Abstract
OBJECTIVE:
To assess the effect of examination time on newborn neurobehavioral examinations administered within 48 hours of delivery and to identify the earliest appropriate time for performing the assessment.
METHODS:
We analyzed data from neurobehavioral examinations on 324 newborns using the NICU Network Neurobehavioral Scale (NNNS). Trends over examination time and cumulative percentage within published normal ranges were analyzed to identify the earliest appropriate time for administering the examination. Ordinal logistic regression and multivariate regression were used for testing and defining the earliest appropriate time for administering the examination without being influenced by acute effects of labor and delivery while controlling for several potential confounding factors.
RESULTS:
The arousal, excitability, lethargy, quality-of-movement, hypotonicity, and nonoptimal-reflexes scales were sensitive to timing of the examination. Results of ordinal logistic regression showed that 20 hours after delivery seemed to be the earliest appropriate time for administering newborn NNNS examinations. The proportion of NNNS scores within the normal range increased with time significantly when the examination was made less than 20 hours after delivery (n = 148) (odds ratio: 1.12 [95% confidence interval: 1.02–1.23]), but there was no longer significant association with time of examination after 20 hours (n = 176) (odds ratio: 1.04 [95% confidence interval: 0.99–1.09]). This result was confirmed by multivariate regression.
CONCLUSIONS:
We recommend 20 hours after delivery as the earliest appropriate time for administering newborn NNNS examinations to obtain results reflecting outcomes that are a representative assessment of newborn neurobehavior and not contaminated by acute effects of labor and delivery.
Keywords: newborns, behavior, assessment, NNNS
WHAT'S KNOWN ON THIS SUBJECT:
The recommended time for assessing infant neurobehavior varies. For early-newborn examinations in hospitals, current practice requires that the infant be examined within ∼48 hours. It is not known how the amount of time since delivery may affect the results.
WHAT THIS STUDY ADDS:
We recommend 20 hours after delivery as the earliest appropriate time for assessing newborn neurobehavior, because it allows accurate assessment of newborn neurobehavior that is less contaminated by the acute effects of delivery.
Newborn neurobehavioral assessment has a history spanning over 30 years, with Brazelton's Neonatal Behavioral Assessment Scale (NBAS)1 as the earliest measure to both assess neurologic reflexes and produce a behavioral profile of the infant. The NBAS still is used extensively today and primarily is intended to measure interactive and adaptive behaviors of the healthy newborn infant in addition to basic neurologic status.
The NICU Network Neurobehavioral Scale (NNNS)2 was developed, with the NBAS as a base, to assess the early neurobehavior of infants at risk because of exposure to illicit drugs, prematurity, or other risk factors. The primary purpose of the Maternal Lifestyles Study,3 for which the NNNS was developed, was to examine the impact of prenatal exposure to cocaine and/or opiates on health and neurobehavioral outcomes from infancy through adolescence.4 Subsequently, the NNNS has been used in studies of prenatal exposure to other substances such as methadone,5 methamphetamine,6 marijuana,7 and tobacco.8–10 It also has been used to identify the impact of being small for gestational age,11 adolescent parenting,12 and maternal depression13 on infant neurobehavior. The instrument has versatility, and additional studies currently are underway using the NNNS to assess early infant neurobehavioral outcomes related to exposure to environmental toxicants. Recent work14 using latent profile analysis establishes the ability of newborn NNNS scores to predict behavior problems, school readiness, and intelligence to 4.5 years of age, demonstrating the invaluable potential to forecast later developmental outcomes during the newborn period using this instrument.
Despite the thorough administration manuals for the NBAS and NNNS, the optimal time for performing newborn neurobehavioral assessment has not been determined. Because newborns typically are uncoordinated and disorganized in the first 48 hours after delivery, Brazleton1 recommended that the NBAS take place on day 3 of life to obtain the most stable and predictive patterns of behavior. Current practices now result in the majority of newborn infants being discharged from the hospital at 48 hours or less, making a third-day assessment difficult for most research studies interested in newborn neurobehavioral outcomes. The original study by Lester et al,4 in which the NNNS was established, examined 1-month-old infants, and an earlier newborn examination was not performed. It is not known how the earlier timing of neurobehavioral assessment, shortly after birth and before hospital discharge, will affect the response of the newborn unrelated to prenatal exposures or other risk factors.
