Abstract
OBJECTIVE:
We compared neurodevelopmental outcomes at 18 to 22 months' corrected age of infants born with extremely low birth weight at an estimated gestational age of <25 weeks during 2 periods: 1999–2001 (epoch 1) and 2002–2004 (epoch 2).
PATIENTS AND METHODS:
We conducted a multicenter, retrospective analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Perinatal and neonatal variables and outcomes were compared between epochs. Neurodevelopmental outcomes at 18 to 22 months' corrected age were evaluated with neurologic exams and Bayley Scales of Infant Development II. Logistic regression analyses determined the independent risk of epoch for adverse outcomes.
RESULTS:
Infant survival was similar between epochs (epoch 1, 35.4%, vs epoch 2, 32.3%; P = .09). A total of 411 of 452 surviving infants in epoch 1 and 405 of 438 surviving infants in epoch 2 were evaluated at 18 to 22 months' corrected age. Cesarean delivery (P = .03), surgery for patent ductus arteriosus (P = .004), and late sepsis (P = .01) were more common in epoch 2, but postnatal steroid use was dramatically reduced (63.5% vs 32.8%; P < .0001). Adverse outcomes at 18 to 22 months' corrected age were common in both epochs. Moderate-to-severe cerebral palsy was diagnosed in 11.1% of surviving infants in epoch 1 and 14.9% in epoch 2 (adjusted odds ratio [OR]: 1.52 [95% confidence interval (CI): 0.86–2.71]; P = .15), the Mental Developmental Index was <70 in 44.9% in epoch 1 and 51% in epoch 2 (OR: 1.30 [95% CI: 0.91–1.87]; P = .15), and neurodevelopmental impairment was diagnosed in 50.1% of surviving infants in epoch 1 and 58.7% in epoch 2 (OR: 1.4 [95% CI: 0.98–2.04]; P = .07).
CONCLUSIONS:
Early-childhood outcomes for infants born at <25 weeks' estimated gestational age were unchanged between the 2 periods.
Keywords: extremely preterm, neurodevelopmental, outcome, cerebral palsy, Bayley Scales of Infant Development II
WHAT'S KNOWN ON THIS SUBJECT:
Early-childhood neurodevelopmental outcomes seem to have improved over the last decade for some groups of preterm infants, but it is not known whether this trend applies to extraordinarily preterm infants who are born at <25 weeks' estimated gestational age.
WHAT THIS STUDY ADDS:
Despite a dramatic reduction in postnatal steroid exposure, neurosensory and cognitive outcomes at 18 to 22 months' corrected age remain guarded and unchanged for infants who are born at <25 weeks' estimated gestational age in the NICHD Neonatal Research Network between 2 recent birth epochs.
Significant advances in perinatal and neonatal care and changes in the approach to immediate resuscitation have led to improved survival rates among preterm infants.1,2 This phenomenon has extended to even extremely preterm infants,3–5 although some analyses6,7 suggest that major in-hospital morbidity rates for these infants may not have improved. The number of extremely preterm infants who survive to discharge has increased over time; however, these children are at high risk for neurodevelopmental sequelae during childhood.
Studies have suggested that some early-childhood neurosensory and developmental outcomes have improved over the last decade for some groups of preterm infants.8–10 However, it is not clear whether this trend applies to the most extremely preterm infants, who are less than 25 weeks' estimated gestational age (EGA). A previous study11 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) demonstrated that 18- to 22-month outcomes for infants born at less than 25 weeks' EGA did not improve between 2 birth cohorts in the 1990s. Hack et al12 presented a single-center analysis that showed no improvement in 20-month outcomes for infants born at 23 to 24 weeks' EGA in 2000–2004 compared with those born in 1995–1999. However, there are no large, recent analyses to examine whether neurodevelopmental outcomes improved, worsened, or remained the same for these most vulnerable preterm infants born since 2000.
The primary objective of our study was to compare 18- to 22-month corrected-age neurodevelopmental outcomes of infants born at less than 25 weeks' EGA in the NICHD NRN during 2 recent birth cohorts: epoch 1, which was from 1999 to 2001, and epoch 2, which was from 2002 to 2004. We also compared survival, perinatal characteristics, and neonatal interventions and morbidities between the 2 epochs. We hypothesized that there would be no significant differences in the rates of neurodevelopmental impairment, moderate-to-severe cerebral palsy, or severe developmental delay between epochs 1 and 2.
METHODS
Study Design and Patient Population
We conducted a retrospective analysis of prospectively collected data from the NICHD NRN Generic Database and Follow-up Study. Infants were included if they were born at less than 25 weeks' EGA, as determined by best obstetrical estimate; were 401 to 1000 g body weight; and were inborn at an NICHD NRN site between January 1, 1999, and December 31, 2001 (epoch 1), and January 1, 2002, to December 31, 2004 (epoch 2). Only centers that were part of the NRN during the entire 6-year study period were included in this analysis. Each center's institutional review board reviewed and approved the data collection procedures.
Research nurses collected demographic, perinatal, and infant data at each center using common definitions, as described in previous publications.1 Antenatal antibiotics were defined as any antibiotics given to the mother during admission that resulted in delivery. Antenatal steroid use was defined as administration of any corticosteroid to accelerate fetal lung maturity in the present pregnancy. Intraventricular hemorrhage was reported according to the classification of Papile et al.13 Cystic periventricular leukomalacia was diagnosed by cranial ultrasound. Early sepsis was defined as culture-proven sepsis in the first 72 hours after birth or treatment with antibiotics for at least 5 days beginning before the age of 72 hours for presumed sepsis regardless of culture result. Late sepsis was defined as culture-proven sepsis at more than 72 hours to discharge or treatment with antibiotics at 72 hours for at least 5 days for presumed sepsis regardless of culture result. Necrotizing enterocolitis was defined as modified Bell's stage IIA or higher14; surgery for necrotizing enterocolitis included both laparotomy and drain. High-frequency ventilation was defined as the use of any high-frequency device during hospitalization. Bronchopulmonary dysplasia was defined as the use of supplemental oxygen at 36 weeks' postmenstrual age. Postnatal steroid use was defined as any corticosteroid given for the prevention or treatment of bronchopulmonary dysplasia. Surgery performed while the infant was in the NICU for patent ductus arteriosus (PDA), necrotizing enterocolitis, or retinopathy of prematurity was noted.
