To the Editor
Gemcitabine is one of first-line therapies for locally advanced pancreatic cancer; however, severe resistance is responsible for less than 6 months of median survival and less than 20% of response rate. Although efforts to overcome gemcitabine resistance have been underway, the only combination treatment that has shown a small, but statistically significant outcome is gemcitabine with erlotinib, an EGFR inhibitor (1).
Improvements in pancreatic cancer therapy can be made by understanding gemcitabine resistance. Currently, expression levels of several proteins such as ENT1 (equilibrative nucleoside transporter-1), MRPs (multidrug resistance-associated proteins), dCK (deoxycytidine kinase) and CDA (cytidine deaminase) have been reported to be involved in gemcitabine resistance (2). However, these are not sufficient to address the full mechanisms of resistance and to direct feasible therapeutic strategies. Instead gemcitabine resistant cells might utilize several biochemical pathways to survive gemcitabine induced cytotoxic or genotoxic damages.
In order to enhance the treatment benefits of conventional therapeutic drugs, targeting protein kinases in combination with DNA damaging drugs has been aggressively attempted for several types of cancers. As part of this effort in pancreatic cancer, we explored 76 protein kinase inhibitors (PKIs) for probing new targetable protein kinases and found that several PKIs possess promising efficacy in combination with gemcitabine (3).
Additionally, parallel investigations on the nature of drug resistance are prerequisite for developing substantial therapeutic strategies in the treatment of pancreatic cancer. Gemcitabine resistant cells may alter the dependency on several key kinases to facilitate cell proliferation and survival. Thus, searching the bypass of biochemical pathways in gemcitabine resistant cells may be achieved by the comparison of sensitivities on PKIs between parental and gemcitabine resistant cells. For efficient isolation of target kinases, we employed a method, in which EC50 ratios were compared.
Materials and Methods
For creating gemcitabine resistant cells, MiaPaCa2 (ATCC, Manassas, VA) cells were exposed to incrementally increasing doses (starting at 0.1 μM) of gemcitabine. When the cells adapted to a dose, the gemcitabine concentration was increased by 0.1 μM. After three months of selection MiaPaCa2 cells surviving at 1.5 μM of gemcitabine were generated.
To determine the EC50 in parental and gemcitabine resistant cells, we measured cell viability using MTT (3-(4,5-dimethyl) ethiazole) assays after treatment with each PKI (from 0.1 to 10 μM). Then EC50 of each PKIs in both cell types were determined using CompuSyn software (ComboSyn, Inc., Paramus, NJ). In order to compare the efficacy of PKIs in gemcitabine resistant cells, we calculated the EC50 ratio [(EC50 in parental cell)/(EC50 in resistant cell)].
Results and Discussion
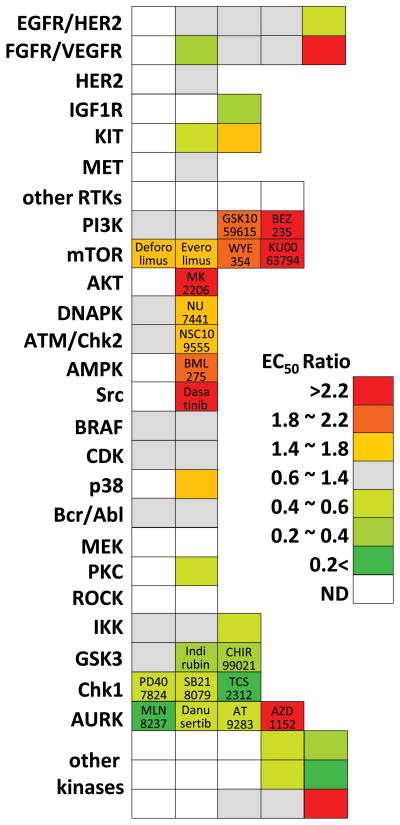
After serial selection with increasing concentrations of gemcitabine, we obtained MiaPaCa2 populations surviving at 1.5 μM gemcitabine. The EC50 of gemcitabine was significantly higher in resistant cells (8.77μM) than in parental cells (0.55 μM). We measured viabilities of gemcitabine resistant and parental cells with 84 PKIs. Comparison of the EC50 ratio revealed that 16 PKIs exhibited high scores (EC50 ratio >1.4) and 18 PKIs showed low scores (EC50 ratio <0.6) (Fig. 1). We could not determine the EC50 of 28 PKIs in both parental and gemcitabine resistant cells (Fig 1).