The reported time at which newborns have been examined in published research using the NBAS and NNNS has varied widely, ranging from several hours to several weeks after delivery and often includes large time spans even within individual studies. No study has attempted to examine the specific effect of postbirth timing of the examination on the assessment or suggest an optimal time for completion of the newborn assessment. To obtain results reflective of the immediate effects of gestational exposure or other risk factors, an examination timed as early as possible after delivery would seem optimal. However, an examination performed too early may produce results reflective of more recent delivery-related influences, such as medications or trauma, and mask the examination objective.
The objective of this study was to assess the effect of examination time on newborn NNNS examinations performed within 48 hours of delivery and thus to define the earliest appropriate time for performing the assessment. The earliest time appropriate for the examination would produce results that are a representative assessment of the effects of prenatal exposure or other risk factors on neurobehavior while minimizing the acute effects of labor and delivery. This also would provide guidance for future neonatal studies and enable more precise comparisons between studies.
METHODS
The study used participants in the Health Outcomes and Measures of the Environment (HOME) study, a prospective cohort study conducted in the greater Cincinnati, Ohio, area. The purpose of the HOME study is to examine the associations between prenatal and postnatal exposure to environmental toxicants and health and developmental outcomes throughout early childhood. Women who were at least 18 years of age were enrolled at 16 ± 3 weeks of pregnancy. Women eligible for study participation resided in homes built before 1978 in 5 preselected Ohio counties, received prenatal care from 1 of 8 participating obstetrical practices, and planned to deliver at 1 of 3 participating hospitals. Details of enrollment have been described elsewhere.10,15,16 Institutional review boards of all the research institutions, hospitals, and laboratories involved approved the study protocol. Written informed consent was obtained from each participant. Of the women enrolled in the HOME study, 389 remained in the study to deliver live-born singletons. The newborn NNNS examinations were administered, at convenience, before discharge from the maternity hospital. The examinations were completed in a quiet room by 1 of 4 examiners trained to reliability by an experienced examiner certified to conduct NNNS training (Dr Yolton). The time of examination was recorded and the age of the newborn was calculated in hours. Of 341 NNNS examinations of newborns completed, we excluded 17 examinations that were administered beyond 48 hours of age because these examinations were mostly conducted at home, a nonstandard setting for the newborn examination. This resulted in a sample of 324 newborns included in our analysis.
The NNNS is a neurobehavioral evaluation that integrates several infant assessment tools with its heaviest influence being from the NBAS.1 The measure involves evaluating the neurologic and behavioral qualities of the newborn as well as observing both overt and subtle signs of stress during the examination. Processing NNNS raw data results in scores on 13 major scales: habituation; attention; arousal; regulation; handling; quality of movement; excitability; lethargy; nonoptimal reflexes; asymmetry; hypertonia; hypotonia; and stress abstinence.2 The habituation package has a sleep requirement that often limits the availability of data (data on habituation was available from 156 of 324 infants in our samples). As with most other published studies, we omitted data from this package. Only a few newborns in our sample (11 of 324) demonstrated hypertonicity, as expected in a normal population. Therefore, the habituation and hypertonicity scales were excluded from the current analysis.
In our initial analytic approach, we examined scatter plots of NNNS scores on each scale by the age of the newborn at the time of the examination, applying local regression as a smoothing technique, to look for a trend in each NNNS over time. We also examined bivariate associations between each NNNS and examination time. For the NNNS that showed significant bivariate associations with examination time, we then further examined the relationship by using the following techniques: (1) percentage of scores within normal range in an 1-hour interval, where the normal range is defined as being within 1 SD of the mean on the basis of a published normative sample of healthy newborns examined between 12 and 56 hours of age17; and (2) plots of the cumulative percentage of normal scores over examination time. We used these methods to visually identify the earliest time at which the newborn NNNS outcomes were no longer affected by the time since delivery. We formally tested the earliest time identified and adjacent time points according to 4-hour increments, using ordinal logistic regression, in which we compared the association between the proportion of scales within the published normal range with the examination time before and after the particular time point being tested. To adjust for potential confounding effects, we included the newborn's gender, anesthesia use during labor and delivery, type of delivery (vaginal versus cesarean delivery), any breastfeeding, and being at risk for neurobehavioral deficits as covariates in the ordinal logistic regression models. Newborns were defined as being at risk for neurobehavioral deficits if they met any of the following criteria: gestational age less than 37 weeks; gestational age more than 41 weeks; being small for gestational age (birth weight less than the 10th percentile at the given gestational age); having a 5-minute Apgar score of less than 7; having a maternal serum cotinine level during pregnancy of more than 10 ng/mL; and having reported maternal marijuana use during pregnancy. Of 324 newborns included in this analysis, 97 were classified as being at risk and 227 were classified as healthy. We also examined the association between the raw scores of the same NNNS with time of examination in a multivariate regression to confirm our findings derived from the ordinal logistic regression. We used SAS 9.2 (SAS Institute, Inc, Cary, NC) for all analyses. For all statistical tests, the level of significance was set at .05.