Neurodevelopmental Assessments
A comprehensive neurodevelopmental assessment was performed on the surviving infants at 18 to 22 months' corrected age. The follow-up visit, as previously described,3,8 consisted of a battery of developmental, neurologic, and behavioral assessments; medical history; and parent interviews. The neuromotor examinations were based on Amiel-Tison assessments,15 and gross motor function was based on the work of Palisano et al.16 Examinations were performed by annually certified examiners who were trained to reliability during a 2-day workshop on neurologic assessment. During the study period, the Bayley Scales of Infant Development II17 were administered, which included determination of the Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI). MDI and PDI scores of 100 ± 15 represent the mean ± 1 SD. The Bayley Scales of Infant Development II was administered by experienced testers, who were annually certified by 1 of 4 gold-standard psychologists.
Cerebral palsy was defined as a nonprogressive central nervous system disorder characterized by abnormal muscle tone in at least 1 extremity and abnormal control of movement and posture that interfered with or prevented age-appropriate motor activity. Children with moderate-to-severe cerebral palsy were nonambulatory or required an assistive device for ambulation. Bilateral severe hearing loss was defined as permanent hearing loss that required amplification in both ears. Bilateral blindness was defined as the absence of functional vision in either eye. Neurodevelopmental impairment was defined as any of the following: moderate-to-severe cerebral palsy; an MDI or PDI of less than 70; deafness; or bilateral blindness. Profound impairment was defined as an MDI of less than 50 or a Gross Motor Function Classification System level of 4 or 5. Unimpaired or minimally impaired was defined as having none of the following: moderate-to-severe cerebral palsy; bilateral severe hearing loss or blindness; an MDI of less than 85; or a PDI of less than 85.
Statistical Analyses
Unadjusted Epoch-related comparative analyses were conducted by using the χ2 or Fisher's exact test for categorical data and the t test for continuous data. Logistic regression models were developed to evaluate the independent risk of epoch 2 versus epoch 1 for neurodevelopmental impairment, an MDI less than 70, and moderate-to-severe cerebral palsy. Model 1 included the following baseline perinatal and case-mix variables: epoch; network center; gender; multiple gestation; cesarean delivery; race; maternal age; body weight; antenatal antibiotic use; and antenatal steroid use. Model 2 included all model 1 variables as well as the following subsequent neonatal morbidities and interventions that could be considered a proxy for severity of illness as well as postdischarge factors: surfactant; high-frequency ventilation; sepsis; necrotizing enterocolitis; grade 3 or 4 intraventricular hemorrhage or cystic periventricular leukomalacia; bronchopulmonary dysplasia; surgery for necrotizing enterocolitis; PDA or retinopathy of prematurity; maternal education less than high school; and age at neurodevelopmental assessment. The rationale for this a priori approach was to differentiate epoch-related odds for adverse outcomes adjusted for changes in baseline factors only from epoch-related odds also adjusted for neonatal variables, complications, and postdischarge factors. Because postnatal steroid exposure during epoch 2 may have been influenced by American Academy of Pediatrics recommendations,18 rather than related to changes in severity of illness between epochs, model 2 was applied both with (model 2 plus postnatal steroid use) and without (model 2) the addition of postnatal steroid use.
RESULTS
The progression of study patients is shown in Fig 1. During the entire study period, 2428 infants born at less than 25 weeks' EGA were inborn at NICHD NRN sites. Of these, 35.4% of infants in epoch 1 and 32.3% in epoch 2 survived until discharge or 1 year (P = .09). In epoch 1, survival according to EGA was as follows: 22 weeks' or less EGA, 12 of 292 (4.1%) infants; 23 weeks' EGA, 101 of 395 (25.6%) infants; and 24 weeks' EGA, 345 of 606 (56.9%) infants. In epoch 2, survival according to EGA was as follows: 22 weeks' or less EGA, 13 of 322 (4.0%) infants; 23 weeks' EGA, 99 of 441 (22.5%) infants; and 24 weeks' EGA 338 of 632 (53.5%) infants. Forty-one infants in epoch 1 and 33 in epoch 2 were lost to follow-up, and a few infants in each epoch died after discharge. This resulted in 411 patients in the epoch 1 and 405 in the epoch 2 follow-up groups. Follow-up rates among survivors were more than 90% in each epoch.
FIGURE 1.
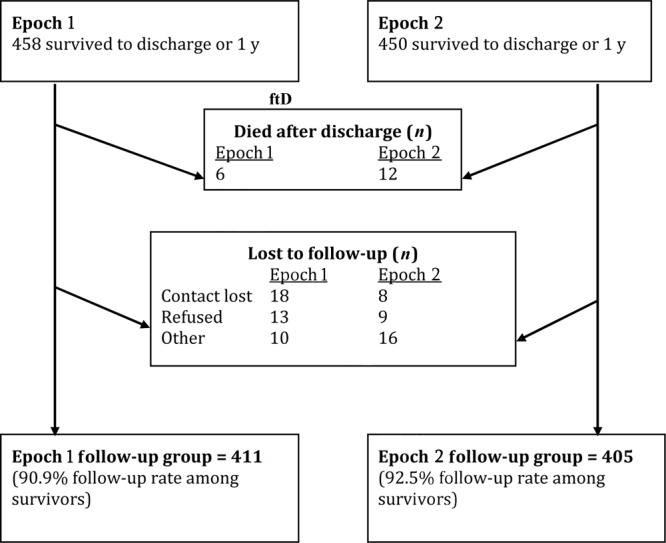
Progression of patients in epochs 1 and 2 from survival to neurodevelopmental follow-up. Epoch 1: born January 1, 1999, to December 31, 2001; epoch 2: born January 1, 2002, to December 31, 2004.
