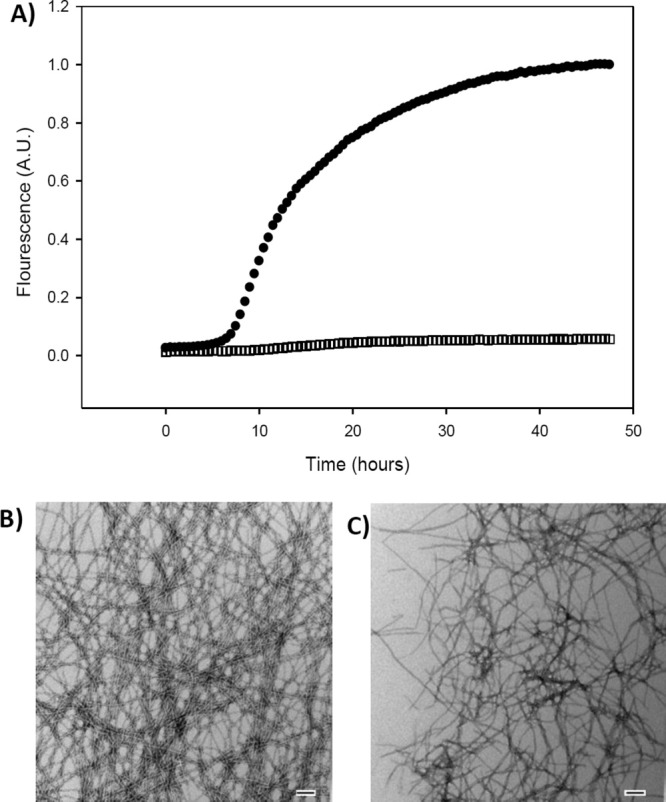
Hydroxyflavones interfere with thioflavin-T-based assays. (A) Thioflavin-T-monitored kinetic experiments. Black circles, IAPP alone. Black open squares, IAPP plus an equimolar amount of Myricetin. (B) TEM image collected at the end of the reaction for the IAPP sample. (C) TEM image collected at the end of the reaction for the 1:1 mixture of IAPP and Myricetin. Scale bars represent 100 nm. Experiments were conducted at 25°C, pH 7.4, 20 mM Tris-HCl, 32 μM IAPP, 0.25% DMSO (v/v).
The compounds under study here have the potential to interfere with thioflavin-T assays. Figure 2 shows the results of a thioflavin-T-monitored kinetic experiment in the presence of Myricetin. In the absence of the compound, a typical IAPP kinetic curve is observed [Fig. 2(A), solid circles]. Very different behavior is observed in its presence. There is only a very small change in fluorescence over the entire time course of the experiment [Fig. 2(A), open square]. Taken alone, these results could imply that Myricetin is a potent amyloid inhibitor; however, transmission electron microscopy (TEM) images collected from samples removed at the end of the reaction revealed the presence of amyloid fibers in the IAPP control sample [Fig. 2(B)] and in the 1:1 mixture of Myricetin and IAPP [Fig. 2(C)]. Thus, Myricetin is actually not an effective IAPP amyloid inhibitor. Similar results were obtained with Kaempferol and Quercetin (Supporting Information). We suspect that these sorts of effects might account for some of the discrepancy in the literature on the potency of this class of compounds in inhibiting other amyloids.44–46 The compounds can also interfere with disaggregation studies. Experiments in which Myricetin is added to a sample, which contains IAPP amyloid fibers and thioflavin-T, show that Myricetin interferes with thioflavin-T-based disaggregation assays. When the compound is added, an immediate drop in fluorescence is observed, but TEM confirms that fibers are still present (Supporting Information).