RESULTS
The demographic characteristics and birth outcomes of 324 newborns are summarized in Table 1. The mothers mostly were White, non-Hispanic, married, employed during pregnancy, had completed a college education or higher, and had private insurance. The median household income was $55 000 annually. The majority of the newborns were born within normal ranges for birth weight and gestational age. The newborn NNNS examinations were administered between 6 and 48 hours of age (median: 20 hours [interquartile range: 16 to 24 hours]).
TABLE 1.
Demographic Characteristics and Birth Outcomes (N = 324)
| Characteristic | Value |
|---|---|
| Newborn age at NNNS examination, median (interquartile range), h | 20 (16–24) |
| Gender of the newborn, male, n (%) | 152 (47) |
| Gestational age, mean (SD), wk | 39 (1) |
| Birth weight, mean (SD), g | 3438 (549) |
| At risk for neurobehavioral deficits, n (%) | 97 (30) |
| Preterm (gestational age < 37 wk) | 20 (6) |
| Postterm (gestational age >41 wk) | 16 (5) |
| Apgar score at 5 min ≤ 7 | 1 (0.3) |
| Small for gestational age | 25 (8) |
| Maternal serum cotinine during pregnancy > 10 ng/mL | 34 (10) |
| Reported marijuana use during pregnancy | 24 (7) |
| Maternal age at delivery, mean (SD), y | 30 (5.6) |
| Maternal race, n (%) | |
| White, non-Hispanic | 211 (65.1) |
| Black, non-Hispanic | 93 (28.7) |
| Other | 18 (5.6) |
| Unknown | 2 (0.6) |
| Marital status at baseline, n (%) | |
| Married | 219 (68) |
| Not married but living with someone | 43 (13.3) |
| Not married and living alone | 60 (18.6) |
| Unknown | 2 (0.6) |
| Household income, median (interquartile range), $ (thousands) | 55 (22.5–85) |
| Mother employed during pregnancy, n (%) | 269 (83) |
| Maternal education, n (%) | |
| Less than high school | 28 (8.6) |
| High school graduate or GED | 43 (13.3) |
| Some college or technical school | 84 (25.9) |
| College graduate | 97 (29.9) |
| Graduate or professional school | 70 (21.6) |
| Unknown | 2 (0.6) |
| Maternal insurance, n (%) | |
| Private | 238 (73.5) |
| Public/none | 84 (25.9) |
| Unknown | 2 (0.6) |
Visual inspection of the scatter plots and local regression smoothing lines showed a preliminary trend over time for the arousal, excitability, lethargy, and nonoptimal-reflex scales. Bivariate regression showed a significant effect of examination time for these 4 scales, as well as for the hypotonia and quality-of-movement scales. Therefore, we further analyzed these 6 scales to determine the earliest appropriate time for the NNNS examination.
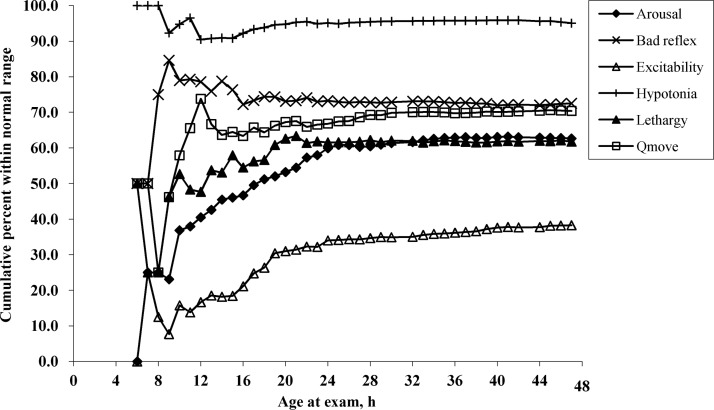
The plots of the hourly cumulative percentages within the normal range for the 6 scales are illustrated in Fig 1. For all 6 scales, the curves for the cumulative percentage within the normal range demonstrated a high variability before 12 hours. After 12 hours, for the arousal, excitability, lethargy, quality-of-movement, and hypotonia scales, the cumulative normal percentage curves showed increasing trends until they became stable by ∼24 hours. The cumulative percentage curve for nonoptimal reflexes exhibited a decreasing trend and also reached a stable level by ∼24 hours. This finding suggests that by 24 hours after birth, NNNS examinations on newborns can be completed without much influence from the examination time.
