Highlights
* Selective attention is the ability to enhance relevant signals and manage distraction. * The neural bases and development of this ability are well-understood. * Further, selective attention appears to impact language, literacy, and math skills. * These impacts can be related to specific neurobiological mechanisms. * Selective attention can also be trained for the better.
Keywords: Selective attention, Distractor suppression, Development, Training
Abstract
To the extent that selective attention skills are relevant for academic foundations and amenable to training, they represent an important focus for the field of education. Here, drawing on research on the neurobiology of attention, we review hypothesized links between selective attention and processing across three domains important to early academic skills. First, we provide a brief review of the neural bases of selective attention, emphasizing the effects of selective attention on neural processing, as well as the neural systems important to deploying selective attention and managing response conflict. Second, we examine the developmental time course of selective attention. It is argued that developmental differences in selective attention are related to the neural systems important for deploying selective attention and managing response conflict. In contrast, once effectively deployed, selective attention acts through very similar neural mechanisms across ages. In the third section, we relate the processes of selective attention to three domains important to academic foundations: language, literacy, and mathematics. Fourth, drawing on recent literatures on the effects of video-game play and mind-brain training on selective attention, we discuss the possibility of training selective attention. The final section examines the application of these principles to educationally-focused attention-training programs for children.
Academic achievement is determined by a variety of factors including educational opportunity, socio-economic status (SES), social aptitudes, personality traits, and cognitive skills (see, for example, Brooks-Gunn and Duncan, 1997, Wentzel, 1991, Wentzel and Caldwell, 2006). Among the latter, the ability to focus on the task at hand and ignore distraction, also termed selective attention, appears to have reverberating effects on several domains important to academic foundations, including language, literacy, and mathematics. While it is important to recognize that many factors determine academic achievement, the focus of this paper will be exclusively on selective attention.
Selective attention refers to the processes that allow an individual to select and focus on particular input for further processing while simultaneously suppressing irrelevant or distracting information. The competing information can occur both externally, as in extraneous auditory or visual stimulation in the environment, or internally, as in distracting thoughts or habitual responses which get in the way of performing the task at hand. As most studies in the literature have focused on the filtering of external information, this review will focus primarily on the ability, when presented with a complex environment, to select the relevant dimensions for the task at hand and respond appropriately. Furthermore, the focus will be on the preschool and early school years, although the considerable neural development occurring during infancy in these domains is acknowledged and discussed elsewhere (e.g., Dehaene, 1997, Kuhl, 2004, Richards, 2003, Sheese et al., 2008, Xu and Spelke, 2000). Drawing on research from cognitive science and cognitive neuroscience, we propose a role for selective attention in three domains important to academic foundations (language, literacy, and mathematics). In the sections below, we posit both possible neural mechanisms linking selective attention to each domain, as well as broader implications for educational and remediation programs based on existing data on the plasticity of selective attention.
1. Neural bases of selective attention in adults
Studies of the neural bases of selective attention in adults provide a useful framework for considering the effects of selective attention on academic foundations during development. These studies have often been divided into three separate sets of questions. One set of questions concerns how selective attention, once deployed, modulates information processing. A second set of questions is focused on the mechanism(s) by which selective attention is deployed, including the neural networks that orient attention to particular aspects of the environment. Finally, a third set of questions relates to the neural mechanisms that actively manage competition from irrelevant stimuli, particularly when these are more salient than the target itself. These three sets of questions are considered in turn below.
1.1. Influence of selective attention on information processing
“Everyone knows what attention is…” wrote William James in 1890. “It is the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought… It implies withdrawal from some things in order to deal effectively with others…” (James, 1890, pp. 403–404). Despite these reassuring words, it has taken the last 50 years of research to understand how attention acts to regulate the flow of information made available in the brain. We are past the raging debate of the 1960s over whether attention operates by applying an early versus late bottleneck on information processing (Broadbent, 1958, Deutsch and Deutsch, 1963). This has been resolved by research showing that the effects of attention can be observed from neural regions supporting early perceptual processing all the way to higher, more integrative decision areas (e.g., Colby and Goldberg, 1999, Martinez et al., 2001, O’Connor et al., 2002).
The first properly controlled study to identify the influences of selective attention on neural processing used a clever experimental paradigm that manipulated the focus of selective attention while holding all else constant (Hillyard et al., 1973). In this pioneering experiment, Steven Hillyard used the event-related brain potential (ERP) technique to examine the temporal dynamics of selective auditory attention. Adult participants were presented with two simultaneous streams of auditory tones delivered via headphones separately to each ear. Participants attended selectively to one ear and detected rare high-frequency tones in the attended channel. Standard (non-target) tones presented to the attended ear elicited larger N1s (negativity between 80 and 120 ms) than the same tones when presented in the unattended channel. This indicated that selective attention modulated early neural processing. Further, as some portion of the attention effect mirrored the distribution of the underlying ERP components, this modulation appeared to act at least in part as a gain control mechanism on input-driven neural activity. This paradigm illustrated what became known as the Hillyard Principle: In order to assess the effects of selective attention, responses should be compared to the same physical stimuli while holding overall arousal level and task demands constant, such that all that differs is the focus of selective attention.
Since this initial report in 1973, several studies have applied the Hillyard Principle to examine the effects of selective attention on information processing in different modalities or when directed to different stimulus attributes (for reviews, see Hillyard et al., 1987, Hopfinger et al., 2004). For example, using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), several studies report attentional modulation throughout multiple cortical and even subcortical processing areas (Corbetta et al., 1999, O’Connor et al., 2002, Schoenfeld et al., 2007—but note that attention effects in the earliest areas may reflect feedback modulation as opposed to modulations of the initial afferent volley, e.g., see Martinez et al., 2001). Further, when selective attention is directed to particular stimulus attributes (e.g., shape, color, or motion) enhanced activity is observed in cortical areas associated with processing that attribute (Corbetta et al., 1999, Motter, 1994, Schoenfeld et al., 2007). Similarly, when attention is directed toward particular object classes associated with specialized cortical areas (e.g., faces, houses), activity is modulated in those object-specific cortical regions (Wojciulik et al., 1998).
The attentional gating observed with non-invasive neuroimaging techniques arises from a push–pull mechanism by which the coding of task-relevant information is enhanced while that of task-irrelevant information is suppressed. Several studies document that attention alters the selectivity of neurons in the hierarchy of visual areas, especially by improving the encoding of task-relevant features (Jehee et al., 2011, Murray and Wojciulik, 2004, Reynolds et al., 2000). Neurophysiological studies from awake behaving monkeys further show that attention acts by allowing neurons to fire as if only the attended object were present in their receptive field, diminishing the effect of distractors falling in the same receptive field (Reynolds and Desimone, 2003, Sundberg et al., 2009, Verghese, 2001). As receptive fields increase in size from early to later cortical processing areas, the modulatory effects of selective attention become more marked, especially in later areas where target and distractors are more likely to fall in the same receptive field given their larger size (Luck et al., 1997). In addition, several recent studies make clear that increasing information processing of the attended stimulus requires engaging neurons that have the best discriminatory power between attended and distracting features. This will not necessarily be the neuronal populations most selective to the attended dimension, but rather may be neuronal populations that fall between attended and distractor features, as initially exemplified by Navalpakkam and Itti (2007) (see also, Ma et al., 2011, Scolari and Serrences, 2010). Thus, it is critical to also consider the nature of distractors and their relationship to the attended target when interpreting attentional modulations.
1.2. Neural systems used to deploy selective attention
The above discussion focused on the effects of selective attention on modulating the flow of information processing. An additional fundamental issue concerns the origin of these attentional modulations, that is, the neural systems engaged to orient selective attention to the location, object, or feature of interest. The guiding of selective attention is commonly agreed to be controlled by both bottom-up signals, with salient information guiding the allocation of attention, as well as in a top-down fashion whereby the goals of the participants also shape attentional allocation (Buschman and Miller, 2007, Corbetta and Shulman, 2002, Desimone and Duncan, 1995, Kastner and Ungerleider, 2000, Yantis and Serences, 2003). Such stimulus-driven and goal-directed ways of controlling attention rely on partially overlapping networks of brain areas, and, while guided by separable operating principles, interact almost constantly to ultimately determine attention allocation.
A fruitful approach to characterizing the operating principles behind the deployment of selective attention is to identify the neural response to preparatory cues that signal the spatial location or stimulus feature to be attended prior to the appearance of the actual target display. Across several single event fMRI studies, preparatory cues have been found to engage a primarily fronto-parietal network (Bressler et al., 2008, Corbetta et al., 2002, Corbetta and Shulman, 2002, Walsh et al., 2011). For example, Hopfinger et al. (2000) examined response to an informative color-coded cue presented at fixation that directed spatial attention to the left or right visual field. Several brain areas were engaged by the instructive cue (but not the subsequent appearance of targets), suggesting their role in top-down attentional control. These regions included the intraparietal sulcus (IPS), frontal eye fields, and additional regions of the anterior frontal gyrus. These regions appear important to the endogenous orienting of selective attention, with some evidence that briefly presented cues that capture attention exogenously recruit a similar neural network (Corbetta et al., 2008, Fan et al., 2005, Peelen et al., 2004—but see also Buschman and Miller, 2007 for differences between top-down and bottom-up sources of attentional control).
While most research has focused on cues that direct selective attention to spatial locations, other studies have investigated cues directing attention to particular stimulus features (e.g., color, shape, or motion). These studies have identified similar fronto-parietal networks associated with orienting selective attention, although there is evidence that the precise regions of the control network may vary somewhat based on the feature or type of selection being made (Corbetta and Shulman, 2002, Giesbrecht et al., 2003). This suggests that while the effects of selective attention are most apparent in the cortical areas associated with the attended stimulus dimension (e.g., modulation of motion-sensitive MT/MST when directing attention to motion), it is a fronto-parietal network that is used across dimensions to deploy selective attention.
1.3. Monitoring and resolving response conflict from competing stimuli
The early attentional filtering reviewed above modulates the amount of processing any distracting information may receive. Distractors may be processed to varying degrees as a function of their physical similarity to the targets, their location in the visual field, and their intrinsic salience, to name a few (see Miller, 1991 for a review). Thus, at times, distracting stimuli may receive enough processing to compete for response selection, triggering the need for response conflict resolution. To assess the influences of such competing distractors, we turn next to a landmark paradigm in the field—the flanker compatibility effect. Flanker tasks evaluate the impact on target processing of distractors flanking a target as a function of whether the flanker is compatible or incompatible with the response associated with the target (Eriksen and Eriksen, 1974).
For example, in the arrow flanker task, participants indicate the direction of a centrally pointing arrow while ignoring the direction that nearby flanker arrows point (Fan et al., 2002). Behaviorally, reaction time is slowed and accuracy decreases when the flanker arrows point in the opposite direction of the central arrow (incompatible condition) compared to in the same direction (compatible condition). This flanker effect is believed to result from the processing of the peripheral, distracting arrows, such that greater regulation of the competing input is required when the flanker arrows point in an incompatible direction due in large part to the competition between the response associated with the target and that with the distractors. Larger reaction time or accuracy differences between compatible and incompatible conditions are typically taken as an index of poorer attentional filtering ability. Other tasks in which a competing dominant response must be inhibited are also taken to measure filtering ability and the management of response conflict, including classic Stroop tasks (e.g., the color-name Stroop, in which participants name the color of ink used to spell color words, such as responding ‘blue’ to the word RED printed in blue ink).