Morin hydrate, but not Myricetin, Kaempferol, or Quercetin is an effective inhibitor of IAPP amyloid fiber formation
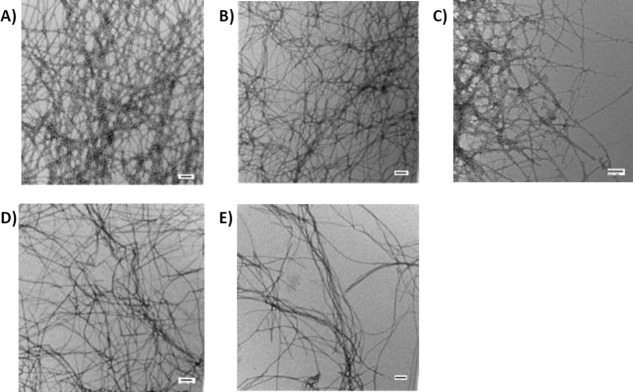
The experiments described in the previous section clearly show that thioflavin-T-based inhibitor assays are not reliable for these hydroxyflavones. Thus, we tested their ability to inhibit IAPP amyloid formation using TEM. Compounds were incubated with IAPP for 48 h and TEM images were recorded. The incubation period is longer than that required for IAPP to form amyloid under these conditions [Fig. 2(A), black circles] and dense collections of amyloid fibers are observed in a control sample of IAPP without compound [Fig. 3(A)]. Some aggregated material is observed in the 1:1 mixtures of IAPP with all four of the compounds [Fig. 3(B–E)]. Amyloid fibers are visible in the TEM images of Kaempferol, Quercetin, and Myricetin [Fig. 3(B–D)]; however, the objects detected in the presence of Morin hydrate are thinner and they appeared to be less abundant [Fig. 3(E)]. These studies encouraged us to examine the effect of different concentrations of Morin hydrate on amyloid formation by IAPP.
Figure 3.
Morin Hydrate, but not Myricetin, Kaempferol, or Quercetin, inhibits amyloid formation by IAPP. TEM images shown are of the samples collected at 48 h after the initiation of amyloid formation. (A) IAPP alone, (B) IAPP:Myricetin = 1:1, (C) IAPP:Kaempferol = 1:1, (D) IAPP:Quercertin = 1:1, and (E) IAPP:Morin hydrate = 1:1. Experiments were conducted at 25°C, pH 7.4, 20 mM Tris-HCl, 32 μM IAPP, 0.25% DMSO (v/v). Inhibitors, when present, were at 32 μM. Scale bars represent 100 nm.
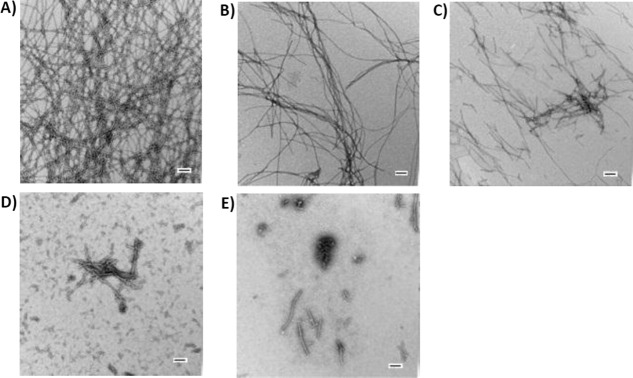
The TEM micrographs shown in Figure 4 compare the results of 1:1, 1:2, 1:5, and 1:10 mixtures of IAPP and Morin hydrate. In all cases, Morin hydrate was added at time equals zero and the samples were incubated for 48 h. Dense mats of fibers are observed in the control sample of IAPP without Morin hydrate [Fig. 4(A)]. Adding Morin hydrate in a twofold excess leads to more pronounced effects than was observed with the 1:1 mixture [compare Fig. 4(B,C)]. The objects which are detected are even thinner and less abundant in the twofold excess sample relative to the 1:1 sample. Addition of a five or tenfold excess of Morin hydrate led to even more pronounced changes. Very short objects together with some amorphous aggregates are detected on the grids [Fig. 4(D,E)]. We also examined the other compounds at higher ratios, but they still failed to inhibit amyloid formation by IAPP (Supporting Information).