Demographic and perinatal characteristics for the follow-up groups are shown in Table 1. There were no significant differences unadjusted between the groups, with the exception of an increase in the proportion of infants born via cesarean delivery from epoch 1 (40.9%) to epoch 2 (48.8%) (P = .03).
TABLE 1.
Demographic and Perinatal Characteristics
| Epoch 1 (N = 411) | Epoch 2 (N = 405) | Pa | |
|---|---|---|---|
| Body weight, mean ± SD, g | 668.6 ± 103.0 | 657.4 ± 99.7 | .114 |
| Body weight 401–500 g, n/N (%) | 19/411 (4.6) | 18/405 (4.4) | NS |
| EGA, mean ± SD, wk | 23.7 ± 0.53 | 23.7 ± 0.52 | NS |
| EGA distribution, n/N (%) | |||
| <22 wk | 1/411 (0.2) | 0 (0.0) | NS |
| 22 wk | 11/411 (2.7) | 12/405 (3.0) | |
| 23 wk | 84/411 (20.4) | 93/405 (23.0) | |
| 24 wk | 315/411 (76.6) | 300/405 (74.1) | |
| Male, n/N (%) | 198/411 (48.2) | 212/405 (52.4) | NS |
| Race, n/N (%) | |||
| Black | 209/411 (50.9) | 178/405 (44.0) | .104 |
| White | 193/411 (47.0) | 213/405 (52.6) | |
| Other | 9/411 (2.2) | 14/405 (3.5) | |
| Rupture of membranes > 24 h, n/N (%) | 120/404 (29.7) | 131/400 (32.8) | NS |
| Multiple birth, n/N (%) | 75/411 (18.3) | 91/405 (22.5) | .158 |
| Antenatal antibiotic use, n/N (%) | 346/410 (84.4) | 323/405 (79.8) | .102 |
| Antenatal steroids use, n/N (%) | 328/411 (79.8) | 332/404 (82.2) | NS |
| Cesarean delivery, n/N (%) | 168/411 (40.9) | 197/404 (48.8) | .028 |
| 5-min Apgar score < 5, n (%) | 97/408 (23.8) | 79/405 (19.5) | .163 |
| Maternal age, mean ± SD, y | 27.2 ± 6.76 | 27.4 ± 6.61 | NS |
| Maternal insurance Medicaid, n/N (%) | 283/409 (69.2) | 301/403 (74.7) | .096 |
P > .2 are reported as not significant.
Common neonatal morbidities and interventions are presented in Table 2. Among the significant epoch-related differences on unadjusted analyses, indomethacin prophylaxis (P = .001), surgery for PDA (P = .004), and late sepsis (P = .01) were more common in epoch 2 than in epoch 1. Surgery during neonatal hospitalization for necrotizing enterocolitis, PDA, or retinopathy of prematurity was slightly more common in epoch 2, with a borderline P value (P = .056). However, the proportion of infants exposed to postnatal steroids in epoch 2 (32.8%) was approximately half that in epoch 1 (63.5%) (P < .0001). It should be noted that there were no significant differences between epoch 1 and epoch 2 in PDA, severe intraventricular hemorrhage, or cystic periventricular leukomalacia, or oxygen use at 36 weeks' postmenstrual age.
TABLE 2.
In-Hospital Morbidities and Interventions and Postdischarge Factors
| Epoch 1 | Epoch 2 | Pa | |
|---|---|---|---|
| Surfactant treatment, n (%) | 364/411 (88.6) | 367/405 (90.6) | NS |
| Any intermittent mandatory ventilation, n (%) | 409/411 (99.5) | 403/405 (99.5) | NS |
| High-frequency ventilation any time during hospitalization, n (%) | 165/411 (40.2) | 184/405 (45.4) | .1460 |
| PDA diagnosed, n (%) | 259/411 (63.0) | 265/405 (65.4) | NS |
| Indomethacin | |||
| Prophylaxis, n (%) | 137/411 (33.3) | 181/405 (44.7) | .0011 |
| Treatment, n (%) | 231/411 (56.2) | 222/405 (54.8) | NS |
| Surgery for PDA, n (%) | 110/411 (26.8) | 147/405 (36.3) | .0040 |
| Sepsis, n (%) | |||
| Early | 5/411 (1.2) | 15/405 (3.7) | .0380 |
| Late | 206/411 (50.1) | 239/405 (59.0) | .0130 |
| Necrotizing enterocolitis, n (%) | 36/411 (8.8) | 40/405 (9.9) | .6682 |
| Surgery for necrotizing enterocolitis (lap or drain), n (%) | 25/411 (50.1) | 27/405 (6.7) | .8430 |
| Grade 3 or 4 intraventricular hemorrhage or cystic periventricular leukomalacia, n (%) | 76/409 (18.6) | 90/404 (22.3) | NS |
| Shunt for hydrocephalus, n (%) | 11/409 (2.7) | 17/403 (4.2) | NS |
| Oxygen at 36 wk postmenstrual age, n (%) | 281/411 (68.4) | 289/405 (71.4) | NS |
| Postnatal steroids, n (%) | 261/411 (63.5) | 133/405 (32.8) | <.0001 |
| Retinopathy of prematurity stage ≥3 with plus, n (%) | 121/409 (29.6) | 122/400 (30.5) | NS |
| Surgery during hospitalization, n (%) | 199/410 (48.5) | 223/402 (55.5) | .0560 |
| Length of stay, median (interquartile range), d | 116 (101–138) | 119 (104–147) | .0230 |
| Maternal education less than high school, n (%) | 87/386 (22.5) | 100/403 (24.8) | NS |
| Corrected age at the time of follow-up visit, mean ± SD, mo | 20.0 ± 2.0 | 20.2 ± 2.9 | NS |
P values of >.2 are reported as not significant (NS).
