Abstract
The causes of obstructive sleep apnea (OSA) are multifactorial. Neural injury affecting the upper airway muscles due to repetitive exposure to intermittent hypoxia and/or mechanical strain resulting from snoring and recurrent upper airway closure have been proposed to contribute to OSA disease progression. Multiple studies have demonstrated altered sensory and motor function in patients with OSA using a variety of neurophysiological and histological approaches. However, the extent to which the alterations contribute to impairments in upper airway muscle function, and thus OSA disease progression, remains uncertain. This brief review, primarily focused on data in humans, summarizes: (1) the evidence for upper airway sensorimotor injury in OSA and (2) current understanding of how these changes affect upper airway function and their potential to change OSA progression. Some unresolved questions including possible treatment targets are noted.
Keywords: sleep apnea, upper airway muscles, neuropathy, myopathy, upper airway physiology, upper airway reflexes
Introduction
In the upper airway there are changes in sensation, muscle properties, and neural drive in patients with obstructive sleep apnea (OSA). These changes are loosely termed airway remodeling and may adversely affect upper airway function during sleep. Changes in nerve and muscle properties may result from vibration through snoring, hypoxia, or both.
The extent to which snoring and hypoxia exacerbate the disease and lead to important damage is unresolved. In this review, some of the more convincing evidence for and against upper airway remodeling in which data have been acquired in both OSA patients and non-OSA controls is highlighted. We review the pathophysiological evidence under three separate headings: (1) anatomical remodeling of the upper airway muscles, (2) efferent changes, and (3) afferent changes (see Figure 1). This encompasses evidence from a variety of neurophysiological approaches including histological, electrophysiological, and physiological studies. The function of upper airway reflexes and tongue force/fatigue characteristics in OSA vs. non-OSA subjects is also briefly reviewed. Finally, we discuss how upper airway remodeling and neural injury might contribute to upper airway closure during sleep.
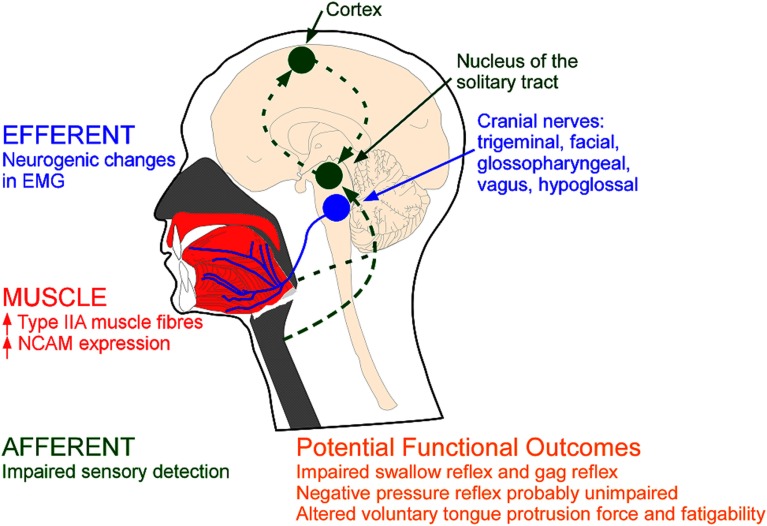
Figure 1.
Types of evidence for neuromuscular pathology in OSA. MUSCLE: Remodelling may be reflected via anatomical changes within the upper airway muscles (red); EFFERENT: changes in the electromyogram (EMG) of the upper airway muscles innervated via the cranial nerves (blue); AFFERENT: changes in sensory pathways (green). NCAM=neural cell adhesion molecule.
Anatomical Structure of Upper Airway Muscles
Several histological studies support the presence of upper airway remodeling in patients with OSA (Tables 1 and 2). Broadly, this includes changes in: muscle fiber type, direction of muscle fibers, and anatomical arrangement of nerve fiber terminals. Sixteen studies are summarized in Table 2. Most of these studies did not rule out OSA in the healthy non-OSA/control groups (full polysomnography evaluations are reported in only four studies). Thus, the reported magnitude of these changes may be an underestimation. The upper airway muscles are composed of a variety of fast and slow muscle fiber types (Table 1) with conventional myosin heavy chain (MHC) isoforms and three primary MHC phenotypes and two subtypes (in order of prevalence for the adult genioglossus muscle in non-OSA subjects MHCIIA > MHCI-IIX > MHCI > MHCI-IIA > MHCIIX; Daugherty et al., 2012). A limitation of biopsies is that they are obtained from a limited area and may not represent the characteristics of the whole muscle. Nonetheless, a number of studies have shown an increase in the percentage of Type IIA muscle fibers in the order of ∼15% in several upper airway muscles in OSA patients including the uvula, genioglossus, medium pharyngeal constrictor, and palatopharyngeus (see Table 1; Smirne et al., 1991; Ferini-Strambi et al., 1998; Carrera et al., 1999; Series et al., 2000; Lindman and Stal, 2002; c.f. Friberg et al., 1998a). Type IIA muscle fibers are fast twitch fibers that can use both aerobic and anaerobic metabolism. While the cause for this increase in fiber type remains unknown, the change is likely to reflect training. Repetitive loading with regular nightly eccentric contractions (neural drive advancing the tongue anteriorly with negative airway pressure pulling the tongue posteriorly) combined with hypoxia may lead to fiber type alterations in the upper airway muscles indicative of an endurance trained muscle (Hildebrand et al., 1991; Pette and Staron, 2001). However, studies to definitively isolate the effects of training on upper airway muscle fiber type have not been done.
Table 1.
Percentage of Type I, Type IIA, and Type IIB muscle fibers in non-OSA and patients with OSA.
| Muscle | Non-OSA % of fibers |
OSA % of fibers |
Author | ||||
|---|---|---|---|---|---|---|---|
| Type I | Type IIA | Type IIB | Type I | Type IIA | Type IIB | ||
| Genioglossus | 61 | 39 | 41 | 59 | Carrera et al. (1999) | ||
| 33.3 | 48.8 | 18.3 | 33 | 53.3 | 11.6 | Series et al. (1996) | |
| Palatopharyngeus | 13.7 | 31.5 | 1.6 | 22.1 | 58.4 | 0.5 | Lindman and Stal (2002) |
| 26 | 77 | 16 | 87 | De Vuono et al. (2007) | |||
| Medium pharyngeal constrictor | 49 | 41.3 | 9.7 | 22.4 | 75.2 | 2.4 | Smirne et al. (1991) |
| 51.9 | 36.1 | 11.8 | 30.7 | 61.8 | 7.5 | Ferini-Strambi et al. (1998) | |
| Uvula | 15.2 | 69.2 | 10.1 | 10.7 | 82.6 | 4.3 | Series et al. (1995) |
| 10.6 | 73.5 | 15.9 | 8.1 | 82.3 | 9.6 | Series et al. (1996) | |
| ∼15 | 76.1 | – | ∼1.8 | 89.4 | – | Series et al. (2000) | |
| 12.8 | 65.3 | 4.8 | 19.5 | 55.8 | 16.1 | Lindman and Stal (2002) | |
Histological biopsy studies have confirmed differences in the muscle properties between non-OSA subjects and patients with OSA. There is an increase in the fraction of Type IIA fiber types in human upper airway musculature. Note: Type IIB fibers in humans are now referred to as Type IIX. Note: some rows do not total 100%. In some cases the number of Type IIB fibers were too low to be considered for calculation (e.g., Series et al., 2000), specialized MHC isoforms were calculated in some cases and are not displayed in the Table (see Lindman and Stal, 2002), and others reflect estimations based on available data.
Table 2.