Figure 4.
The effects of Morin hydrate are concentration dependent. TEM images shown are of the samples collected at 48 h after the initiation of amyloid formation. (A) IAPP alone, (B) 1:1 mixture of IAPP and Morin hydrate, (C) 1:2 mixture of IAPP and Morin hydrate, (D) 1:5 mixture of IAPP and Morin hydrate, and (E) 1:10 mixture of IAPP and Morin hydrate. Experiments were conducted at 25°C, pH 7.4, 20 mM Tris-HCl, 32 μM IAPP, 0.25% DMSO (v/v). The concentration of Morin hydrate, when present, ranged from 32 to 320 μM. Scale bars represent 100 nm.
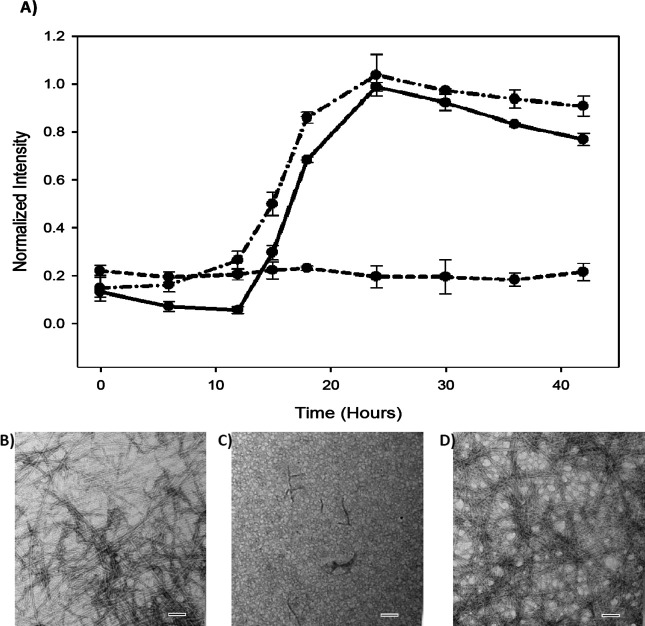
We conducted right-angle light scattering experiments to obtain an independent test of the ability of Morin hydrate to inhibit IAPP amyloid formation and to confirm that these compounds can interfere with thioflavin-T assays. The curve recorded in the absence of inhibitor shows a sigmoidal transition which closely follows the time course of the thioflavin-T studies [Fig. 5(A)]. TEM confirms that amyloid fibers are formed [Fig. 5(B)]. Very different results were obtained when Morin hydrate was present at a fivefold excess relative to IAPP. No change in signal was observed over the course of the experiment, and TEM confirmed the lack of amyloid [Fig. 5(A,C)]. We also used right-angle light scattering to examine the effects of Myricetin on IAPP amyloid formation. The light scattering curve recorded in the presence of Myricetin resembles the curve obtained from the sample of pure IAPP and is very different from the data obtained in the presence of Morin hydrate. TEM confirms that amyloid fibers are formed [Fig. 5(D)]. These experiments further verify that Myricetin is not an effective inhibitor of IAPP amyloid formation and, even more importantly, confirm that Morin hydrate is an inhibitor.
Figure 5.
Right-angle light scattering confirms that Morin hydrate, but not Myricetin, inhibits IAPP amyloid formation. (A) Light scattering: solid line (—) IAPP alone, dashed line (----) IAPP plus Morin hydrate, and dashed-dotted line (–·–·–) IAPP plus Myricetin. (B) TEM image of the sample of IAPP without compound. Sample was removed at 42 h. (C) TEM image of a sample from the experiment conducted in the presence of Morin hydrate in fivefold excess. Sample was removed at 42 h. (D) TEM image of a sample from the experiment conducted in the presence of Myricetin in fivefold excess. Sample was removed at 42 h. All samples contained 32 μM IAPP, 0.25% DMSO (v/v) 20 mM Tris-HCl, pH 7.4. The signal was monitored at 500 nm. Experiments were conducted at 25°C. Scale bars in TEM images represent 100 nm. The light scattering data are the result of three separate measurements for each sample. The error bars represent the standard deviation of each data point.