Using flanker and Stroop tasks, neural networks associated with the managing of response conflict have been identified by subtracting neural activity in response to compatible trials from incompatible trials. These include the anterior cingulate cortex (ACC), thalamus, and bilateral frontal regions, as well as portions of the fusiform gyrus (Casey et al., 2000, Fan et al., 2005, MacDonald et al., 2000). Several studies suggest that the ACC is involved primarily in the monitoring of response conflict, which in turn signals frontal regions to resolve the conflict (Botvinick et al., 2004, Bush et al., 2000, MacDonald et al., 2000). Interestingly, ACC recruitment appears specific to situations involving potential response conflict (as occurs in flanker and Stroop tasks), whereas increasing competing stimuli that are not associated with a competing response (as achieved with increased white noise or task-irrelevant signals) instead recruits posterior parietal cortex (PPC) and frontal regions (Liston et al., 2006). This suggests that the fronto-parietal network described earlier may be involved in narrowing the focus of selective attention, whereas the ACC-frontal network is specifically engaged to manage response conflict.
In support of the key role of frontal regions in regulating response conflict, greater activity in DLFPC has been specifically associated with reduced interference on Stroop tasks (MacDonald et al., 2000). Further, transient disruption of right inferior frontal junction (IFJ) using transcranial magnetic stimulation impairs both attentional modulation and subsequent memory performance in selective attention/working memory paradigms (Zanto et al., 2011). This finding highlights the causal role of regions of the frontal cortex in selective attention, as well as the critical role of selective attention in supporting working memory performance, a topic to which we will return later.
Taken together, these data indicate that selective attention is initially deployed using a largely fronto-parietal network. When this network is effectively deployed, early modulation of neural activity is observed during the first few hundred milliseconds of neural processing, with the effects of selective attention percolating throughout multiple cortical processing areas. These modulations allow a first filtering of task-relevant information; in addition, when competing sources of information lead to response conflict, the ACC and associated frontal areas become recruited to exert greater attentional control.
2. Development of selective attention
The above section focused on the neural systems mediating selective attention in the mature adult. However, many of the neural structures implicated above in effectively deploying selective attention and resolving response conflict, including regions of the prefrontal and parietal cortex, show a protracted period of postnatal structural development lasting into at least the third decade of life (Giedd et al., 1999, Gogtay et al., 2004, Sowell et al., 2001, Tsujimoto, 2008—see also Sowell et al., 2002 suggesting the cingulate cortex shows more rapid structural maturation relative to frontal regions). This slow maturation of the basic neural architecture important to selective attention suggests that functional changes in selective attention may also occur throughout childhood and adolescence. As in the discussion of selective attention in the mature adult, we consider the maturation of both (1) the influences of selective attention on neural processing and (2) the neural systems that deploy and control selective attention.
Behaviorally, while some aspects of attention are clearly present in some form in infancy (Gomes et al., 2000, Richards, 2004), the ability to deploy and control selective attention continues to develop into early adulthood (for reviews, see: Plude et al., 1994, Ridderinkhof and van der Stelt, 2000). For example, background noise creates greater interference effects for younger children and adolescents (Cherry, 1981, Doyle, 1973, Pearson and Lane, 1991, Plude et al., 1994), who also show larger effects of flanker stimuli relative to adults (Plude et al., 1994, Rueda et al., 2004).
Interestingly, despite generally poorer selective attention skills, there are some conditions under which children can achieve adult-like performance. For example, while children may be less efficient at filtering information, if a spatial (i.e., exogenous orienting) pre-cue is used to focus children's attention before appearance of the target, children perform like adults (Akhtar and Enns, 1989). Also, while children generally show larger flanker effects than adults, children and adults can be made to show equivalent flanker effects when attentional demands are high (Huang-Pollack et al., 2002). This latter finding has been interpreted in the framework of the perceptual load theory of attention (Lavie, 2005), in which the processing of flanker stimuli arises from untapped attentional resources spilling over to distracting information. As attentional demands are increased (by varying perceptual load, for example), most available attentional resources are devoted to the target stimulus, leaving little to no resources for distractor processing. As a result, the size of the flanker effect decreases. Accordingly, Huang-Pollack et al. (2002) found that flanker interference decreased for both children and adults as perceptual load of the central task increased. This effect was greater for children than adults such that under conditions of higher perceptual load, there were no longer differences in flanker effects between children and adults.
These findings have important implications for conceptualizing how selective attention develops. In a recent review, Ridderinkhof and van der Stelt (2000) proposed that the abilities to select among competing stimuli and preferentially process more relevant information are essentially available in very young children, but that the speed and efficiency of these behaviors improve as children develop. Reports of poor selective attention skills in older children may thus be a reflection of broad, inflexible, or poorly tuned attention control rather than an inability to differentially process attended and unattended stimuli after attention has been deployed. The implication is that selective attention can be recruited, even in young children, if sufficient cues are provided to direct selective attention. With respect to the neural bases of selective attention described above, this suggests that attentional modulation of sensory processing may be possible for children – including early modulation of sensory processing – but that the frontal-parietal structures guiding the control of selective attention and the resolving of response conflict may show a more protracted period of development.
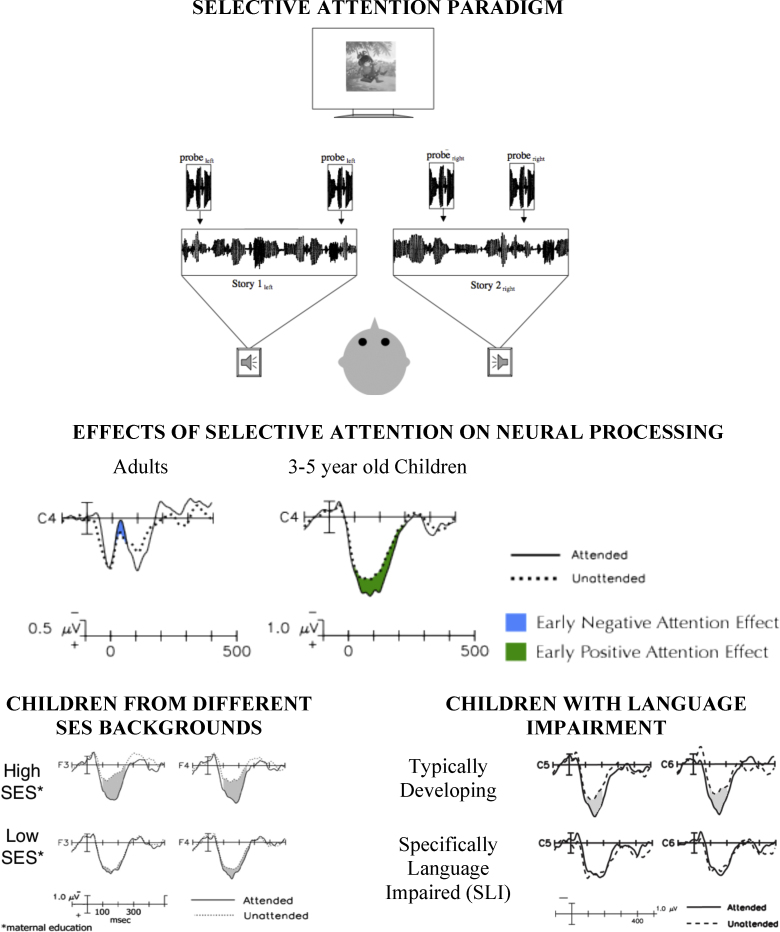
Within this framework, it would be predicted that when children do deploy selective attention, the influences on neural processing would be similar to those observed in adults. For example, it might be expected that children, like adults in the studies described above, can show attentional modulation of neural processing that occurs within the first 100 ms of stimulus presentation. To address this question, we recently developed a child-friendly version of the Hillyard selective auditory attention paradigm described above (Coch et al., 2005, Sanders et al., 2006). In this modified paradigm, children listened to one of two narratives played simultaneously from separate speakers located to the left and right of the child. Children were cued to attend to one of the two stories, and ERPs to identical probe stimuli embedded in the attended versus unattended channel were recorded (see Fig. 1, top panel). First, we tested whether adults showed typical attention effects in this child-friendly paradigm. Remarkably, even though adults were not actually listening to the probe stimuli (they were merely embedded in the story), they showed typical effects of selective attention on ERP responses. Specifically, by 100 ms post-stimulus onset, ERPs in response to probe stimuli embedded in the attended channel showed an increased negativity compared to responses to identical probes embedded in the unattended channel. When this task was used with children as young as three years of age, probe stimuli elicited a broad positivity from about 100–300 ms post-stimulus onset, rather than the typical P1-N1 morphology observed in adults. However, as in adults, the neural response in children was modulated by selective attention within 100 ms, such that the broad positivity was amplified in response to probes embedded in the attended as compared to unattended channel (see Fig. 1, middle panel).
Fig. 1.
Effects of selective auditory attention on neural processing in adults and children. Top panel: Schematic representation of the experimental paradigm. Children were instructed to attend to the story presented from either the left or right speaker. ERPs were recorded to probe stimuli superimposed on both the attended and ignored narrative. Middle panel: Both children and adults showed attentional modulation of early (∼100 ms) sensory ERP components, though the morphology of the underlying evoked potential differed. Bottom panel: Early attentional modulation of evoked potentials is reduced children from lower socio-economic backgrounds (left box) or with specific language impairment (right box).
Data taken from: Sanders et al. (2006), Stevens et al., 2009, Stevens et al., 2006.
These findings provide compelling support for the argument that, with sufficient cues, children can show impressive attentional modulations. However, not all children show this robust effect of selective attention on early neural processing. For example, children from lower socio-economic backgrounds show reduced effects of selective attention on early neural processing relative to their peers from higher socioeconomic backgrounds (Stevens et al., 2009) (see Fig. 1, bottom left panel). Similar results were recently reported using a similar paradigm with adolescents from different socioeconomic backgrounds (D’Angiulli et al., 2008). This suggests that individual differences during development exist in the capacity to deploy selective attention and, as a result, modulate early neural processing. More generally, the findings suggest that the ability to control the deployment of attention is fragile in development, and while essentially available, attentional modulation may not be harnessed to the same degree by all children. At stake, then, is the issue of why selective attention is not always deployed effectively in children, as proper deployment has a cascade of beneficial consequences on neural processing. It is likely that these differences reflect less mature control structures, or sources of attentional modulation, as well as greater difficulty handling response conflict. As mentioned earlier, some of the prefrontal and parietal areas implicated in attentional control are structurally the latest developing neural structures, and these regions may show similarly protracted periods of functional development.
To our knowledge, no studies have examined developmental changes in the neural response to endogenous orienting cues. However, one fMRI study examined developmental changes in an exogenous cueing paradigm, in which a spatial pre-cue exogenously oriented attention to a spatial location either congruent (valid) or incongruent (invalid) with the subsequent appearance of a target (Konrad et al., 2005). In comparison to adults, children age 8–12 years showed larger reaction time differences between valid and invalid cue trials, as well as less recruitment of the right temporo-parietal junction (TPJ) relative to adults. The right TPJ is believed to be particularly important to disengaging attention from a previously attended location, suggesting that the ability to dynamically re-allocate attention as task demands change may also be a slower maturing process.