Anatomical structural changes.
| Reference | Main findings | Comments |
|---|---|---|
| Woodson et al. (1991) | Soft palate and uvula biopsy; histological differences in soft palate and uvula in OSA vs. snorers and non-snorers measured via electron microscopy | Notes A and B |
| • Focal degeneration of myelinated nerve fibers and axons in OSA | ||
| • Focal muscle fiber atrophy in snorers and OSA | ||
| • Muscle bundle disruption and fiber degeneration in all snorers and OSA | ||
| Edstrom et al. (1992) | Palatopharyngeal muscle biopsy; neurogenic changes in OSA vs. non-OSA | Note A |
| • ↑ Number of angulated atrophic fibers | ||
| • Fiber type grouping | ||
| • Muscle fiber atrophy | ||
| Series et al. (1995) | Uvula biopsy; altered anatomy and function in OSA patients vs. snorers | All subjects had sleep studies |
| • ↑ Cross-sectional area and Type IIA muscle fiber area in OSA | No healthy control group | |
| • Creative kinase, GADPH, phosphofructokinase, and phosphorylase ↑ in OSA (all elements in the anaerobic pathway) | Findings consistent with a muscle training effect | |
| • ↑ Twitch and tetanic tension in OSA | ||
| • Fatigability index similar between the two groups | ||
| Series et al. (1996) | Uvula and genioglossus biopsies; altered anatomy in OSA patients vs. snorers | All subjects had sleep studies |
| • Uvula ↑ glycolytic, glycogenolytic, and anaerobic enzyme activities | No healthy control group | |
| • No difference in genioglossus glycolytic and anaerobic enzyme activities | Snorers had a low AHI | |
| • Uvula and genioglossus: ↑ Type IIA, ↓ Type IIB muscle fiber%, and no change in muscle fiber area | Findings consistent with a muscle training effect | |
| Friberg et al. (1997) | Soft palate mucosa biopsy; neurogenic changes in OSA vs. snorers and non-snorers | Note A |
| • ↑ Density of afferent nerve endings in epithelium | Non-OSA = chronic tonsillitis and one pharyngeal tumor | |
| • ↑ Number of varicose nerve endings in epithelium | ||
| • ↑ Nerves containing neuropeptides (↑ PGP9.5 density indicating increased neurons and nerve fibers) | Epithelium thicker in OSA | |
| Ferini-Strambi et al. (1998) | Medium pharyngeal constrictor and quadricep biopsies; altered anatomy of pharyngeal constrictor, but, not quadricep muscle in OSA vs. non-OSA | OSA based on clinical symptoms and portable monitor |
| • ↑ % Type I, ↑ % Type IIA, and ↓ % Type IIB | ||
| • ↑ Type IIA fiber diameter | ||
| Friberg et al. (1998a) | Palatopharyngeal and anterior tibial biopsies; altered anatomy of palatopharyngeal, but, not in anterior tibial muscle in OSA vs. non-OSA | Note A, interviewed history, and spouses |
| • Similar palatopharyngeal muscle fiber type distribution | Non-OSA = tonsillectomy chronic tonsillitis, 2 for tumor | |
| • ↑ Palatopharyngeal muscle fiber size variations | ||
| • Rounded atrophic palatopharyngeal muscle fibers | ||
| • ↑ Incidence of centrally located nuclei and split palatopharyngeal muscle fibers | ||
| • Palatopharyngeal muscle fiber type grouping | ||
| Carrera et al. (1999) | Genioglossus biopsy; altered anatomy and function in OSA vs. non-OSA | Note A |
| • Untreated OSA patients ↑ % Type II muscle fibers, and ↑ fatigability | No break down of muscle fibers into Type IIA and B | |
| • No differences in muscle fiber distribution and fatigability in a separate analysis of CPAP treated patients vs. non-OSA | Not the same subjects pre- and post-treatment | |
| Series et al. (2000) | Uvula muscle biopsy; altered anatomy in OSA patients vs. snorers | All subjects had sleep studies |
| • ↑ % Type IIA muscle fibers | No control group | |
| • No difference in muscle fiber area in Type I or II, thus, no evidence for atrophy or hypertrophy | ||
| Paulsen et al. (2002) | Uvula mucosa biopsy; altered anatomy in OSA patients vs. snorers and non-OSA. Measured via electron microscopy | Note B |
| • Absence of connective tissue papillae | ||
| • ↓ Cytokeratins | ||
| • Acanthosis of epithelium | ||
| • ↑ Leukocytes inside the lamina propria | ||
| Lindman and Stal (2002) | Uvula and palatopharyngeus biopsies; altered anatomy in OSA patients vs. non-OSA | Note B |
| • ↓ Ratio of area of palatopharyngeus muscle fibers to connective tissue in OSA patients | ||
| • ↑ Palatopharyngeus muscle Type I, ↑ Type IIA, and ↓ Type IIAB and IIB muscle fibers in OSA patients | ||
| • ↓ Uvula muscle fiber diameters for all fiber types except Type IIC/IM | ||
| Boyd et al. (2004) | Soft palate and/or tonsillar pillar biopsies; denervation in OSA patients vs. non-OSA | Note A, screened via questionnaire |
| • ↑ PGP9.5 specifically expressed in the neurons and nerve fibers | ||
| • ↑ Sarcolemmal expression of N-CAM 16 vs. 1% | ||
| De Vuono et al. (2007) | Palatopharyngeal biopsy; similar findings in children with OSA, vs. snorers vs. controls with the presence of each of the following in similar proportions | All subjects had sleep studies Findings suggest that these histological features may reflect a “normal” muscle rather than neurogenic or myopathic pathology |
| • Muscle fiber size variability | ||
| • Rounded atrophic muscle fibers | ||
| • ↑ Type II muscle fibers | ||
| • Irregular muscle fiber architecture “moth-eaten” | ||
| Stål et al. (2009) | Uvula and palatopharyngeaus biopsies; altered anatomy in OSA patients vs. non-OSA | Note A, no history of any significant disease Note B |
| • ↓ Capillary density per muscle fiber area | ||
| • Histopathological in all OSA muscle biopsies: fibrosis, variability in fiber size, developmental MyHC isoforms | ||
| Bassiouny et al. (2009) | Uvula biopsies; degenerative anatomical changes to both myelinated and unmyelinated nerve endings in OSA patients vs. snorers and non-OSA | Note A, confirmed via partners and medical history |
| • Myelin sheath had obvious signs of: degenerated and irregular Schwann cells, lamellated bodies, neurofilament loss, and fatty degeneration | Note B | |
| Non-OSA died from causes unrelated to head and neck | ||
| Stål and Johansson (2012) | Uvula and palatopharyngeaus biopsies; altered anatomy in OSA patients vs. non-OSA | Note A, previously physically healthy Note B |
| • ↓ Capillaries around abnormal muscle fibers | ||
| • Abnormal mitochondrial distribution | ||
| • Uvula ↑ fiber hypertrophy | ||
| • Palatopharyngeus muscle fibers display signs of both atrophy and hypertrophy in OSA vs. controls |
Note A: indicates that overnight sleep studies were not performed in the non-OSA group.
Note B: indicates that samples from the non-OSA group were obtained via autopsy.
Anatomical changes consistent with upper airway muscle remodeling in OSA in adults include: increased prevalence of angulated muscle fibers, increased diameter of muscle fibers (Smirne et al., 1991; Ferini-Strambi et al., 1998; Series et al., 2000; Sauleda et al., 2003; Svanborg, 2005; De Vuono et al., 2007; Stål et al., 2009), atrophied muscles fibers (Edstrom et al., 1992; Friberg et al., 1998a), changes in capillary density and mitochondria content (Stål et al., 2009; Stål and Johansson, 2012), fiber type grouping (Edstrom et al., 1992; Ferini-Strambi et al., 1998; see Table 2), and increased neural cell adhesion molecule (N-CAM) expression (Boyd et al., 2004). However, one study found similarly widespread neurogenic changes in children both with and without OSA (De Vuono et al., 2007). Thus, questions remain as to what degree of chronic partial denervation is “normal” for upper airway muscles.
Snoring is a key feature of OSA. The vibration induced by snoring and the mechanical strain caused by repetitive upper airway closure is associated with inflammatory changes to soft tissue structures of the upper airway (Berger et al., 2002; Boyd et al., 2004). The local inflammation increases the thickness of the surrounding tissues and may narrow the upper airway to exacerbate obstructive apneas (Rubinstein, 1995; Sekosan et al., 1996; Berger et al., 2002). Patients with severe snoring have soft palate swelling in the morning that recedes while awake (Sekosan et al., 1996).
There is also increased fat in and around the muscles of the upper airway in patients with OSA (Horner et al., 1989; Stauffer et al., 1989; Schwartz et al., 1991; Zohar et al., 1998b; Berger et al., 2002). The importance of increased fat is highlighted by the observation that weight loss in obese OSA patients can eliminate airway obstruction. While infiltration of fat is not a result of neural changes, it will alter the dynamics of the upper airway. With increased intramuscular fat, contractile performance may change via altered loading of individual muscle fibers and altered “passive” properties (Busha et al., 2002). Given that some of the tension developed in one part of the muscle can be transmitted via shear links to other parts of the muscle (Kjaer, 2004), increased fat may alter the force distribution across muscle fibers in OSA patients. Nevertheless, maximal voluntary tongue protrusion force is the same, or increased in OSA patients (e.g., Mezzanotte et al., 1992; Mortimore et al., 2000; Eckert et al., 2011).