Morin hydrate disaggregates IAPP amyloid fibers
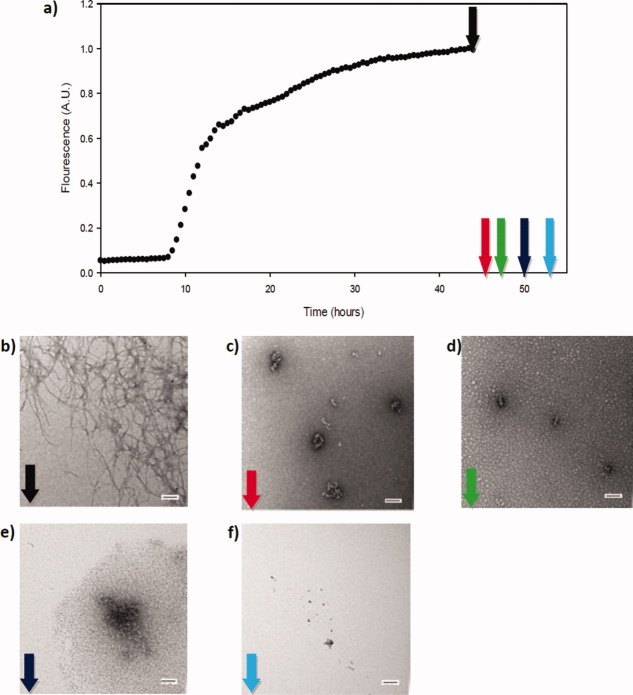
We next examined the ability of Morin hydrate to disaggregate preformed IAPP fibers (Fig. 6). A sample of IAPP was incubated for 45 h and a fivefold excess of Morin hydrate was added [Fig. 6(A), black arrow]. The thioflavin-T curve is included simply to illustrate that the amyloid reaction had reached the plateau region before the compound was added. Thioflavin-T fluorescence cannot be used to follow the kinetics of disassembly because the hydroxyflavones interfere with thioflavin-T assays. TEM images of a sample removed just before the addition of the compound revealed dense mats of amyloid fibers [Fig. 6(B)]. Additional aliquots were removed for TEM analysis starting 1 h after addition of Morin hydrate [Fig. 6(A), colored arrows]. No fibers were detected in the TEM images of these samples (Fig. 6C-F).
Figure 6.
Morin hydrate disaggregates human IAPP amyloid fibers. (A) A thioflavin-T-monitored kinetic experiment in the absence of Morin hydrate. Morin hydrate was added in fivefold excess at the time point indicated by the black arrow. (B) TEM image of a sample removed just prior to the addition of Morin hydrate (black arrow). (C) TEM image 1 h after addition of Morin hydrate (red arrow). (D) TEM image 2 h after addition of Morin hydrate (green arrow). (E) TEM image 4 h after addition of Morin hydrate (dark blue arrow). (F) TEM image 6 h after addition of Morin hydrate (light blue arrow). Experiments were conducted at 25°C, pH 7.4, 20 mM Tris-HCl, 32 μM IAPP, 0.5% DMSO (v/v). The concentration of Morin hydrate was 160 μM. Scale bars represent 100 nm.