The ability to handle response conflict may also be particularly slow to develop in children. Several studies have examined developmental changes in the neural systems important for handling response conflict, as arises in Stroop and flanker tasks. Using various color-word Stroop tasks, age-related increases in prefrontal cortex activity have been reported for incongruent relative to neutral or congruent trials (Adleman et al., 2002, Marsh et al., 2006, Schroeter et al., 2004), with younger children also showing a delay in prefrontal deployment by approximately two seconds relative to adults (Schroeter et al., 2004). There is also evidence to suggest greater recruitment of parietal regions with increasing age during Stroop tasks, though these differences may be present only between children and adolescents and show little development from adolescence to adulthood (Adleman et al., 2002). Less consistent have been findings for age-related changes in the ACC during Stroop interference, with reports of both increased (Adleman et al., 2002) and decreased (Marsh et al., 2006) activity with age. A separate study examined neural activity to congruent relative to neutral conditions in a flanker arrow task in children and adults (Bunge et al., 2002). Children age 8–12 years showed atypical recruitment of prefrontal cortex relative to adults. Specifically, children showed greater left PFC activity whereas adults showed greater right PFC activity. These differences in laterality were taken to reflect possible changes in attentional strategy, with children perhaps more reliant on the verbal coding of arrow directions to perform the task.
Taken together, studies of the development of selective attention suggest both similarities and differences between children and adults. Clearly selective attention can influence neural processing – and do so even at early stages of perceptual processing – in children. This suggests that, once deployed, comparable neural mechanisms mediate attentional modulations in children and adults. In contrast, the control structures guiding both the deployment of selective attention (i.e., fronto-parietal networks) and the resolving of response conflict arising from competing stimuli (i.e., ACC-frontal regions) show maturation that continues through adolescence. Next, we consider the impacts of compromised selective attention on foundational processes important to three academic domains.
3. Role of selective attention on domains important to academic performance
The above discussion has highlighted the impact of selective attention on multiple stages of neural processing and demonstrated that this mechanism of attention is essentially available to very young children. By what specific mechanisms might selective attention have wide-ranging impacts on domains considered essential to academic achievement? Below, we examine three traditional domains relevant to the education literature, including language, literacy, and mathematics, positing possible neurobiological mechanisms linking selective attention to performance in each domain.
3.1. Selective attention & language processing
From the first day of kindergarten, children receive instruction and interact with classmates via spoken language. This renders early success in the classroom largely contingent on entering the school system with a basic facility in oral language. Here, we consider how selective attention may be implicated in language processing. This consideration begins with the observation that while auditory speech is perceived as a series of discrete words, the actual acoustic signal is a complex, rapidly changing stream of information with few objective boundaries. Further, the acoustic boundaries that do exist seldom coincide with actual word boundaries (see Fig. 2, top panel). From this continuous stream of auditory input, then, the listener faces the challenge of parsing word boundaries and extracting meaning. Complicating this task, many speech sounds are differentiated based only on subtle spectral or temporal differences on the order of tens of milliseconds, and many morphemes have low perceptual salience in the context of the continuous speech stream.
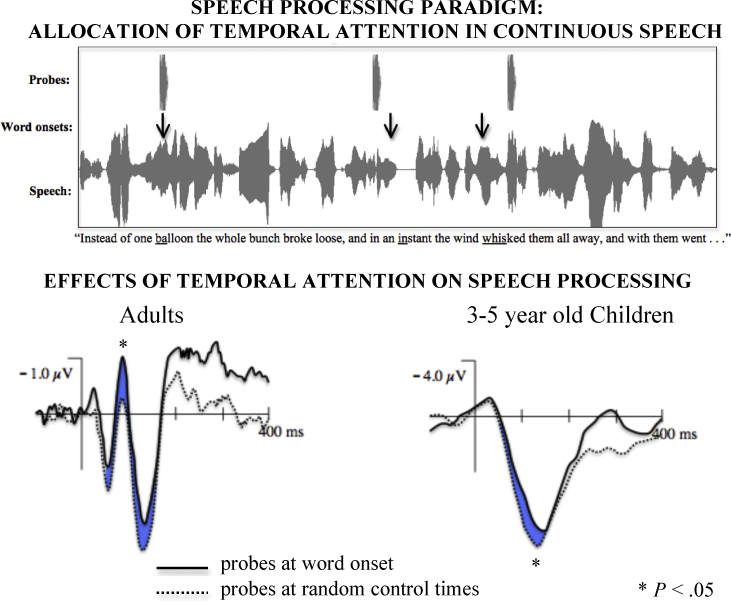
Fig. 2.
Effects of temporal selective attention on neural processing of continuous speech in adults and children. Top panel: Schematic representation of experimental paradigm. Attention probes, 50 ms excerpts of the narrator saying “ba”, were presented during acoustically matched portions of continuous speech. The probes were presented concurrently with word onsets and at random control times among other conditions. Bottom panel: Probes presented during the initial portions of words elicited a larger negativity by 80 ms after onset in both adults and 3–5-year-old children over anterior and medial regions.
Data taken from: Astheimer and Sanders, 2009, Astheimer and Sanders, 2011.
Given the complex nature of the speech signal, it is reasonable to ask what role selective attention might have in facilitating language processing. Whereas this review has up until now focused on selectively attending when competing visual or auditory information is presented simultaneously, temporal selective attention involves identifying and selecting particular points in time for further processing. Several recent ERP studies document that humans can allocate attention to particular points in time and that the effects on neural processing are markedly similar to those observed with selection based on other stimulus features, with attentional enhancement evidenced as an increased neural response within 100 ms of processing (Griffin et al., 2002, Lange et al., 2003).
The link between temporal selective attention and speech processing has recently been explored in an elegant series of ERP studies. In one study, participants listened to sentences, and ERPs were recorded during word-initial as compared to word-medial syllables within the sentences, with the syllables equated for loudness, length, and phonemic content (Sanders and Neville, 2003). To ensure that participants were attending to the sentences, they were occasionally queried as to whether the preceding sentence contained a particular word. Adults showed a larger N1 to word-initial as compared to word-medial syllables, suggesting temporal selective attention has been allocated to the initial portions of the words. Similar results have been reported for word-initial and word-medial syllables embedded in continuous strings of novel psuedowords among participants who are able to learn the novel words in a separate task prior to ERP testing (Sanders et al., 2002). Similar results are also reported when ERPs are recorded to identical probe stimuli superimposed on continuous narratives, such that probes presented at or immediately following word onsets elicit an enhanced N1 compared to probes embedded in other locations matched for acoustic properties (Astheimer and Sanders, 2009—see Fig. 2, bottom left panel). This early enhancement suggests that when processing speech, listeners learn to identify and predict word initial segments and selectively direct attention to those points in time to aid in processing. Although not explored, similar processes may enable listeners to direct attention to other points in time with high information value, e.g., the points in time where inflectional morphemes might appear. Thus, deploying temporal selective attention strategically may allow the listener to select and amplify processing of the portions of the speech signal most critical for comprehension.
If temporal selective attention were important to language learning, we would expect to find that young children are able to deploy this mechanism during language acquisition. In a recent study, Astheimer and Sanders (2011) examined whether children age 3–5 years show evidence of temporal selective attention during speech processing, similar to that observed in adults in the studies described above. ERPs were compared to linguistic probe stimuli embedded in an ongoing children's narrative, either concurrent with word onsets or at other control times matched for basic acoustic properties. Results indicated that children, like adults, deployed temporally selective attention to modulate early sensory processing of linguistic probes presented coincident with word onsets relative to control times (see Fig. 2, bottom right panel). These findings indicate that early enhancement of word-initial processing is a neural mechanism available to young children and thus a candidate critical mechanism for parsing and processing the continuous speech stream. To the extent that children have difficulty selectively attending – or using cues to identify which regions should be attended – they may face difficulties parsing and processing the speech stream. Indeed, other research indicates that selective attention may be important to shaping auditory sound processing during development, including aspects of sound processing important to language development (Sussman and Steinschneider, 2009).
In support of the role of selective attention in language processing, several studies have examined whether children with Specific Language Impairment (SLI) exhibit deficits in selective attention. SLI is a developmental disorder marked by delayed and atypical language ability that cannot be explained by a child's age, general intelligence, or educational opportunity (Leonard, 1998). Several behavioral studies report deficits in aspects of selective attention among children with SLI (Noterdaeme et al., 2000, Spaulding et al., 2008, Ziegler et al., 2005). However, as behavioral tasks represent the summed activity of multiple stages of processing, it is unclear whether the locus of the attention deficit arises in attentional modulation of early sensory processing.
To examine whether the early neural effects of selective attention are compromised in children with SLI, we recently compared a group of children with SLI to a control group matched for age, gender, nonverbal IQ, and socioeconomic status using the child-friendly ERP selective auditory attention task described above (Stevens et al., 2006). In this task, children attended selectively to one of two narratives presented from separate speakers, and ERPs were recorded to probe stimuli superimposed on the attended and unattended stories. Although the children with SLI were willing and able to perform the selective attention task (as indicated by performance on comprehension questions about the attended story), they did not show early effects of attention on neural processing (see Fig. 1, bottom right panel). Furthermore, these performance deficits were associated specifically with a reduced ability to enhance the neural response to the attended stimuli (i.e., a problem with signal enhancement), rather than difficulty suppressing the unattended response. It is an ongoing question whether children with SLI show the same deficits in temporal selective attention during online language processing. However, the striking deficits in selective auditory attention suggest this may be a critical link between selective attention and language processing difficulties in at least some children with SLI. It remains for future investigations to further assess the strength of such a link.
3.2. Selective attention & literacy
The emergence of written symbol scripts during human history had the advantage of transforming the ephemeral speech signal into a durable visual form. This form is widely used in academic contexts as the medium for communicating information. However, printed text brought with it new demands on visuo-spatial attention. Unlike language processing, where auditory selective attention must be directed to critical points in time, reading requires that visual selective attention be focused spatially. Without this ability, the printed page would be a sea of visual clutter. For example, all scripts require that selective attention move serially in some ordered fashion (e.g., from left to right across a page and/or from top to bottom), and there is evidence that learning to read biases both the gradient of visual attention and scan patterns to align with the demands of the script being learned (Ferretti et al., 2008). For alphabetic scripts, attention must be further focused, particularly during the early learning stages, on letters and letter clusters in order to acquire the mappings between graphemes and speech sounds, i.e., knowledge of the alphabetic principle. Below, evidence for the role of selective attention on literacy development is reviewed, with an emphasis on the impact of attentional focus during alphabetic script learning on the development of neural systems important for fast and accurate reading.
As a relatively recent cultural invention, reading is a form of perceptual expertise that is unlikely to be innately specified in the brain (Cohen and Dehaene, 2004). Thus, over the course of literacy acquisition, the brain must adapt processing systems to support the fast, accurate identification of written symbol strings. Although neuroimaging studies have identified a set of left-hemisphere regions important to skilled reading (e.g., for a review see Pugh et al., 2000), we focus here on a region of left extrastriate cortex, often referred to as the visual word form area (VWFA) (Cohen et al., 2000, McCandliss et al., 2003b). This area is believed to respond preferentially during tasks that involve the automatic conversion of a visual form to a linguistic form, most typically with printed words but also observed in other tasks including naming visual presentations of objects and colors (e.g., see Price and Devlin, 2003, Wright et al., 2008). Intracranial recordings and ERP studies localize the rapid timecourse of neural specialization in the VWFA, with the N170 larger in amplitude over left posterior regions in response to words relative to other classes of visual stimuli and likely arising from the activity of neurons in the VWFA (e.g., Allison et al., 1994, Bentin et al., 1999, McCandliss et al., 2003b).