Evidence for Upper Airway Efferent Changes
In addition to changes in the soft tissues and muscle fibers, there are also electrophysiological changes in the muscles of patients with OSA (Table 3). During wakefulness patients with OSA have higher levels of multiunit electromyographic activity (EMG) recorded in the upper airway muscles compared to healthy control subjects (Mezzanotte et al., 1992, 1996; Fogel et al., 2001; c.f. Series et al., 2009). The apparent increase in drive was ascribed to a neural compensation for a narrow upper airway. This concept became widely accepted. The neural compensation was thought to be analogous to that in diaphragm and other obligatory inspiratory muscles of patients with chronic obstructive pulmonary disease where more motor units are recruited and their average discharge frequencies are higher (De Troyer et al., 1997; Gorman et al., 2005) because of the increased resistive load. Recent studies indicate that motor unit discharge frequencies also increase with higher neural drive to the genioglossus (Bailey et al., 2007; Saboisky et al., 2010). However, there is currently no strong evidence to support an overall increase in the discharge frequencies, or recruitment of additional motor units in the genioglossus in OSA during wakefulness (Saboisky et al., 2007, 2012). During the pre-inspiratory phase of the respiratory cycle the overall excitability of the genioglossus motoneuron pool appears to be increased in OSA patients vs. controls. The timing of the initial activation of phasic inspiratory motor units is earlier (Saboisky et al., 2007). Similarly, during late expiration, the conduction times of motor evoked potentials elicited via transcranial magnetic stimulation of the motor cortex are shorter in the genioglossus in OSA patients than in controls (Wang et al., 2010). Thus, while there is currently no evidence for increased discharge frequencies or additional recruitment of motor units there are quantifiable changes in the timing of neural drive to genioglossus in OSA. Therefore, how do the new findings fit with these earlier studies?
Table 3.
Efferent changes.
| Reference | Main findings | Comments |
|---|---|---|
| Mezzanotte et al. (1992) | Fine-wire intramuscular multiunit genioglossus electromyography; increased neural drive in OSA vs. non-OSA | Note A |
| • ↑ Peak EMG during quiet breathing | ||
| Fogel et al. (2001) | Fine-wire intramuscular genioglossus electromyography; increased neural drive in OSA vs. non-OSA | Note A |
| • ↑ EMG during wakefulness (↑ tonic activation and ↑ negative-epiglottic pressure during entrained iron-lung ventilation in inspiration) | Primary analysis included older OSA patients | |
| • No difference in mean EMG/Pepi relationship in OSA and controls under all conditions (basal breathing, iron-lung ventilation and heliox breathing) | Not matched for BMI | |
| • Age matched sub-analysis EMG not increased (but trended ↑ in OSA tonic, peak, and phasic) | ||
| Svanborg (2005) | Concentric needle single motor unit palatopharyngeus electromyography; neurogenic changes in OSA patients vs. snorers and controls | Note A |
| • 10/12 OSA evidence of lesions: polyphasic potentials, 3/15 snorers exhibited “moderate” lesions | ||
| Saboisky et al. (2007) | Monopolar needle single motor unit genioglossus electromyography; neurogenic changes in OSA patients vs. controls | Note B Not matched for BMI |
| • ↑ Area and longer duration action potentials | ||
| • Distribution of six unit types identical between groups | ||
| • Inspiratory units recruited earlier in OSA patients | ||
| • ↑ Peak discharge frequencies of Inspiratory Phasic units but, inspiratory tonic ↓ in OSA | ||
| Series et al. (2009) | Transcrainal magnetic stimulation and surface genioglossus and diaphragm electromyography; different responses in OSA patients vs. controls | Note B |
| • ↓ Latency of genioglossus MEPs during tongue protraction | ||
| • Negative correlation between genioglossus MEP latency and AHI | ||
| • Similar baseline genioglossus EMG activity in OSA patients and controls during quiet breathing | ||
| Ramchandren et al. (2010) | Hypoglossal nerve conduction; abnormalities in patients with OSA vs. controls | Note A |
| • ↓ Compound muscle action potential amplitudes | Treated OSA subjects | |
| Saboisky et al. (2012) | Concentric needle MACRO and single motor unit genioglossus electromyography; neurogenic changes in OSA patients vs. controls | Note B Large sample: no timing data minimal frequency data No snoring data BMI matched subjects |
| • ↑ Duration, size index, relative irregularity coefficient, phases, turns, area to amplitude ratio area/phase, and MACRO duration amplitude and area | ||
| • No difference in peak to peak amplitudes | ||
| • Irregularity coefficient correlated with minimal overnight oxygen saturation |
Note A: indicates that overnight sleep studies were not performed in the non-OSA group.
Note B: indicates that both the OSA and non-OSA group had overnight sleep studies.
An alternative explanation is that the elevated EMG in the upper airway muscles in patients with OSA is secondary to neurogenic remodeling. This is characterized as chronic partial denervation of muscle fibers, with reinnervation of the orphaned muscle fibers by collateral sprouting of surviving motor axons (Boyd et al., 2004; Gonzalez-Forero et al., 2004). The overall duration of motor unit potentials increase, often with increased amplitude (Bertorini et al., 1994; Preston and Shapiro, 2002). Recent investigations using single motor unit techniques have shown that the motor unit potentials of upper airway muscles in OSA patients are larger in area, longer in duration, and more complex (Svanborg, 2005; Saboisky et al., 2007, 2012; see also Podnar and Dolenc Groselj, 2010). These changes could contribute to the increased multiunit EMG in OSA. Thus, active remodeling may help to preserve the functional capacity of the muscles. However, the presence of denervation and subsequent axonal sprouting may lead to changes in fine motor control such as speech (Goldshtein et al., 2011). An additional potential problem with the initial reports of increased multiunit EMG in OSA (awake) is that values were normalized to the EMG produced during a maximal volitional task and may not be comparable between OSA patients and controls.
Evidence for Upper Airway Afferent Changes
If the anatomically deeper upper airway motor axons are affected by vibration, sensory afferents, closer to the airway surface, should also be impaired. However, the evidence supporting sensory nerve impairment in OSA is less convincing than that for motor nerves. If sensory nerves are affected, this may impair normal reflex mechanisms which contribute to upper airway function.
A variety of techniques have revealed potential sensory impairments in OSA. Two-point discrimination and detection of vibration, airflow, and temperature for the upper airway are all altered in OSA patients (see Table 4). The ability to detect and rate the size of inspiratory resistive loads to breathing in patients with OSA is impaired (McNicholas et al., 1984; Tun et al., 2000; c.f. Clerk et al., 1994). However, cognitive processing is important for these measurements and they may be independently affected by sleepiness and prior respiratory loading. Airway edema is present in untreated OSA patients and may impair upper airway sensation. Thus, separating the relative contribution of airway edema and sleepiness and cognitive processing vs. sensory nerve injury is difficult. One strategy to overcome some of these confounders is to repeat measurements after an acute period of continuous positive airway pressure (CPAP) treatment. Here, edema and sleepiness are likely to resolve more rapidly than potential nerve injury changes. In support of a role of long-term sensory injury in OSA, one study revealed only partial resolution of oropharynx sensation with vibration detection and two-point discrimination after 4 months of CPAP therapy (Kimoff et al., 2001). However, in another study, impaired sensation to loaded breathing was corrected after 2 weeks of CPAP therapy (Tun et al., 2000). In untreated OSA patients breathing responses to respiratory stimuli measured during wakefulness vary. Impaired respiratory load detection is associated with a reduction in the ventilatory response to hypercapnia (McNicholas et al., 1984). Conversely, the ability to adjust breathing (e.g., respiratory timing and minute ventilation) in response to respiratory loading may be unaltered in OSA (Hlavac et al., 2007).
Table 4.