Major neurosensory outcomes for all epoch 1 and epoch 2 infants, stratified according to EGA (≤23 and 24–24 weeks), are shown in Table 3. There were no significant differences between epochs. Developmental and composite outcomes are shown in Table 4. Rates of PDI less than 70 and neurodevelopmental impairment were higher in epoch 2 compared with epoch 1 on unadjusted analyses. The proportion of children who were profoundly impaired did not differ significantly between epochs, nor did the proportion of those who where unimpaired or minimally impaired. Of note, approximately one-fifth of children born before 25 weeks' EGA were found to be unimpaired or minimally impaired at 18 to 22 months in both epochs, and this proportion did not change between epochs (21.8% and 21.9% in Epochs 1 and 2, respectively).
TABLE 3.
Cerebral Palsy, Deafness, and Blindness at 18 to 22 Months of Age, Corrected for Prematurity
| Epoch 1 | Epoch 2 | Pa | Epoch 1 |
Epoch 2 |
|||
|---|---|---|---|---|---|---|---|
| ≤23 wk | 24 wk | ≤23 wk | 24 wk | ||||
| Cerebral palsy, n | 407 | 403 | 96 | 311 | 105 | 298 | |
| Moderate to severe, n (%) | 45 (11.1) | 60 (14.9) | .129 | 15 (15.6) | 30 (9.7) | 19 (18.1) | 41 (13.8) |
| Severe, n (%) | 25 (6.1) | 25 (6.2) | NS | 8 (8.3) | 17 (5.5) | 9 (8.6) | 16 (5.4) |
| Hearing, n | 406 | 400 | 93 | 313 | 103 | 297 | |
| Severe hearing loss, bilateralb | 8 (2.0) | 17 (4.3) | .096 | 3 (3.2) | 5 (1.6) | 5 (4.9) | 12 (4.0) |
| Vision, n | 408 | 405 | — | 96 | 312 | 105 | 300 |
| Blind, bilateral, n (%)c | 8 (2.0) | 9 (2.2) | NS | 3 (3.1) | 5 (1.6) | 3 (2.9) | 6 (2.0) |
P pertains to comparisons of all epoch 1 versus epoch 2. P values of >.2 are reported as not significant (NS).
Severe hearing loss, bilateral indicates bilateral permanent hearing loss that requires amplification in both ears.
Blind, bilateral indicates no functional vision in either eye.
TABLE 4.
Bayley Scales of Infant Development 2 Developmental and Composite Outcomes at 18 to 22 Months of Age, Corrected for Prematurity
| Epoch 1, Total | Epoch 2, Total | Pa | Epoch 1 |
Epoch 2 |
|||
|---|---|---|---|---|---|---|---|
| ≤23 wk | 24 wk | ≤23 wk | 24 wk | ||||
| MDI, nb | 374 | 384 | 89 | 285 | 102 | 282 | |
| MDI < 70, n (%) | 168 (44.9) | 196 (51.0) | .107 | 52 (58.4) | 116 (40.7) | 63 (61.8) | 133 (47.2) |
| MDI < 50, n (%) | 60 (16.0) | 66 (17.2) | NS | 21 (23.6) | 39 (13.7) | 26 (25.5) | 40 (14.2) |
| PDI, nb | 369 | 384 | — | 88 | 281 | 104 | 280 |
| PDI < 70, n (%) | 103 (27.9) | 134 (34.9) | .047 | 31 (35.2) | 72 (25.6) | 46 (44.2) | 88 (31.4) |
| PDI < 50, n (%) | 59 (16.0) | 73 (19.0) | NS | 23 (26.1) | 36 (12.8) | 27 (26.0) | 46 (16.4) |
| Neurodevelopmental impairment, n/N (%) | 186/371 (50.1) | 227/387 (58.7) | .023 | 57/89 (64.0) | 129/282 (45.7) | 72/103 (69.9) | 155/284 (54.6) |
| Profound impairment, n/N (%) | 63/376 (16.8) | 67/383 (17.5) | NS | 21/89 (23.6) | 42/287 (14.6) | 27/102 (26.5) | 40/281 (14.2) |
| Unimpaired/minimally impaired, n/N (%) | 82/376 (21.8) | 85/389 (21.9) | NS | 8/89 (9.0) | 74/287 (25.8) | 14/104 (13.5) | 71/285 (24.9) |
P values pertain to comparisons of all epoch 1 versus epoch 2. P values of >.2 are reported as not significant (NS).
Because of acute illness, language, behavioral problems, sensory impairment, and other reasons, there were 37 children without an MDI and 42 children without a PDI in epoch 1 and 21 children without an MDI and 21 children without a PDI in epoch 2.
Table 5 shows adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for major adverse outcomes at 18 to 22 months of age, corrected for prematurity. Epoch was not independently associated with neurodevelopmental impairment, an MDI of less than 70, or moderate-to-severe cerebral palsy, after adjusting for either differences in baseline and case-mix variables alone (model 1) or after inclusion of neonatal morbidities and postdischarge influences (model 2), with or without postnatal steroid use in the model. Only the unadjusted odds of neurodevelopmental impairment were greater in epoch 2 compared with epoch 1. Variables independently associated with neurodevelopmental impairment in multivariate regression included male gender (OR: 1.8 [95% CI: 1.29–2.60]), sepsis (OR: 1.75 [95% CI: 1.23–2.48]), any high-frequency ventilation during hospitalization (OR: 2.16 [95% CI: 1.44–3.23]), grade 3 or 4 intraventricular hemorrhage or cystic periventricular leukomalacia (OR: 1.67 [95% CI: 1.09–2.56]), surgery (OR: 2.69 [95% CI: 1.85–3.90]), and postnatal steroid use (OR: 1.52 [95% CI: 1.02–2.26]). Variables inversely associated with neurodevelopmental impairment included antenatal antibiotic exposure (OR: 0.58 [95% CI: 0.35–0.95]) and birth weight (OR: 0.81 [95% CI: 0.68–0.96] for each 100-g increase in body weight).
