Abstract
Serum samples, 100 in the total number, were collected from different laboratories in Tehran, Iran and tested for anti-Toxoplasma specific IgG and IgM antibodies using indirect immunofluorescent antibody test (IFAT). Using the IgG (chronic) and IgM (acute) positive samples, the IgG avidity test was performed by ELISA in duplicate rows of 96-well microtiter plates. One row was washed with 6 M urea and the other with PBS (pH 7.2), then the avidity index (AI) was calculated. Sixteen out of 18 (88.9%) sera with acute toxoplasmosis showed low avidity levels (AI≤50), and 76 out of 82 (92.7%) sera in chronic phase of infection showed high avidity index (AI>60). Six sera had borderline ranges of AI. The results showed that the IgG avidity test by ELISA could distinguish the acute and chronic stages of toxoplasmosis in humans.
Keywords: Toxoplasma gondii, IgG avidity test, ELISA, IgM, indirect immunofluorescent antibody test
INTRODUCTION
Toxoplasmosis is usually asymptomatic and distributed worldwide [1]. Serological studies show variable prevalence of 7.5-95.0% in different parts of the world [2]. The practical ways of transmission are eating undercooked meat of infected animals, through food or water contaminated with cat feces, and mother-to-fetus transmission [2,3]. Toxoplasmic infection of immunocompetent individuals is an asymptomatic and self-limiting illness [4]. However, it is potentially severe in 2 situations; pregnancy because of a risk of congenital toxoplasmosis and profound cellular immune deficiency status such as AIDS infection, transplantation, and malignant diseases that receive immune suppressive therapy [3]. When primary infection occurs during the first trimester of pregnancy, the disease can lead to significant morbidity and mortality in the developing fetus [1-2]. If the transmission occurs in the last trimester, it can cause clinical sings (mainly chorioretinitis) of congenital infection during the first 2 decades of life [3,5]. In immunodeficiency patients, the disease can lead to cerebral or extra-cerebral toxoplasmosis [6]. Therefore, it is important to distinguish between acute and chronic stages of infection for treatment and limitation of the effects, especially during the pregnancy [7]. It is difficult to isolate the parasite, so the diagnosis is usually based on serological methods [4,6-8], with detection of specific Toxoplasma IgG, IgM, and IgA antibodies [3].
There are some limitations in this protocol; significant rise of IgG titer is not always observed especially in children and adolescents with ocular manifestation of congenital toxoplasmosis [9]. Toxoplasma IgM antibody is present in some cases for years after primary infection (residual IgM) [8], and it can be detected in some diseases that have rheumatoid factors and antinuclear antibodies (ANA) [8]. Also, specific IgA antibodies can be detected after 45 months of a documented seroconversion [4,9]. On the other hand, it is well known that the strength of the bond between the antibody and epitope increases with the duration of infection [10]. The IgG avidity ELISA test could measure the avidity of specific IgG in acute and chronic phases of toxoplasmosis [10].
The avidity of IgG is low in acute phase and high in chronic phase of toxoplasmosis [2-6]; therefore, detection of a low IgG avidity is a reliable indicator for recent toxoplasmosis, whereas a high avidity shows that the infection is occurred in the previous 3-5 months [11,12]. The aim of this study was to perform IgG avidity test for detection of acute toxoplasmosis in correlation with IgM and IgG ELISA test.
MATERIALS AND METHODS
Serum sample collection
The present case-control study was carried out from 2009 to 2010. A total of 100 human serum samples were collected from different laboratories in Tehran, Iran. Sera were stored at -20℃ until use. At first, all the sera were checked for anti-Toxoplasma IgG and IgM antibodies with the immunofluorescent antibody (IFA) test. Then, they were divided into 2 separate groups:
Group I consisted of 18 serum samples from patients in acute phase of Toxoplasma infection, in which the presence of specific IgM antibodies was confirmed by IgM-IFA. All the patients had the sign of lymphadenopathy.
Group II consisted of 82 serum samples prepared from patients in chronic phase of Toxoplasma infection, and the presence of specific IgG antibodies was detected by IgG-IFA.
Antigen preparation
Tachyzoites of T. gondii (RH strain) were collected from the peritoneal cavity of mice that were injected 3 days earlier. Tachyzoites were washed with PBS (pH.7.2) 3 times, sonicated, and centrifuged at 12,000 g for 1 hr, and the supernatant was collected as the soluble antigen. The protein content was measured by the method of Bradford. The 96-well microtiter plates (Nunc Inc., Rochester, New York, USA) were coated with 5 µg/ml of diluted protein in carbonate buffer (pH 9.6). Coated plates were placed at 4℃ over night, then washed and stored at -20℃ until use.