Afferent changes.
| Reference | Main findings | Comments |
|---|---|---|
| McNicholas et al. (1984) | Inspiratory resistive load detection thresholds; decreased ability to detect inspiratory resistive loads in OSA patients vs. non-OSA | Note C |
| Clerk et al. (1994) | Inspiratory resistive loads detection thresholds; not different in normal weight OSA patients vs. non-OSA | Note C |
| Friberg et al. (1998b) | Soft palate mucosa Laser Doppler perfusion/electrical stimulation; altered afferent nerve regulation in OSA Patients vs. snores and non-OSA | Note A |
| • ↓ Vasodilatation of the peripheral mucosa of severe OSA patients vs. non-OSA | ||
| Tun et al. (2000) | Inspiratory resistive load magnitude perception; reduced in untreated OSA vs. non-OSA | Notes A and C |
| • Reversed after 2 weeks CPAP therapy | ||
| Kimoff et al. (2001) | Two-point discrimination and vibration sensation in the oropharynx, lip, and hand; reduced oropharynx, but, not lip and hand sensation in OSA and non-apneic snorers vs. controls | Note B and C Not matched for BMI |
| • Partial reversibility of changes post-CPAP in OSA, but, still impaired | ||
| • Anesthesia abolished two point discrimination in all patients and ↓ vibratory sensation | ||
| • Similar impairment in both snorers and OSA | ||
| Guilleminault et al. (2002) | Two-point palatal discrimination; reduced in OSA vs. UARS and controls | Notes B and C |
| Gora et al. (2002) | Respiratory-related evoked potentials (RREP); no difference in P1 amplitude or latency in OSA vs. non-OSA | Note A |
| Akay et al. (2003) | Respiratory-related evoked potentials; reductions in early RREP activity in OSA vs. non-OSA | Note A |
| Predominantly CPAP treated OSA patients | ||
| Afifi et al. (2003) | Respiratory and auditory evoked potentials; no difference in early waveform components in OSA vs. non-OSA | Note B, RDI defined using portable monitor |
| Nguyen et al. (2005) | Sensory detection thresholds for air-pressure pulses delivered to the oropharynx, velopharynx, hypopharynx, and larynx determined via endoscope; specific alternations in certain OSA patients vs. controls | Notes B and C Reproducible measures after 3 months |
| • Two subgroups of patients with and without abnormal sensory measures at multiple upper airway sites | ||
| • Correlations between laryngeal sensory measures and apnea severity and nadir SaO2 | ||
| Dematteis et al. (2005) | Sensory perception thresholds at the soft palate to varying airflow rates; reduced sensitivity in OSA patients vs. controls | Notes B and C OSA patients untreated |
| • Pharyngeal sensitivity correlated with OSA severity | ||
| Hlavac et al. (2007) | Inspiratory resistive load magnitude perception; reduced in untreated OSA vs. controls | Notes B and C |
| Donzel-Raynaud et al. (2009) | Respiratory-related evoked potentials; no difference in P1 amplitude or latency in OSA vs. controls | Note B |
| Eckert et al., 2011 | Respiratory-related evoked potentials and genioglossus and tensor palatini electromyography; no difference in early P1 component and reflex onset latency to brief negative upper airway pressure pulses in untreated OSA patients vs. controls | Note B |
| Sunnergren et al. (2011) | Soft palate and lip temperature detection thresholds; impaired in OSA patients vs. controls | Notes B and C |
| • Soft palate thresholds: 6.6°C OSA, 5.1°C Snorers, 2.8°C non-snorers | ||
| Grippo et al. (2011) | Respiratory-related evoked potentials; reductions in early RREP activity in OSA vs. controls | Note B |
| OSA patients untreated |
Note A: indicates that overnight sleep studies were not performed in the non-OSA group.
Note B: indicates that both the OSA and non-OSA group had overnight sleep studies.
Note C: indicates that finding may not represent primary sensory impairment; potentially influenced by confounders such as impairments in cognitive processing or edema related to OSA.
Cortical evoked potentials can provide information about peripheral and central transmission as well as cortical processing of sensory information. Delays in the timing and/or reductions in amplitude of the early components of evoked potentials may reflect afferent impairment. The early parameters do not require a perceptual decision and are less affected by sleepiness. Measured during wakefulness, brainstem auditory evoked responses do not appear to be abnormal in OSA (e.g., Mosko et al., 1981; Karnaze et al., 1984). Similarly, the latency and amplitude of the early N1 component of the cortical evoked response to auditory stimuli are not altered in OSA patients (Afifi et al., 2003; Vakulin et al., 2012).
Of relevance to the upper airway, several studies have examined respiratory-related evoked potentials (RREPs) during wakefulness in OSA. Consistent with impaired sensory transmission to respiratory stimuli, two studies revealed a reduction in the amplitude but not the latency of the early RREP components to brief negative pressures during inspiration (Akay et al., 2003) and expiration (Grippo et al., 2011). However, other studies have shown no difference in P1 amplitude or latency (reflecting the arrival of the sensory information to the cortex) of the RREP to inspiratory occlusions and negative pressures in OSA patients (Gora et al., 2002; Afifi et al., 2003; Donzel-Raynaud et al., 2009; Eckert et al., 2011). During sleep, P1 does not appear to be different between OSA patients and controls (Gora et al., 2002; Afifi et al., 2003). However, the amplitude of the latter N550 component (reflecting sensory processing) is reduced in the OSA patients (Gora et al., 2002; Afifi et al., 2003).
While recordings of peripheral sensory and motor nerve potentials are affected by obesity, several findings consistent with sensory neuropathy have been observed in nerves innervating limb muscles and organs in patients with OSA (Mayer et al., 1999; Fanfulla et al., 2000; Lüdemann et al., 2001; Dziewas et al., 2007). OSA patients show evidence of a mild axonal neuropathy in the sural, median, and ulnar nerves (Mayer et al., 1999; Dematteis et al., 2001; Lüdemann et al., 2001; Dziewas et al., 2007). Two studies report slower sensory conduction and smaller amplitude responses (Mayer et al., 1999; Fanfulla et al., 2000). In addition, it is notable that comparable changes have been observed for the compound muscle action potential in the median, sural, and ulnar nerves (Mayer et al., 1999; Lüdemann et al., 2001; Dziewas et al., 2007). This finding occurs when controlling for the increased body mass index in OSA (Dziewas et al., 2007). Thus, intermittent hypoxia may mediate systemic neural changes in OSA that are not isolated to the upper airway.
In summary, these findings reveal varying levels of sensory impairment to certain sensory stimuli in OSA. While the precise pathophysiological role of sensory impairment remains uncertain, sensory testing using calibrated airflow has been proposed as a screening test for OSA (Dematteis et al., 2005). There may also be different populations of OSA patients; those with sensory impairments and those patients without (Nguyen et al., 2005). Thus, while sensory impairments may play a key role to obstructions in some patients with OSA, neuromuscular, and anatomical influences may be more important in others.
Function
Upper airway reflexes
The integrity of the swallowing reflex is impaired in OSA patients and snorers compared to controls (Table 5). A greater bolus volume is required to elicit the swallowing reflex, swallowing onset latency is delayed, and bolus leakage throughout the pharynx occurs more frequently in OSA patients and snorers compared to controls (Zohar et al., 1998a; Teramoto et al., 1999; Jäghagen et al., 2000; Levring Jaghagen et al., 2003; Valbuza et al., 2011b). Inhibition of inspiration associated with swallowing is also less pronounced in OSA (Teramoto et al., 1999). While these changes would favor aspiration laryngeal penetration was uncommon. This indicates that alterations in swallowing are predominantly subclinical in OSA. CPAP may improve subclinical swallowing dysfunction in OSA (Okada et al., 2000). Palatal reflexes to tactile stimuli such as the gag reflex are diminished, particularly in severe OSA (Valbuza et al., 2011a). The extent to which these changes are caused by sensory impairments or efferent changes and whether or not they influence OSA severity remains unknown.
Table 5.
Functional changes.