Left-lateralization of the N170 is observed not only during explicit reading, but also during implicit processing tasks that do not require reading, such as one-back repetition detection tasks. This observation has led to the phonological mapping hypothesis (Maurer and McCandliss, 2007), which proposes that left-lateralization of the N170 to words reflects automatic links between orthography and left-lateralized phonological systems. Indeed, there is evidence for developmental shifts in the lateralization of the N170 to words during reading acquisition. During the initial stages of reading acquisition, children show a bilateral or even right-lateralized N170 enhancement to words relative to false-font strings in one-back tasks (Maurer et al., 2005, Maurer et al., 2007). Initial right-lateralization of the N170 is hypothesized to reflect a visual expertise effect, similar to that observed for face stimuli or novel trained objects (Rossion et al., 2002), that does not become left-lateralized until words are automatically mapped to left-lateralized phonological systems. In this context, it is reasonable to assume that these automatic mappings are facilitated when attention is directed to smaller units of orthographic analysis during early literacy acquisition.
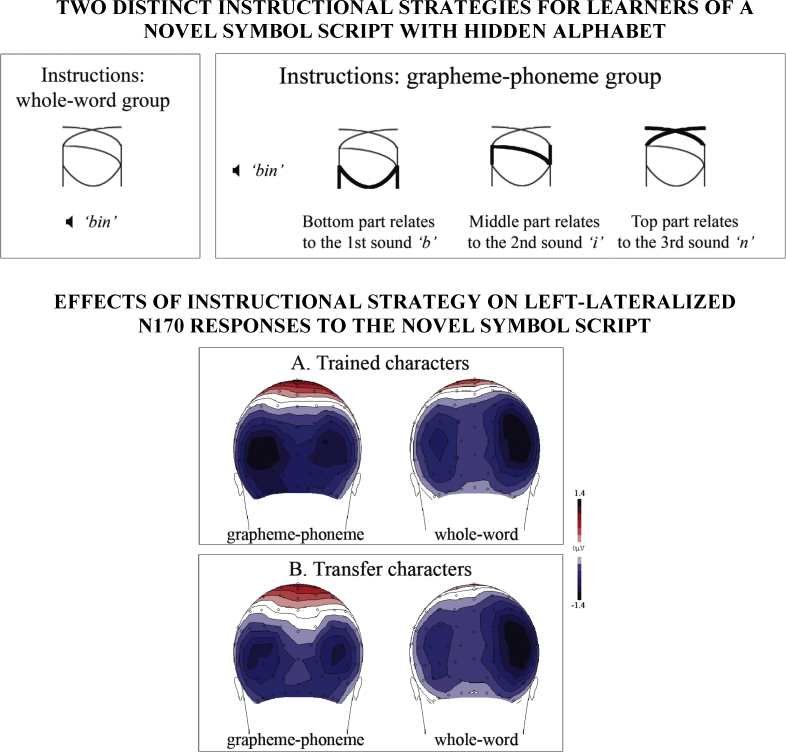
In support of this hypothesis, a recent study examined whether the N170 lateralization in response to a novel symbol script was influenced by attentional focus during the learning phase. In this study, a group of adults received a brief, 20-min training session with a novel symbol script in which portions of holistic word symbols consistently corresponded to particular phonemes (see Fig. 3). Attentional focus during training was manipulated by instructing half of participants to focus on the symbol as a holistic unit and the other half to focus on mapping portions of the symbol to individual phonemes (i.e., explicit attention to the alphabetic principle), also shown in Fig. 3. Following the training, ERPs were recorded while participants completed an explicit phonology-based task in which they indicated whether a visually presented symbol matched an auditorily presented word (both trained and untrained symbols were used, with similar results across stimulus types). Results indicated that N170 responses to the visual symbols varied as a function of attentional focus during training: adults instructed to focus on the symbols holistically showed a bilateral N170 response to the symbols whereas adults trained to map the symbols to phonemes showed a left-lateralized N170 response (Yoncheva et al., 2010). Interestingly, neither group showed a left-lateralized N170 to the newly learned symbols during an implicit processing one-back task (Maurer et al., 2010). The differentiation across tasks is hypothesized to be a consequence of the brief 20-min training, with left-lateralization not yet apparent in the implicit processing one-back task. Longer-term training data will help further evaluate this hypothesis. However, the most striking finding across these studies was that left-lateralization of the N170 to words only occurred for the group of participants who attended to the embedded alphabetic principle within the novel symbols. This suggests that attentional focus, and the ability to identify and process smaller orthographic-to-phonological units of words, may facilitate the development of rapid, left-lateralized neural responses important to fluent reading.
Fig. 3.
Effects of attentional focus during a novel word learning task on lateralization of the N170. Top panel: Participants received a 20-min training on a novel symbol script. The script contained a hidden alphabet, in which portions of the symbol corresponded to individual speech sounds (see right side, bold for illustration only). During the training, participants were either instructed to attend to the symbol holistically (right side) or to the grapheme-phoneme mapping (right side). Bottom panel: ERPs recorded to both trained and transfer characters during a test of word learning showed left-lateralized N170 responses in the grapheme-phoneme group only, whereas the whole-group group showed a bilateral response.
Data taken from: Yoncheva et al. (2010).
A recent fMRI study further supports the relationship between selective attention and development of the VWFA (Vogel et al., 2011). This study used resting state functional connectivity MRI to examine the functional relationship between the VWFA and other brain regions. The logic of the analysis was that brain areas that show correlated slow fluctuations of BOLD activity during rest likely share a history of use-dependent co-activation. Although the VWFA is considered a key part of the reading network, its slow fluctuations of BOLD activity showed little correlation with other regions of the reading network (left SMG, AG, and ITG). Instead, large correlations were observed between activity in the VWFA and fronto-parietal regions associated with orienting selective attention (bilaterial IPS, MT+, and frontal eye fields). Further, the strength of these associations increased with age and reading experience, particularly in the IPS. These findings suggest that selective attention may be critical to development of the VWFA, perhaps through the role of fronto-parietal attention networks in shifting the focus of selective attention to different unit sizes (words, letters, or bigrams/trigrams) during literacy acquisition.
These data are suggestive that selective attention may be important for establishing the neural circuits important for efficient reading, and in particular the development of responses in the VWFA to word strings. A related question, then, is whether there is evidence that individuals with Specific Reading Disorder (dyslexia) show atypical selective attention. Indeed, multiple studies have documented deficits in aspects of visual selective attention among individuals with reading disorder, using both linguistic and nonlinguistic stimuli (Atkinson, 1991, Bosse and Valdois, 2009, Sperling et al., 2005, Spinelli et al., 2002—but see also Shovman and Ahissar, 2006). Further, the relationship between measures of attention and literacy skill may be more pronounced during the early years of literacy acquisition (Bosse and Valdois, 2009), and several recent reviews have proposed that selective attention is critical to early literacy acquisition (Valdois et al., 2004, Vidyasagar, 2005, Vidyasagar and Pammer, 2009). Further, there is evidence to suggest that while children with dyslexia easily focus attention on letters in the word-initial position, they have particular difficulty focusing attention on letters in other positions within the word (McCandliss et al., 2003a). As well, using the ERP selective auditory attention paradigm described earlier, in which children attend selectively to one of two simultaneously presented narratives, we have recently shown that five-year-old children with poor pre-literacy skills show reduced effects of selective attention on early neural processing (Stevens et al., 2011).
These findings indicate deficits in selective attention among individuals with reading disorder, but a remaining question is whether or how these attention deficits are related to the development of the neural circuits important to efficient reading. Several studies document under-activation of left posterior regions of the reading network in adults and children with dyslexia, including the VWFA (for reviews, see McCandliss and Noble, 2003, Pugh et al., 2000). Interestingly, while training studies have demonstrated the ability to improve decoding skill among individuals with dyslexia, including increased activity in the VWFA evident with fMRI (Shaywitz et al., 2004), the rapid timecourse of neural activation in some neural areas, evident with magnetoencephalographic imaging, can remain delayed even following intervention (Simos et al., 2002). This finding parallels behavioral results showing improved (non-timed) decoding skills, but relative intractability of reading fluency (i.e., reading that is both fast and accurate) (Shaywitz et al., 2004). It is possible that attentional control may be critical to driving the automatization indexed by left-lateralization of the N170, and in turn fast and accurate reading. Although only suggestive at this point, one study demonstrated that prior attention training translated to increased benefits of a subsequent remedial writing intervention for adolescents with dyslexia (Chenault et al., 2006). However, to date no studies have examined how prior (or concomitant) attention training may impact the efficacy of reading interventions, including the recruitment and timecourse of activation of neural systems important for reading. This will represent an important direction for future research and contribute to our understanding of the relationship between selective attention and the development of neural circuits supporting reading.
3.3. Selective attention & mathematics
Mathematics is a broad domain, including several distinct skills. Here, we consider the role of selective attention on a particular class of mathematics tasks referred to as word problems. We acknoweldge however that other aspects of math skill may be impacted by selective attention (e.g., see Piazza and Dehaene, 2004). In word problems, an individual must hold on to and retain relevant information presented linguistically for making a computation while not being distracted by extraneous information irrelevant to the computation. Furthermore, the relevant information must then be effectively manipulated to arrive at a computational solution. Intuitively, word problems place high demands on working memory, which in turn is influenced by selective attention. Indeed, it has been proposed that working memory skills are particularly important during word problems in order to form a conceptual problem model, as opposed to approaching word problems with rote, direct-translation strategies (Hegarty et al., 1995, Jonassen, 2003, LeBlanc and Weber-Russell, 1996). Importantly, this begins to highlight the interaction between working memory and selective attention. Although these have traditionally been treated as distinct mental capacities, as detailed below there is considerable interactions between these processes, both in terms of functional significance and neural bases.
Behavioral studies have associated poor performance on mathematics word problems with poorer working memory performance (Andersson and Lyxell, 2007, Fuchs et al., 2006, Swanson and Beebe-Frankenberger, 2004, Swanson and Jerman, 2006). Importantly, the working memory deficits extend beyond numerical content to visuo-spatial and verbal working memory tasks (Wilson and Swanson, 2001), and the zero-order correlations between working memory and word problems are higher than correlations between working memory and basic arithmetic or computation skills (Fuchs et al., 2006). Similarly, errors in mathematical computation are often attributed to deficits in working memory, rather than procedural knowledge (Ayres, 2001), and somewhat ironically, mathematics anxiety is associated with a concomitant reduction in working memory capacity and mathematics performance (Ashcraft and Krause, 2007). However, studies that include multiple predictors of word problem performance simultaneously in a model often report reduced or eliminated effects of working memory on word problem performance (e.g., Fuchs et al., 2006, Kail and Hall, 1999). When associations with working memory are reduced or eliminated in study results, this may reflect the selection of particular working memory measures (see Fuchs et al., 2006 for a discussion) or may be the result of shared variance across predictors entered in the model (e.g., between working memory and verbal processes, inattention, and processing speed, see, for example, Fuchs et al., 2006, Kail and Hall, 1999).
Interestingly, recent data suggest that working memory deficits among individuals with mathematics difficulty are related to impairments in ignoring irrelevant or no longer relevant information during memory tasks. This is seen in a high number of intrusion errors of previously presented, but currently task-irrelevant content during working memory tasks (Passolunghi et al., 1999, Passolunghi and Siegel, 2001). This pattern of intrusion errors of to-be-ignored information suggests a difficulty suppressing items that are either irrelevant or that have been processed but are now irrelevant to the task at hand. Although few studies have directly assessed aspects of attention in relation to mathematics performance, one recent study reported that the degree of interference in a flanker arrow task is associated with poorer mathematics performance in a sample of 12 year olds (Checa and Rueda, 2011).