Avidity ELISA
Microtiter plates previously coated with Toxoplasma antigens were washed 3 times with PBS plus 0.05% tween 20 (PBST). Serum samples were diluted 1/200 and added (100 µl/well) on 2 rows of a plate (row A and row B), after incubation for 45 min at 37℃; the row B was washed 3 times with PBST, and the row A was washed 3 times with the modified PBST buffer containing 6 M urea and a fourth time with PBST. The anti-human IgG conjugated with horseradish peroxidase (HRP) (Dako, Glostrup, Denmark) was added with the dilution of 1/1,000 in PBST.
After incubation and washing, the chromogenic substrate, o-phenylenediamine (OPD) Merk, Darmstadt, Germany), was added. The reaction was stopped by addition of sulfuric acid 20%. The absorbance (Abs) was read by an automated ELISA reader (BIOTEC, LX800, Winooski, Vermont, USA) at 492 nm. Avidity index (AI; %) was calculated as the result of Abs of wells washed with PBS-urea (U+), divided by the Abs of wells washed with PBST (U-), and multiplied with 100, based on the formula; AI=Abs(U+)/Abs(U-)×100.
IgM ELISA
Anti-Toxoplasma IgM antibodies were tested by IgM-ELISA. Briefly, sera were diluted serially and added to the T. gondii antigen-coated microtiter plate and then anti-human IgM antibodies conjugated with HRP was added. After incubation and washing, the chromogenic substrate OPD was added, and the optical density was read by means of an automated ELISA-reader.
RESULTS
Acute toxoplasmosis group
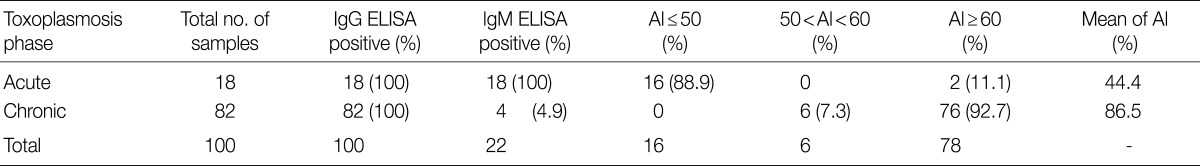
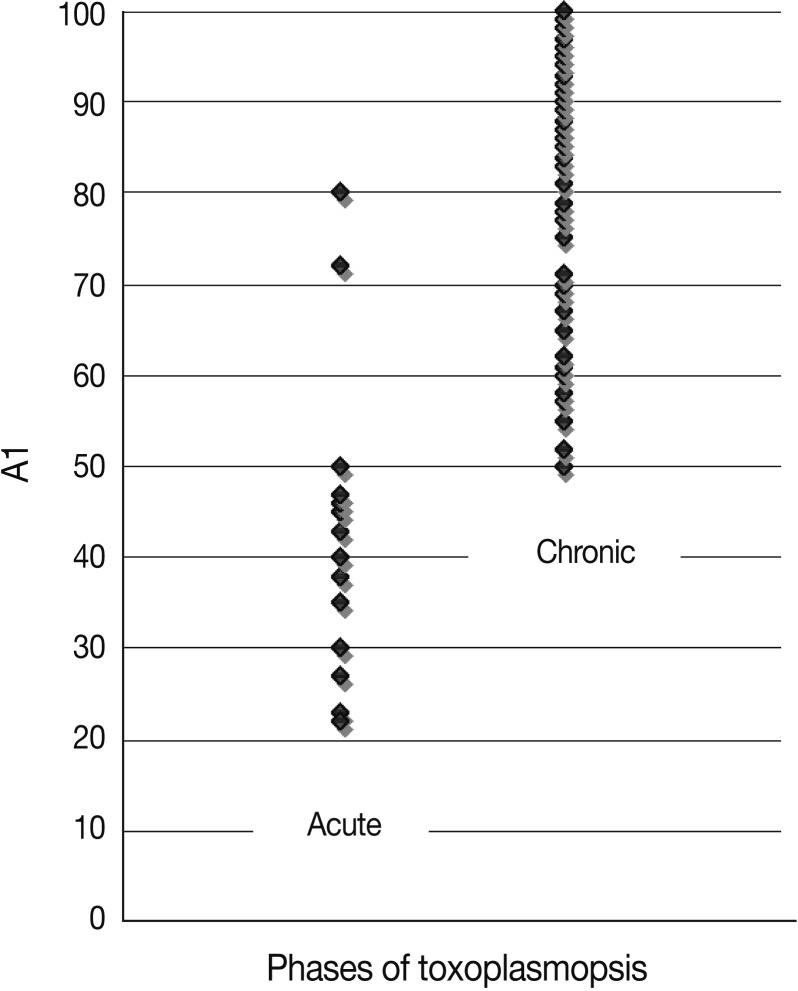
Among 18 patients with acute toxoplasmosis, 16 (88.9%) had the AI as 50% and lower, and 2 (11.1%) showed the greater AI than 50%. The mean AI for these 16 sera was 44.4%. All of the sera were positive for Toxoplasma specific IgG and IgM antibodies (Table 1; Fig. 1). The statistical analysis showed that there was a significant correlation between high IgM ELISA titers and low avidity of IgG (P<0.05).