Figure 1.
The EC50 ratio [(EC50 in parental cell)/(EC50 in resistant cell)] was calculated and is illustrated as a heat map. Each block represents a PKI classified into each target kinase. Because most PKIs have multiple targets, we have classified them by their most representative target. PKIs described in the results and discussion sections are indicated.
One of the most well characterized pathways in cell survival and drug resistance is the PI3K/AKT/mTOR axis. This axis is frequently activated in pancreatic cancer (5) and several clinical trials targeting this axis are underway. Inhibitors for the PI3K/AKT/mTOR signaling axis showed relatively high scores, i.e., they are more effective in gemcitabine resistant cells than in parental cells. Among them MK2206, an AKT inhibitor, exhibited the best efficacy (EC50 ratio = 6.52). Since we observed elevated levels of phosphorylated AKT in gemcitabine resistant cells (data not shown), this result might indicate that the cells became more dependent on AKT signaling for survival. Inhibitors of PI3K, BEZ235 (EC50 ratio = 2.51) and GSK1059615 (EC50 ratio = 2.10), also exhibited preferential cytotoxic effect on gemcitabine resistant cells. All mTOR specific inhibitors also showed a high EC50 ratio. Interestingly, mTOR complex 1 and 2 (mTORC1 and mTORC2) dual inhibitors (KU0063794 and WYE354) showed better efficacy than mTORC1 inhibitors (Everolimus and Deforolimus). Although mTORC2 is known to mediate cell proliferation and survival, nothing has been reported about its correlation with drug resistance. Thus, further investigation on the role of mTORC2 is needed to elucidate its correlations with gemcitabine resistance.
Inhibition of DNAPK, a member of the PI3K-related kinase subfamily, displayed a high EC50 ratio (NU7441, 1.49). Previously, we observed that NU7441 can preferentially potentiate the efficacy of gemcitabine (3). As the main function of DNAPK is non-homologous end-joining upon a DNA double strand break, the efficient utilization of this kinase might be necessary to escape the gemcitabine induced DNA damage. In this context, modulation of cell cycle regulating systems upon DNA damage might be another strategy to avoid gemcitabine induced genotoxic stress. The EC50 of the Chk2 inhibitor, NSC109555, is significantly reduced in gemcitabine resistant cells. However, the EC50 of Chk1 inhibitors (PD407824, SB218078, and TCS2312) are significantly elevated in gemcitabine resistant cells compared to parental cells. Targeting Chk1 as well as ATR for gemcitabine sensitization is well documented including our own observations (3). Since ATR/Chk1 has been reported to be more essential than ATM/Chk2 in the response to gemcitabine induced DNA damage (6), these data might indicate that tight regulation of ATR/Chk1 has been nullified by cells while becoming gemcitabine resistant.
Our data also suggest that the AMPK inhibitor, BML275, can be a promising therapeutic candidate. Given that AMPK activation upon oxidative stress serves as a key regulator of cell survival, gemcitabine induced oxidative stress may be mitigated when AMPK is substantially activated. Duxbury et al. reported that inhibition of Src impairs gemcitabine resistance in PANC1 cells (7). However, the efficiency of targeting Src signaling remains unclear: we found that only Dasatinib, which is considered a multi-targeting inhibitor rather than a Src-specific PKI, showed prominent efficacy.
The function of GSK3 in cell survival and drug resistance is quite controversial. On the one hand, GSK3β directly phosphorylates the oncoprotein, β-catenin, with subsequent degradation. On the other hand, GSK3β deficiency in mice leads to an intrinsic defect in the activation of NFκB, which shares some responsibility for the drug resistance mechanism in pancreatic cancer. However, inhibition of GSK3 (Indirubin and CHIR99021) revealed little inhibition and limited efficacy in previous reports (8). In addition, indirect targeting of NFκB with IKK inhibitors was not effective in gemcitabine resistant cells. Thus, the NFκB pathway may not be a determinant factor in cell survival of our gemcitabine resistant cell model.