Morin hydrate also disaggregates IAPP amyloid fibers when it is added at a ratio of 1:1. In this case, the dense collection of amyloid fibers is disrupted to produce a smaller amount of thinner and shorter fibers (Supporting Information) which bear some resemblance to the species observed when Morin hydrate is added to an IAPP solution at time equals zero. However, disaggregation does not have to be the reverse of amyloid fiber formation and has been shown to be different for at least one inhibitor.18
Discussion
The results presented here highlight the difficulty of using thioflavin-T-based assays to test for amyloid inhibitors and for compounds which disaggregate amyloid fibers. They reiterate the importance of conducting experiments which directly test for the presence of amyloid fibers. Here, we used TEM to test for the presence of amyloid and light scattering to monitor the kinetics of aggregation. The data show that Morin hydrate inhibits amyloid formation by IAPP and demonstrate that it can disassemble IAPP amyloid fibers. The compound represents a new class of IAPP amyloid inhibitors. The ability of this class of compounds to inhibit amyloid formation by IAPP appears to be strongly dependent on the substitution pattern in the B-ring because the other compounds tested were much less effective.
There are compounds that inhibit amyloid formation by both IAPP and Aβ.4, 13, 18 In contrast, some of the hydroxyflavones studies here have different effects on the Aβ peptide. In particular, Myricetin has been reported to inhibit Aβ amyloid formation,44–46 but the data presented here show that it is not effective against IAPP. Several hydroxyflavones, in addition to Myricetin, have been tested for their ability to inhibit amyloid formation by the Aβ peptide and there are differing reports of their effectiveness.44–46 The difficulty of quantitatively interpreting thioflavin-T fluorescence intensities in the presence of the hydroxyflavones may contribute to the apparent differences. These compounds interfere with thioflavin-T assays of amyloid formation by IAPP, thus perhaps they could also interfere or partially interfere with the assays of Aβ amyloid formation.
Materials and Methods
Peptide synthesis
IAPP was synthesized using a CEM Liberty Automatic Microwave Peptide Synthesizer and 9-fluorenylmethoxycarbonyl (Fmoc) solid-phase peptide chemistry. FMOC-PAL-Polyethylene-Glycol-Polystyrene (FMOC-PAL-PEG-PS) resin was used and standard Fmoc reaction cycles were employed. Pseudoprolines were used to assist the synthesis as described previously.50 The peptide was cleaved from the resin using standard trifluoroacetic acid (TFA) methods and was then lyophilized for 24–48 h. To increase the solubility of the peptides for later stages of purification, the crude material was dissolved in 20% acetic acid for 2–3 h and lyophilized. The crude peptide was oxidized in dimethyl sulfoxide (DMSO).51 Peptides were purified using reversed-phase high-performance liquid chromatography (HPLC) using a Vydac C18 preparative column with a 2-buffer system. Buffer A contained 0.045% HCl in distilled and deionized (DDI) water and buffer B contained 0.045% HCl in 80% acetonitrile (ACN) and 20% DDI water. The identity of the pure peptide was confirmed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). IAPP expected, 3903.3 Da; observed, 3903.9 Da.
Myricetin, Quercetin, Morin hydrate, and Kaempferol were obtained from Sigma-Aldrich and dissolved in DMSO at a concentration of 15.8 mM for the experiments in which the compound was added at a ratio of 1:1 and 130 mM for experiments in which it was added at a higher ratio. The small molecules were dissolved in DMSO on the day of the experiment.
Thioflavin-T fluorescence assays
Fluorescence experiments were performed using a Beckman model D880 plate reader. The thioflavin-T studies used excitation and emission filters of 430 and 485 nm, respectively. The experiments were performed in 96-well plates at 25°C.
IAPP was dissolved in hexafluoroisopropanol (HFIP) at a final concentration of 1.58 mM. The stock solution of IAPP in HFIP was filtered using a 0.45-μm syringe filter. Aliquots of filtered stock solution were pipetted into Eppendorf tubes and lyophilized. All of the thioflavin-T assays were prepared by dissolving the dried samples of IAPP in Tris-HCl buffer (20 mM, pH = 7.4) immediately before measurements. The final peptide concentration was 32 μM in 0.25% DMSO, 20 mM Tris-HCl buffer at pH 7.4, and 32 μM thioflavin-T with or without inhibitors. For experiments with the inhibitor, concentrated stock solutions of compounds in DMSO were diluted into the thioflavin-T IAPP solution. The data were analyzed using Sigma-Plot v11.