TABLE 5.
Unadjusted and Adjusted ORs for Major Adverse Outcomes at 18 to 22 Months of Age Corrected for Prematurity in Epoch 2 Compared With Epoch 1
| Model | Epoch 2 vs 1, OR (95% CI) | P |
|---|---|---|
| Neurodevelopmental Impairment | ||
| Unadjusted | 1.41 (1.06–1.88) | .019 |
| Model 1 | 1.30 (0.96–1.77) | .096 |
| Model 2 | 1.22 (0.87–1.72) | .246 |
| Model 2 plus PNS | 1.41 (0.98–2.04) | .066 |
| MDI < 70 | ||
| Unadjusted | 1.28 (0.96–1.70) | .092 |
| Model 1 | 1.21 (0.89–1.65) | .220 |
| Model 2 | 1.12 (0.80–1.56) | .513 |
| Model 2 plus PNS | 1.30 (0.91–1.87) | .154 |
| Moderate-to-severe cerebral palsy | ||
| Unadjusted | 1.41 (0.93–2.13) | .105 |
| Model 1 | 1.23 (0.79–1.90) | .354 |
| Model 2 | 1.22 (0.72–2.09) | .463 |
| Model 2 plus PNS | 1.52 (0.86–2.71) | .152 |
PNS indicates postnatal steroid use.
DISCUSSION
This analysis is the largest to date to examine early-childhood outcomes among preterm infants born at less than 25 weeks' EGA during 2 epochs in the recent era. We found that survival rates had not changed from epoch 1 to epoch 2. There were no significant differences between epochs in rates of blindness, severe hearing loss, moderate-to-severe cerebral palsy, or an MDI less than 70. Although the absolute rate of neurodevelopmental impairment was greater in the more recent period, epoch was not independently associated with neurodevelopmental impairment based on multivariable analysis. Despite a dramatic reduction in postnatal steroid exposure, neurodevelopmental outcomes in early childhood for this group of extraordinarily preterm infants remain unchanged.
Our results may seem to conflict with previous studies that demonstrated improvements in some neurodevelopmental outcomes for some groups of preterm infants. However, our current analysis focused on the most extremely preterm infants, who may be uniquely vulnerable and developmentally distinct from more advanced preterm infants. Vohr et al8 reported that rates of severe sequelae at 18 to 22 months of age, including an MDI of less than 70 (41.8%–37.2%) and neurodevelopmental impairment (50.2%–44.6%) had significantly decreased over 3 time periods among infants born at 22 to 26 weeks' EGA in the NICHD NRN, independent of confounding variables. But that analysis included infants born at 25 and 26 weeks' EGA. In a single-center study, Wilson-Costello et al9 compared 20-month outcomes of infants with extremely low birth weight who were born between 2000 and 2002 to those born during the 2 previous periods. The authors found that the rates of any neurosensory abnormality moved from 18% to 23% to 9% over the 3 time periods (P = .001), and cerebral palsy rates moved from 8% to 13% to 5% (P = .01). Survival without impairment increased from 1990–1999 to 2000–2002. However, mean body weight among survivors was 90 g greater and mean EGA was ∼2 weeks more advanced than in our cohort.
Significant concerns often have been raised regarding mortality and short-term morbidities of infants considered to be at the “border of viability.”19 However, few recent studies have focused on the early-childhood outcomes of infants born at less than 25 weeks' EGA, likely because of small patient numbers. A previous NICHD NRN study, which described 18- to 22-month outcomes of more than 700 infants born at less than 25 weeks' EGA during 2 periods in the 1990s, failed to demonstrate improved neurodevelopmental outcomes over time despite more consistently proactive perinatal and neonatal management.11 In a single-center study, Hack et al12 compared 20-month outcomes of infants born at 23 to 24 weeks' EGA in 1995–1999 to those born during 2000–2004 (n = 50 in each time period). The results, which demonstrated no improvement with regard to an MDI less than 70 (42% vs 54%), an MDI less than 50 (10% vs 12%), or cerebral palsy (6% vs 12%), are consistent with those of our study. The Victorian Infant Collaborative Study Group reported that for infants born at less than 26 weeks' EGA, neurosensory outcomes at 2 years had not improved among those born in 1997 compared with those born between 1991 and 1992.5 But a recent study10, which includes the 2005 Victorian Infant Collaborative Study birth cohort, was more encouraging. Although survival rates and quality-adjusted survival rates had not improved from the 1997 to 2005 cohorts of infants born at less than 28 weeks' EGA overall, the mean utility per survivor was higher (better) at each week of gestation for the 2005 cohort compared with either cohort from the 1990s. There were only 7 survivors from those born at 23 weeks' EGA and 22 survivors from those born at 24 weeks' EGA in the 2005 cohort; therefore, extrapolation to extraordinarily preterm infants should generally be viewed with caution. Unlike the Victorian Infant Collaborative Study Group, we did not include a normal-birth-weight control group, and ours was not a population-based cohort, both of which are limitations of our study. A strength of our analysis is, however, that it included a large number of extraordinarily preterm infants. However, our analysis included a large number of extraordinarily preterm infants, which would not have been possible without the benefit of a multicenter network. This is a strength of our analysis.