| Reference | Main findings | Comments | |
|---|---|---|---|
| UPPER AIRWAY REFLEXES | |||
| Mortimore and Douglas (1997) | Fine-wire intramuscular multiunit levator palatini and palatoglossus electromyography; reduced EMG responses to negative upper airway pressure pulses in OSA patients vs. non-OSA | Note A Large MTA (100 ms) not appropriate for examining reflexes | |
| • CPAP treated patients had similar EMG responses vs. non-OSA | |||
| EMG latencies unknown | |||
| Zohar et al. (1998a) | Swallowing provocation test and scintigraphy testing; altered swallowing reflex in OSA | Minimal sleep monitoring (sound and O2) in non-OSA | |
| • Impaired bolus clearance in the oral cavity | |||
| • Surgery (UPPP) improved bolus clearance but was not related to reductions in RDI | |||
| Teramoto et al. (1999) | Swallowing provocation test; altered swallowing reflex in OSA vs. age, gender, and BMI matched controls | Note B | |
| • ↓ Onset latency | |||
| • ↓ Inspiratory suppression time from the termination of swallowing to the next onset of inspiration | |||
| • ↑ Provocant required to elicit swallow reflex | |||
| Jäghagen et al. (2000) | Swallowing provocation and videoradiographic testing; altered swallowing function in OSA patients and snorers vs. non-OSA | Note A | |
| • Impaired swallowing function in 54 vs. 7% in the controls | |||
| • No correlation between swallowing function and severity of snoring | |||
| Levring Jaghagen et al. (2003) | Swallowing provocation and videoradiographic testing; altered swallowing function in OSA patients and snorers vs. controls | Note A | |
| • Dysfunction in 50% with AHI of >30, 61% AHI of 5–29, and 43% with AHI of <5 events/h | |||
| Berry et al. (2003) | Multiunit surface and intramuscular genioglossus electromyography; slightly increased amplitude of EMG response to brief negative pressure pulses of moderate but not large magnitude | Note A OSA treated |
|
| MTA not appropriate for examining reflexes per se | |||
| Eckert et al. (2011) | Fine-wire intramuscular multiunit genioglossus and tensor palatini electromyography; similar EMG responses in OSA patients vs. controls | Note B Not matched for BMI | |
| • No difference in short latency reflex timing or amplitude to negative pressure pulses | |||
| • Peak genioglossus EMG and epiglottic pressure not different during passive iron-lung ventilation | |||
| Valbuza et al. (2011b) | Swallowing provocation and nasal fibroscopy; Swallowing dysfunction in OSA vs. controls | Note B | |
| • 64% had premature oral leakage to a food/liquid bolus | |||
| Valbuza et al. (2011a) | Palatal and gag reflexes; absence of reflexes in moderate and severe OSA patients vs. controls | Note B Pressure not standardized |
|
| VOLUNTARY TONGUE PROTRUSION FORCE/FATIGABLITY | |||
| Mezzanotte et al. (1992) | Maximal tongue protrusion force; not different in four very severe OSA patients vs. four non-OSA (30 vs. 38 N) | Note A Likely underpowered |
|
| Mortimore et al. (2000) | Tongue protrusion tasks; similar in OSA vs. non-OSA | Note A | |
| • No difference in maximal protrusion strength | Max tongue force only explained ∼4% of the variance in AHI | ||
| • No difference in tongue fatigability measured as time to hold a 50% of maximal contraction | |||
| • No change over the course of a night (AM vs. PM) in strength or fatigability | |||
| • ↓ Tongue strength with ↑ age, ↑ BMI, ↑AHI, and ↑neck size | |||
| Busha et al. (2002) | Tongue protrusion tasks; similar in OSA vs. non-OSA | Note A | |
| • Optimum length of the genioglossus longer in OSA patients vs. non-OSA | |||
| • At longer muscle lengths ↑ maximal protrusion force in OSA patients | |||
| • Endurance times similar between the two groups | |||
| Blumen et al. (2004) | Tongue protrusion tasks; recovery longer in OSA vs. controls | Note B | |
| • No change in endurance time or fatigability in OSA patients | |||
| • ↑ Time to recovery of initial maximal force in the OSA patients | |||
| • Final EMG median frequency was significantly higher and the final low-frequency EMG component smaller in the OSA patients | |||
| • Mean endurance time in the OSA patients 75% shorter | |||
| Shepherd et al. (2006) | Maximal tongue protrusion force; increased in severe OSA (AHI 49 h) vs. mild/non-OSA (AHI 8 h) supine but not seated | Note B | |
| • Tongue strength not related to AHI | |||
| Eckert et al. (2011) | Tongue protrusion tasks; altered voluntary tongue protrusion force and fatigability in OSA patients vs. controls | Note B | |
| • ↑ Maximal protrusion force | |||
| • ↓ Time to task failure (70% maximal force 5 s on 5 s off) | |||
Note A: indicates that overnight sleep studies were not performed in the non-OSA group.
Note B: indicates that both the OSA and non-OSA group had overnight sleep studies.
Conversely, the upper airway negative pressure reflex, crucial for maintaining airway patency, does not appear to be impaired in OSA patients during wakefulness. Studies that were not optimally designed to measure reflex responses have shown reduced EMG activation in the palatal muscles (Mortimore and Douglas, 1997), comparable genioglossus EMG activation to high levels of negative pressure (−20 cm H2O), and elevated genioglossus EMG activity to moderate suction pressures (−10 to −13 cm H2O; Berry et al., 2003). However, a recent study using sensitive neurophysiological techniques found no differences in the timing or the amplitude of the genioglossus and tensor palatini negative pressure reflex during wakefulness between OSA patients and controls (Eckert et al., 2011).
Tongue Protrusion Force and Fatigability
Maximum voluntary force of the tongue protuders is comparable (Mezzanotte et al., 1992; Mortimore et al., 2000; Busha et al., 2002; Blumen et al., 2004) or increased (Shepherd et al., 2006; Eckert et al., 2011) in OSA patients. While the maximal force generating capacity in the tongue protuders does not appear to be impaired in OSA, whether or not they are more vulnerable to fatigue remains uncertain. Fatigability, quantified as time to task failure during sustained isometric tongue protrusion tasks at 30, 50, and 80% of maximum, were not different between OSA patients and controls (Mortimore et al., 2000; Blumen et al., 2004). Conversely, time to task failure during an intermittent isometric tongue protrusion task (5 s on 5 s off at 70% maximal force) occurred approximately twice as quickly in OSA patients vs. controls (Eckert et al., 2011). Similarly, recovery of maximal force capacity following submaximal isometric tongue protrusion tasks was prolonged in OSA patients (Blumen et al., 2004). How various voluntary tongue protrusion measures during wakefulness relate to upper airway patency during sleep and OSA severity remains unclear. In an earlier study, maximal tongue protrusion force positively correlated with OSA severity, albeit it only weakly (r2 = 0.04), whereas fatigability to a submaximal isometric sustained task did not (Mortimore et al., 2000). In a subsequent study, there was no correlation between maximal tongue protrusion force and OSA severity (Shepherd et al., 2006).
Summary and Potential Role of Neural Injury in OSA
Remodeling of the upper airway muscles occurs in OSA. The most commonly reported finding is an increase in the proportion of Type IIA muscle fibers, a change which is consistent with muscle training. Changes in multiunit EMG are difficult to interpret. Recent single motor unit studies reveal chronic partial denervation in OSA. Upper airway sensory impairments to a range of stimuli also occur in OSA. However, it remains challenging to differentiate the relative contribution of afferent neural injury vs. the confounding influences of OSA (e.g., edema) to primary impairments in sensation.
Given that long-term vibration causes neurogenic changes in limb muscles (Takeuchi et al., 1986; Dahlin and Lundborg, 2001), snoring and its mechanical effects are likely to contribute to the observed upper airway anatomical, efferent, and afferent changes. However, intermittent hypoxia may also impair peripheral nerves in OSA. Thus, there is the potential for synergistic effects to mediate upper airway remodeling in OSA. Some of the changes in muscle properties and sensory impairments resolve, at least in part, with CPAP treatment (e.g., Carrera et al., 1999; Mayer et al., 1999; Tun et al., 2000; Kimoff et al., 2001; Nguyen et al., 2005; Dziewas et al., 2007).
The evidence for a link between upper airway remodeling and the integrity of upper airway reflexes is limited. The swallow and gag reflexes are impaired in patients with OSA. However, the existing data does not support a link between the presence of swallowing impairment and OSA severity (Jäghagen et al., 2000) and the potential for an impaired gag reflex to influence OSA severity is uncertain. Other subtle alterations, such as changes in speech, may also occur in OSA. Importantly, there is no strong evidence to suggest that key protective reflexes involved in maintaining upper airway patency are impaired in OSA. In addition, the ability of the tongue to generate volitional protrusion force is similar or enhanced in OSA. However, despite the consistent observation of increased Type IIA fibers in the upper airway muscles of OSA patients, which would be predicted to increase resistance to muscle fatigue, the upper airway muscles may be more prone to fatigue. However, the laboratory task used to measure performance does not exactly mimic the repetitive movements associated with intermittent obstructions in OSA.