Right-angle light scattering
Experiments were conducted using an Applied Phototechnology Fluorescence Spectrophotometer at 500 nm. This wavelength was chosen because none of the compounds has any absorbance in this region. Experiments were conducted at 25°C, pH 7.4, 20 mM Tris-HCl, 32 μM IAPP, 0.25% DMSO (v/v).
TEM
Aliquots were removed from the fluorescence assays and were analyzed by TEM. Samples were prepared by pipetting 15 μL of the solution onto carbon-coated Formvar 300 mesh copper grids. The grids were stained using saturated uranyl acetate for 1 min. An FEI Technai12 BioTwinG2 Transmission Electron Microscope was used and the grids were viewed at a magnification of 98,000× (high voltage, 80 kV).
Amyloid disaggregation experiments
Normal procedures for forming fibers were performed as described above. Morin hydrate was added at approximately 45 h after the initiation of fiber formation. Aliquots of the assay were periodically removed from the solution for TEM analysis.
Glossary
Abbreviations:
- DDI
distilled and deionized
- Fmoc
9-fluorenylmethoxycarbonyl
- IAPP
islet amyloid polypeptide
- MALDI-TOF MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- PAL-PEG
5-(4′-Fmoc-aminomethyl-3′,5-dimethoxyphenyl) valeric acid
- TEM
transmission electron microscopy
- TFA
trifluoroacetic acid.
Supplementary material
Additional Supporting Information may be found in the online version of this article.
References
- 1.Sipe JD. Amyloidosis. Crit Rev Clin Lab Sci. 1994;31:325–354. doi: 10.3109/10408369409084679. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 3.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 4.Yan LM, Tatarek-Nossol M, Velkova A, Kazantzis A, Kapurniotu A. Design of a mimic of nonamyloidogenic and bioactive human islet amyloid polypeptide (IAPP) as nanomolar affinity inhibitor of IAPP cytotoxic fibrillogenesis. Proc Natl Acad Sci USA. 2006;103:2046–2051. doi: 10.1073/pnas.0507471103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng BY, Toyama BH, Wille H, Colby DW, Collins SR, May BCH, Prusiner SB, Weissman J, Shoichet BK. Small-molecule aggregates inhibit amyloid polymerization. Nat Chem Biol. 2008;4:197–199. doi: 10.1038/nchembio.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blazer LL, Neubig RR. Small molecule protein-protein interaction inhibitors as CNS therapeutic agents: current progress and future hurdles. Neuropsychopharmacology. 2009;34:126–141. doi: 10.1038/npp.2008.151. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Mihara H. Peptide and protein mimetics inhibiting amyloid beta-peptide aggregation. Acc Chem Res. 2008;41:1309–1318. doi: 10.1021/ar8000475. [DOI] [PubMed] [Google Scholar]
- 8.Abedini A, Meng F, Raleigh DP. A single-point mutation converts the highly amyloidogenic human islet amyloid polypeptide into a potent fibrillization inhibitor. J Am Chem Soc. 2007;129:11300–11301. doi: 10.1021/ja072157y. [DOI] [PubMed] [Google Scholar]
- 9.Scrocchi LA, Chen Y, Wang F, Han K, Ha K, Wu L, Fraser PE. Inhibitors of islet amyloid polypeptide fibrillogenesis, and the treatment of type-2 diabetes. Lett Pept Sci. 2003;10:545–551. [Google Scholar]
- 10.Gazit E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 11.Mishra R, Bulic B, Sellin D, Jha S, Waldmann H, Winter R. Small-molecule inhibitors of islet amyloid polypeptide fibril formation. Angew Chem Int Ed Engl. 2008;47:4679–4682. doi: 10.1002/anie.200705372. [DOI] [PubMed] [Google Scholar]