These findings suggest a link between attention and mathematics word problems skills that is mediated by the effect of selective attention on working memory. This hypothesis is yet to be tested directly. However, several neuroimaging studies support the role of selective attention, and distractor suppression in particular, in effective working memory performance. For example, in a recent study by Vogel et al. (2005), working memory capacity was related to a neurophysiological measure of attentional selection. Individuals with higher working memory capacity were better able to suppress irrelevant items and encode only the relevant items presented in a multi-stimulus display. In contrast, individuals with poorer working memory capacity encoded more information from the display, including irrelevant items. This suggests that selective attention, and distractor suppression in particular, is important for regulating access to working memory and optimizing working memory capacity (Cowan and Morey, 2006).
Additional support for the relationship between selective attention and working memory was found in a recent study by Zanto and Gazzaley (2009). Participants in this study observed a series of four multi-dot images, alternating between stationary single-colored dots and coherently moving grey dots. In different blocks, participants were instructed to attend either to the moving dot images, the stationary colored dot images, or both. After a delay period, a probe image appeared and participants indicated whether it had appeared in the four-item sequence. ERPs of identical dot images in the attended as compared to the unattended position were compared, separately for trials considered to have high as compared to low performance based on a median split of reaction time. Evidence was found for larger effects of attention on neural processing in the first few hundred milliseconds after stimulus onset for high as compared to low performance trials. Further, the differences across trial types were specific to better suppression of irrelevant stimulus displays (as opposed to greater signal enhancement of the to-be-attended displays), further suggesting that distractor suppression is a key mechanism associated with improved working memory performance. Taken together, these data suggest that effective allocation of selective attention during encoding, and in particular the suppression of irrelevant information, helps optimize working memory performance.
The links between selective attention and mathematics performance are clearly more speculative than those linking selective attention with language and literacy. However, the above data suggest interesting links between selective attention and mathematics performance, mediated by the effect of selective attention on working memory. Future studies can test this hypothesis directly. As well, cleverly designed neuroimaging studies may be able to assess the distribution of attention across information in word problems using paradigms parallel to those described earlier.
4. Malleability of selective attention: Insights from adult populations
Given the vulnerability of selective attention skills and their proposed relevance for processing across several academic domains, it is reasonable to ask whether selective attention itself can be trained. If so, such training might be expected to act as a force-multiplier, leading to improvements across a range of domains. Two lines of research demonstrate the capacity of selective attention to be enhanced, the first one concerns action game play and the second mind-body training.
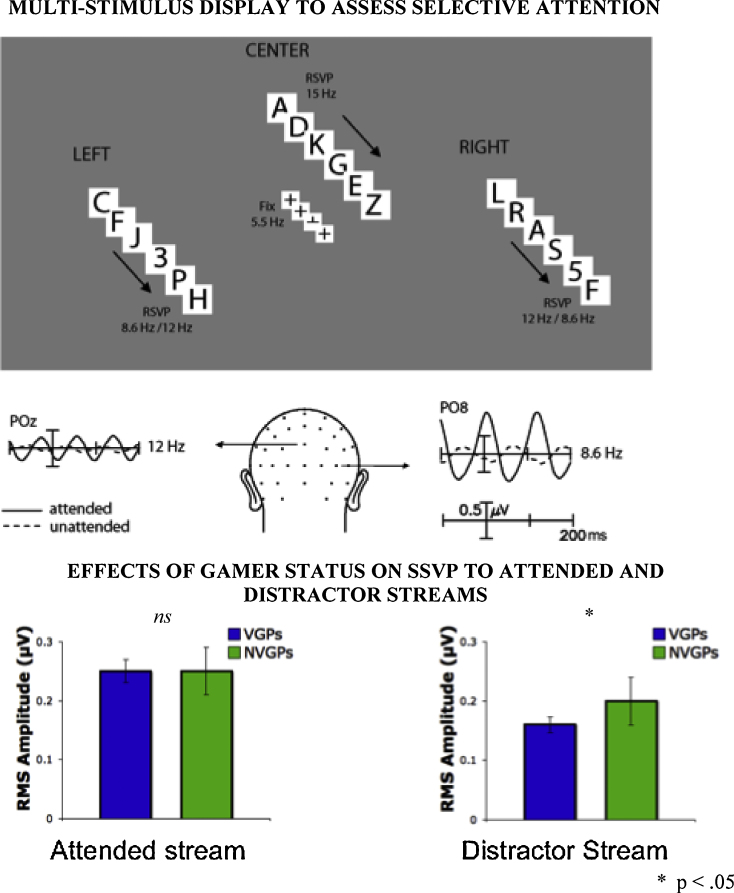
Across several studies, action game play is related to enhancements in various aspects of attention, including selective attention over space, time, or objects (for a review, see Hubert-Wallander et al., 2010). Recently, Mishra et al. (2011) made use of the steady-state visual evoked potentials technique to understand the neural bases of the attentional enhancement noted in action gamers (see Fig. 4). They found that action gamers more efficiently suppress unattended, potentially distracting information. Participants viewed four different streams of rapidly flashed alphanumeric characters. Each stream flashed at a distinct temporal frequency allowing retrieval of the brain signals evoked by each stream independently at all times. Thus, not only could the brain activation evoked by the attended stream be retrieved, but also those evoked by each of the unattended and potentially distracting streams. Action gamers suppressed irrelevant streams to a greater extent than non-gamers, and the extent of the suppression predicted the speed of their response. This finding suggests that action video-game play sharpens selective attention by allowing players to better focus on the task at hand by ignoring other sources of potentially distracting information.
Fig. 4.
Plasticity of distractor suppression mechanisms in action video game players (VGPs) relative to non action video game players (NVGPs). Participants viewed different streams of rapidly flashed alphanumeric characters. Each stream flashed at a distinct temporal frequency, and steady state visual evoked potentials (SSVEPs) were recorded at each frequency. Action gamers suppressed irrelevant streams to a greater extent than non-gamers and the extent of the suppression predicted the speed of their response.
Data taken from: Mishra et al. (2011).
A second line of research has examined changes in attention abilities among experienced meditators. While there is some evidence that different styles of meditation are associated with unique changes at both a behavioral and neural level (e.g., see Jha et al., 2007, Lutz et al., 2008), across several studies of different meditation styles, experienced meditators show enhancements in several aspects of selective attention (Jha et al., 2007, Kubose, 1976, Linden, 1973, Slagter et al., 2007), similar to that reported in action gamers. It has been proposed that meditation improves attention in a practice-related manner, with the act of meditation engaging neural systems supporting selective attention. In support of this hypothesis, several neuroimaging studies document engagement of fronto-parietal regions associated with deploying selective attention during meditation (Brefczynski-Lewis et al., 2007, Cahn and Polich, 2006), with similar regions recruited across a variety of meditative styles (Cahn and Polich, 2006). However, as a relatively young field, most studies have not differentiated specific subregions of frontal or parietal cortex.
The study of attentional changes in action video gamers and experienced meditators provide both a “proof of concept” for the malleability of attention and also underscore several important methodological points. First, both lines of research provide causal evidence for the role of experience in shaping attention through the inclusion of training studies with random assignment to conditions. In these studies, previously inexperienced gamers or meditators show improvements following training in their respective domain relative to comparison control groups (e.g., see Feng et al., 2007, Green and Bavelier, 2003, Green and Bavelier, 2006a, Green and Bavelier, 2006b, Green and Bavelier, 2007, Jha et al., 2010, Spence et al., 2009, Tang et al., 2007). However, within the study of action video gamers, the enhancements in attention appear most marked when the experience is with action video games (e.g., Medal of Honor, Call of Duty, Unreal Tournament). Similar effects are not observed with other types of games tested (e.g., Tetris, Restaurant Empire, the Sims). Thus, at present this particular line of research has limited utility ethically as an attention training program for children.
Second, these studies provide insights into the nature of neural changes that take place in attentional systems. For example, it should be noted that not all aspects of attention seem equally enhanced by action game play. While top-down attentional mechanisms are altered for the better, there is little evidence for changes in bottom-up mechanisms, at least as measured by the automatic orienting an exogenous cue produces (Castel et al., 2005, Dye et al., 2009a, Hubert-Wallander et al., 2011).
Third, when changes are observed, these changes are not always linear in nature. For example, Brefczynski-Lewis et al. (2007) examined the effects of auditory distractor sounds on neural processing while participants meditated on a visual stimulus. Compared to inexperienced meditators, participants with some meditation experience showed greater activity in fronto-parietal regions in response to distracting auditory sounds. Yet, expert meditators showed a striking down-regulation of activation in these regions under the same condition. This inverted U-shape pattern is consistent with the view that meditation experience initially enables greater recruitment of attentional control systems as the attentional task becomes more effortful; but as expertise develops with extra training, meditation expertise may allow one to achieve a focused state of attentional control more automatically, therefore calling upon fewer attentional resources (see also Petersen et al., 1998 for reductions and changes in neural recruitment as automaticity sets in). The possibility of increasing attentional allocation and its automaticity is mirrored in a recent report documenting reduced recruitment of fronto-parietal networks in action gamers as compared to non-gamers as attentional demands were increased (Bavelier et al., 2011). Further studies are needed to link changes in the fronto-parietal network with behavioral enhancement, however this work already highlights the importance of considering non-linear changes in the dynamics of selective attention with training, including changes in processing resources, primary task demands, and automaticity with training (see Lustig et al., 2009 for similar considerations about working memory training).
Finally, this line of research has highlighted challenges with some measures of attention currently used in the developmental literature. In particular, this work demonstrates that interpretation of flanker compatibility effects such as those measured in the ANT paradigm (Fan et al., 2002, Rueda et al., 2004) should not be considered as an absolute measure of the ability to deploy efficient filtering. In several studies, action video game players were found to have larger filtering scores than nonaction game players (Dye et al., 2009b, Green and Bavelier, 2003, Green and Bavelier, 2006a). While this would typically be interpreted as a lack of attentional control in the developmental literature, gamers concurrently show reaction times on incompatible trials that are faster than those of non-gamers on compatible trials. In short, they excel at the target task while exhibiting greater spill-over of unused resources to distractors. This suggests that a subtraction score can be influenced by overall reaction time differences across groups and/or by differences across groups in the difficulty of the central task, i.e., differences in perceptual load of the primary task (see Dye et al., 2009b for a discussion of this issue), and should thus be interpreted cautiously. This is of special concern in developmental studies in which typically groups of different ages or etiology show major differences also in basic reaction times.
Taken together, the literature on attentional enhancements in adults provides a proof of concept for the malleability of selective attention, and in particular improvements in attentional allocation and distractor suppression. Although activities such as action video-game play and intense meditation may seem quite disparate at first sight, they may evoke shared mechanisms by enabling greater automatization and flexibility of attentional control. The key component that a training regimen would need to include to achieve such changes is an extremely active area of research. Next, we consider the implications of these benefits for training programs in an educational context.
5. Malleability of selective attention: Implications for childhood education
Given the evidence that some aspects of attention can be trained, it is natural to ask whether such benefits can be observed following more academic-focused training with children, as this may have important implications for educational practice. Interestingly, we first observed such attentional enhancements in an education setting with children in response to proposed domain-specific interventions targeting auditory processing and/or literacy skills. In one study (Stevens et al., 2008), we examined whether six weeks of high-intensity training (100 min/day) with a computerized intervention program designed to improve language skills would influence the neural mechanism of selective auditory attention we had previously shown to be deficient in children with SLI. Following training, both children with SLI and typically developing children receiving the training showed larger effects of selective attention on neural processing. Similar changes were not observed in a no-treatment control group. In a second study (Stevens et al., 2011), we examined five-year-old children either on-track in preliteracy skills or at-risk for reading difficulties both before and after the first semester of kindergarten. The at-risk group also received supplemental instruction with the Early Reading Intervention (ERI, an evidence-based reading intervention, Simmons et al., 2007, Simmons et al., 2003) in addition to the regular kindergarten curriculum. Children receiving the supplemental reading instruction showed increases in the effects of selective attention on neural processing from the beginning to end of the first semester of kindergarten. In both of the studies described above, increases in the effects of selective attention on sensorineural processing were accompanied by behavioral changes in other domains that were targeted specifically by the training programs, including language and preliteracy skills, respectively. These findings are in line with recent proposals suggesting that some interventions designed to improve language skills might also target or train selective attention (Gillam, 1999, Gillam et al., 2001a, Gillam et al., 2001b, Hari and Renvall, 2001, Sundberg and Lacerda, 2003).