FIGURE 1.
Cumulative percentage within the normal range in 1-hour intervals. Qmove indicates quality of movement.
We formally tested the 24-hour assessment time using ordinal logistic regression, comparing the association between the proportion of scales within the normal range with the time of examination before and after 24 hours. We also tested adjacent time points to see whether an earlier or later lower bound existed. For simplicity purposes, and to minimize the influence of multiple comparisons, we tested the time points in 4-hour increments from the 24-hour time point. In all analyses, we controlled for the following potentially confounding factors: gender; anesthesia use during labor and delivery; type of delivery; any breastfeeding; and status of being at risk for neurobehavioral deficits. Results from ordinal logistic regression showed that newborns examined less than 24 hours after birth (n = 233) had a significantly higher odds (odds ratio [OR]: 1.08 [95% confidence interval (CI): 1.02–1.14]) of a greater proportion of NNNSs being within the normal range as the age at time of examination increased, whereas newborns examined more than 24 hours (n = 91) after birth did not show a significant association between the proportion of NNNSs within the normal range with time of examination (OR: 1.01 [95% CI: 0.94–1.07]). When testing the 28-hour assessment time, results were similar: there was a significant effect of examination time on the proportion of scales within the normal range before 28 hours (n = 277) (OR: 1.08 [95% CI: 1.03–1.13]) but not after 28 hours (n = 47) (OR: 0.97 [95% CI: 0.88–1.07]). When we tested an earlier time point at 20 hours, there still was a significant effect of examination time on the proportion of scales within the normal range before 20 hours (n = 148) (OR: 1.12 [95% CI: 1.02–1.23]); however, this was not statistically significant after 20 hours (n = 176) (OR: 1.04 [95% CI: 0.99–1.09]). This identified a potential new lower bound of 20 hours. To confirm 20 hours as the lower bound, we tested the 16-hour time and found a significant effect of examination time after 16 hours (n = 248) (OR: 1.04 [95% CI: 1.00–1.08]).
Results from multivariate regression were confirmatory, showing that the association between time of the examination and NNNS performance was not statistically significant after 20 hours after delivery. We conclude that 20 hours is the earliest appropriate time to perform an NNNS examination without significant variability introduced by time of examination. Finally, we produced a normative table summarizing the NNNS scores of newborns examined between 20 and 48 hours of age in this study (Table 2).
TABLE 2.
Normative NNNS Scores Based on Newborns Examined Between 20 and 48 Hours of Age (N = 176)
| NNNS (Birth) | Descriptive |
Percentile |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Minimum | Maximum | P5 | P10 | P25 | P50 | P75 | P90 | P95 | |
| Habituationa | 77 | 6.00 | 1.85 | 2.00 | 9.00 | 3.00 | 3.67 | 4.00 | 6.00 | 7.67 | 8.33 | 9.00 |
| Attentiona | 145 | 5.36 | 1.02 | 2.60 | 7.43 | 3.29 | 4.00 | 4.71 | 5.43 | 6.00 | 6.71 | 7.00 |
| Arousal | 176 | 3.80 | 0.64 | 1.86 | 5.29 | 2.86 | 3.00 | 3.43 | 3.71 | 4.29 | 4.71 | 5.00 |
| Regulationa | 161 | 5.28 | 0.69 | 3.00 | 6.77 | 4.18 | 4.43 | 4.83 | 5.29 | 5.77 | 6.15 | 6.42 |
| Handlinga | 151 | 0.28 | 0.21 | 0.00 | 0.88 | 0.00 | 0.00 | 0.13 | 0.25 | 0.38 | 0.50 | 0.75 |
| Quality of movement | 176 | 4.30 | 0.53 | 2.50 | 5.67 | 3.50 | 3.67 | 4.00 | 4.33 | 4.50 | 5.00 | 5.33 |
| Excitability | 176 | 2.71 | 1.90 | 0.00 | 7.00 | 0.00 | 0.00 | 1.00 | 2.00 | 4.00 | 5.00 | 6.00 |
| Lethargy | 176 | 5.22 | 2.66 | 1.00 | 13.00 | 2.00 | 3.00 | 3.00 | 5.00 | 6.00 | 10.00 | 11.00 |
| Nonoptimal reflexes | 176 | 3.64 | 1.65 | 0.00 | 9.00 | 1.00 | 1.00 | 3.00 | 4.00 | 5.00 | 6.00 | 7.00 |
| Asymmetry total | 176 | 0.98 | 0.95 | 0.00 | 4.00 | 0.00 | 0.00 | 0.00 | 1.00 | 2.00 | 2.00 | 3.00 |
| Hypertonia total | 176 | 0.03 | 0.18 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hypotonia total | 176 | 0.30 | 0.68 | 0.00 | 4.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 1.00 |
| Stress abstinence | 176 | 0.11 | 0.04 | 0.02 | 0.27 | 0.04 | 0.06 | 0.08 | 0.10 | 0.12 | 0.16 | 0.18 |
NNNSs that require a minimum of items completed to be scored.