Given the current uncertainty as to the links between changes in sensorimotor function and the upper airway in OSA and sleep-disordered breathing, how novel therapeutic targets might modify these upper airway pathophysiological changes is unclear. Nonetheless, recent studies have demonstrated that novel exercise training techniques (e.g., didgeridoo playing and a battery of tasks designed by a speech pathologist) can reduce OSA severity (e.g., Puhan et al., 2006; Guimarães et al., 2009). Recent studies using implantable devices to electrically stimulate the genioglossus muscle during sleep have shown reductions in OSA severity (e.g., Eastwood et al., 2011; Oliven, 2011). However, consistent with the varying causes of OSA, treatment response varies between patients (Dotan et al., 2011; Eastwood et al., 2011). The upper airway muscles are stronger and have an increased proportion of Type IIA muscle fibers in OSA. These findings suggest that the upper airway muscles are highly trained by chronic overnight loading and/or hypoxia. Nonetheless, targeted exercise tasks performed during wakefulness may further enhance these changes and potentially lead to improved neuromechanical performance during sleep. The use of some of the measurement techniques outlined in this review to study the effects before and after training would help address some of this uncertainty and may provide novel therapeutic targets.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Afifi L., Guilleminault C., Colrain I. M. (2003). Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir. Physiol. Neurobiol. 136, 221–234 10.1016/S1569-9048(03)00084-3 [DOI] [PubMed] [Google Scholar]
- Akay M., Leiter J. C., Daubenspeck J. A. (2003). Reduced respiratory-related evoked activity in subjects with obstructive sleep apnea syndrome. J. Appl. Physiol. 94, 429–438 [DOI] [PubMed] [Google Scholar]
- Bailey E. F., Rice A. D., Fuglevand A. J. (2007). Firing patterns of human genioglossus motor units during voluntary tongue movement. J. Neurophysiol. 97, 933–936 10.1152/jn.00737.2006 [DOI] [PubMed] [Google Scholar]
- Bassiouny A., Nasr S., Mashaly M., Ayad E., Qotb M., Atef A. (2009). Electron microscopy study of peripheral nerves in the uvulae of snorers and obstructive sleep apnoea patients. J. Laryngol. Otol. 123, 203–207 10.1017/S0022215108002971 [DOI] [PubMed] [Google Scholar]
- Berger G., Gilbey P., Hammel I., Ophir D. (2002). Histopathology of the uvula and the soft palate in patients with mild, moderate, and severe obstructive sleep apnea. Laryngoscope 112, 357–363 10.1097/00005537-200204000-00026 [DOI] [PubMed] [Google Scholar]
- Berry R. B., White D. P., Roper J., Pillar G., Fogel R. B., Stanchina M., Malhotra A. (2003). Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J. Appl. Physiol. 94, 1875–1882 [DOI] [PubMed] [Google Scholar]
- Bertorini T. E., Stalberg E., Yuson C. P., Engel W. K. (1994). Single-fiber electromyography in neuromuscular disorders: correlation of muscle histochemistry, single-fiber electromyography, and clinical findings. Muscle Nerve 17, 345–353 10.1002/mus.880170312 [DOI] [PubMed] [Google Scholar]
- Blumen M. B., De La Sota A. P., Quera-Salva M. A., Frachet B., Chabolle F., Lofaso F. (2004). Tongue mechanical characteristics and genioglossus muscle EMG in obstructive sleep apnoea patients. Respir. Physiol. Neurobiol. 140, 155–164 10.1016/j.resp.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Boyd J. H., Petrof B. J., Hamid Q., Fraser R., Kimoff R. J. (2004). Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 170, 541–546 10.1164/rccm.200308-1100OC [DOI] [PubMed] [Google Scholar]
- Busha B. F., Strobel R. J., England S. J. (2002). The length-force relationship of the human genioglossus in patients with obstructive sleep apnea. Respir. Physiol. Neurobiol 130, 161–168 10.1016/S0034-5687(01)00340-1 [DOI] [PubMed] [Google Scholar]
- Carrera M., Barbe F., Sauleda J., Tomas M., Gomez C., Agusti A. G. (1999). Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am. J. Respir. Crit. Care Med. 159, 1960–1966 [DOI] [PubMed] [Google Scholar]
- Clerk A. A., Dunan S. R., Guilleminault C. (1994). Load detection in subjects with sleep-induced upper airway obstruction. Am. J. Respir. Crit. Care Med. 149, 727–730 [DOI] [PubMed] [Google Scholar]
- Dahlin L. B., Lundborg G. (2001). Vibration-induced hand problems: role of the peripheral nerves in the pathophysiology. Scand. J. Plast. Reconstr. Surg. Hand. Surg. 35, 225–232 [DOI] [PubMed] [Google Scholar]
- Daugherty M., Luo Q., Sokoloff A. J. (2012). Myosin heavy chain composition of the human genioglossus muscle. J. Speech Lang. Hear. Res. 55, 609–625 10.1044/1092-4388(2011/10-0287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A., Leeper J. B., McKenzie D. K., Gandevia S. C. (1997). Neural drive to the diaphragm in patients with severe COPD. Am. J. Respir. Crit. Care Med. 155, 1335–1340 [DOI] [PubMed] [Google Scholar]
- De Vuono I. M., Zanoteli E., Oliveira A. S., Fujita R. R., Pignatari S. S., Pizarro G. U., Pradelle-Hallinan M. L., Moreira G. A. (2007). Histological analysis of palatopharyngeal muscle from children with snoring and obstructive sleep apnea syndrome. Int. J. Pediatr. Otorhinolaryngol. 71, 283–290 10.1016/j.ijporl.2006.10.019 [DOI] [PubMed] [Google Scholar]
- Dematteis M., Levy P., Pepin J. L. (2005). A simple procedure for measuring pharyngeal sensitivity: a contribution to the diagnosis of sleep apnoea. Thorax 60, 418–426 10.1136/thx.2003.015032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dematteis M., Pepin J. L., Jeanmart M., Deschaux C., Labarre-Vila A., Levy P. (2001). Charcot-Marie-Tooth disease and sleep apnoea syndrome: a family study. Lancet 357, 267–272 10.1016/S0140-6736(00)03614-X [DOI] [PubMed] [Google Scholar]
- Donzel-Raynaud C., Redolfi S., Arnulf I., Similowski T., Straus C. (2009). Abnormal respiratory-related evoked potentials in untreated awake patients with severe obstructive sleep apnoea syndrome. Clin. Physiol. Funct. Imaging 29, 10–17 10.1111/j.1475-097X.2008.00830.x [DOI] [PubMed] [Google Scholar]
- Dotan Y., Golibroda T., Oliven R., Netzer A., Gaitini L., Toubi A., Oliven A. (2011). Parameters affecting pharyngeal response to genioglossus stimulation in sleep apnoea. Eur. Respir. J. 38, 338–347 10.1183/09031936.00125810 [DOI] [PubMed] [Google Scholar]
- Dziewas R., Schilling M., Engel P., Boentert M., Hor H., Okegwo A., Ludemann P., Ringelstein E. B., Young P. (2007). Treatment for obstructive sleep apnoea: effect on peripheral nerve function. J. Neurol. Neurosurg. Psychiatr. 78, 295–297 10.1136/jnnp.2006.102806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood P. R., Barnes M., Walsh J. H., Maddison K. J., Hee G., Schwartz A. R., Smith P. L., Malhotra A., McEvoy R. D., Wheatley J. R., O’Donoghue F. J., Rochford P. D., Churchward T., Campbell M. C., Palme C. E., Robinson S., Goding G. S., Eckert D. J., Jordan A. S., Catcheside P. G., Tyler L., Antic N. A., Worsnop C. J., Kezirian E. J., Hillman D. R. (2011). Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep 34, 1479–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert D. J., Lo Y. L., Saboisky J. P., Jordan A. S., White D. P., Malhotra A. (2011). Sensori-motor function of the upper airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J. Appl. Physiol. 111, 1644–1653 10.1152/japplphysiol.00653.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edstrom L., Larsson H., Larsson L. (1992). Neurogenic effects on the palatopharyngeal muscle in patients with obstructive sleep apnoea: a muscle biopsy study. J. Neurol. Neurosurg. Psychiatr. 55, 916–920 10.1136/jnnp.55.10.916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanfulla F., Malaguti S., Montagna T., Salvini S., Bruschi C., Crotti P., Casale R., Rampulla C. (2000). Erectile dysfunction in men with obstructive sleep apnea: an early sign of nerve involvement. Sleep 23, 775–781 [PubMed] [Google Scholar]