These data suggest that modifications in behavior can arise alongside changes in the early neural mechanisms of attention. However, these studies did not target attention directly. In our most recent research, we have been investigating the possibility that attention itself might be trainable, and that this training can impact processing in a number of academic domains. Indeed, in his seminal work, Principles of Psychology, William James raised the idea of attention training for children, proposing that this would be “the education par excellence” (James, 1890, italics original). While James went on to say that such an education is difficult to define and bring about, the design of such programs has become precisely the focus of recent research in cognitive science and cognitive neuroscience.
For example, in a pre/post training study, Rueda et al. (2005) developed a computerized attention training program for young children involving five sessions administered over a two to three week period. While not an educational setting per se, this study used computerized activities that could easily be incorporated into an educational setting. Children in the intervention engaged in interactive, adaptive computer games that trained several aspects of attention. Children randomly assigned to the control group also visited the laboratory but instead watched videos that paused intermittently, and which they could advance by pressing a remote controller. Results indicated that children receiving the attention training showed evidence of improvements in a neurophysiological measure of selective attention with a child-friendly flanker arrow task, as well as on standardized measures of IQ that were distinct from the training exercises.
While attention training programs such as this one are promising, they emphasize direct child training only and do not harness the larger context of child development, including the school and home settings. To provide more integrated attention training, we have recently developed an eight-week preschool attention training program that includes both child training activities and home-based strategies for parents. Preliminary results indicate that after eight weeks of such training, parenting practices improve, parental stress decreases, and children's behavior, cognition, and brain functions supporting attention improve significantly compared to children and parents randomly assigned to a more child-focused, contrasting intervention (Neville et al., under review, Stevens et al., 2010). These results suggest that attention training can be very effective when targeting multiple contexts, with benefits extending across multiple domains.
5.1. Conclusions and future directions
To the extent that selective attention skills are both relevant for academic foundations and amenable to training, they represent an important focus for the field of education. Here, drawing on research on the neurobiology of attention, we have posited specific links between selective attention and processing across three academic domains. Further, although not the focus of this review, a separate review paper has recently related aspects of attention to self-regulation, with implications for academic and socio-emotional functioning (Rueda et al., 2010).
Within the research community, the data reviewed here raise important considerations for future research. First, the findings on selective attention point to the importance of separately assessing distractor suppression and signal enhancement. These two aspects of selective attention can operate independently, and each may have unique relationships to particular academic skills. The data also point to the importance of being careful in the use of attention assessment measures. Quick assessments based on reaction times may be misleading in some cases, particularly when overall reaction time differences exist between populations. As well, some changes with training may follow non-linear trajectories. If there is a larger moral here, it is that the interpretation of selective attention measures is more complex than it appears at first blush, and researchers must be careful in their use and interpretation of such data (see Mishra et al., submitted for publication, for a review of methodological issues in measures of attention and working memory).
In a larger classroom context, data on the development and trainability of attention raises important considerations for supporting children's selective attention skills. Some children may need more cues to support their ability to selectively attend. This may, for some children, involve limiting distractors or presenting a longer opportunity to orient so that a child is prepared to deal with distractions. However, selective attention is also highly malleable, showing enhancements under some conditions. Training data indicate that attention skills can be enhanced, and distractor suppression may be especially modifiable. In a classroom context, there may be large benefits to incorporating attention-training activities into the school context, and indeed some classroom-based interventions include such activities, with evidence for improvements in children's selective attention (Diamond et al., 2007). Indeed, the history of such “mental orthopedics exercises,” or teaching children how to learn through training attention, self-discipline, and memory, dates at least as far back as Binet's special education courses in Paris (Binet, 1911/1975). As noted then, some classroom visitors found these exercises unusual or non-academic, yet their power to influence students’ learning outcomes – to teach them to “learn to learn” – was evidenced in the children's progress in traditional academic content.
In an educational era concerned with letters and numbers and the easy evaluation of skill sets, it is important to consider how domain-specific skills may critically harness domain-general selective attention skills. To the extent that training and support for selective attention is valued, it may be leveraged as a force-multiplier across domains. In an age of accountability, this also puts pressure on the research community to develop valid and reliable measures of specific aspects of attention that will be sensitive to developmental change on the time scales used for educational and intervention evaluation.
Conflict of interest
None.
Acknowledgements
We thank Betsy McDonald for help in editing. DB was supported by grants NIDCD DC04418, NEI EY01688 and the Charles A. Dana Foundation.
Contributor Information
Courtney Stevens, Email: cstevens@willamette.edu.
Daphne Bavelier, Email: daphne@cvs.rochester.edu.
References
- Adleman N., Menon V., Blasey C., White C.D., Warsofsky I., Glover G. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Akhtar N., Enns J. Relations between covert orienting and filtering in the development of visual attention. Journal of Experimental Child Psychology. 1989;48:315–334. doi: 10.1016/0022-0965(89)90008-8. [DOI] [PubMed] [Google Scholar]
- Allison T., McCarthy G., Nobre A., Puce A., Perez E., McCarthy G. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cerebral Cortex. 1994;4:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- Andersson U., Lyxell B. Working memory deficit in children with mathematical difficulties: a general or specific deficit? Journal of Experimental Child Psychology. 2007;96:197–228. doi: 10.1016/j.jecp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Ashcraft M., Krause J. Working memory, math performance, and math anxiety. Psychonomic Bulletin & Review. 2007;14:243–248. doi: 10.3758/bf03194059. [DOI] [PubMed] [Google Scholar]
- Astheimer L., Sanders L. Listeners modulate temporally selective attention during natural speech processing. Biological Psychology. 2009;80:23–34. doi: 10.1016/j.biopsycho.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astheimer, L., Sanders, L. Temporally selective attention supports speech processing in 3- to 5-year-old children, 2011. Developmental Cognitive Neuroscience. [DOI] [PMC free article] [PubMed]
- Atkinson, J., 1991. Review of human visual development: Crowding and dyslexia. In: Cronly-Dillon, J., Stein, J. (Eds.), Vision & Visual Dysfunction, vol. 13: Vision & Visual Dyslexia, pp. 44–57.
- Ayres P. Systematic mathematical errors and cognitive load. Contemporary Educational Psychology. 2001;26:227–248. doi: 10.1006/ceps.2000.1051. [DOI] [PubMed] [Google Scholar]
- Bavelier D., Achtman R., Mani M., Fockert J. Neural bases of selective attention in action video game players. Vision Research. 2011 doi: 10.1016/j.visres.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S., Mouchetant-Rostaing Y., Giard M., Echallier J., Pernier J. ERP manifestations of processing printed words at different psycholinguistic levels: time course and scalp distribution. Journal of Cognitive Neuroscience. 1999;11:235–260. doi: 10.1162/089892999563373. [DOI] [PubMed] [Google Scholar]
- Binet A. S. Heisler, Trans; Menlo Park, California: 1911/1975. Modern Ideas About Children. [Google Scholar]
- Bosse M., Valdois S. Influence of the visual attention span on child reading performance: a cross-sectional study. Journal of Research in Reading. 2009;32:230–253. [Google Scholar]
- Botvinick M., Cohen J., Carter C. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brefczynski-Lewis J., Lutz A., Schaefer H., Levinson D., Davidson R. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of the National Academy of Science. 2007;104:11483–11488. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler S., Tang W., Sylvester C., Shulman G.L., Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. Journal of Neuroscience. 2008;28:10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent D. Pergamon Press; London: 1958. Perception and Communication. [Google Scholar]
- Brooks-Gunn J., Duncan G. The effects of poverty on children. The Future of Children. 1997;7:55–71. [PubMed] [Google Scholar]
- Bunge S., Dudukovic N., Thomason M., Vaidya C., Gabrielli J. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman T., Miller E. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Bush G., Luu P., Posner M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cahn B., Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Thomas K., Welsh T., Badgaiyan R.D., Eccard C., Jennings J.R. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Science. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel A., Pratt J., Drummond E. The effects of action video game experience on the time course of inhibition of return and the efficiency of visual search. Acta Psychologica. 2005;119:217–230. doi: 10.1016/j.actpsy.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Checa P., Rueda M.R. Behavioral and brain measures of executive attention and school competence in late childhood. Developmental Neuropsychology. 2011;36:1018–1032. doi: 10.1080/87565641.2011.591857. [DOI] [PubMed] [Google Scholar]
- Chenault B., Thomson J., Abbott R.D., Berninger V.W. Effects of prior attention training on child dyslexics’ response to composition instruction. Developmental Neuropsychology. 2006;29:243–260. doi: 10.1207/s15326942dn2901_12. [DOI] [PubMed] [Google Scholar]
- Cherry R. Development of selective auditory attention skills in children. Perceptual and Motor Skills. 1981;52:379–385. doi: 10.2466/pms.1981.52.2.379. [DOI] [PubMed] [Google Scholar]
- Coch D., Sanders L., Neville H. An event-related potential study of selective auditory attention in children and adults. Journal of Cognitive Neuroscience. 2005;17:605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S., Naccache L., Lehéricy S., Dehaene-Lambertz G., Hénaff M. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Colby C., Goldberg M. Space and attention in parietal cortex. Annual Review of Neuroscience. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Kincade J., Shulman G.L. Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Miezin F.M., Dobmeyer S., Shulman G.L., Petersen S.E. Attentional modulation of neural processing of shape, color, and velocity in humans. Science. 1999;248:1556–1559. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cowan N., Morey C. Visual working memory depends on attentional filtering. Trends in Cognitive Science. 2006;10:139–141. doi: 10.1016/j.tics.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angiulli A., Herdman A., Stapells D., Hertzman C. Children's event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22:293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- Dehaene S. Oxford University Press; 1997. The Number Sense: How the Mind Creates Mathematics. [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Deutsch J., Deutsch D. Attention: some theoretical considerations. Psychology Review. 1963;70:80–90. doi: 10.1037/h0039515. [DOI] [PubMed] [Google Scholar]