AURKA, which has a central role in mitotic entry and bipolar spindle assembly, is actively targeted in combination with Taxane (9). However, we previously reported that combinational treatment of AURKA inhibitors with gemcitabine showed the lowest synergism (3). Consistently, our data revealed that gemcitabine resistant cells exhibited rather resistant to AURKA inhibitors (MLN8237, Danusertib and AT9283) compared to parental cells. On the other hand, synergism of AZD1152, an AURKB inhibitor, with gemcitabine was already validated in a xenograft model (10). Our data also demonstrated the potency of AZD1152 implying that the functions of AURKA and AURKB are quite different in gemcitabine resistance.
Unveiling the acquired biochemical bypasses during survival against chemotherapeutic drugs might provide significant clues for enhancement of pancreatic cancer therapy. In comparison with previous study (3), we can postulate that PI3K/AKT/mTOR and DNAPK/Chk2 are inevitable and pivotal pathways for cell survival over gemcitabine and that ATR/Chk1 and AURKA are abrogated pathways to facilitate cell proliferation. Further demonstration of the roles of their target kinases based on our new findings might generate further information and direct new strategies for the treatment of advanced pancreatic cancer.
Acknowledgments
This work was partially supported by Susan G. Komen for the Cure (FAS0703858) and the National Institutes of Health (1R03CA152530). This research was also supported by the WCU (World Class University) Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (R31-10069).
Footnotes
The authors have no conflicts of interest to disclose.
Contributor Information
Yeon Jeong Kim, Department of Nanobiomedical Science and WCU Research Center of Nanobiomedical Science, Dankook University, Cheonan, Republic of Korea.
Young Bin Hong, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC. Department of Nanobiomedical Science and WCU Research Center of Nanobiomedical Science, Dankook University, Cheonan, Republic of Korea.
Chi Heum Cho, Department of Obstetrics and Gynecology, Keimyung University, School of Medicine, Daegu, Korea.
Yeon-Sun Seong, Department of Nanobiomedical Science and WCU Research Center of Nanobiomedical Science, Dankook University, Cheonan, Republic of Korea.
Insoo Bae, Department of Oncology and Department of Radiation Medicine, Lombardi Comprehensive Cancer Center, Georgetown University, Washington DC. Department of Nanobiomedical Science and WCU Research Center of Nanobiomedical Science, Dankook University, Cheonan, Republic of Korea.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Nishio R, Tsuchiya H, Yasui T, Matsuura S, Kanki K, Kurimasa A, Hisatome I, Shiota G. Disrupted plasma membrane localization of equilibrative nucleoside transporter 2 in the chemoresistance of human pancreatic cells to gemcitabine (dFdCyd) Cancer Sci. 2011;102:622–629. doi: 10.1111/j.1349-7006.2010.01837.x. [DOI] [PubMed] [Google Scholar]
- 3.Hong YB, Kim JS, Yi YW, Seong YS, Bae I. Exploring Protein Kinase Inhibitors: Potentiating Gemcitabine Efficacy in Pancreatic Cancer. Pancreas. 2011 doi: 10.1097/MPA.0b013e318230f71a. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 5.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated Akt in pancreas cancer. Br J Cancer. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azorsa DO, Gonzales IM, Basu GD, Choudhary A, Arora S, Bisanz KM, Kiefer JA, Henderson MC, Trent JM, Von Hoff DD, Mousses S. Synthetic lethal RNAi screening identifies sensitizing targets for gemcitabine therapy in pancreatic cancer. J Transl Med. 2009;7:43. doi: 10.1186/1479-5876-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res. 2004;10:2307–2318. doi: 10.1158/1078-0432.ccr-1183-3. [DOI] [PubMed] [Google Scholar]
- 8.Mamaghani S, Patel S, Hedley DW. Glycogen synthase kinase-3 inhibition disrupts nuclear factor-kappaB activity in pancreatic cancer, but fails to sensitize to gemcitabine chemotherapy. BMC Cancer. 2009;9:132. doi: 10.1186/1471-2407-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapenna S, Giordano A. Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009;8:547–566. doi: 10.1038/nrd2907. [DOI] [PubMed] [Google Scholar]
- 10.Azzariti A, Bocci G, Porcelli L, Fioravanti A, Sini P, Simone GM, Quatrale AE, Chiarappa P, Mangia A, Sebastian S, Del Bufalo D, Del Tacca M, Paradiso A. Aurora B kinase inhibitor AZD1152: determinants of action and ability to enhance chemotherapeutics effectiveness in pancreatic and colon cancer. Br J Cancer. 2011;104:769–780. doi: 10.1038/bjc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]