Table 1.
Avidity index (AI) and anti-T. gondii antibodies
Fig. 1.
Values of avidity index (AI; %) in acute and chronic toxoplasmosis patients.
Chronic toxoplasmosis group
Seventy six (92.7%) of 82 sera with chronic toxoplasmosis had the AI greater than 60%, and 6 (7.3%) of them had the AI among 50-60% (Fig. 1; Table 1). The mean of AI for this group was 86.5%. All the sera from this group had high titers of IgG antibodies by IgG-ELISA method, and 4 (4.9%) of them had acceptable titers of IgM-ELISA (Table 1; Fig. 1). No significant correlation was found between IgG concentration and avidity index in 2 group samples.
DISCUSSION
Distinguishing of acute and chronic phases of toxoplasmosis has critical importance in pregnant women and immunocompromised patients. Some assays have been used to measure Toxoplasma specific IgM antibodies as indicators of the acute phase infection [7,13-15]. Toxoplasma IgM antibodies can be detected for 1 year or longer in some cases, so in asymptomatic individuals with stable titers of Toxoplasma IgG antibodies, positive IgM results are not easy to interpret [8].
The IgG avidity assay that had been started in the 1980s for diagnosis of rubella and hepatitis C is now used to distinguish between acute and chronic toxoplasmosis [9]. This method was originally developed by Hedman et al. [10] in Finland. It is based on dissociation of hydrogen bond between antigen and antibody with urea [8]. High avidity (AI≥60%) means that Toxoplasma infection was acquired before 3 months ago, whereas borderline avidity (50%<AI<60%) means infection at an indeterminate period, and low avidity (AI≤50%) means that the infection was acquired within the last 3 months.
According to the present study, 88.9% of patients with acute toxoplasmosis had low avidity (AI≤50%) and 92.7% of individuals with chronic infection had high avidity ranges (AI≥60%). It was suggested that Toxoplasma specific IgG avidity assay that is performed on specific IgM positive samples could diagnose acute and chronic phases of toxoplasmosis [6,16]. Candolfi et al. [3] also demonstrated that measuring of IgG avidity could differentiate between acute and chronic phases of toxoplasmosis. Our results showed that there was no significant relation between the concentration of IgG antibody and the level of AI. Therefore, the strength of links between antibody and antigen is not dependent on antibody concentration, which was confirmed by other investigators [3]. Meanwhile, there are a converse relationship between IgM titer and levels of IgG AI in this study (P<0.05). On the other hand, Remington et al. [17] suggested that IgG avidity ELISA test is not enough to confirm acute toxoplasmosis, and it should be done in addition to IgM ELISA test. Many investigators had mentioned the role of IgM detection as a key for diagnosis of acute toxoplasmosis [1,2,6]. One of the limitations of IgG avidity method is detection of reactivated toxoplasmosis in immunocompromised patients [18]. In the present study, both IgM ELISA and IgG avidity ELISA tests together could confirm the phases of infection absolutely.
ACKNOWLEDGMENTS
This study was financially supported by Vice Chancellors for Education of Tehran University of Medical Sciences and approved by Ethical Committee of Tehran University of Medical Sciences. The authors wish to thank Miss Roshanak R. Shahsavandi for her assistance.