It should also be acknowledged that neurodevelopmental follow-up at 18 to 22 months' corrected age is a very early window into childhood outcomes. Previous research has shown that profound disability in early childhood is a good predictor of moderate or severe disability at early school age.20 However, for those with mild or moderate disability in early childhood, anticipating later outcomes is much more challenging.20,21 The Bayley Scales of Infant Development II scores in early childhood have been shown to be poorly predictive of cognitive outcomes at 8 years.22 However, despite difficulties in predicting outcomes precisely, it is clear that extremely preterm infants continue to have substantial impairments in cognitive, motor, and executive function through childhood.20,23,24 A recent report25 of 8-year outcomes among infants born in 1997 at less than 28 weeks' EGA from the Victorian Infant Collaborative Study demonstrated that moderate to severe disability was seen in 19% and mild disability in 40% of the cohort of 142 children. Seminal and truly long-term follow-up to young adulthood has shown that neurodevelopmental impairments persist among very-low-birth-weight infants, although many are able to overcome their initial challenges, and risk-taking behavior is less common than among normal-birth-weight control subjects.26,27 Evaluation at a later age allows for the identification and delineation of a broader range of concerns and for longitudinal analysis to determine the predictive value of early disability. What is considered to be the “border of viability” has shifted substantially over recent decades; infants born at less than 25 weeks' EGA are now more routinely offered intensive care.5 It is crucial, therefore, to understand neurologic and cognitive outcomes, both in early and later childhood, to properly counsel families and prepare for supports and services the children may require.
CONCLUSIONS
Rates of survival and major neurodevelopmental outcomes at 18 to 22 months' corrected age for infants born at less than 25 weeks' EGA born in the NICHD NRN did not improve between 2 recent birth epochs. Our results highlight the importance of ongoing research and evidence-based efforts to achieve the ultimate goal of preventing extremely preterm birth.28 Nevertheless, opportunities to reduce neonatal morbidities associated with adverse early-childhood neurodevelopmental outcomes, including sepsis, must be pursued. Our results clearly underscore the unique vulnerability of these infants; to substantially improve early-childhood outcomes will likely require steps beyond traditional quality-improvement approaches. Although the majority of studies have focused on the association of perinatal and neonatal factors and events with early-childhood outcomes, it is critically important to evaluate the potential for postdischarge developmental interventions to improve outcomes for these high-risk extremely preterm infants.29
ACKNOWLEDGMENTS
The National Institutes of Health and the NICHD provided grant support for the NRN′s generic database and follow-up studies. Data collected at participating sites of the NICHD NRN were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Dr Abhik Das (DCC Principal Investigator) and Mr Douglas Kendrick (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
The following investigators (and their National Institutes of Health grant numbers), in addition to those listed as authors, participated in this study: Alan H. Jobe, MD, PhD, University of Cincinnati (NRN Chair); Alpert Medical School of Brown University and Women and Infants Hospital of Rhode Island (U10 HD27904): William Oh, MD, Abbot R. Laptook, MD, Barbara Alksninis, PNP, Angelita M. Hensman, RN, BSN, Teresa M. Leach, Med, CAES, Martha R. Leonard, BA, BS, Lucy Noel, RN, Rachel A. Vogt, MD, and Victoria E. Watson, MS, CAS; Case Western Reserve University, Rainbow Babies & Children's Hospital (GCRC M01 RR80 and U10 HD21364): Michele C. Walsh, MD, MS, Avroy A. Fanaroff, MD, Nancy S. Newman, RN, Bonnie S. Siner, RN, and Harriet G. Friedman, MA; Cincinnati Children's Hospital Medical Center, University of Cincinnati Hospital and Good Samaritan Hospital (GCRC M01 RR8084 and U10 HD27853): Edward F. Donovan, MD, Jean J. Steichen, MD, Barbara Alexander, RN, Cathy Grisby, BSN, CCRC, Marcia Worley Mersmann, RN, Holly L. Mincey, RN, BSN, Jody Hessling, RN, and Teresa L. Gratton, PA; Emory University Children's Health Care of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (GCRC M01 RR39 and U10 HD27851): Barbara J. Stoll, MD, Ira Adams-Chapman, MD, Sheena Carter, PhD, Elisabeth Dinkins, PNP, Ellen C. Hale, RN, BS, CCRC, Maureen Mulligan LaRossa, RN, and Gloria V. Smikle, PNP, MSN; NICHD: Linda L. Wright, MD, and Elizabeth M. McClure, Med; Indiana University, Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (GCRC M01 RR750 and U10 HD27856): Brenda B. Poindexter, MD, MS, James A. Lemons, MD, Marilyn Bull, MD, Anna M. Dusick, MD, FAAP, Darlene Kardatzke, MD, Carolyn Lytle, MD, MPH, Diana D. Appel, RN, BSN, Lon G. Bohnke, MS, Greg Eaken, PhD, Dianne E. Herron, RN, Lucy C. Miller, RN, BSN, CCRC, Heike M. Minnich, PsyD, HSPP, Leslie Richard, RN, and Leslie Dawn Wilson, BSN, CCRC; RTI