- Ferini-Strambi L. J., Smirne S., Moz U., Sferrazza B., Iannaccone S. (1998). Muscle fibre type and obstructive sleep apnea. Sleep Res. Online 1, 24–27 [PubMed] [Google Scholar]
- Fogel R. B., Malhotra A., Giora P., Edwards J. K., Beauregard J., Shea S. A., White D. P. (2001). Genioglossal activation in paitents with obstructive sleep apnea versus control subjects. Am. J. Respir. Crit. Care Med. 164, 2025–2030 [DOI] [PubMed] [Google Scholar]
- Friberg D., Ansved T., Borg K., Carlsson-Nordlander B., Larsson H., Svanborg E. (1998a). Histological indications of a progressive snorers disease in an upper airway muscle. Am. J. Respir. Crit. Care Med. 157, 586–593 [DOI] [PubMed] [Google Scholar]
- Friberg D., Gazelius B., Lindblad L. E., Nordlander B. (1998b). Habitual snorers and sleep apnoics have abnormal vascular reactions of the soft palatal mucosa on afferent nerve stimulation. Laryngoscope 108, 431–436 10.1097/00005537-199803000-00022 [DOI] [PubMed] [Google Scholar]
- Friberg D., Gazelius B., Hokfelt T., Nordlander B. (1997). Abnormal afferent nerve endings in the soft palatal mucosa of sleep apnoics and habitual snorers. Regul. Pept. 71, 29–36 10.1016/S0167-0115(97)01016-1 [DOI] [PubMed] [Google Scholar]
- Goldshtein E., Tarasiuk A., Zigel Y. (2011). Automatic detection of obstructive sleep apnea using speech signals. IEEE Eng. Med. Biol. Mag. 58, 1373–1382 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Forero D., Portillo F., Sunico C. R., Moreno-Lopez B. (2004). Nerve injury reduces responses of hypoglossal motoneurones to baseline and chemoreceptor-modulated inspiratory drive in the adult rat. J. Physiol. 557, 991–1011 10.1113/jphysiol.2003.059972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gora J., Trinder J., Pierce R., Colrain I. M. (2002). Evidence of a sleep-specific blunted cortical response to inspiratory occlusions in mild obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 166, 1225–1234 10.1164/rccm.2106005 [DOI] [PubMed] [Google Scholar]
- Gorman R. B., McKenzie D. K., Butler J. E., Tolman J. F., Gandevia S. C. (2005). Diaphragm length and neural drive after lung volume reduction surgery. Am. J. Respir. Crit. Care Med. 172, 1259–1266 10.1164/rccm.200412-1695OC [DOI] [PubMed] [Google Scholar]
- Grippo A., Carrai R., Romagnoli I., Pinto F., Fanfulla F., Sanna A. (2011). Blunted respiratory-related evoked potential in awake obstructive sleep apnoea subjects: a NEP technique study. Clin. Neurophysiol. 122, 1562–1568 10.1016/j.clinph.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Guilleminault C., Li K., Chen N. H., Poyares D. (2002). Two-point palatal discrimination in patients with upper airway resistance syndrome, obstructive sleep apnea syndrome, and normal control subjects. Chest 122, 866–870 10.1378/chest.122.3.866 [DOI] [PubMed] [Google Scholar]
- Guimarães K. C., Drager L. F., Genta P. R., Marcondes B. F., Lorenzi-Filho G. (2009). Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 179, 962–966 10.1164/rccm.200806-981OC [DOI] [PubMed] [Google Scholar]
- Hildebrand I. L., Sylven C., Esbjornsson M., Hellstrom K., Jansson E. (1991). Does chronic hypoxaemia induce transformations of fibre types? Acta Physiol. Scand. 141, 435–439 10.1111/j.1748-1716.1991.tb09102.x [DOI] [PubMed] [Google Scholar]
- Hlavac M. C., Catcheside P. G., Adams A., Eckert D. J., McEvoy R. D. (2007). The effects of hypoxia on load compensation during sustained incremental resistive loading in patients with obstructive sleep apnea. J. Appl. Physiol. 103, 234–239 10.1152/japplphysiol.01618.2005 [DOI] [PubMed] [Google Scholar]
- Horner R. L., Mohiaddin R. H., Lowell D. G., Shea S. A., Burman E. D., Longmore D. B., Guz A. (1989). Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur. Respir. J. 2, 613–622 [PubMed] [Google Scholar]
- Jäghagen E. L., Berggren D., Isberg A. (2000). Swallowing dysfunction related to snoring: a videoradiographic study. Acta. Otolaryngol. 120, 438–443 10.1080/000164800750000702 [DOI] [PubMed] [Google Scholar]
- Karnaze D., Gott P., Mitchell F., Loftin J. (1984). Brainstem auditory evoked potentials are normal in idiopathic sleep apnea. Ann. Neurol. 15, 406. 10.1002/ana.410150422 [DOI] [PubMed] [Google Scholar]
- Kimoff R. J., Sforza E., Champagne V., Ofiara L., Gendron D. (2001). Upper airway sensation in snoring and obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 164, 250–255 [DOI] [PubMed] [Google Scholar]
- Kjaer M. (2004). Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84, 649–698 10.1152/physrev.00031.2003 [DOI] [PubMed] [Google Scholar]
- Levring Jaghagen E., Franklin K. A., Isberg A. (2003). Snoring, sleep apnoea and swallowing dysfunction: a videoradiographic study. Dentomaxillofac. Radiol. 32, 311–316 10.1259/dmfr/29209140 [DOI] [PubMed] [Google Scholar]
- Lindman R., Stal P. S. (2002). Abnormal palatopharyngeal muscle morphology in sleep-disordered breathing. J. Neuro. Sci. 195, 11–23 10.1016/S0022-510X(01)00676-1 [DOI] [PubMed] [Google Scholar]
- Lüdemann P., Dziewas R., Soros P., Happe S., Frese A. (2001). Axonal polyneuropathy in obstructive sleep apnoea. J. Neurol. Neurosurg. Psychiatr. 70, 685–687 10.1136/jnnp.70.5.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer P., Dematteis M., Pépin J. L., Wuyam B., Veale D., Vila A., Lévy P. (1999). Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am. J. Respir. Crit. Care Med. 159, 213–219 [DOI] [PubMed] [Google Scholar]
- McNicholas W. T., Bowes G., Zamel N., Phillipson E. A. (1984). Impaired detection of added inspiratory resistance in patients with obstructive sleep apnea. Am. Rev. Respir. Dis. 129, 45–48 [DOI] [PubMed] [Google Scholar]
- Mezzanotte W. S., Tangel D. J., White D. P. (1992). Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J. Clin. Invest. 89, 1571–1579 10.1172/JCI115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte W. S., Tangel D. J., White D. P. (1996). Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am. J. Respir. Crit. Care Med. 153, 1880–1887 [DOI] [PubMed] [Google Scholar]
- Mortimore I. L., Bennett S. P., Douglas N. J. (2000). Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J. Sleep Res. 9, 389–393 10.1046/j.1365-2869.2000.00222.x [DOI] [PubMed] [Google Scholar]
- Mortimore I. L., Douglas N. J. (1997). Palatal muscle EMG response to negative pressure in awake sleep apneic and control subjects. Am. J. Respir. Crit. Care Med. 156, 867–873 [DOI] [PubMed] [Google Scholar]
- Mosko S. S., Pierce S., Holowach J., Sassin J. F. (1981). Normal brain stem auditory evoked potentials recorded in sleep apneics during waking and as a function of arterial oxygen saturation during sleep. Electroencephalogr. Clin. Neurophysiol. 51, 477–482 10.1016/0013-4694(81)90224-8 [DOI] [PubMed] [Google Scholar]
- Nguyen A. T., Jobin V., Payne R., Beauregard J., Naor N., Kimoff R. J. (2005). Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep 28, 585–593 [DOI] [PubMed] [Google Scholar]
- Okada S., Ouchi Y., Teramoto S. (2000). Nasal continuous positive airway pressure and weight loss improve swallowing reflex in patients with obstructive sleep apnea syndrome. Respiration 67, 464–466 10.1159/000029551 [DOI] [PubMed] [Google Scholar]
- Oliven A. (2011). Treating obstructive sleep apnea with hypoglossal nerve stimulation. Curr. Opin. Pulm. Med. 17, 419–424 [DOI] [PubMed] [Google Scholar]
- Paulsen F. P., Steven P., Tsokos M., Jungmann K., Muller A., Verse T., Pirsig W. (2002). Upper airway epithelial structural changes in obstructive sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 166, 501–509 10.1164/rccm.2109099 [DOI] [PubMed] [Google Scholar]
- Pette D., Staron R. S. (2001). Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 115, 359–372 [DOI] [PubMed] [Google Scholar]
- Podnar S., Dolenc Groselj L. (2010). “Neuropathic changes of the genioglossus muscle in patients with snoring and obstructive sleep apnoea,” in 20th Congress of the European Sleep Research Society, Lisbon [Google Scholar]