DISCUSSION
The NNNS was initially developed to study the impact of prenatal exposure to illicit drugs on early neurobehavior among infants,2 with the first published study using the instrument at 4 weeks after delivery.4 More recently, the NNNS has been used to describe neurobehavior among infants with other prenatal exposures or risk factors.2,5–13 The time of newborn examination reported in published studies ranges widely from several hours to several weeks, and no recommendation has been made as to the earliest age of reliable assessment. An early examination seems desirable because it may provide more valuable information on the immediate effects of prenatal exposures or other risk factors. However, an examination administered too close to the time of delivery may produce results more reflective of the acute effects of labor and delivery and mask the examination objectives. We aimed to examine the effect of examination time on the early postbirth assessment and to identify the earliest appropriate time for administering the examination to obtain results accurately representing newborn neurobehavior without influence from labor and delivery factors.
Six of 11 NNNSs that we studied were sensitive to time of examination including arousal, excitability, lethargy, nonoptimal reflexes, hypotonia, and quality of movement. We further analyzed these 6 scales in an attempt to identify the earliest time appropriate for the NNNS examination. There is a dearth of literature on the duration of labor-and-delivery effects on newborn neurobehavior. However, it is reasonable to assume that the acute effects of labor and delivery on newborn neurobehavior would diminish over time. Therefore, we were looking for a time beyond which the NNNS examination was no longer significantly influenced by time of the examination, while controlling for other factors that could influence the outcome.
In searching for the earliest appropriate time for administering the NNNS, we examined how the cumulative percentage of scales within the normal range changed over time. High temporal variability of the cumulative percentage of the scales within the normal range at those very early hours suggested that newborns who were examined too early might not perform in a consistent fashion because of the acute effects of labor and delivery. The increasing trend of the cumulative percentage within the normal range for the arousal, excitability, lethargy, nonoptimal reflexes, and hypotonia indicated that factors related to labor and delivery may be influential in rating newborn neurobehavior during the early hours after delivery and that as this influence diminishes over time, the newborn neurobehavioral performance stabilized. At ∼24 hours, the cumulative percentage within the normal range reached a plateau, initially suggesting that 24 hours is the earliest appropriate time for a newborn NNNS examination without being affected by factors related to labor and delivery.
Ordinal logistic regression was used to formally test the 24-hour time along with adjacent time points in 4-hour increments. This allowed evaluation of the association of the proportion of scales (all 6 scales) within the normal range, with time of the examination, while controlling for potential confounding factors. As a confirmatory analysis, we also tested the same time points using multivariate regression and examined the associations between the raw scores of the same 6 NNNSs tested in the ordinal logistic regression and time of examination. Results from both ordinal logistic regression and multivariate regression indicated that newborn NNNSs were significantly influenced by time of examination at the early hours immediately after the delivery. The effect of time of examination was no longer significant after at least 20 hours after delivery. We concluded that 20 hours after delivery is the earliest appropriate time to administer an NNNS examination on a newborn infant without obtaining results influenced by the acute effects of labor and delivery.
There are several limitations of this study. First, the number of NNNS examinations was unevenly distributed over time. The NNNS examinations were administered at convenience before the newborn was discharge from the maternity hospital. The majority of newborns were assessed between 16 and 24 hours, with fewer newborns being assessed at the earliest and latest time periods. However, with the 20-hour time identified as the earliest appropriate for administering the examination, there were 148 and 176 newborns assessed before and after 20 hours, respectively. This provided similar sample sizes for comparison; therefore, it is less likely that the 20-hour time was influenced by the uneven distribution of the number of examinations over time. Second, when examining the association between the proportion of NNNSs within the normal range with time of examination, we controlled for several potential effect modifiers or confounding factors including the newborn infant's gender, anesthesia use during labor and delivery, type of delivery, any breastfeeding, and being at risk for neurobehavioral deficits. There might be additional effect modifiers or confounding factors that we have not measured. Third, we defined the normal range of NNNSs on the basis of published normative data.17 Newborns in that normative sample were assessed between 12 and 56 hours. On the basis of our analysis, some of these normative data were collected before the earliest time that we consider appropriate for the newborn examination.