International (U01 HD36790): W. Kenneth Poole, PhD, Betty Hastings, Elizabeth M. McClure, Med, Jeanette O'Donnell Auman, BS, Carolyn M. Petrie Huitema, MS, and Scott E. Schaefer, MS; Stanford University, Lucile Packard Children's Hospital and California Pacific Medical Center (GCRC M01 RR70 and U10 HD27880): David K. Stevenson, MD, Krisa P. Van Meurs, MD, Susan Hintz, MD, MSEpi, Charles E. Ahlfors, MD, Robert D. Stebbins, MD, Jean G. Kohn, MD, MPH, Dharshi Sivakumar, MD, MRCP, M. Bethany Ball, BS, CCRC, Carol G. Kuelper, PhD, Julie C. Lee-Ancajas, PhD, Joan M. Baran, PhD, Lori E. Bond, PhD, Ginger K. Brudos, PhD, Anne M. DeBattista, RN, PNP, Renee P. Pyle, PhD, and Nicholas H. St John, PhD; University of Alabama at Birmingham Health System and Children's Hospital of Alabama (GCRC M01 RR32 and U10 HD34216): Waldemar A. Carlo, MD, Myriam Peralta-Carcelen, MD, MPH, Kathleen G. Nelson, MD, Kirstin J. Bailey, PhD, Fred J. Biasini, PhD, Stephanie A. Chopko, PhD, Monica V. Collins, RN, BSN, MaEd, Shirley S. Cosby, RN, BSN, Mary Beth Moses, PT, MS, PCS, Vivien A. Phillips, RN, BSN, Julie Preskitt, MSOT, MPH, Richard V. Rector, PhD, and Sally Whitley, MA, OTR-L, FAOTA; University of Miami Holtz Children's Hospital (GCRC M01 RR16587 and U10 HD21397): Shahnaz Duara, MD, Charles R. Bauer, MD, Mary Allison, RN, Ruth Everett-Thomas, RN, MSN, Alexis N. Diaz, BA, Elaine O. Mathews, RN, Kasey Hamlin-Smith, PhD, Lisa Jean-Gilles, BA, Maria Calejo, MS, Silvia M. Frade Eguaras, BA, Silvia Hiriart-Fajardo, MD, and Yamiley C. Gideon, BA; University of Texas Southwestern Medical Center at Dallas Parkland Health and Hospital System and Children's Medical Center Dallas (GCRC M01 RR633 and U10 HD40689): Abbot R. Laptook, MD, Charles R. Rosenfeld, MD, Walid A. Salhab, MD, Roy J. Heyne, MD, R. Sue Broyles, MD, Sally S. Adams, PNP, Cathy Twell Boatman, MS, Cristin Dooley, PhD, LSSP, Alicia Guzman, Gaynelle Hensley, RN, Elizabeth Heyne, PA-C, Jackie F Hickman, RN, Linda A. Madden, BSN, RN, CPNP, Nancy A. Miller, RN, Janet S. Morgan, RN, and Susie Madison, RN; University of Texas Medical School, Children's Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373): Jon E. Tyson, MD, MPH, Kathleen A. Kennedy, MD, MPH, Esther G. Akpa, RN, BSN, Patty A. Cluff, RN, Claudia Y. Franco, RN, BSN, Anna E. Lis, RN, BSN, Georgia E. McDavid, RN, Patti L. Tate, RCP, Nora I. Alaniz, BS, Pamela J. Bradt, MD, MPH, Magda Cedillo, Susan Dieterich, PhD, Patricia Evans, MD, Terri Major-Kincade, MD, MPH, Brenda H. Morris, MD, Maegan C. Simmons, RN, Laura L. Whitely, MD, and Sharon L. Wright, MT; Wayne State University Hutzel Women's Hospital and Children's Hospital of Michigan (U10 HD21385): Seetha Shankaran, MD, Athina Pappas, MD, Yvette R. Johnson, MD, MPH, Virginia Delaney-Black, MD, MPH, Rebecca Bara, RN, BSN, Debra Driscoll, RN, BSN, Laura Goldston, MA, Deborah Kennedy, RN, BSN, Geraldine Muran, RN, BSN, Yale University Yale–New Haven Children's Hospital (general clinical research center) (M01 RR6022 and U10 HD27871): Richard A. Ehrenkranz, MD, Patricia Gettner, RN, Nancy Close, PhD, Walter Gilliam, PhD, Monica Konstantino, RN, BSN, JoAnn Poulsen, RN, Elaine Romano, MSN, Janet Taft, RN, BSN, and Joanne Williams, RN, BSN.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Biography
COMMERCIALS FOR SCHOOLS: For those of us who watch a bit of college football, every weekend we are treated not only to a head-to-head battle between two football teams but their respective universities. As reported on The Wall Street Journal website (November 18, 2010:1–3), as part of the TV contract between the network and the schools and conferences, the schools competing in the televised football game may air a free 30-second commercial. As commercial sponsors may pay $100,000 for that time, these “institutionals” tend to air at times when viewers attention might have lapsed, e.g. during half time. Most university commercials tend to look similar emphasizing a collection of researchers, musicians, and happy students. To learn which of the commercials were most effective, The Wall Street Journal asked four “experts” to review 112 of the 120 “institutionals” produced by schools in the NCAA's Football Bowl Subdivision. Experts included an advertising executive, a film instructor, and two students. Grading criteria included strength of message, technical merit, and whether they made the students want to attend the school. Evidently, too many schools cannot decide on a single message and instead try all at the same time to draw teens to apply, build alumni support, and prove to the community their commitment to the local economy. Celebrity appearances or voiceovers seem effective. The highest-rated commercials tended to focus on a single theme without using buzz words or hackneyed images. The BCS (Bowl Championship Series) championship football game will be played in January, but according to the experts, the champion self-promoter is the University of Minnesota. The spot features Massoud Amin, a professor of electrical and computer engineering, speaking about the importance of creating a better power grid. Rarely particularly successful on the football field, the win is a welcome victory for the Golden Gophers.