- 12.Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 13.Meng F, Abedini A, Raleigh DP. Combination of kinetically selected inhibitors in trans leads to highly effective inhibition of amyloid formation. J Am Chem Soc. 2010;132:14340–14342. doi: 10.1021/ja1046186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, Wanker EE. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 15.Tatzelt J, Rambold AS, Miesbauer M, Olschewski D, Seidel R, Riemer C, Smale L, Brumm L, Levy M, Gazit E, Oesterhelt D, Baier M, Becker CFW, Engelhard M, Winklhofer KF. Green tea extracts interfere with the stress-protective activity of PrP(C) and the formation of PrP(Sc) J Neurochem. 2008;107:218–229. doi: 10.1111/j.1471-4159.2008.05611.x. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa M, Masuda M, Suzuki N, Taniguchi S, Oikawa T, Nonaka T, Iwatsubo T, Hisanaga S, Goedert M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry. 2006;45:6085–6094. doi: 10.1021/bi0600749. [DOI] [PubMed] [Google Scholar]
- 17.Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, Roark M, Legleiter J, Marsh JL, Thompson LM, Lindquist S, Muchowski PJ, Wanker EE. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Hum Mol Gen. 2006;15:2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 18.Meng F, Abedini A, Plesner A, Verchere CB, Raleigh DP. The Flavanol (−)-Epigallocatechin 3-Gallate inhibits amyloid formation by islet amyloid polypeptide, disaggregates amyloid fibrils, and protects cultured cells against IAPP-induced toxicity. Biochemistry. 2010;49:8127–8133. doi: 10.1021/bi100939a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandrashekaran IR, Adda CG, MacRaild CA, Anders RF, Norton RS. EGCG disaggregates amyloid-like fibrils formed by Plasmodium falciparum merozoite surface protein 2. Arch Biochem Biophys. 2011;513:153–157. doi: 10.1016/j.abb.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci USA. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc Natl Acad Sci USA. 2009;106:9033–9038. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa M, Masuda M, Nonaka T, Oikawa T, Yonetani M, Yamaguchi Y, Kato K, Hisanaga S, Goedert M. Inhibition of alpha-synuclein fibril assembly by small molecules: analysis using epitope-specific antibodies. FEBS Lett. 2009;583:787–791. doi: 10.1016/j.febslet.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 23.Aitken J, Loomes K, Konarkowska B, Cooper GJS. Suppression by polycyclic compounds of the conversion of human amylin into insoluble amyloid. Biochem J. 2003;374:779–784. doi: 10.1042/BJ20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lansbury PT, Lashuel HA. A century-old debate on protein aggregation and neurodegeneration enters the clinic. Nat Cell Biol. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 25.Ray S, Nowak R, Brown R, Lansbury P. Small-molecule-mediated stabilization of familial amyotrophic lateral sclerosis-linked superoxide dismutase mutants against unfolding and aggregation. Proc Natl Acad Sci USA. 2005;102:3639–3644. doi: 10.1073/pnas.0408277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Armstrong AH, Koehler AN, Hecht MH. Small molecule microarrays enable the discovery of compounds that bind the Alzheimer's Aβ peptide and reduce its cytotoxicity. J Am Chem Soc. 2010;132:17015–17022. doi: 10.1021/ja107552s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng F, Abedini A, Plesner A, Middleton CT, Potter KJ, Zanni MT, Verchere CB, Raleigh DP. The sulfated triphenyl methane derivative acid fuchsin is a potent inhibitor of amyloid formation by human islet amyloid polypeptide and protects against the toxic effects of amyloid formation. J Mol Biol. 2010;400:555–566. doi: 10.1016/j.jmb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westermark P, Wernstedt C, Wilander E, Hayden DW, Obrien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purification and characterization of a peptide from amyloid-rich pancreases of type-2 diabetic-patients. Proc Natl Acad Sci USA. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper GJS. Amylin compared with calcitonin-gene-related peptide—structure, biology, and relevance to metabolic disease. Endocrine Rev. 1994;15:163–201. doi: 10.1210/edrv-15-2-163. [DOI] [PubMed] [Google Scholar]
- 31.Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Amylin receptors: molecular composition and pharmacology. Biochem Soc Trans. 2004;32:865–867. doi: 10.1042/BST0320865. [DOI] [PubMed] [Google Scholar]