- Diamond A., Barnett W., Thomas J., Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A. Listening to distraction: a developmental study of selective attention. Journal of Experimental Child Psychology. 1973;15:100–115. doi: 10.1016/0022-0965(73)90134-3. [DOI] [PubMed] [Google Scholar]
- Dye M., Green C., Bavelier D. Increasing speed of processing with action video games. Current Directions in Psychological Science. 2009;18:321–326. doi: 10.1111/j.1467-8721.2009.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye M., Green C., Bavelier D. The development of attention skills in action videogame players. Neuropsychologia. 2009;47:1780–1789. doi: 10.1016/j.neuropsychologia.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B., Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Fan J., McCandliss B., Fossella J., Flombaum J., Posner M.I. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J., McCandliss B., Sommer T., Raz A., Posner M.I. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Feng J., Spence I., Pratt J. Playing an action video game reduces gender differences in spatial cognition. Psychological Science. 2007;18:850–855. doi: 10.1111/j.1467-9280.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- Ferretti G., Mazzotti S., Brizzolara D. Visual scanning and reading ability in normal and dyslexic children. Behavioral Neurology. 2008;19:87–92. doi: 10.1155/2008/564561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs L., Fuchs D., Compton D., Powell S., Seethaler P., Capizzi A. The cognitive correlates of third-grade skill in arithmetic, algorithmic computation, and arithmetic word problems. Journal of Educational Psychology. 2006;98:29–43. [Google Scholar]
- Giedd J., Blumenthal J., Jeffries N., Castellanos F., Liu H., Zijdenbos A. Brain development during childhood and adolescence: a longitudinal MRI study. Nature. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giesbrecht G., Woldoff M., Song A., Mangun G. Neural mechanisms of top-down control during spatial and feature attention. NeuroImage. 2003;19:496–512. doi: 10.1016/s1053-8119(03)00162-9. [DOI] [PubMed] [Google Scholar]
- Gillam R. Computer assisted language intervention using Fast ForWord: theoretical and empirical considerations for clinical decision-making. Language, Speech, and Hearing Services in Schools. 1999;30:363–370. doi: 10.1044/0161-1461.3004.363. [DOI] [PubMed] [Google Scholar]
- Gillam R., Crofford J., Gale M., Hoffman L. Language change following computer-assisted language instruction with Fast ForWrod or Laureate Learning Systems software. American Journal of Speech-Language Pathology. 2001;10:231–247. [Google Scholar]
- Gillam R., Loeb D., Friel-Patti S. Looking back: a summary of five exploratory studies of Fast ForWord. American Journal of Speech-Language Pathology. 2001;10:269–273. [Google Scholar]
- Gogtay N., Giedd J., Lusk L., Hayashi K., Greenstein D., Vaituzis A. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Science. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes H., Molholm S., Christodoulou C., Ritter W., Cowan N. The development of auditory attention in children. Frontiers in Bioscience. 2000;5:108–120. doi: 10.2741/gomes. [DOI] [PubMed] [Google Scholar]
- Green C., Bavelier D. Action video game modifies visual attention. Nature. 2003;423:534–537. doi: 10.1038/nature01647. [DOI] [PubMed] [Google Scholar]
- Green C., Bavelier D. Effects of action video games on the spatial distribution of visuospatial attention. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:1465–1478. doi: 10.1037/0096-1523.32.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C., Bavelier D. Enumeration versus multiple object tracking: the case of action video game players. Cognition. 2006;101:217–245. doi: 10.1016/j.cognition.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C., Bavelier D. Action-video-game experience alters the spatial resolution of vision. Psychological Science. 2007;18:88–94. doi: 10.1111/j.1467-9280.2007.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin I., Miniussi C., Nobre A. Multiple mechanisms of selective attention: differential modulation of stimulus processing by attention to space or time. Neuropsychologia. 2002;40:2325–2340. doi: 10.1016/s0028-3932(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Hari R., Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends in Cognitive Sciences. 2001;5:525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- Hegarty M., Mayer R., Monk C. Comprehension of arithmetic word problems: a comparison of successful and unsuccessful problem solvers. Journal of Educational Psychology. 1995;87:18–32. [Google Scholar]
- Hillyard S.A., Hink R., Schwent V., Picton T. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hillyard S.A., Woldoff M., Mangun G., Hansen J. Mechanisms of early selective attention in auditory and visual modalities. The London Symposia, EEG supplement. 1987;39:317–324. [PubMed] [Google Scholar]
- Hopfinger J., Buonocore M., Mangun G. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hopfinger J., Luck S., Hillyard S. Selective attention: electrophysiological and neuromagnetic studies. In: Gazzaniga M., editor. The Cognitive Neurosciences III. MIT Pres; 2004. pp. 561–574. [Google Scholar]
- Huang-Pollack C., Carr T., Nigg J. Development of selective attention: perceptual load influences early versus late attentional selection in children and adults. Developmental Psychology. 2002;38:363–375. [PubMed] [Google Scholar]
- Hubert-Wallander B., Green C., Bavelier D. Stretching the limits of visual attention: the case of action video games. WIREs Cognitive Science. 2010;1:1–9. doi: 10.1002/wcs.116. [DOI] [PubMed] [Google Scholar]
- Hubert-Wallander, B., Green, C.S., Sugarman, M., Bavelier, D., 2011. Changes in search rate but not in the dynamics of exogenous attention in action video game players. Attention, Perception and Performance. [DOI] [PubMed]
- James W. Henry Holt and Co.; New York: 1890. Principles of Psychology. [Google Scholar]
- Jehee J., Brady D., Tong F. Attention improves encoding of task-relevant features in the human visual cortex. Journal of Neuroscience. 2011;31:8210–8219. doi: 10.1523/JNEUROSCI.6153-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A., Krompinger J., Baime M. Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Jha A., Stanley E., Kiyonaga A., Wong L., Gelfand L. Examing the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10:54–64. doi: 10.1037/a0018438. [DOI] [PubMed] [Google Scholar]
- Jonassen D. Designing research-based instruction for story-problems. Educational Psychology Review. 2003;15:267–296. [Google Scholar]
- Kail R., Hall L. Sources of developmental change in children's word-problem performance. Journal of Educational Psychology. 1999;91:660–668. [Google Scholar]
- Kastner S., Ungerleider L.G. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Thiel C., Specht K., Hanisch C., Fan J. Development of attentional networks: an fMRI study with children and adults. NeuroImage. 2005;28:429–439. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Kubose S. An experimental investigation of psychological aspects of meditation. Psychologia. 1976;19:1–10. [Google Scholar]
- Kuhl P. Early language acquisition: cracking the speech code. Nature Reviews Neuroscience. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Lange K., Rösler F., Röder B. Early processing stages are modulated when auditory stimuli are presented at an attended moment in time: an event-related potential study. Psychophysiology. 2003;40:806–817. doi: 10.1111/1469-8986.00081. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends in Cognitive Science. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- LeBlanc M., Weber-Russell S. Text integration and mathematical connections: a computer model of arithmetic word problem solving. Cognitive Science. 1996;20:357–407. [Google Scholar]
- Leonard L. Massachusetts Institute of Technology; Cambridge, MA: 1998. Children with Specific Language Impairment. [Google Scholar]
- Linden W. Practicing of meditation by school children and their levels of field dependence-independence, test anxiety, and reading achievement. Journal of Consulting and Clinical Psychology. 1973;41:139–143. doi: 10.1037/h0035638. [DOI] [PubMed] [Google Scholar]
- Liston C., Matalon S., Hare T., Davidson M., Casey B.J. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron. 2006;50:643–653. doi: 10.1016/j.neuron.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Luck S., Chelazzi L., Hillyard S., Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. Journal of Neurophysiology. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Lustig C., Shah P., Seidler R., Reuter-Lorenz P. Aging, training, and the brain: a review and future directions. Neuropsychology Review. 2009;19:504–522. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A., Slagter H., Dunne J., Davidson R. Attention regulation and monitoring in meditation. Trends in Cognitive Science. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Navalpakkam V., Beck J., Berg R., Pouget A. Behavior and neural basis of near-optimal visual search. Nature Neuroscience. 2011;14:783–790. doi: 10.1038/nn.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald A., III, Cohen J., Stenger V., Carter C. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Marsh R., Zhu H., Schultz R., Quackenbush G., Royal J., Skudlarksi P. A developmental fMRI study of self-regulatory control. Human Brain Mapping. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Di Russo F., Anllo-Vento L., Sereno M., Buxton R., Hillyard S. Putting spatial attention on the map: timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Research. 2001;41:1437–1457. doi: 10.1016/s0042-6989(00)00267-4. [DOI] [PubMed] [Google Scholar]
- Maurer U., Blau V., Yoncheva Y., McCandliss B. Development of visual expertise for reading: rapid emergence of visual familiarity for an artificial script. Developmental Neuropsychology. 2010;35:404–422. doi: 10.1080/87565641.2010.480916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U., Brem S., Bucher K., Brandeis D. Emerging neurophysiological specialization for letter strings. Journal of Cognitive Neuroscience. 2005;17:1532–1552. doi: 10.1162/089892905774597218. [DOI] [PubMed] [Google Scholar]
- Maurer U., Brem S., Kranz F., Bucher K., Benz R., Halder P. Coarse neural tuning for print peaks when children learn to read. NeuroImage. 2007;33:749–758. doi: 10.1016/j.neuroimage.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Maurer U., McCandliss B. The development of visual expertise for words: the contribution of electrophysiology. In: Grigorenko E., Naples A., editors. Single-Word Reading: Biological and Behavioral Perspectives. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. pp. 43–64. [Google Scholar]
- McCandliss B., Beck I., Sandak R., Perfetti C. Focusing attention on decoding for children with poor reading skills: design and preliminary tests of the Word Building intervention. Scientific Studies of Reading. 2003;7:75–104. [Google Scholar]
- McCandliss B., Cohen L., Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McCandliss B., Noble K. The development of reading impairment: a cognitive neuroscience model. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9:196–205. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- Miller J. The flanker compatibility effect as a function of visual angle, attentional focus, visual transients, and perceptual load: a search for boundary conditions. Attention, Perception, & Psychophysics. 1991;49:270–288. doi: 10.3758/bf03214311. [DOI] [PubMed] [Google Scholar]
- Mishra, J., Bavelier, D., Gazzaley, A. Probing the plasticity of attention and working memory, submitted for publication.
- Mishra J., Zinni M., Bavelier D., Hillyard S. Neural basis of superior performance of action videograme players in an attention-demanding task. Journal of Neuroscience. 2011;31:992–998. doi: 10.1523/JNEUROSCI.4834-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter B. Neural correlates of attentive selection for color or luminance in extrastriate area V4. Journal of Neuroscience. 1994;14:2178–2189. doi: 10.1523/JNEUROSCI.14-04-02178.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S., Wojciulik E. Attention increases neural selectivity in the human lateral occipital complex. Nature Neuroscience. 2004;7:70–74. doi: 10.1038/nn1161. [DOI] [PubMed] [Google Scholar]
- Navalpakkam V., Itti L. Search goal tunes visual features optimally. Neuron. 2007;53:605–617. doi: 10.1016/j.neuron.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Neville, H., Stevens, C., Fanning, J., Klein, S., Bell, T., & Pakulak, E. Training improves behavior, cognition, and brain functions supporting attention in lower SES children, under review.