Noted by WVR, MD
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- EGA
- estimated gestational age
- NICHD
- Eunice Kennedy Shriver National Institute of Child Health and Human Development
- NRN
- Neonatal Research Network
- PDA
- patent ductus arteriosus
- MDI
- Mental Developmental Index
- PDI
- Psychomotor Developmental Index
- OR
- odds ratio
- CI
- confidence interval
REFERENCES
- 1. Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196(2):147.e1–147.e8 [DOI] [PubMed] [Google Scholar]
- 2. Hakansson S, Farooqui A, Homgren PA, Serenius F, Hogberg U. Proactive management promotes outcome in extremely preterm infants: a population-based comparison of two perinatal management strategies. Pediatrics. 2004;114(1):58–64 [DOI] [PubMed] [Google Scholar]
- 3. Hintz SR, Poole WK, Fanaroff AA, et al. Changes in mortality and morbidity among infants born at less than 25 weeks during the post-surfactant era. Arch Dis Child Fetal Neonatal Ed. 2005;90(2):F128–F133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serenius F, Ewald U, Farooqui A, Holmgren PA, Hakansson S, Sedin G. Short-term outcome after active perinatal management at 23–25 weeks of gestation: a study from two Swedish tertiary care centres. Part 2: infant survival. Acta Paediatr. 2004;93(8):1081–1089 [PubMed] [Google Scholar]
- 5. Doyle LW; Victorian Infant Collaborative Study Group Neonatal intensive care at borderline viability: is it worth it? Early Hum Dev. 2004;80(2):103–113 [DOI] [PubMed] [Google Scholar]
- 6. Costeloe KL, Hennessy EM, Myles J, Draper ES. EPICure 2: survival and early morbidity of extremely preterm babies in England: changes since. E-PAS2008:5365.1. Available at: http://www.abstracts2view.com/pasall Accessed November 16, 2010
- 7. Fischer N, Steurer MA, Adams M, Berger TM, the Swiss Neonatal Network Survival rates of extremely preterm infants (gestational age <26 weeks) in Switzerland: impact of the Swiss guidelines for the care of infants born at the limit of viability. Arch Dis Child Fetal Neonatal Ed. 2009;94(6):F407–F413 [DOI] [PubMed] [Google Scholar]
- 8. Vohr BR, Wright LL, Poole SK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks' gestation between 1993 and 1998. Pediatrics. 2005;116(3):635–643 [DOI] [PubMed] [Google Scholar]
- 9. Wilson-Costello D, Friedman H, Minich N, et al. Improved neurodevelopmental outcomes of extremely low birth weight infants in 2000–2002. Pediatrics. 2007;119(1):37–45 [DOI] [PubMed] [Google Scholar]
- 10. Doyle LW, Roberts G, Anderson PJ; Victorian Infant Collaborative Study Group Outcomes at age 2 years of infants <28 weeks' gestational age born in Victoria in 2005. J Pediatr. 2010;156(1):49.e1–53.e1 [DOI] [PubMed] [Google Scholar]
- 11. Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins R. Changes in neurodevelopmental outcomes at 18–22 months' corrected age among infants born at less than 25 weeks' gestation 1993–1999. Pediatrics. 2005;115(6):1645–1651 [DOI] [PubMed] [Google Scholar]
- 12. Hack M, Wilson Costello D, Friedman H, Minich N, Siner B. Early childhood outcomes of infants born at the limits of viability have not improved in 2000–2004. E-PAS 2008:5365.21. Available at: http://www.abstracts2view.com/pasall Accessed November 16, 2010
- 13. Papile L-A, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a case study of infants with birth weights less than 1500 g. J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 14. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin N Am. 1986;33(1):179–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amiel-Tison C. Neuromotor status. In: Taeusch HW, Yogman MW, eds. Follow-up Management of the High-Risk Infant. Boston MA: Little, Brown and Company; 1987:115–126 [Google Scholar]
- 16. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223 [DOI] [PubMed] [Google Scholar]
- 17. Bayley N. Bayley Scales of Infant Development II. San Antonio, TX: Psychological Corp; 1993 [Google Scholar]
- 18. Committee on Fetus and Newborn Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109(2):330–338 [DOI] [PubMed] [Google Scholar]
- 19. Lucey JF, Rowan CA, Shiono P, et al. Fetal infants: the fate of 4172 infants with birth weights of 401–500 grams: the Vermont Oxford Network Experience (1996–2000). Pediatrics. 2004;113(6):1559–1566 [DOI] [PubMed] [Google Scholar]
- 20. Marlow N, Wolke D, Bracewell MA, Samara M, the EPICure Study Group Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19 [DOI] [PubMed] [Google Scholar]
- 21. Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289(6):705–711 [DOI] [PubMed] [Google Scholar]
- 22. Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116(2):333–341 [DOI] [PubMed] [Google Scholar]
- 23. Woodward LJ, Moor S, Hood KM, et al. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed. 2009;94(5):F339–F344 [DOI] [PubMed] [Google Scholar]
- 24. Marlow N, Hennessy EM, Bracewell MA, Wolke D, the EPICure Study Group Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120(4):793–804 [DOI] [PubMed] [Google Scholar]
- 25. Roberts G, Anderson PJ, De Luca C, Doyle LW, the Victorian infant Collaborative Study Group Changes in neurodevelopmental outcome at age eight in geographic cohorts of children born at 22–27 weeks' gestational age during the 1990s. Arch Dis Child Fetal Neonatal Ed. 2010;95(2):F90–F94 [DOI] [PubMed] [Google Scholar]
- 26. Hack M. Young adult outcomes of very low birth weight children. Semin Fetal Neonatal Med. 2006;11(2):127–137 [DOI] [PubMed] [Google Scholar]
- 27. Saigal S, Stoskopf B, Streiner D, et al. Transition of extremely low-birth-weight infants from adolescence to young adulthood. Comparison with normal birth-weight controls. JAMA. 2006;295(6):667–675 [DOI] [PubMed] [Google Scholar]
- 28. Costeloe K; EPICure Study Group EPICure: facts and figures: why preterm labour should be treated. BJOG. 2006;113(suppl 3):10–12 [DOI] [PubMed] [Google Scholar]
- 29. Spittle AJ, Ferretti C, Anderson PJ, et al. Improving the outcome of infants born at <30 weeks' gestation- a randomized controlled trial of preventative care at home. BMC Pediatr. 2009;9:73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]