- 32.Nishi M, Sanke T, Nagamatsu S, Bell GI, Steiner DF. Islet amyloid polypeptide—a new beta-cell secretory product related to islet amyloid deposits. J Biol Chem. 1990;265:4173–4176. [PubMed] [Google Scholar]
- 33.Hutton JC. The insulin secretory granule. Diabetologia. 1989;32:271–281. doi: 10.1007/BF00265542. [DOI] [PubMed] [Google Scholar]
- 34.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 35.Clark A, Lewis CE, Willis AC, Cooper GJS, Morris JF, Reid KBM, Turner RC. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987;2:231–234. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- 36.Kahn SE, Hull RL, Westermark GT, Westermark P. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–3643. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 37.Marzban L, Park K, Verchere CB. Islet amyloid polypeptide and type 2 diabetes. Exp Geront. 2003;38:347–351. doi: 10.1016/s0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 38.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic-islet cell toxicity of amylin associated with type-2 diabetes-mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 39.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJS, Holman RR, Turner RC. Islet amyloid, increased alpha-cells, reduced beta-cells and exocrine fibrosis—quantitative changes in the pancreas in type-2 diabetes. Diabetes Res Clin Pract. 1988;9:151–159. [PubMed] [Google Scholar]
- 40.Butler PC, Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 41.Westermark GT, Westermark P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 42.Teplow DB, Kirkitadze MD, Bitan G. Paradigm shifts in Alzheimer's disease and other neuro degenerative disorders: the emerging role of oligomeric assemblies. J Neurosci Res. 2002;69:567–577. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 43.Potter KJ, Abedini A, Marek P, Klimek AM, Butterworth S, Driscoll M, Baker R, Nilsson MR, Warnock GL, Oberholzer J, Bertera S, Trucco M, Korbutt GS, Fraser PE, Raleigh DP, Verchere CB. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci USA. 2010;107:4305–4310. doi: 10.1073/pnas.0909024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akaishi T, Morimoto T, Shibao M, Watanabe S, Sakai-Kato K, Utsunomiya-Tate N, Abe K. Structural requirements for the flavonoid fisetin in inhibiting fibril formation of amyloid β protein. Neurosci Lett. 2008;444:280–285. doi: 10.1016/j.neulet.2008.08.052. [DOI] [PubMed] [Google Scholar]
- 45.Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent antiamyloidogenic and firbil-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim H, Park B, Lee K, Choi C, Jang S, Kim Y, Lee S. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J Agric Food Chem. 2005;53:8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 47.Levine H. Thioflavine-T interaction with amyloid beta-sheet structures. Amyloid. 1995;2:1–6. [Google Scholar]
- 48.Meng F, Marek P, Potter KJ, Verchere CB, Raleigh DP. Rifampicin does not prevent amyloid fibril formation by human islet amyloid polypeptide but does inhibit fibril thioflavin-T interactions: implications for mechanistic studies beta-cell death. Biochemistry. 2008;47:6016–6024. doi: 10.1021/bi702518m. [DOI] [PubMed] [Google Scholar]
- 49.Hudson SA, Ecroyd H, Kee TW, Carver JA. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS Lett. 2009;276:5960–5972. doi: 10.1111/j.1742-4658.2009.07307.x. [DOI] [PubMed] [Google Scholar]
- 50.Abedini A, Raleigh D. Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregation-prone polypeptide. Org Lett. 2005;7:693–696. doi: 10.1021/ol047480+. [DOI] [PubMed] [Google Scholar]
- 51.Abedini A, Singh G, Raleigh DP. Recovery and purification of highly aggregation-prone disulfide-containing peptides: application to islet amyloid polypeptide. Anal Biochem. 2006;351:181–186. doi: 10.1016/j.ab.2005.11.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.