References
- 1.Leite M, Siciliano S, Rocha LS, Justa MT, César KR, Granato CF. Correlation between specific IgM levels and percentage IgG-class antibody avidity to Toxoplasma gondii. Rev Inst Med Trop Sao Paulo. 2008;50:237–242. doi: 10.1590/s0036-46652008000400010. [DOI] [PubMed] [Google Scholar]
- 2.Iqbal J, Khalid N. Detection of acute Toxoplasma gondii infection in early pregnancy by IgG avidity and PCR analysis. J Med Microbiol. 2007;56(Pt 11):1495–1499. doi: 10.1099/jmm.0.47260-0. [DOI] [PubMed] [Google Scholar]
- 3.Candolfi E, Pastor R, Huber R, Filisetti D, Villard O. IgG avidity assay firms up the diagnosis of acute toxoplasmosis on the first serum sample in immunocompetent pregnant women. Diagn Microbiol Infect Dis. 2007;58:83–88. doi: 10.1016/j.diagmicrobio.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Crucerescu E, Lovin DR. Study on specific IgG avidity as a tool for recent primary Toxoplasma gondii infection diagnosis. J Prev Med. 2002;10:56–62. [Google Scholar]
- 5.Roberts A, Hedman K, Luyasu V, Zufferey J, Bessières MH, Blatz RM, Candolfi E, Decoster A, Enders G, Gross U, Guy E, Hayde M, Ho-Yen D, Johnson J, Lécolier B, Naessens A, Pelloux H, Thulliez P, Petersen E. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur J Clin Microbiol Infect Dis. 2001;20:467–474. doi: 10.1007/pl00011289. [DOI] [PubMed] [Google Scholar]
- 6.Hedman K, Lappalainen M, Sönderlund M, Hedman L. Avidity of IgG in serodiagnosis of infectious diseases. Rev Med Microbiol. 1993;4:123–129. [Google Scholar]
- 7.Marcolino PT, Silva DAO, Leser PG, Camargo ME, Mineo JR. Molecular markers in acute and chronic phases of human toxoplasmosis: determination of immunoglobulin G avidity by Western blotting. Clin Diagn Lab Immunol. 2000;7:384–389. doi: 10.1128/cdli.7.3.384-389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedman K, Lappalainen M, Seppäiä I, Mäkelä O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. doi: 10.1093/infdis/159.4.736. [DOI] [PubMed] [Google Scholar]
- 9.Camargo ME, da Silva SM, Leser PG, Granato CH. Avidity of specific IgG antibodies as markers of recent primary infection caused by Toxoplasma gondii. Rev Inst Med Trop Sao Paulo. 1991;33:213–218. [PubMed] [Google Scholar]
- 10.Hedman K, Seppälä I. Recent rubella virus infection indicated by a low avidity of specific IgG. J Clin Immunol. 1988;8:214–221. doi: 10.1007/BF00917569. [DOI] [PubMed] [Google Scholar]
- 11.Flori P, Bellete B, Crampe C, Maudry A, Patural H, Chauleur C, Hafid J, Raberin H, Tran Manh Sung R. A technique for dating toxoplasmosis in pregnancy and comparison with the Vidas anti-Toxoplasma IgG avidity test. Clin Microbiol Infect. 2008;14:242–249. doi: 10.1111/j.1469-0691.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 12.Paul M. Immunoglobulin G avidity in diagnosis of toxoplasmic lymphadenopathy and ocular toxoplasmosis. Clin Diagn Lab Immunol. 1999;6:514–518. doi: 10.1128/cdli.6.4.514-518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joynson DH, Payne RA, Rawal BK. Potential role of IgG avidity for diagnosing toxoplasmosis. J Clin Pathol. 1990;43:1032–1033. doi: 10.1136/jcp.43.12.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montoya JG, Huffman HB, Remington JS. Evaluation of the immunoglobulin G avidity test for diagnosis of toxoplasmic lymphadenopathy. J Clin Microbiol. 2004;42:4627–4631. doi: 10.1128/JCM.42.10.4627-4631.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikawa A, Yamada H, Yamamoto T, Mizue Y, Akashi Y, Hayashi T, Nihei T, Nishiwaki M, Nishihira J. A case of congenital toxoplasmosis whose mother demonstrated serum low IgG avidity and positive tests for multiplex-nested PCR in the amniotic fluid. J Obstet Gynaecol Res. 2009;35:372–378. doi: 10.1111/j.1447-0756.2008.00953.x. [DOI] [PubMed] [Google Scholar]
- 16.Press C, Montoya JG, Remington JS. Use of a single serum sample for diagnosis of acute toxoplasmosis in pregnant women and other adults. J Clin Microbiol. 2005;43:3481–3483. doi: 10.1128/JCM.43.7.3481-3483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Remington JS, Thulliez P, Montoya JG. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol. 2004;42:941–945. doi: 10.1128/JCM.42.3.941-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mechain B, Garin YJ, Robert-Gangneux F, Dupouy-Camet J, Derouin F. Lack of utility of specific immunoglobulin G antibody avidity for serodiagnosis of reactivated toxoplasmosis in immunocompromised patients. Clin Diagn Lab Immunol. 2000;7:703–705. doi: 10.1128/cdli.7.4.703-705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]