CONCLUSIONS
Several scales of the newborn NNNS examination are sensitive to the timing of the examination. In an examination performed too soon after delivery, true neurobehavioral outcomes could be masked by factors related to labor and delivery. Our analysis showed that examinations performed at or after 20 hours after delivery were not significantly affected by timing of examination in contrast to examinations performed before 20 hours. We recommend 20 hours after delivery as the earliest appropriate time for administering the newborn NNNS examination to obtain neurobehavioral evaluations that are not influenced by the acute effects of labor and delivery and thus most accurately reflective of newborn neurobehavior. This recommendation will assist in setting a standardized time for the assessment that can be used in future studies using newborn neurobehavioral assessment and allow better comparability across studies.
ACKNOWLEDGMENTS
This work was partially supported by a grant from the Flight Attendant Medical Research Institute (062620_CIA) and from Children's Environmental Health Center grant PO1 ES11261 from the National Institute of Environmental Health Sciences and the Environmental Protection Agency.
We also recognize the support of Dr Bruce Lanphear during the completion of this project.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
- NBAS
- Neonatal Behavioral Assessment Scale
- NNNS
- NICU Network Neurobehavioral Scale
- OR
- odds ratio
- CI
- confidence interval
REFERENCES
- 1. Brazelton TB. Neonatal Behavioral Assessment Scale. 2nd ed Philadelphia, PA: J. B. Lippincott Co; 1984 [Google Scholar]
- 2. Lester BM, Tronick EZ. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 pt 2):634–640 [PubMed] [Google Scholar]
- 3. Lester BM. The Maternal Lifestyles Study. Ann N Y Acad Sci. 1998;846:296–305 [PubMed] [Google Scholar]
- 4. Lester BM, Tronick EZ, LaGasse L, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110(6):1182–1192 [DOI] [PubMed] [Google Scholar]
- 5. Velez ML, Jansson LM, Schroeder J, Williams E. Prenatal methadone exposure and neonatal neurobehavioral functioning. Pediatr Res. 2009;66(6):704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith LM, Lagasse LL, Derauf C, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30(1):20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Moraes Barros MC, Guinsburg R, de Araujo Peres C, Mitsuhiro S, Chalem E, Laranjeira RR. Exposure to marijuana during pregnancy alters neurobehavior in the early neonatal period. J Pediatr. 2006;149(6):781–787 [DOI] [PubMed] [Google Scholar]
- 8. Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111(6 pt 1):1318–1323 [DOI] [PubMed] [Google Scholar]
- 9. Stroud LR, Paster RL, Papandonatos GD, et al. Maternal smoking during pregnancy and newborn neurobehavior: effects at 10 to 27 days. J Pediatr. 2009;154(1):10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yolton K, Khoury J, Xu Y, et al. Low-level prenatal exposure to nicotine and infant neurobehavior. Neurotoxicol Teratol. 2009;31(6):356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Moraes Barros MC, Guinsburg R, Mitsuhiro SS, Chalem E, Laranjeira RR. Neurobehavior of full-term small for gestational age newborn infants of adolescent mothers [in Portuguese]. J Pediatr (Rio J). 2008;84(3):217–223 [DOI] [PubMed] [Google Scholar]
- 12. de Moraes Barros MC, Guinsburg R, Mitsuhiro S, Chalem E, Laranjeira RR. Neurobehavioral profile of healthy full-term newborn infants of adolescent mothers. Early Hum Dev. 2008;84(5):281–287 [DOI] [PubMed] [Google Scholar]
- 13. Salisbury AL, Lester BM, Seifer R, et al. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007;29(3):331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J, Bann C, Lester B, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125(1). Available at: www.pediatrics.org/cgi/content/full/125/1/e90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geraghty SR, Khoury JC, Morrow AL, Lanphear BP. Reporting individual test results of environmental chemicals in breastmilk: potential for premature weaning. Breastfeed Med. 2008;3(4):207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Phelan KJ, Khoury J, Xu Y, Lanphear B. Validation of a HOME Injury Survey. Inj Prev. 2009;15(5):300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 pt 2):676–678 [PubMed] [Google Scholar]