- Noterdaeme M., Amorosa H., Mildenberger K., Sitter S., Minow F. Evaluation of attention problems in children with autism and children with specific language disorder. European Child & Adolescent Psychology. 2000;10:58–66. doi: 10.1007/s007870170048. [DOI] [PubMed] [Google Scholar]
- O’Connor D., Fukui M., Pinsk M., Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nature Neuroscience. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Passolunghi M., Cornoldi C., De Liberto S. Working memory and intrusions of irrelevant information in a group of specific poor problem solvers. Memory & Cognition. 1999;27:779–790. doi: 10.3758/bf03198531. [DOI] [PubMed] [Google Scholar]
- Passolunghi M., Siegel L. Short-term memory, working memory, and inhibitory control in children with difficulties in arithmetic problem solving. Journal of Experimental Child Psychology. 2001;80:44–57. doi: 10.1006/jecp.2000.2626. [DOI] [PubMed] [Google Scholar]
- Pearson D., Lane D. Auditory attention switching: a developmental study. Journal of Experimental Child Psychology. 1991;51:320–334. doi: 10.1016/0022-0965(91)90039-u. [DOI] [PubMed] [Google Scholar]
- Peelen M., Heslenfeld D., Theeuwes J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. NeuroImage. 2004;22:822–830. doi: 10.1016/j.neuroimage.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Mier H., Fiez J., Raichle M. The effects of practice on the functional anatomy of task performance. Proceedings of the National Academy of Science. 1998;95:853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza M., Dehaene S. From number neurons to mental arithmetic: the cognitive neuroscience of number sense. In: Gazzaniga M., editor. The Cognitive Neurosciences III. MIT Press; Cambridge: 2004. pp. 865–876. [Google Scholar]
- Plude D., Enns J., Brodeur D. The development of selective attention: a life-span overview. Acta Psychologica. 1994;86:227–272. doi: 10.1016/0001-6918(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Price C., Devlin J. The myth of the visual word form area. NeuroImage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Pugh K., Mencl W., Jenner A., Katz L., Frost S., Lee J. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Reynolds J., Desimone R. Interacting roles of attention and visual salience in V4. Neuron. 2003;37:853–863. doi: 10.1016/s0896-6273(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Reynolds J., Pasternak T., Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Richards J. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Developmental Science. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. Attention. In: Hopkins B., Barr R., Michel G., Rochat P., editors. The Cambridge Encylopedia of Child Development. Cambridge University Press; United Kingdom: 2004. pp. 282–286. [Google Scholar]
- Ridderinkhof K., van der Stelt O. Attention and selection in the growing child: views derived from developmental psychophysiology. Biological Psychology. 2000;54:55–106. doi: 10.1016/s0301-0511(00)00053-3. [DOI] [PubMed] [Google Scholar]
- Rossion B., Gauthier I., Goffaux V., Tarr M., Crommelinck M. Expertise training with novel objects leads to left-lateralized facelike electrophysiological responses. Psychological Science. 2002;13:250–257. doi: 10.1111/1467-9280.00446. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Checa P., Rothbart M. Contributions of attentional control to socioemotional and academic development. Early Education & Development. 2010;21:744–764. [Google Scholar]
- Rueda M.R., Fan J., McCandliss B., Halparin J., Gruber D., Lercari L. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Rothbart M., McCandliss B., Saccomanno L., Posner M.I. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the USA. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L., Neville H. An ERP study of continuous speech processing. I. Segmentation, semantics, and syntax in native speakers. Cognitive Brain Research. 2003;15:228–240. doi: 10.1016/s0926-6410(02)00195-7. [DOI] [PubMed] [Google Scholar]
- Sanders L., Newport E., Neville H. Segmenting nonsense: an event-related potential index of perceived onsets in continuous speech. Nature Neuroscience. 2002;5:700–703. doi: 10.1038/nn873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders L., Stevens C., Coch D., Neville H. Selective auditory attention in 3- to 5-year-old children: an event-related potential study. Neuropsychologia. 2006;44:2126–2138. doi: 10.1016/j.neuropsychologia.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Schoenfeld M., Hopf J., Martinez A., Mai H., Sattler C., Gasde A. Spatio-temporal analysis of feature-based attention. Cerebral Cortex. 2007;17:2468–2477. doi: 10.1093/cercor/bhl154. [DOI] [PubMed] [Google Scholar]
- Schroeter M., Zysset S., Wahl M., Von Cramon D. Prefrontal activation due to Stroop interference increases during development—an event-related fNIRS study. NeuroImage. 2004;23:1317–1325. doi: 10.1016/j.neuroimage.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Scolari M., Serrences J. Basing perceptual decisions on the most informative sensory neurons. Journal of Neurophysiology. 2010;104:2266–2273. doi: 10.1152/jn.00273.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz B., Shaywitz S., Blachman B., Pugh K., Fulbright R., Skudlarksi P. Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Sheese B., Rothbart M., Posner M.I., White L., Fraundorf H. Executive attention and self-regulation in infancy. Infant Behavior & Development. 2008;31:501–510. doi: 10.1016/j.infbeh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Shovman M., Ahissar M. Isolating the impact of visual perception on dyslexics’ reading ability. Vision Research. 2006;46:3514–3525. doi: 10.1016/j.visres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Simmons D., Kame'enui E., Harn B., Coyne M., Stoolmiller M., Edwards L. Attributes of effective and economic kindergarten reading intervention: an examination of instructional time and design specificity. Journal of Learning Disabilities. 2007;40:331–347. doi: 10.1177/00222194070400040401. [DOI] [PubMed] [Google Scholar]
- Simmons D., Kame'enui E., Stoolmiller M., Coyne M., Harn B. Accelerating growth and maintaining proficiency: a two-year intervention study of kindergarten and first-grade children at risk for reading difficulties. In: Foorman B., editor. Preventing and Remediating Reading Difficulties: Bringing Science to Scale. York Press; Timonium, MD: 2003. pp. 197–228. [Google Scholar]
- Simos P., Fletcher J., Bergman E., Breier J., Foorman B., Castillo E. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Slagter H., Lutz A., Greischar L., Francis A., Nieuwenhuis S., Davis J. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5:1228–1235. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E., Thompson P., Tessner K., Toga A. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E., Trauner D., Gamst A., Jernigan T. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spaulding T., Plante E., Vance R. Sustained selective attention skills of preschool children with Specific Language Impairment: evidence for separate attentional capacities. Journal of Speech, Language, & Hearing Research. 2008;51:16–34. doi: 10.1044/1092-4388(2008/002). [DOI] [PubMed] [Google Scholar]
- Spence I., Yu J., Feng J., Marshman J. Women match men when learning a spatial skill. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35:1097–1103. doi: 10.1037/a0015641. [DOI] [PubMed] [Google Scholar]
- Sperling A.J., Lu Z.-L., Manis F., Seidenberg M. Deficits in perceptual noise exclusion in developmental dyslexia. Nature Neuroscience. 2005;8:862–863. doi: 10.1038/nn1474. [DOI] [PubMed] [Google Scholar]
- Spinelli D., De Luca M., Judica A., Zaccolotti P. Crowding effects on word identification in developmental dyslexia. Cortex. 2002;38:179–200. doi: 10.1016/s0010-9452(08)70649-x. [DOI] [PubMed] [Google Scholar]
- Stevens C., Fanning J., Coch D., Sanders L., Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: electrophysiological evidence from language-impaired and typically developing children. Brain Research. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, C., Fanning, J., Klein, S., Neville, H., 2010. Development and comparison of two models of attention training. Poster Presented at the Annual Meeting of the Institute of Education Sciences. National Harbor, MD.
- Stevens C., Harn B., Chard D., Currin J., Parisi D., Neville H. Examining the role of attention and instruction in at-risk kindergarteners: electrophysiological measures of selective auditory attention before and after an early literacy intervention. Journal of Learning Disabilities. 2011 doi: 10.1177/0022219411417877. [DOI] [PubMed] [Google Scholar]
- Stevens C., Lauinger B., Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Developmental Science. 2009;12:634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Sanders L., Neville H. Neurophysiological evidence for selective auditory attention deficits in children with specific language impairment. Brain Research. 2006;1111:143–152. doi: 10.1016/j.brainres.2006.06.114. [DOI] [PubMed] [Google Scholar]
- Sundberg K., Mitchell J., Reynolds J. Spatial attention modulates center-surround interactions in macaque visual area V4. Neuron. 2009;61:1–12. doi: 10.1016/j.neuron.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg, U., Lacerda, F., 2003. Does training with manipulated stimuli improve auditory perception in non-typical language learning children? Reports in Phonetics Umeå University, PHONUM 9, Proceedings från FONETIK 2003. Lövånger June 2–4, 2003, Umeå University, Sweden. pp. 13–16.
- Sussman E., Steinschneider M. Attention effects on auditory scene analysis in children. Neuropsychologia. 2009;47:771–785. doi: 10.1016/j.neuropsychologia.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson H.L., Beebe-Frankenberger M. The relationship between working memory and mathematical problem solving in children at risk and not at risk for serious math difficulties. Journal of Educational Psychology. 2004;96:471–491. [Google Scholar]
- Swanson H.L., Jerman O. Math disabilities: a selective meta-analysis of the literature. Review of Educational Research. 2006;76:249–274. [Google Scholar]
- Tang Y., Ma Y., Wang J., Fan Y., Feng S., Lu Q. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Science. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: functional neural development during early childhood. The Neuroscientist. 2008;14:345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Valdois S., Boss M., Tainturier M. The cognitive deficits responsible for developmental dyslexia: review of evidence for a selective visual attentional disorder. Dyslexia. 2004;10:339–363. doi: 10.1002/dys.284. [DOI] [PubMed] [Google Scholar]
- Verghese P. Visual search and attention: a signal detection theory approach. Neuron. 2001;31:523–535. doi: 10.1016/s0896-6273(01)00392-0. [DOI] [PubMed] [Google Scholar]
- Vidyasagar T.R. Attentional gating in primary visual cortex: a physiological basis for dyslexia. Perception. 2005;34:903–911. doi: 10.1068/p5332. [DOI] [PubMed] [Google Scholar]
- Vidyasagar T.R., Pammer K. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends in Cognitive Science. 2009;14:57–63. doi: 10.1016/j.tics.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Vogel, A., Miezin, F.M., Petersen, S.E., Schlaggar, B., 2011. The putative visual word form area is functionally connected to the dorsal attention network. Cerebral Cortex. [DOI] [PMC free article] [PubMed]
- Vogel E., McCollough A., Machizawa M. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Walsh B., Buonocore M., Carter C., Mangun G. Integrating conflict detection and attentional control mechanisms. Journal of Cognitive Neuroscience. 2011;23:2211–2221. doi: 10.1162/jocn.2010.21595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzel K. Relations between social competence and academic achievement in early adolescence. Child Development. 1991;62:1066–1078. doi: 10.1111/j.1467-8624.1991.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Wentzel K., Caldwell K. Friendships, peer acceptance, and group membership: relations to academic achievement in middle school. Child Development. 2006;68:1198–1209. doi: 10.1111/j.1467-8624.1997.tb01994.x. [DOI] [PubMed] [Google Scholar]
- Wilson K., Swanson H.L. Are mathematics disabilities due to a domain-general or domain-specific working memory deficit? Journal of Learning Disabilities. 2001;34:237–248. doi: 10.1177/002221940103400304. [DOI] [PubMed] [Google Scholar]
- Wojciulik E., Kanwisher N., Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyurs: fMRI study. Journal of Neurophysiology. 1998;79:1574–1578. doi: 10.1152/jn.1998.79.3.1574. [DOI] [PubMed] [Google Scholar]
- Wright N., Mechelli A., Noppeney U., Veltman D., Rombouts S., Glensman J. Selective activation around the left occipito-temporal sulcus for words relative to pictures: individual variability or false positives? Human Brain Mapping. 2008;29:986–1000. doi: 10.1002/hbm.20443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Spelke E. Large number discrimination in 6-month-old infants. Cognition. 2000;74:B1–B11. doi: 10.1016/s0010-0277(99)00066-9. [DOI] [PubMed] [Google Scholar]
- Yantis S., Serences J.T. Cortical mechanisms of space-based and object-based attentional control. Current Opinion in Neurobiology. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Yoncheva Y., Blau V., Maurer U., McCandliss B. Attentional focus during learning impacts N170 ERP responses to an artificial script. Developmental Neuropsychology. 2010;35:423–445. doi: 10.1080/87565641.2010.480918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto T., Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. The Journal of Neuroscience. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto T., Rubens M., Thangavel A., Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature Neuroscience. 2011;5:656–663. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J., Pech-Georgel C., George F., Alario F., Lorenzi C. Deficits in speech perception predict language learning impairment. Proceedings of the National Academy of Science. 2005;102:14110–14115. doi: 10.1073/pnas.0504446102. [DOI] [PMC free article] [PubMed] [Google Scholar]