- Preston D. C., Shapiro B. E. (2002). Needle electromyography. Fundamentals, normal and abnormal patterns. Neurol. Clin. 20, 361–396 10.1016/S0733-8619(01)00005-6 [DOI] [PubMed] [Google Scholar]
- Puhan M. A., Suarez A., Lo Cascio C., Zahn A., Heitz M., Braendli O. (2006). Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. Br. Med. J. (Clin. Res. Ed.) 332, 266–270 10.1136/bmj.38705.470590.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandren S., Gruis K. L., Chervin R. D., Lisabeth L. D., Concannon M., Wolfe J., Albers J. W., Brown D. L. (2010). Hypoglossal nerve conduction findings in obstructive sleep apnea. Muscle Nerve 42, 257–261 10.1002/mus.21690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein I. (1995). Nasal inflammation in patients with obstructive sleep apnea. Laryngoscope 105, 175–177 10.1288/00005537-199502000-00012 [DOI] [PubMed] [Google Scholar]
- Saboisky J. P., Butler J. E., McKenzie D. K., Gorman R. B., Trinder J. A., White D. P., Gandevia S. C. (2007). Neural drive to human genioglossus in obstructive sleep apnoea. J. Physiol. 585, 135–146 10.1113/jphysiol.2007.139584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky J. P., Jordan A. S., Eckert D. J., White D. P., Trinder J. A., Nicholas C. L., Gautam S., Malhotra A. (2010). Recruitment and rate-coding strategies of the human genioglossus muscle. J. Appl. Physiol. 109, 1939–1949 10.1152/japplphysiol.00812.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky J. P., Stashuk D. W., Hamilton-Wright A., Carusona A. L., Campana L. M., Trinder J., Eckert D. J., Jordan A. S., McSharry D. G., White D. P., Nandedkar S., David W. S., Malhotra A. (2012). Neurogenic changes in the upper airway of obstructive sleep apnea patients. Am. J. Respir. Crit. Care Med. 185, 322–329 10.1164/rccm.201106-1058OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauleda J., Garcia-Palmer F. J., Tarraga S., Maimo A., Palou A., Agusti A. G. (2003). Skeletal muscle changes in patients with obstructive sleep apnoea syndrome. Respir. Med. 97, 804–810 10.1016/S0954-6111(03)00034-9 [DOI] [PubMed] [Google Scholar]
- Schwartz A. R., Gold A. R., Schubert N., Stryzak A., Wise R. A., Permutt S., Smith P. L. (1991). Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am. Rev. Respir. Dis. 144, 494–498 10.1164/ajrccm/144.3_Pt_1.494 [DOI] [PubMed] [Google Scholar]
- Sekosan M., Zakkar M., Wenig B. L., Olopade C. O., Rubinstein I. (1996). Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope 106, 1018–1020 10.1097/00005537-199608000-00021 [DOI] [PubMed] [Google Scholar]
- Series F., Cote C., Simoneau J. A., Gelinas Y., St Pierre S., Leclerc J., Ferland R., Marc I. (1995). Physiologic, metabolic, and muscle fiber type characteristics of musculus uvulae in sleep apnea hypopnea syndrome and in snorers. J. Clin. Invest. 95, 20–25 10.1172/JCI117640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Series F., Simoneau J. A., St Pierre S. (2000). Muscle fiber area distribution of musculus uvulae in obstructive sleep apnea and non-apneic snorers. Int. J. Obes. Relat. Metab. Disord. 24, 410–415 10.1038/sj.ijo.0801172 [DOI] [PubMed] [Google Scholar]
- Series F., Wang W., Similowski T. (2009). Corticomotor control of the genioglossus in awake OSAS patients: a transcranial magnetic stimulation study. Respir. Res. 10, 74. 10.1186/1465-9921-10-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Series F. J., Simoneau S. A., St Pierre S., Marc I. (1996). Characteristics of the genioglossus and musculus uvulae in sleep apnea hypopnea syndrome and in snorers. Am. J. Respir. Crit. Care Med. 153, 1870–1874 [DOI] [PubMed] [Google Scholar]
- Shepherd K. L., Jensen C. M., Maddison K. J., Hillman D. R., Eastwood P. R. (2006). Relationship between upper airway and inspiratory pump muscle force in obstructive sleep apnea. Chest 130, 1757–1764 10.1378/chest.130.6.1757 [DOI] [PubMed] [Google Scholar]
- Smirne S., Iannaccone S., Ferini-Strambi L., Comola M., Colombo E., Nemni R. (1991). Muscle fibre type and habitual snoring. Lancet 337, 597–599 10.1016/0140-6736(91)91651-A [DOI] [PubMed] [Google Scholar]
- Stål P. S., Johansson B. (2012). Abnormal mitochondria organisation and oxidative activity in palate muscles of long-term snorers with obstructive sleep apnea. Respiration 83, 407–417 10.1159/000336040 [DOI] [PubMed] [Google Scholar]
- Stål P. S., Lindman R., Johansson B. (2009). Capillary supply of the soft palate muscles is reduced in long-term habitual snorers. Respiration 77, 303–310 10.1159/000197975 [DOI] [PubMed] [Google Scholar]
- Stauffer J. L., Buick M. K., Bixler E. O., Sharkey F. E., Abt A. B., Manders E. K., Kales A., Cadieux R. J., Barry J. D., Zwillich C. W. (1989). Morphology of the uvula in obstructive sleep apnea. Am. Rev. Respir. Dis. 140, 724–728 [DOI] [PubMed] [Google Scholar]
- Sunnergren O., Brostrom A., Svanborg E. (2011). Soft palate sensory neuropathy in the pathogenesis of obstructive sleep apnea. Laryngoscope 121, 451–456 10.1002/lary.21371 [DOI] [PubMed] [Google Scholar]
- Svanborg E. (2005). Impact of obstructive apnea syndrome on upper airway respiratory muscles. Respir. Physiol. Neurobiol. 147, 263–272 10.1016/j.resp.2005.06.012 [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Futatsuka M., Imanishi H., Yamada S. (1986). Pathological changes observed in the finger biopsy of patients with vibration-induced white finger. Scand. J. Work Environ. Health 12, 280–283 10.5271/sjweh.2140 [DOI] [PubMed] [Google Scholar]
- Teramoto S., Sudo E., Matsuse T., Ohga E., Ishii T., Ouchi Y., Fukuchi Y. (1999). Impaired swallowing reflex in patients with obstructive sleep apnea syndrome. Chest 116, 17–21 10.1378/chest.116.4.1145-a [DOI] [PubMed] [Google Scholar]
- Tun Y., Hida W., Okabe S., Kikuchi Y., Kurosawa H., Tabata M., Shirato K. (2000). Inspiratory effort sensation to added resistive loading in patients with obstructive sleep apnea. Chest 118, 1332–1338 10.1378/chest.118.5.1332 [DOI] [PubMed] [Google Scholar]
- Vakulin A., Catcheside P. G., Baulk S. D., Antic N. A., Van Den Heuvel C. J., Banks S., McEvoy R. D. (2012). Auditory evoked potentials remain abnormal after CPAP treatment in patients with severe obstructive sleep apnoea. Clin. Neurophysiol. 123, 310–317 10.1016/j.clinph.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Valbuza J. S., De Oliveira M. M., Conti C. F., Prado L. B., Carvalho L. B., Do Prado G. F. (2011a). Oropharyngeal examination as a predictor of obstructive sleep apnea: pilot study of gag reflex and palatal reflex. Arq. Neuropsiquiatr. 69, 805–808 10.1590/S0004-282X2011000600015 [DOI] [PubMed] [Google Scholar]
- Valbuza J. S., De Oliveira M. M., Zancanella E., Conti C. F., Prado L. B., Carvalho L. B., Do Prado G. F. (2011b). Swallowing dysfunction related to obstructive sleep apnea: a nasal fibroscopy pilot study. Sleep Breath. 15, 209–213 10.1007/s11325-010-0474-9 [DOI] [PubMed] [Google Scholar]
- Wang W., Kang J., Kong D. (2010). The central motor conductivity of genioglossus in obstructive sleep apnea. Respirology 15, 1209–1214 10.1111/j.1440-1843.2009.01667.x [DOI] [PubMed] [Google Scholar]
- Woodson B. T., Garancis J. C., Toohill R. J. (1991). Histopathologic changes in snoring and obstructive sleep apnea syndrome. Laryngoscope 101, 1318–1322 10.1002/lary.5541011211 [DOI] [PubMed] [Google Scholar]
- Zohar Y., Grusko I., Sulkes J., Melloul M. M. (1998a). Oropharyngeal scintigraphy: a computerized analysis of swallowing in patients with obstructive sleep apnea. Laryngoscope 108, 37–41 10.1097/00005537-199801000-00009 [DOI] [PubMed] [Google Scholar]
- Zohar Y., Sabo R., Strauss M., Schwartz A., Gal R., Oksenberg A. (1998b). Oropharyngeal fatty infiltration in obstructive sleep apnea patients: a histologic study. Ann. Otol. Rhinol. Laryngol. 107, 170–174 [DOI] [PubMed] [Google Scholar]